Abstract
Background
Despite evidence that CNS treatment is associated with cognitive and academic impairment, interventions to prevent or mitigate these problems are limited. The purpose was to determine if early intervention can prevent declines in mathematics abilities.
Procedures
Fifty-seven children with ALL were enrolled and randomized to a Mathematics Intervention or Standard Care. Subjects completed neurocognitive assessments prior to the intervention, post intervention, and one year later. Parents received written results and recommendations for use with their school. The Mathematics Intervention was based on Multiple Representation Theory and delivered individually over one year.
Results
Thirty-two of 57 subjects completed the study and were included in data analyses. These 32 subjects completed all neurocognitive assessments and, for those in the intervention group, 40–50 hours of the mathematics intervention. There were no group differences on relevant demographic variables; risk stratification; number of intrathecal methotrexate injections or high dose systemic methotrexate. Significant improvements in calculation and applied mathematics from baseline to post-intervention (p = 0.003 and 0.002, respectively) and in visual working memory from baseline to one year follow-up (p = 0.02) were observed in the Intervention but not the Standard Care group. Results from repeated measures ANOVA demonstrated significant between group differences for applied mathematics (F[2, 29] 12.47, p<0.001) and visual working memory (F[2 29]= 5.53, p=0.009).
Conclusions
The Mathematics Intervention improved mathematics abilities and visual working memory compared to standard care. Future studies are needed to translate the Mathematics Intervention into a “virtual” delivery method more readily available to parents and children.
Keywords: mathematics intervention, childhood leukemia, prevention, neurocognitive sequelae
Background
Leukemia is the most common form of childhood cancer, accounting for roughly 30% of all cancers occurring before 20 years of age.[1] Improvements in the treatment of acute lymphoblastic leukemia (ALL) have led to a remarkable increase in the survival rate for children younger than 15 years of age, which currently exceeds 90%.[2] Neurocognitive impairment are increasingly recognized as a relatively common consequence of childhood leukemia treatment. While the adverse effects of central nervous system (CNS) radiation are well documented, [3–7] intrathecal and systemic methotrexate are also associated with neuroanatomical changes, [8–10] and academic and cognitive problems.[11–16] Recent work also demonstrates systemic methotrexate therapy is associated with impairment in working memory and visual-spatial organization and reasoning.[17] Two meta-analytic reviews confirm the existence of significant neurocognitive sequelae in children treated for leukemia with and without radiation.[15,18]
Neurocognitive impairment is most often characterized by deficits in attention/concentration, visual/motor integration and visual-spatial awareness, information processing speed, and working memory. Studies of long-term survivors who received chemotherapy for CNS treatment report declines in performance on tests of intellectual function, [11,19,20] as well as problems with attention, [21,22]working memory, [3,17,23] visual spatial abilities, [16,24] and executive function.[15,25]Declines in mathematics continue to be the most frequently documented academic problem.[12,13,26] A number of investigators found statistically significant declines in mathematics achievement scores three or more years following diagnosis. Our research team reported that children with ALL receiving methotrexate for CNS treatment had an average decline of 0.63 standard deviation (SD) in mathematics abilities, 0.80 SD in visual spatial skills, and 1.07 SD in verbal fluency.[13,14,26]
Despite a growing body of literature documenting the adverse effects of CNS treatment on cognitive and academic abilities of children with ALL, there are very few published reports on interventions designed to address this clinically important problem.[27] To date, strategies to mitigate the impact of cancer therapy on brain function have included psychopharmacologic treatment and cognitive rehabilitation. Treatment of attention problems with methylphenidate has demonstrated limited efficacy in improving specific cognitive skills in survivors of pediatric cancer.[28–30] While methylphenidate may enhance behavior and learning strategies, such treatment has no demonstrated impact on academic outcomes such as mathematical problem solving. Cognitive rehabilitation strategies include direct intervention on specific areas of impairment and the integration of problem solving strategies.[31] Butler and Copeland developed a cognitive remediation program (CRP) based on the literature from brain injury rehabilitation, special education psychology and clinical pediatric psychology.[32] Essential components of the CRP include repetitive practices that exercise attentional process related to executive function, cognitive strategies related to task preparedness, and behavioral interventions that support a positive, realistic therapeutic environment that promotes skill acquisition. A multicenter, randomized clinical trial of the CRP enrolled 161 survivors of childhood cancer who were 6–17 years of age and experienced an attention deficit. Two-thirds of the group participated in the CRP. Participants had small improvement in academic achievement (0.20 SD) however there were no significant benefits to neurocognitive function that included attention, working memory, memory recall, and vigilance. Researchers have also reported performance gains in a group of 12 survivors who completed a 12 week cognitive and problem solving program; however low participation rates raised concerns regarding acceptance of this type of training efforts.[33] Hardy and colleagues developed a home-based computerized cognitive training program for leukemia and brain tumor survivors. They found good feasibility and acceptability associated with the program with significant increases in working memory and parent reports of decreased attention problems following the 12 week program.[34]
As both psychopharmacologic and cognitive rehabilitative treatment approaches have been initiated after the onset of late effects, the interventions may occur too late in the process of development of neurocognitive problems. Early stimulation of “at risk” cognitive abilities, prior to the onset of “impairment” may enhance neural networks and reduce the eventual decline during long-term survivorship. The goal for neurocognitive interventions for children treated for leukemia must promote developmental and adaptive progress and address skill or functional deficits.[35] Interventions should focus not only on remediating a single skill deficit but also problem solving strategies and establish a context that facilitates optimal adjustment.
We initially tested the feasibility of a Multiple Representation Theory[36,37] based Mathematics Intervention that focused on learning problem solving skills for survivors of childhood leukemia with documented academic problems.[14] Mathematics concepts were presented using a variety of symbolic, visual, and concrete representations in a systematic skill acquisition method. This method has been effectively used with children who have non-verbal learning disabilities and/or mathematics difficulties.[36] There were no group differences with respect to age or mathematics abilities at the time of ALL diagnosis. Children in the mathematics intervention group demonstrated significant gains in their performance on measures of mathematics achievement, while children in the Comparison Group did not show similar improvement. .[14] As a result of these encouraging preliminary findings, we prospectively evaluated the efficacy of the Mathematics Intervention in a randomized clinical trial.
The aim of the current study was to determine if the Mathematics Intervention was effective in preventing declines in mathematics abilities among children with newly diagnosed ALL. Children were randomized to either the Mathematics Intervention or to the Standard Care Group during the continuation phase of chemotherapy for childhood ALL. Neurocognitive assessments were conducted at baseline, at the end of the intervention, and one year following the completion of the intervention. We hypothesized that mean scores on mathematics achievement tests would be significantly higher in the Intervention Group than the Standard Care Group, and that this difference in mean scores would be maintained at the one-year follow-up interval.
Methods
Participants
Fifty-seven children with ALL (32 females and 25 males) were recruited through one of two childhood cancer centers in the Southwest U.S. and enrolled at the time of ALL diagnosis. All children were being treated on a standard protocol for childhood leukemia, which involved either a short high dose intravenous methotrexate (HD IV-MTX) infusion (i.e. 2 g/m2 over 4 hr) or a long HD IV-MTX infusion (i.e. 1 g/m2 over 24 hr) during the consolidation phase of treatment. All patients were also treated with IT MTX at age-titrated doses according to the same dose schedule. No child included in the study received cranial radiation therapy (CRT). All children were required to be at least five years of age at the time of the initiation of the intervention, given the content of therapeutic training exercises, and had entered the continuation phase of chemotherapy. Exclusion criteria included non-English-speaking children, and previous diagnoses of learning disability, Attention-Deficit Hyperactivity Disorder, mental retardation, psychiatric disorder, neurological disorder, or traumatic brain injury associated with an alteration in consciousness. Protocols were approved by the Institutional Review Boards of both institutions. Informed consent was obtained from all parents and assent for participation was obtained from the children.
Evaluation Measures
Subjects in the Mathematics Intervention and the Standard Care groups were evaluated according to the same neurocognitive assessment schedule. Baseline assessments were done 12 months after completion of induction therapy. The Post-Intervention assessment occurred one year later and corresponded to completion of the Intervention. The Follow-up assessment was done 1 year after the Post-Intervention assessment. This final assessment occurred 12 months after the end of the Intervention and approximately at the end of ALL therapy. The neurocognitive evaluation included assessment of general intellectual functioning using the four factor Full Scale IQ index from the Wechsler Abbreviated Scale of Intelligence.[38] Processing speed was assessed with the Processing Speed index from the Wechsler Intelligence Scale for Children – Third Edition.[39] Verbal and nonverbal working memory were assessed using the Sentence and Bead Memory subtests from the Stanford-Binet Fourth Edition.[40] Visual-motor integration skills were assessed using the visual-motor integration index from the Beery-Buktenica Developmental Test of Visual-Motor Integration.[41] Fine motor speed and dexterity was assessed using the Purdue Pegboard test.[42] Finally, the Woodcock-Johnson-R Tests of Academic Achievement was used to assess reading (Letter/Word identification subtest), spelling (Spelling subtest), mathematical calculations (Calculations subtest), and applied mathematics (Applied Problems subtest).[43] All of the measures standardized and norm referenced, and commonly employed in clinical assessments.
Intervention Procedure
Patients were randomized to either the Mathematics Intervention (n = 24) or to Standard Care (n = 33) Group using a computer-generated randomization table. 57 patients were originally recruited and enrolled in the trial, although 4 were subsequently determined to be ineligible due to a lack of proficiency with the English language. Of the 53 truly eligible patients, 32 (60.4%) completed the intervention and all three evaluation time-points (Baseline, Post-Intervention, Follow-up). Completion of the intervention was defined as receiving 40–50 hours of individualized mathematics intervention during a one-year period. Reasons for non-completion of the remaining 21 patients include elective withdrawal (n = 9), disease relapse (n = 6), family relocation (n=4), and study funding concluded prior to completion of chemotherapy and evaluation time-points (n = 2).
Both the Standard Care and the Mathematics Intervention Groups received the same neurocognitive evaluations, with results and relevant recommendations provided in a written report for them to use with their local school system. All evaluations and reports were completed under the direct supervision of a licensed clinical neuropsychologist.
The intervention was delivered individually approximately one to two hours per week. Children in the Math Intervention Group were scheduled for intervention sessions either directly before or after their medical clinic appointments. The sessions occurred near the clinic in a designated space that was converted into a mathematics learning center. The room was decorated with educational posters of mathematics and colorful bins filled with concrete manipulatives, such as base-10 number blocks, tiles, cubes, counting chips, number lines, etc., specifically designed for mathematics instruction.
The intervention sessions were designed to have the children explore, ask questions, discuss, solve problems, and explain their solutions using multi-modalities, such as pictures, abstract symbolism (numbers and operation symbols), contexts, mathematical language, and concrete manipulatives. Although the problem solving component of the curriculum was designed specifically for application in mathematical contexts, the goal was for transferability to general problem solving skills. Intervention subjects were placed in the curriculum according to baseline skills and abilities demonstrated on the diagnostic placement evaluation. The curriculum was designed with various entry points for students in mathematical content and process standards as recommended by the National Council of Teachers of Mathematics (NCTM)[44] which, in turn, are reflected in the 2010 Common Core State Standards for Mathematics.[45]
The multi-modality approach for teaching and learning mathematics during the intervention sessions was used because Multiple Representation Theory postulates that strengthening the ability to move between and among representations improves the growth of students’ understanding of mathematical concepts.[37] The curriculum was developed and teacher training was conducted by one of the co-authors (C.A.). Teachers implemented the intervention and were required to have prior experience in teaching elementary, middle or high school students and have background knowledge in multiple representations for teaching and learning mathematics.
Statistical Analyses
Independent samples T-tests or Chi-Square, where appropriate, were used to examine group differences at Baseline on demographic and treatment characteristics. Performances on all neurocognitive measures were transformed into age-adjusted standard scores, based on nationally normative data. Repeated measures analysis of variance (ANOVA) was employed to examine the change in performance within and between groups at Baseline, Post-Intervention, and Follow-up. As outlined above, only participants who completed the intervention were included in analyses, and evaluations for both groups were conducted at fixed intervals. This resulted in a balanced study design, making a repeated measures analytic approach appropriate. Paired samples t-tests were conducted to compare change at Post-Intervention and Follow-up time-points, with both referenced to Baseline performance.
Results
As seen in Table I, no group differences were evident in age at diagnosis, sex, mothers’ or father’s education, or grade placement of the subjects prior to intervention. Groups were equivalent on risk stratification, total number of intrathecal injections and number of patients randomized to high dose intravenous methotrexate therapy arms. None of the subjects received cranial radiation. The mean (SD) age at the time of ALL diagnosis was 6.7 (1.75) and 6.5 (2.71) years in the Intervention and Standard Care Groups, respectively, which is slightly higher than incidence data given the inclusion criteria and need to engage in mathematical concepts and activities during the intervention. There were no differences between those who completed (n = 32) and did not complete (n = 25) the study on age at diagnosis (p = 0.67), grade at time of study recruitment (p = 0.46), mother’s education (p = 0.78), father’s education (p = 0.35) risk stratification (p = 0.60), IV methotrexate treatment regimen (p = 0.14) or assignment to Intervention or Standard Care Group (p = 0.33).
Table I.
Group Demographic and Treatment Characteristics
| Intervention n=15 | Standard Care n=17 | p value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age @ Diagnosis | 6.7 | 1.75 | 6.5 | 2.71 | 0.765 |
| Sex | % | % | 0.314 | ||
| Female | 53.3 | 29.4 | |||
| Male | 46.7 | 70.6 | |||
| Grade @ study recruitment | 2 | 1.81 | 2.8 | 2.88 | 0.323 |
| Mother’s Education (yrs) | 14.1 | 1.90 | 14 | 2.39 | 0.922 |
| Father’s Education (yrs) | 13.6 | 1.75 | 13.6 | 3.02 | 0.972 |
| Risk Stratification | % | % | 0.77 | ||
| Low | 53.3 | 58.8 | |||
| Standard | 40 | 29.4 | |||
| High | 6.7 | 11.8 | |||
| Total # IT MTX Injections | 13.6 | 2.13 | 14.9 | 2.93 | 0.09 |
| HD IV MTX treatment arm | % | % | 0.916 | ||
| 1gm/m2 | 75.0 | 73.3 | |||
| 2gm/m2 | 25.0 | 26.7 | |||
Table II presents a comparison between groups on Baseline neurocognitive evaluations. Although assignment to groups was randomized, the Baseline performance on applied mathematics was higher for the Standard Care Group (M = 114.6, SD = 12.17) compared to the Intervention Group (M = 99.5, SD = 16.53), p=0.006, though performance in both Groups was in the average range. A similar pattern was observed on the measure of verbal working memory, which was higher for the Standard Care Group (M = 100.8, SD = 12.45) compared to the Intervention Group (M = 90.3, SD = 15.01), p=0.015, though again performance in both groups was in the average range. Of note, both groups scored slightly lower on measures of fine motor dexterity, consistent with our previous report of early onset problems in this skill.[46]
Table II.
Baseline Neurocognitive Functioning by Group
| Intervention n=15 | Standard Care n=17 | p value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Intellectual Functioning | |||||
| Verbal1 | 97.9 | 12.78 | 106.0 | 14.60 | 0.11 |
| Perceptual1 | 102.5 | 18.56 | 111.8 | 14.80 | 0.13 |
| Processing Speed2 | 106.2 | 16.21 | 114.7 | 15.84 | 0.21 |
| Academic Functioning | |||||
| Reading3 | 100.7 | 16.80 | 108.3 | 15.68 | 0.19 |
| Calculation3 | 97.8 | 23.82 | 101.6 | 14.21 | 0.64 |
| Applied Mathematics3 | 99.5 | 16.53 | 114.6 | 12.17 | 0.006* |
| Spelling3 | 93.0 | 15.01 | 102.1 | 12.63 | 0.08 |
| Working Memory | |||||
| Verbal4 | 90.3 | 12.75 | 100.8 | 12.45 | 0.015* |
| Visual4 | 92.8 | 19.17 | 98.9 | 12.69 | 0.69 |
| Visual and Motor | |||||
| Dexterity Dominant Hand5 | 87.4 | 14.18 | 95.7 | 20.95 | 0.21 |
| Visual-Motor Integration6 | 96.6 | 18.94 | 100.4 | 12.91 | 0.51 |
Values of statistical significance are given in bold.
Independent samples T-test;
Wechsler Abbreviated Scale of Intelligence[38];
Wechsler Intelligence Scale for Children - Third Edition[39];
Woodcock-Johnson III: Tests of Achievement[43];
Stanford-Binet Intelligence Scale: Fourth Edition[40];
Purdue Pegboard[42];
Beery-Buktenica Developmental Test of Visual-Motor Integration[41]
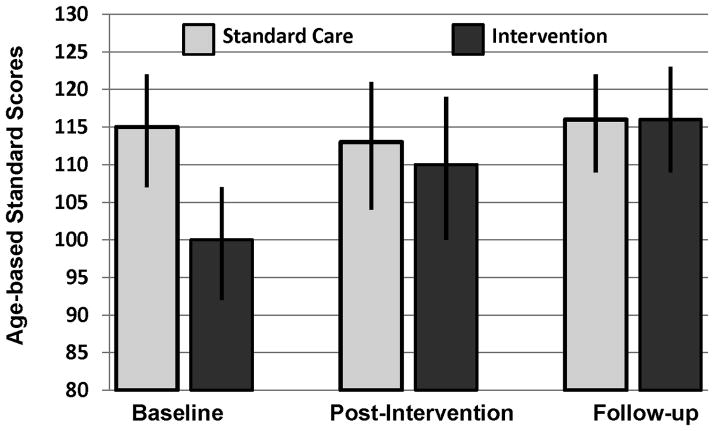
The results of the repeated measures ANOVA demonstrated signifcant interactions between assessment time point and group membership. A significant interaction was displayed for applied mathematics, F(2, 29) = 12.47, p<0.001, with a trend for improvement in calculation skills over time, F(2, 29) = 3.00, p=0.07. As displayed in Table III, the intervention procedure was effective at increasing applied mathematics at the post-intervention time (p=0.002) and the follow-up time (p=0.001). Significant increases from baseline to post-intervention were demonstrated in the Intervention Group on measures of calculations (p=0.003), though by follow-up performance returned toward baseline (p=0.32). No significant change was noted on the measures of mathematics in the Standard Care Group and no differences were noted in either group on measures of reading or spelling [F(2, 29) = 0.20, p=0.82 for reading; and F(2, 29) = 1.03, p=0.37 for spelling]. This pattern demonstrates specific efficacy in improving mathematical problem solving, with maintanance of the primary intervention strategy (applied mathematics) over time. Figure 1 presents mean group performance, with 95% confidence intervals, for applied mathematics. As seen in that figure, the Intervention Group demonstrates signfiicant improvement over time, to the point that these subjects no longer fall below those in the Standard Care Group at the post-intervention and followup time intervals.
Table III.
Neurocognitive Abilities over Time
| Intervention Group (n=15) | Standard Care Group (n=17) | Between Group*** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-Intervention* Change Score | Follow-up** Change Score | Post-Intervention* Change Score | Follow-up** Change Score | ||||||||||
| Mean | SD | p value | Mean | SD | p value | Mean | SD | p value | Mean | SD | p value | p value | |
| Intellectual Functioning | |||||||||||||
| Verbal1 | 2.8 | 11.58 | 0.37 | 3.7 | 7.98 | 0.09 | −3.5 | 10.45 | 0.19 | 0.1 | 15.18 | 0.98 | 0.30 |
| Perceptual1 | −1.2 | 8.60 | 0.60 | 2.9 | 13.41 | 0.41 | −2.5 | 7.93 | 0.21 | −6.5 | 16.37 | 0.12 | 0.24 |
| Processing Speed2 | −3.1 | 13.88 | 0.44 | −4.2 | 12.74 | 0.25 | −5.5 | 9.05 | 0.7 | −8.3 | 10.91 | 0.03a | 0.68 |
| Academic Functioning | |||||||||||||
| Reading3 | 2.1 | 11.05 | 0.47 | 1.6 | 9.37 | 0.60 | 0.4 | 16.69 | 0.92 | 0.5 | 15.46 | 0.91 | 0.20 |
| Calculation3 | 12.1 | 11.79 | 0.003a | 6.0 | 17.05 | 0.32 | −1.36 | 15.47 | 0.78 | −4.4 | 10.20 | 0.29 | 0.07 |
| Applied Mathematics3 | 10.0 | 9.89 | 0.002 a | 11.5 | 7.26 | 0.001 a | −1.9 | 6.02 | 0.20 | −1.1 | 14.45 | 0.81 | <0.001b |
| Spelling3 | 2.6 | 11.96 | 0.41 | −2.9 | 9.93 | 0.38 | −1.4 | 9.71 | 0.56 | −6.0 | 14.70 | 0.21 | 0.37 |
| Working Memory | |||||||||||||
| Verbal4 | 1.4 | 9.64 | 0.55 | 3.5 | 13.03 | 0.31 | −1.9 | 10.65 | 0.46 | −1.0 | 11.09 | 0.72 | 0.65 |
| Visual4 | 2.20 | 13.92 | 0.56 | 11.9 | 17.25 | 0.02 a | 1.4 | 14.79 | 0.70 | −5.0 | 14.33 | 0.17 | 0.009 b |
| Visual and Motor | |||||||||||||
| Motor Dexterity5 | 0.5 | 19.73 | 0.93 | 1.6 | 19.30 | 0.75 | 0.5 | 21.53 | 0.93 | −2.0 | 20.74 | 0.70 | 0.84 |
| Visual-Motor Integration6 | 1.2 | 15.48 | 0.77 | −6.2 | 16.54 | 0.17 | −0.1 | 9.41 | 0.96 | −8.7 | 14.13 | 0.02 a | 0.35 |
Post-Intervention* p-values reflect comparison between Baseline and Post-Intervention score. Follow-up** p-values reflect comparison between Baseline and Follow-up score. Between Group*** p-value associated with repeated measures analysis of variance for between group effect. Values of statistical significance are given in bold.
Paired sample T-test.
Repeated measures analysis of variance.
Wechsler Abbreviated Scale of Intelligence[38];
Wechsler Intelligence Scale for Children - Third Edition[39];
Woodcock-Johnson III: Tests of Achievement[43];
Stanford-Binet Intelligence Scale: Fourth Edition[40];
Purdue Pegboard[42];
Beery-Buktenica Developmental Test of Visual-Motor Integration[41]
Figure 1.
Applied Mathematical Problem Solving Age Adjusted Standard Scores (mean = 100, standard deviation =15) in the Intervention and Standard Care Groups. Results of repeated measures ANOVA demonstrate a significant group by time interaction (F [2, 29] = 12.47, p <0.001).
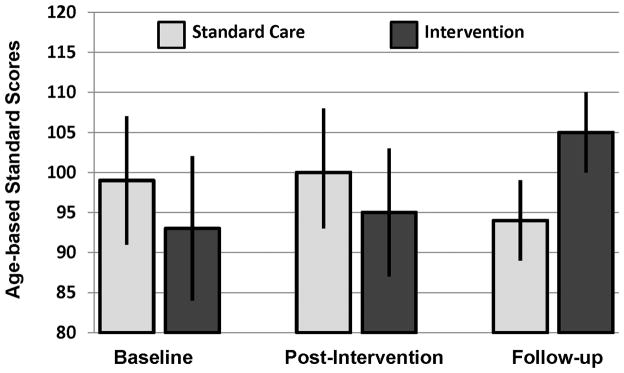
In addition to improving targeted academic skills, improvement was seen in visual working memory, F(2, 29) = 5.53, p=0.009. A significant improvement was observed in the Intervention Group at the follow-up evalaution (p=0.02). Over the course of the intervention, there was a gradual increase in visual working memory in the Intervention Group and a gradual decrease in the Standard Care Group (see Figure 2). No differences overall differences were observed for verbal intelligence, F(2, 29) = 1.26, p=0.30, perceptual intelligence, F(2, 29) = 1.52, p=0.24, visual motor integration, F(2, 29) = 0.35, p=0.71, verbal working memory, F(2, 29) = 0.65, p=0.53, or fine motor dexterity, interaction F(2, 29) = 0.17, p=0.84. Processing speed did decline at the Follow-Up assessment in the Standard Care Group (p=0.03), although the overall group effect was non-significant, F(2, 29) = 0.39, p=0.68.
Figure 2.
Visual Working Memory Age Adjusted Standard Scores (mean = 100, standard deviation =15) in the Intervention and Standard Care Groups. Results of repeated measures ANOVA demonstrate a significant change over time in Intervention Group (F [2, 29] = 5.53, p < =.009).
Discussion
Results support our hypothesis that a targeted Mathematics Intervention based on Multiple Representation Theory was effective in improving mathematics abilities compared to standard care. The intervention also improved visual working memory, a skill necessary for mathematical reasoning and continued development of intellectual abilities. To our knowledge this is one of the first studies that employed an early intervention approach that focused on prevention rather than remediation of mathematical problems. Intervention at this early phase resulted in an increase of roughly one standard deviation in applied mathematics, substantially higher than the 0.20 standard deviation demonstrated in the intensive CRP study described above.
The intervention appeared to have an immediate effect on improving calculation skills and a prolonged effect on enhancing applied mathematical problem solving. Applied mathematical problem solving continued to increase after the intervention ended. This dual impact is of interest, as the training was targeted to age appropriate skills. As the child increases in age, the expectations for applied mathematical problem solving advance as well. The ability of the children in the Intervention Group to demonstrate continued improvement in problem solving approaches two years after the start of the intervention suggests that they have developed strategies that carry forward into more advanced problem sets. Although regression to the mean could be initially considered as a potential factor in explaining differential changes on applied mathematics in group changes over time, this explanation is inadequate as the significant impact of the intervention moves the Intervention Group away from the mean (i.e. mean standard score of 99.5 at Baseline to 110.5 at Post-Intervention and 112.0 at Follow-up).
The improvement in working memory suggests that the early intervention approach may also have a more robust effect and generalize to other cognitive abilities. Given that mathematical problem solving requires working memory skills, training global problem solving may have inadvertently enhanced these more basic processes. This may suggest that a top-down approach (i.e. teaching problem solving to enhance more basic skills) to neurocognitive intervention may be as beneficial, if not more so, than a bottom-up approach (i.e. stimulating basic skills in the hope that they generalize to more advanced problem solving abilities). Additional studies are needed to evaluate the potential benefits of early cognitive stimulation in children receiving CNS therapy.
Mechanisms by which targeted interventions improve cognitive abilities are poorly understood. One possible explanation is enhancing plasticity and neural pathways important for complex cognitive abilities. Future studies that include neuro-imaging measures could advance knowledge about the biological benefits of behavioral interventions to improve cognitive and academic abilities.
Limitations of the current study should be acknowledged. The sample size is relatively small given the national prevalence of children diagnosed with ALL. This, in part, was impacted by the resources available to provide an intense intervention at two participating sites within a limited time frame. This smaller sample was associated with a second limitation involving a chance difference in groups at the Baseline assessment. Specifically, the Standard Care Group demonstrated significantly higher performance on a measure of applied mathematics, in the “high average range”, and verbal working memory, in the “average range”. Verbal working memory did not change in either group over the course of the study, while children in the Intervention Group demonstrated a significant improvement in applied mathematics. The “high average” performance on applied mathematics in the Standard Care Group may have limited the ability for that group to improve over time. Regardless, the joint improvement demonstrated by the Intervention Group in applied mathematics and visual working memory, which did not differ between groups at Baseline, would suggest a shared impact of the intervention and not a simple ceiling effect.
One of the challenges of this project was attrition due to issues such as competing demands of treatment regimens and other family obligations. Despite the positive outcomes of the intervention on mathematical abilities and working memory, it is important to translate the Mathematics Intervention into a delivery method that is more readily available to parents and children. Our goal is to develop a virtual intervention that can be delivered in home the environment with the availability of online assistance as needed.
Acknowledgments
This work was supported by the National Institute of Nursing Research grant NR 04905 (I. M. Moore). Support provided to K. R. Krull by the National Cancer Institute Cancer Center Support (CORE) grant (CA 21765) and by the American, Lebanese, Syrian Associated Charities (ALSAC).
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. Report. http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copeland DR, Fletcher JM, Pfefferbaum-Levine B, et al. Neuropsychological sequelae of childhood cancer in long-term survivors. Pediatrics. 1985;75(4):745–753. [PubMed] [Google Scholar]
- 4.Halberg FE, Kramer JH, Moore IM, et al. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 1992;22(1):13–16. doi: 10.1016/0360-3016(92)90976-o. [DOI] [PubMed] [Google Scholar]
- 5.Moore IM, Kramer JH, Wara W, et al. Cognitive function in children with leukemia. Effect of radiation dose and time since irradiation. Cancer. 1991;68(9):1913–1917. doi: 10.1002/1097-0142(19911101)68:9<1913::aid-cncr2820680912>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Mulhern RK, Wasserman AL, Fairclough D, et al. Memory function in disease-free survivors of childhood acute lymphocytic leukemia given CNS prophylaxis with or without 1,800 cGy cranial irradiation. J Clin Oncol. 1988;6(2):315–320. doi: 10.1200/JCO.1988.6.2.315. [DOI] [PubMed] [Google Scholar]
- 7.Paakko E, Harila-Saari A, Vanionpaa L, et al. White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: correlation with neuropsychological findings. Med Pediatr Oncol. 2000;35(5):456–461. doi: 10.1002/1096-911x(20001101)35:5<456::aid-mpo3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Davidson A, Payne G, Leach MO, et al. Proton magnetic resonance spectroscopy ((1)H-MRS) of the brain following high-dose methotrexate treatment for childhood cancer. Med Pediatr Oncol. 2000;35(1):28–34. doi: 10.1002/1096-911x(200007)35:1<28::aid-mpo5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Seidel H, Nygaard R, Haave I, et al. Magnetic resonance imaging and neurological evaluation after treatment with high-dose methotrexate for acute lymphocytic leukaemia in young children. Acta Paediatr. 1996;85(4):450–453. doi: 10.1111/j.1651-2227.1996.tb14059.x. [DOI] [PubMed] [Google Scholar]
- 10.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26(5):1263–1269. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RT, Madan-Swain A, Pais R, et al. Chemotherapy for acute lymphocytic leukemia: cognitive and academic sequelae. J Pediatr. 1992;121(6):885–889. doi: 10.1016/s0022-3476(05)80333-6. [DOI] [PubMed] [Google Scholar]
- 12.Brown RT, Sawyer MB, Antoniou G, et al. A 3-year follow-up of the intellectual and academic functioning of children receiving central nervous system prophylactic chemotherapy for leukemia. J Dev Behav Pediatr. 1996;17(6):392–398. doi: 10.1097/00004703-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kaemingk KL, Carey ME, Moore IM, et al. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychology. 2004;10(1):14–23. doi: 10.1076/chin.10.1.14.26240. [DOI] [PubMed] [Google Scholar]
- 14.Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16(4):279–290. doi: 10.1053/sonu.2000.16582. discussion 291–279. [DOI] [PubMed] [Google Scholar]
- 15.Peterson CC, Johnson CE, Ramirez LY, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008 doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 16.Kadan-Lottick NS, Brouwers P, Breiger D, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(35):5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey ME, Hockenberry MJ, Moore IM, et al. Brief report: effect of intravenous methotrexate dose and infusion rate on neuropsychological function one year after diagnosis of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32(2):189–193. doi: 10.1093/jpepsy/jsj114. [DOI] [PubMed] [Google Scholar]
- 18.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49(1):65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 19.Moore IM, Kramer J, Ablin A. Late effects of central nervous system prophylactic leukemia therapy on cognitive functioning. Oncol Nurs Forum. 1986;13(4):45–51. [PubMed] [Google Scholar]
- 20.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age-and sex-related differences. Eur J Cancer. 2003;39(3):359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 21.Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: a literature review and the development of a therapeutic approach. J Int Neuropsychol Soc. 2002;8(1):115–124. [PubMed] [Google Scholar]
- 22.Brouwers P, Riccardi R, Poplack D, et al. Attentional deficits in long-term survivors of childhood acute lymphoblastic leukemia (ALL) J Clin Neuropsychol. 1984;6(3):325–336. doi: 10.1080/01688638408401222. [DOI] [PubMed] [Google Scholar]
- 23.Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatr Blood Cancer. 2010;54(4):585–590. doi: 10.1002/pbc.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schatz J, Kramer JH, Ablin AR, et al. Visual attention in long-term survivors of leukemia receiving cranial radiation therapy. J Int Neuropsychol Soc. 2004;10(2):211–220. doi: 10.1017/S1355617704102075. [DOI] [PubMed] [Google Scholar]
- 25.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26(25):4138–4143. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 26.Espy KA, Moore IM, Kaufmann PM, et al. Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: a prospective study. J Pediatr Psychol. 2001;26(1):1–9. doi: 10.1093/jpepsy/26.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Askins MA, Moore BD., 3rd Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23(10):1160–1171. doi: 10.1177/0883073808321065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19(6):1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- 29.Mulhern RK, Khan RB, Kaplan S, et al. Short-term efficacy of methylphenidate: a randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. J Clin Oncol. 2004;22(23):4795–4803. doi: 10.1200/JCO.2004.04.128. [DOI] [PubMed] [Google Scholar]
- 30.Conklin HM, Helton S, Ashford J, et al. Predicting methylphenidate response in long-term survivors of childhood cancer: a randomized, double-blind, placebo-controlled, crossover trial. J Pediatr Psychol. 2010;35(2):144–155. doi: 10.1093/jpepsy/jsp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30(1):65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 32.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76(3):367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SK, Katz ER, Richardson R, et al. Cognitive and problem solving training in children with cancer: a pilot project. J Pediatr Hematol Oncol. 2009;31(9):670–677. doi: 10.1097/MPH.0b013e3181b25a1d. [DOI] [PubMed] [Google Scholar]
- 34.Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: a pilot study. J Pediatr Oncol Nurs. 2011;28(1):27–33. doi: 10.1177/1043454210377178. [DOI] [PubMed] [Google Scholar]
- 35.Rey-Casserly C, Meadows ME. Developmental perspectives on optimizing educational and vocational outcomes in child and adult survivors of cancer. Dev Disabil Res Rev. 2008;14(3):243–250. doi: 10.1002/ddrr.31. [DOI] [PubMed] [Google Scholar]
- 36.Cicerone KD, Dahlberg C, Kalmar K, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81(12):1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 37.Lesh R, Post T, Behr M. Representations and Translations among Representations in Mathematics Learning and Problem Solving. In: Janvier C, editor. Problems of Representations in the Teaching and Learning of Mathematics. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 33–40. [Google Scholar]
- 38.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 39.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 40.Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. 4. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- 41.Beery KE. The Beery-Buktenica Developmental Test of Visual-Motor Integration: administration, scoring, and teaching manual. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- 42.Tiffin J. Purdue Pegboard: examiner’s manual. Chicago: Science Research Associates; 1968. [Google Scholar]
- 43.Woodcock RW, McGrew KS, Mather N, et al. Woodcock-Johnson III: Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 44.National Council of Teachers of Mathematics. Principles and standards in school mathematics. Reston, VA: NCTM; 2000. [Google Scholar]
- 45.Council of Chief State School Officers. 2010 < http://www.corestandards.org/assets/CCSSI_Math%20Standards.pdf, retrieved August 7, 2011.
- 46.Hockenberry M, Krull K, Moore K, et al. Longitudinal evaluation of fine motor skills in children with leukemia. J Pediatr Hematol Oncol. 2007;29(8):535–539. doi: 10.1097/MPH.0b013e3180f61b92. [DOI] [PubMed] [Google Scholar]