Abstract
Background and purpose
Cerebral preconditioning provides insights into endogenous mechanisms that protect the brain from ischemic injury. Hypoxia and the anesthetic isoflurane are powerful preconditioning agents. Recent data show that sphingosine 1-phosphate (S1P) receptor stimulation improves outcome in rodent models of stroke. Endogenous S1P levels are controlled by the expression and activity of sphingosine kinases (SPK). We hypothesize that SPK up-regulation mediates preconditioning induced by isoflurane and hypoxia and reduces ischemic injury.
Methods
Male wild-type C57BL/J, SPK1−/− and SPK2−/− mice were exposed to isoflurane (IsoPC) or hypoxia preconditioning (HPC) before transient middle cerebral artery occlusion. Infarct volume and neurological outcome were measured 24 hours later. SPK inhibitors (SKI-II and ABC294640) were used to test the involvement of SPK2. Expressions of SPK1, SPK2 and HIF1α were determined. Primary cultures of mouse cortical neurons were exposed to isoflurane before glutamate- or hydrogen peroxide-induced cell death.
Results
IsoPC and HPC significantly reduced infarct volume and improved neurological outcome in wild-type and SPK1−/− mice, but not in SPK2−/− mice. Pretreatment with SKI-II or ABC294640 abolished the IsoPC-induced tolerance. Western blot showed a rapid and sustained increase in SPK2 level, whereas SPK1 level was similar between preconditioned mice and controls. HIF1α was up-regulated in wild-type IsoPC mice, but not in SPK2−/−. IsoPC protected primary neurons against cell death, which was abolished in ABC294640-treated cells.
Conclusions
Applying genetic and pharmacological approaches, we demonstrate that neuronal SPK2 isoform plays an important role in cerebral preconditioning.
Keywords: Sphingosine kinase 2, preconditioning, isoflurane, hypoxia, cerebral ischemia, neurons, cell death
Introduction
Cerebral preconditioning is a procedure by which a noxious stimulus is applied to a tissue or organ below the threshold of damage. After a recovery period, organs such as brain develop a tolerance to the same or even different noxious stimuli given above the threshold of damage1, 2. Studying cerebral preconditioning may provide insight into endogenous protective mechanisms that could be exploited therapeutically. Known preconditioning stimuli include isoflurane3–5, hypoxia6–8, cortical spreading depression9, 10, and pro-inflammatory agents (such as lipopolysaccharide)11, 12. Isoflurane, an inhalational anesthetic used widely and safely in surgical procedures, induces tolerance to ischemia in many organs, including brain5, 13, 14, heart15 and kidney16, 17.
Stroke is the leading cause of death and disability in developed countries. Despite the accumulating knowledge on the cellular and molecular mechanisms underlying ischemia/reperfusion injury, there is still a lack of effective treatment for stroke18. The sphingosine 1-phosphate (S1P) receptor agonist Fingolimod (FTY720) has been shown to be protective in several animal models of cerebral ischemia19–21. FTY720 is phosphorylated by sphingosine kinase (SPK) into the active compound phospho-FTY720, which then acts on four of the five known S1P receptor subtypes22. In the central nervous system, S1P regulates multiple cellular processes, including proliferation, survival and migration of neurons23. Intracellular S1P is tightly regulated by the expression and activity of SPK. Previous reports suggested that SPK plays a role in heart24, 25, kidney16, 17 and brain preconditioning8. We previously showed that SPK2 is the predominant isoform in brain26. The aim of this study was to test the hypothesis that SPK2 is a universal mediator of both isoflurane- and hypoxia-induced preconditioning. In order to test the hypothesis that neuronal SPK2 accounted for preconditioning, we used primary cultures of mouse cortical neurons to examine whether pre-treatment with specific SPK2 inhibitor could block IsoPC-induced protection against cell death in vitro.
Materials and methods
Animals
Male C57BL/J mice (23–25 g, Charles River) and age-matched wild-type, SPK1−/−27 and SPK2−/−28 mice were maintained on a 12/12 hours light/dark cycle and fed ad libitum. Experiments were conducted according to protocols approved by the Animal Research Committee of Massachusetts General Hospital and NIH guide for the Care and Use of Laboratory Animals. Mice were randomly allocated; after preconditioning or drug treatments, their identity and genotype were coded with tail marks in order to blind the investigators to the treatment groups; cerebral ischemia, infarct volumes measurement and neurological deficit evaluations were performed in a blinded fashion. Total number of mice included and mortality during surgery are summarized in supplementary tables 1 and 2.
Cerebral preconditioning
For isoflurane preconditioning (IsoPC), mice were exposed to 1% isoflurane (in 70% nitrogen and 30% oxygen) for 3 hours in an air-tight chamber. Mice were allowed to recover in an incubator (at 28°C) for ~30 minutes and then for 24 hours in their original cages5. For hypoxic preconditioning (HPC), mice were kept in an air-tight chamber flushed with 8% O2 for 4 hours. Mice were allowed to recover for 72 hours7, 8, 29. Naïve mice were placed in the air-tight chamber flushed with air for the same duration of time.
Treatment with SPK inhibitors
Fifteen min before IsoPC, mice were administered a specific SPK inhibitor (SKI-II, Chembridge30) or an isoform-selective SPK2 inhibitor (ABC294640, Apogee Biotechnology Corporation31) at 100 mg/kg via oral gavage (dissolved in 100 μl of polyethylene glycol 400). Dosage, solvent and route of administration were based on the published pharmacokinetics17, 30, 31.
Transient middle cerebral artery occlusion (MCAo) model
MCA was occluded for 90 min using a commercially available coated monofilament (Doccol Corporation) as reported previously8, 21, 26 (online supplement).
Expression studies
Mice were exposed to isoflurane (1% in 70% N2 + 30%O2, 3 hours) and sacrificed at the following time points: immediately after isoflurane exposure (t = 0), or 1, 6, 24, 48 hours after exposure. Mice were perfused transcardially with cold saline as described before26 (online supplement).
Primary culture of neurons and IsoPC
Primary cultures of neurons were exposed to IsoPC. The extent of glutamate-and hydrogen peroxide-induced cell death in control and preconditioned neurons was compared (online supplement).
Statistical analysis
Data are expressed as mean±SD. For infarct volumes and cell viability assay, statistical difference between groups was calculated by ANOVA. Neurological deficit score was compared using Mann-Whitney U test. Gene and protein expression levels were compared to control by one-way ANOVA. p<0.05 was considered statistically significant.
Results
IsoPC reduced infarct volumes and improved neurological outcomes
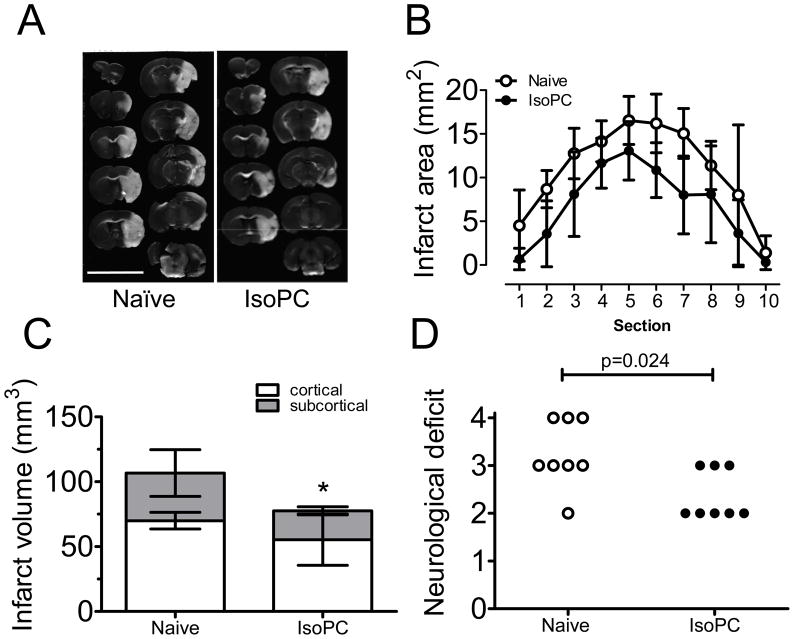
IsoPC significantly protected brain from transient MCAo, as shown in a representative TTC staining (Fig. 1A). Serial quantitative analysis of infarct volumes revealed that the induced tolerance was observed at all rostro-caudal levels (Fig. 1B), resulting in a smaller total infarct volumes in preconditioned mice (74.5±19.8 vs. 104.5±18.8 mm3, p<0.05, Fig. 1C). IsoPC also improved neurological score (p<0.05) in mice at 24 hours after transient MCAo (Fig. 1D). Median values of neurological deficit score of naïve and preconditioned mice were 3 and 2 respectively.
Figure 1.
Effect of isoflurane preconditioning on infarct volumes and neurological deficit scores in mice that underwent a 90-min middle cerebral artery occlusion (MCAo). A, Representative pictures of 2,3,5-triphenyltetrazolium chloride (TTC)-stained coronal brain slides (1 mm-thick each) from naïve and preconditioned (IsoPC) mice. B, Infarct areas in consecutive coronal slices. C, Cortical and subcortical infarct volumes in naïve and preconditioned mice were measured and compared. Data are mean±SD (n=8). P value for cortical, subcortical and total infarct volumes were 0.063, 0.041 and 0.026 respectively. D, Neurological deficit was evaluated and scored based on four categories: grade 0: no observable neurological deficit (normal); grade 1: failure to extend forepaw fully on lifting the whole body by the tail (mild); grade 2: circling to the contralateral side (moderate); grade 3: falling to one side (severe); grade 4: no spontaneous walking, depressed level of consciousness (very severe).
Expression of SPK1 and SPK2
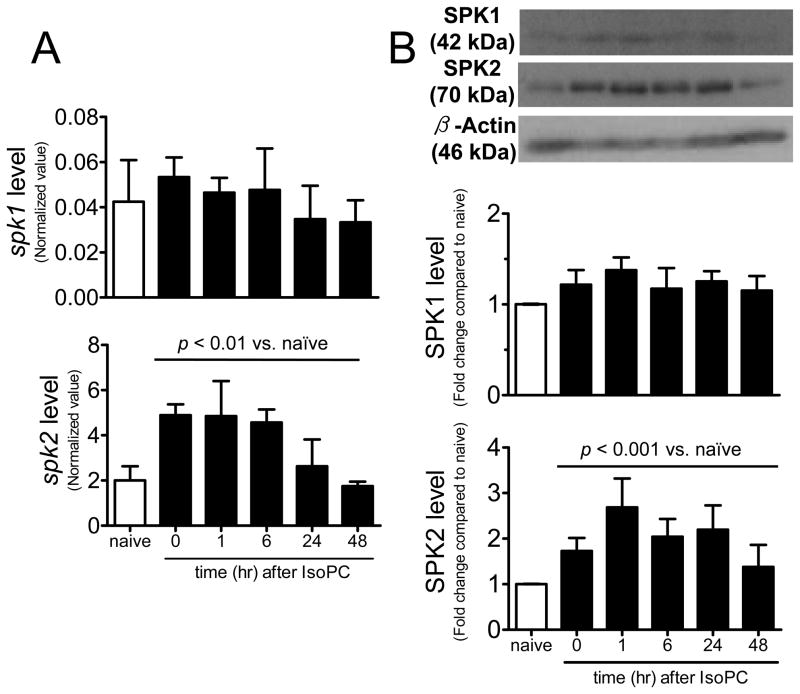
In isoflurane-preconditioned mice, cortical spk2 mRNA was up-regulated (peak level of approximately 2.4 fold increase at t=0 and 1 hour) in preconditioned mice (Fig. 2A). SPK2 protein was rapidly up-regulated (about 1.7 fold increase at t=0, i.e. immediately after the 3-hour isoflurane exposure) and the peak SPK2 level was found at 1 hour after isoflurane exposure (2.7 fold increase). The up-regulated SPK2 expression was still 2.2 times higher than control at 24 hours (the time at which MCAo was induced) (Fig. 2B). In contrast, cerebral SPK1 mRNA (p=0.467) and protein (p=0.053) expression remained unchanged at the different time points examined after IsoPC.
Figure 2.
Expression of sphingosine kinase isoforms in mouse cortex extracts after isoflurane exposure. A, spk1 and spk2 mRNA levels were normalized to 18S RNA (n=3). B, Protein levels were normalized to loading control (β-actin) and fold changes compared to control were calculated (n=4). Data are mean±SD. Expressions were compared to naïve control by one-way ANOVA and p values as indicated.
Pharmacological approaches
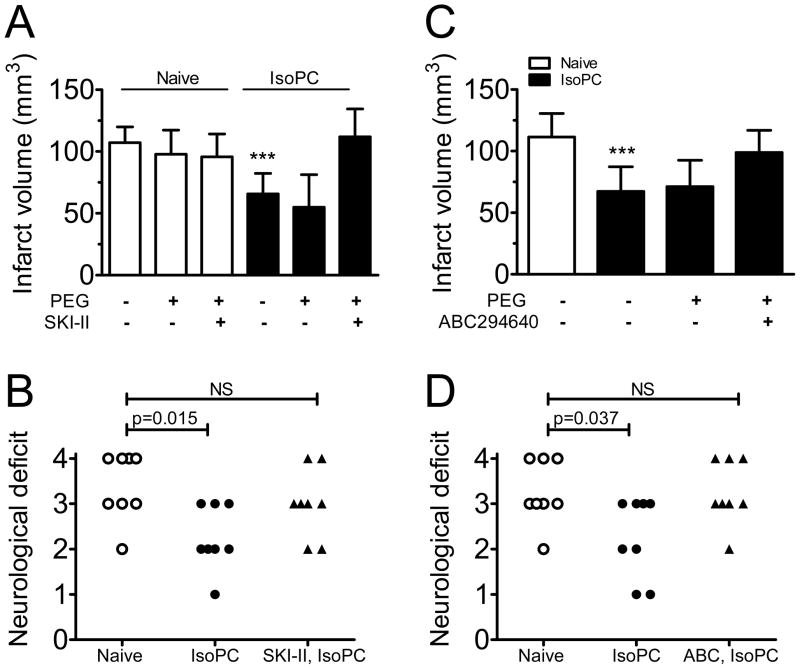
We first established that infarct volumes were unaffected in naïve mice treated with a specific SPK inhibitor (SKI-II at 100 mg/kg, oral gavage) or vehicle (PEG400) 24 hours before cerebral ischemia (Fig. 3A). SKI-II treatment (15 min before isoflurane exposure) abolished the protective effect of preconditioning, resulting in infarct volumes comparable to those seen in naïve mice (111.9±22.6 vs. 107.2±12.8 mm3 in naïve, Fig. 3A). SKI-II treatment also prevented IsoPC-induced improvement in neurological outcomes (Fig. 3B).
Figure 3.
Treatment with two SPK inhibitors abolished the protective effects of isoflurane preconditioning. A specific SPK inhibitor (SKI-II, A and B) and an SPK2 isoform-selective inhibitor (ABC294640, C and D) were used to verify the role of SPK2 in cerebral preconditioning. Mice were treated with either inhibitor at 100 mg/kg or vehicle (PEG400) by oral gavage at 15 min before preconditioning (IsoPC) and allowed to recover for 24 hours before a 90 min-MCAo. Neurological scores were evaluated at 24 hours after reperfusion (B and D). Data are mean±SD (n=8). *** indicates p<0.001 when compared to naïve mice. NS indicates not statistically significant.
ABC294640 is a novel isoform-selective inhibitor for SPK231. In a preliminary study, we investigated whether this compound was neuroprotective and found similar infarct volumes in mice treated with 100 mg/kg ABC294640 either 24 hours before MCAo, or 30 min after reperfusion (see supplementary figure 1).
Pretreatment with ABC294640 15 min before isoflurane exposure blocked the protective effect of IsoPC; infarct volumes were similar in IsoPC mice treated with ABC294640 and naïve mice (99.0±17.9 vs. 111.5±19.1 mm3, Fig 3C). ABC294640 also blocked the improvement in neurological score in preconditioned mice (Fig. 3D).
Genetic tools
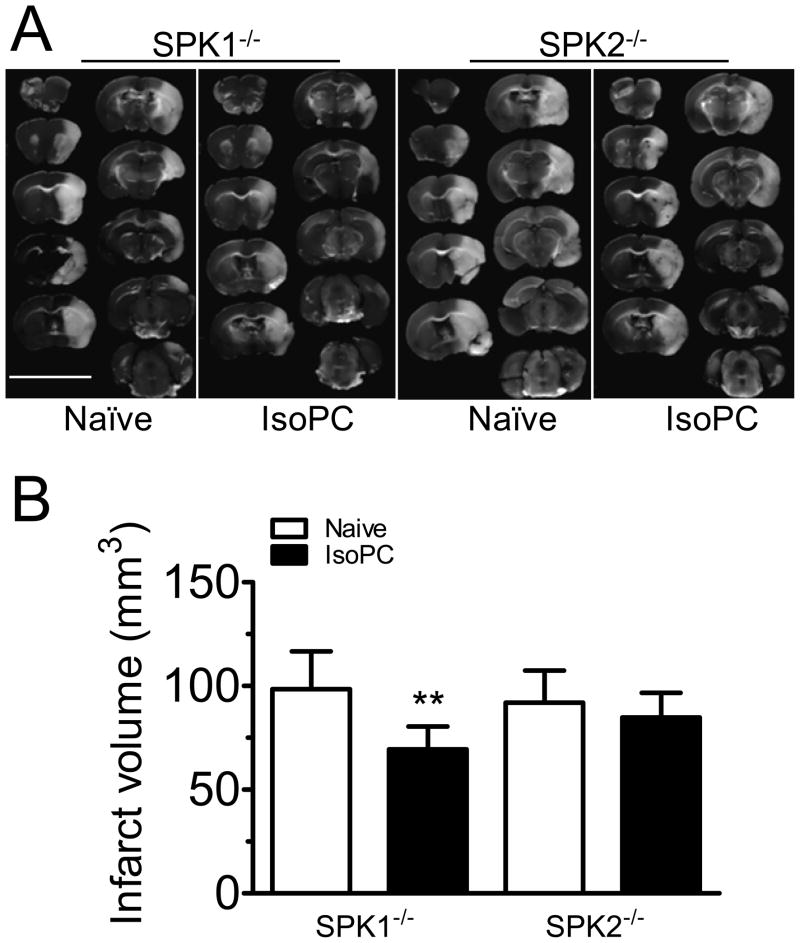
The circle of Willis did not show obvious differences in the three mouse strains investigated and naïve wild-type. The change in relative cerebral blood flow during MCAo and reperfusion were similar (supplementary table 3), and SPK1−/− and SPK2−/− mice had similar infarct volumes (104.5±15.3, 98.5±18.2 and 91.9±15.5 mm3 respectively, Supplementary figure 2). IsoPC reduced infarct volumes in SPK1−/− mice (69.4±10.9 vs. 98.5±18.2 mm3, p<0.005, Fig. 4B) by 30%, comparable to that observed in WT mice. In contrast, infarct volumes in naïve and preconditioned SPK2−/− mice did not differ (91.9±15.5 vs. 84.9±11.9 mm3, Fig. 4B).
Figure 4.
Effect of IsoPC on infarct volumes in SPK knockout mice. A, Representative pictures of TTC-stained brain sections. B, Summarized data for infarct volumes from naïve and preconditioned mice lacking either SPK isoforms. Data are mean±SD (n=7–8). ** indicates p<0.01 compared to naïve SPK1−/− mice.
In vitro IsoPC
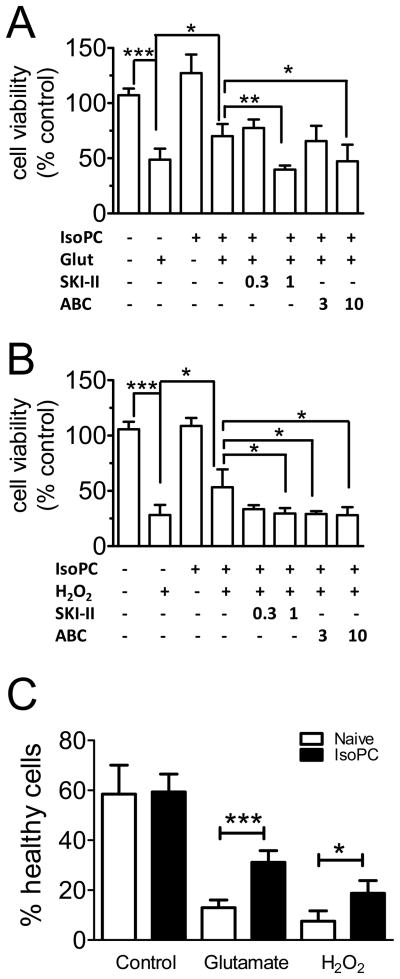
The extent of cell death was measured by MTT assay. Glutamate (Fig. 5A) and H2O2 (Fig. 5B) induced cell death in primary culture of mouse cortical neurons, which were significantly prevented by IsoPC. This protection was not observed when cells were pretreated (30 min before IsoPC) with 1 μmol/L SKI-II or 10 μmol/L ABC294640.
Figure 5.
SPK2 mediates isoflurane preconditioning in primary culture of mouse cortical neurons. Glutamate- (A) and hydrogen peroxide-induced (B) cell death in naïve and preconditioned (IsoPC) neurons were measured by MTT assay. Neurons were pre-treated with SPK inhibitors (0.3 and 1 μmol/L SKI-II or 3 and 10 μmol/L ABC294640) 30 min before IsoPC. C, percentage of cell death was summarized. Bars are mean±SD (n=4). Statistical significance among the groups was measured and calculated as shown. *p<0.05, **p<0.01, ***p<0.001 compared to corresponding naïve control. Glut, glutamate; H2O2, hydrogen peroxide; ABC, ABC294640
The degree of cell death was also quantified by Hoechst 33342 staining (Supplementary Fig. 3). Neurons with condensed nuclei (i.e. undergoing apoptosis) were counted (Fig. 5C), providing results similar to MTT measurements.
SPK2 – a general mediator for cerebral preconditioning
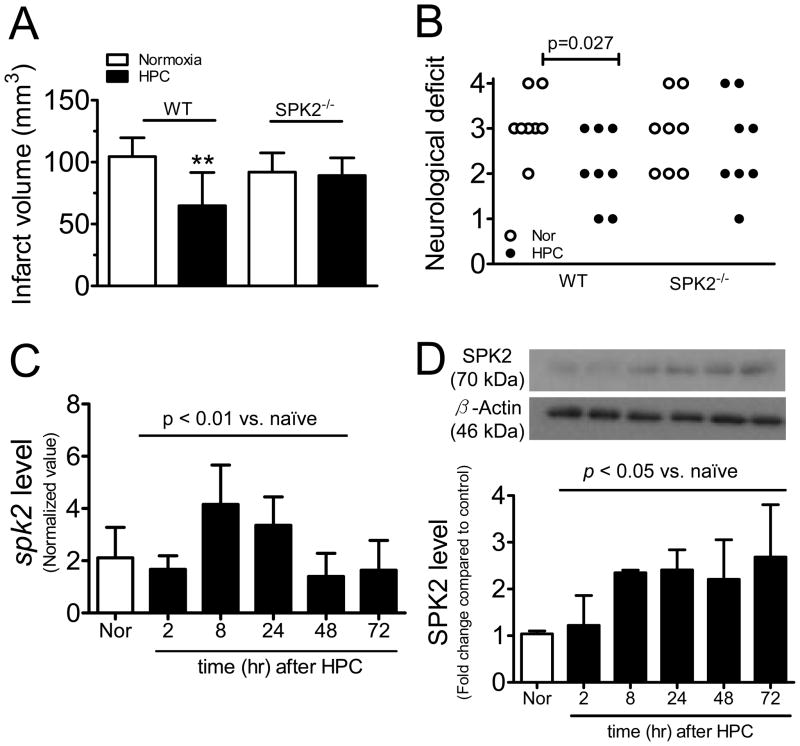
Hypoxic preconditioning significantly reduced infarct volumes in WT mice (64.8±26.7 vs. 104.5±15.3 mm3, p<0.01, Fig. 6A), whereas infarct volumes were similar in naïve and preconditioned SPK2−/− mice (91.9±15.5 vs. 89.1±14.4 mm3, Fig. 6A). Neurological outcome was significantly improved in hypoxia-preconditioned WT mice, but not in SPK2−/− mice (Fig. 6B). Cerebral spk2 mRNA level was up-regulated (Fig. 6C). Western blot revealed an elevated SPK2 protein expression starting at 2 hours, and maintained up to 72 hours after HPC (Fig 6D).
Figure 6.
SPK2 also mediates hypoxic preconditioning (HPC). A, Infarct volumes in naïve and preconditioned wild-type (WT) and SPK2−/− mice (n=8, **p<0.01). B, Neurological score was evaluated at 24 hours after MCAo. C, spk2 mRNA level were normalized to 18s (n=4). D, SPK2 expression was quantified (n=3). Data are mean±SD. mRNA and protein levels were compared to control by one-way ANOVA.
Discussion
Cerebral preconditioning elicited a global neuroprotective effect and reduced infarct volumes. We observed up-regulated cerebral SPK2, but not SPK1 protein expression in preconditioned mice, suggesting that the former isoform may play a role in preconditioning. Indeed, we also show that the reduced infarct volumes and improved neurological outcomes are absent in mice treated with a selective SPK2 inhibitor or in mice lacking SPK2. As SPK inhibition blocked IsoPC-induced tolerance in primary neurons, we conclude that up-regulation and activation of neuronal SPK2 is essential in cerebral preconditioning and protects the brain against ischemic injury and cell death.
The two SPK isoforms share high sequence homology (80% amino acid homology), yet differ in the central regions and N-termini32. SPK1 and SPK2 show different subcellular localizations and enzymatic properties, as well as different expression in various tissues32. We have previously observed that the SPK2 isoform predominates in different regions and cell types in the mouse brain26. But SPK1 is the more abundant isoform in renal proximal tubules and cardiomyocytes, and it was shown to be up-regulated and activated in preconditioning in the kidney and heart16, 17, 25. In contrast, an effect of SPK2 activation in preconditioning has been reported in one study of brain ischemia, which suggests a role of cerebral microvessel SPK2 in hypoxia preconditioning8. This study reported that SPK inhibition abolished the induced ischemic tolerance8. However the SPK inhibitor used in this study, dimethylsphingosine, is known to inhibit the SPK1 isoform31, 33, and possibly other enzymes, such as protein kinase C25. Following up on this study, we used knockout mice lacking either one of the SPK isoforms and a new selective SPK2 inhibitor, ABC294640, which dose-dependently inhibits SPK2 with an IC50 of approximately 60 μmol/L, without affecting the activity of SPK1 at concentrations up to at least 100 μmol/L31. Taken together, our study adds further support to the notion that SPK2 is a general mediator in cerebral preconditioning in vivo and in vitro.
The present data reveal a rapid and sustained up-regulation of SPK2 protein expression in cortical samples in preconditioned mice (~2.2-fold increase at 24 hours after IsoPC and ~2.5-fold increase at 72 hours after HPC). Taking into account that the published therapeutic windows for IsoPC and HPC are 24 hours5, 13, 14 and 48–72 hours7, 29 respectively, our data strongly suggest a functional role of SPK2 in mediating preconditioning. Interestingly, Wacker et al., 2009 showed an elevated SPK2 protein expression (1.7-fold increase at 2 hours after HPC) in microvessel-enriched brain extracts, suggesting that the endothelium of cerebral microvessels is the major cellular source for SPK during hypoxia preconditioning8. However, this SPK2 up-regulation was transient, as it declined back to baseline in 24 hours in hypoxia-preconditioned mice8. We previously observed an elevated spk2 mRNA expression in neurons treated with oxygen-glucose deprivation26. We now show that selective SPK2 inhibition (by ABC294640) suppresses the neuroprotective effect of IsoPC in these cells, suggesting an autocrine effect of neuronal SPK2 in response to preconditioning. Cerebral preconditioning requires gene and protein synthesis34. IsoPC up-regulates anti-apoptotic protein (Bcl-2)13 and vascular endothelium growth factor (VEGF)35 in brain, while hypoxia inducible factor-1alpha (HIF1α) has been shown to mediate HPC36. As a master regulator of transcription, HIF binds to hypoxia responsive elements of hypoxia-inducible genes32. Hypoxia up-regulates spk1 in cancer37 and endothelial cells38. However, less is known about the transcription regulation of spk2. In attempt to explore the role of HIF1α in cerebral preconditioning, we find an up-regulated HIF1α in cerebral cortex after IsoPC in wild-type mice, but not in SPK2−/− (Supplementary figures 4–5). This supports the previous observations that SPK is activated by hypoxia and SPK stabilizes HIF1α expression.39, 40 Taken together, our findings pinpoint the crucial role of neuronal SPK as a universal regulator that mediates preconditioning and protects the brain against ischemic injury.
Cerebral ischemia/reperfusion triggers acute cellular injury (for example neuronal cell death took place within hours) and late phase tissue damage (such as inflammatory responses progress and peak in days after ischemic insult)18. Although numerous reports support the notion that preconditioning could protect brain cells (including neurons, endothelial cells and astrocyte) from cell death, there is limited information regarding the role of SPK in mediating the acute neuroprotection by preconditioning. To this end, we evaluate the effects of cerebral preconditioning on stroke outcomes (infarct volumes and neurological deficit score) at 24 hrs after transient MCAo. It will be interesting to follow-up on the potential roles of SPK in mediating long-term neuroprotection (such as angiogenesis and neurogenesis) that may lead to improved motor function and recovery.
A review of preconditioning literature finds that a large number of pathways seem to mediate this phenomenon1, 2. This suggests that these pathways might act via common mediators to induce tolerance. Interestingly, SPK is known to be activated by a wide array of stimuli, including cell depolarization, G protein receptor agonists (muscarinic receptor agonists, formyl peptide, nucleotides, bradykinin, cannabinoids, lysophosphatidic acid and S1P), agonists at receptor tyrosine kinases (PDGF, EGF, NGF, VEGF), cross-linking of immunoglobulin receptors, TNF-α, TGF-β, interleukins, Ca2+ increasing agents and phorbol ester41. Furthermore, sphingolipids stimulate many signaling pathways (including HIF signaling39, 40, see above) and modulate most cellular functions42. It is therefore tempting to speculate that sphingolipid signaling plays a central role in the many pathways involved in preconditioning.
In summary, the present data demonstrates that SPK2 is a universal mediator in isoflurane- and hypoxia-induced preconditioning. Further investigation of the cross-talk between the SPK/S1P axis and HIF is likely to provide insights into the endogenous signaling that could protect the brain against ischemia/reperfusion injury.
Supplementary Material
Acknowledgments
We thank Drs. Michael Moskowitz and Cenk Ayata for discussion and comments, and Dr. Zhongcong Xie for assistance in isoflurane preconditioning in vitro.
Sources of funding
This work was supported by NIH grants (NS049263 and NS055104) to Christian Waeber and grant P30NS045776 (Interdepartmental Neuroscience Center).
Footnotes
Disclosures
C.S. is CEO and President of Apogee Biotechnology Corp.
References
- 1.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Kitano H, Hurn PD, Murphy SJ. Estradiol attenuates neuroprotective benefits of isoflurane preconditioning in ischemic mouse brain. J Cereb Blood Flow Metab. 2008;28:1824–1834. doi: 10.1038/jcbfm.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–1386. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: A redox sensitive mechanism? Neuroreport. 2002;13:1431–1435. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- 6.Bickler PE, Fahlman CS. Expression of signal transduction genes differs after hypoxic or isoflurane preconditioning of rat hippocampal slice cultures. Anesthesiology. 2009;111:258–266. doi: 10.1097/ALN.0b013e3181a8647f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 2001;12:1663–1669. doi: 10.1097/00001756-200106130-00030. [DOI] [PubMed] [Google Scholar]
- 8.Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: Role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otori T, Greenberg JH, Welsh FA. Cortical spreading depression causes a long-lasting decrease in cerebral blood flow and induces tolerance to permanent focal ischemia in rat brain. J Cereb Blood Flow Metab. 2003;23:43–50. doi: 10.1097/01.WCB.0000035180.38851.38. [DOI] [PubMed] [Google Scholar]
- 10.Douen AG, Akiyama K, Hogan MJ, Wang F, Dong L, Chow AK, Hakim A. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebal cortex plasma membranes. J Neurochem. 2000;75:812–818. doi: 10.1046/j.1471-4159.2000.0750812.x. [DOI] [PubMed] [Google Scholar]
- 11.Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: A critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orio M, Kunz A, Kawano T, Anrather J, Zhou P, Iadecola C. Lipopolysaccharide induces early tolerance to excitotoxicity via nitric oxide and cGMP. Stroke. 2007;38:2812–2817. doi: 10.1161/STROKEAHA.107.486837. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–113. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–425. doi: 10.1097/ALN.0b013e318164cab1. [DOI] [PubMed] [Google Scholar]
- 16.Song JH, Kim M, Park SW, Chen SW, Pitson SM, Lee HT. Isoflurane via TGF-β1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules. Am J Physiol Renal Physiol. 2010;298:F1041–50. doi: 10.1152/ajprenal.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, et al. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Annals of Neurology. 2011;69:119–29. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 23.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88:1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 24.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 26.Blondeau N, Lai Y, Tyndall S, Popolo M, Topalkara K, Pru JK, et al. Distribution of sphingosine kinase activity and mrna in rodent brain. J Neurochem. 2007;103:509–517. doi: 10.1111/j.1471-4159.2007.04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 28.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 30.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 31.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–39. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 34.Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Weihrauch D, Schwabe DA, Bienengraeber M, Warltier DC, Kersten JR, et al. Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1α and vascular endothelial growth factor expression in rats. Anesth Analg. 2006;103:281–288. doi: 10.1213/01.ane.0000226094.94877.98. table of contents. [DOI] [PubMed] [Google Scholar]
- 36.Li QF, Zhu YS, Jiang H. Isoflurane preconditioning activates HIF-1α, iNOS and ERK1/2 and protects against oxygen-glucose deprivation neuronal injury. Brain Res. 2008;1245:26–35. doi: 10.1016/j.brainres.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 37.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 38.Schwalm S, Doll F, Romer I, Bubnova S, Pfeilschifter J, Huwiler A. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun. 2008;368:1020–1025. doi: 10.1016/j.bbrc.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 39.Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: A new modulator of hypoxia inducible factor 1α during hypoxia in human cancer cells. Cancer Res. 2008;68:8635–8642. doi: 10.1158/0008-5472.CAN-08-0917. [DOI] [PubMed] [Google Scholar]
- 40.Ader I, Malavaud B, Cuvillier O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: New targets for cancer therapy. Cancer Res. 2009;69:3723–3726. doi: 10.1158/0008-5472.CAN-09-0389. [DOI] [PubMed] [Google Scholar]
- 41.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 42.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.