Abstract
The ability of mitochondria to take up Ca2+ was discovered 50 years ago. This calcium uptake, through a mitochondrial calcium uniporter (MCU), is important not only for the regulation of cellular ATP concentration but also for more complex pathways such as shaping Ca2+ signals and activation of programmed cell death. The molecular nature of the uniporter remained unknown for decades. By a comparative study of mitochondrial protein profiles of organisms lacking or possessing MCU, such as yeast in the former case and vertebrates and trypanosomes in the latter, two groups recently found the protein that possesses all the characteristics of the MCU. These results add another success story to the already substantial contributions of trypanosomes to mammalian biochemistry.
Mitochondrial discovery
Mitochondria have a central role in intracellular Ca2+ homeostasis, and it is well established that intramitochondrial Ca2+ concentration can reach micromolar values of tens to hundreds upon a few micromolar rise in cytosolic Ca2+ [1,2]. This is because mitochondria are exposed to microdomains of high Ca2+ concentration in proximity to sites of Ca2+ release at the endoplasmic reticulum, or to Ca2+ channels at the plasma membrane [1–6]. This Ca2+ uptake is important for shaping the amplitude and spacio-temporal patterns of cytosolic Ca2+ increases [7–9] and for regulating the activity of three mitochondrial dehydrogenases. Intramitochondrial Ca2+ stimulates a pyruvate dehydrogenase phosphatase that activates the pyruvate dehydrogenase or allosterically activates 2-oxoglutarate- and isocitrate-dehydrogenases, resulting in increased ATP production [10–15]. Activation by Ca2+ of metabolite carriers on the external face of the mitochondrial inner membrane also facilitates this stimulation of energy production [16,17]. Excessive Ca2+ uptake, however, favors the formation of the ‘permeability transition pore’, leading to the release of pro-apoptotic factors in the cytosol and cell death (reviewed in [18]).
Under physiological conditions, mitochondrial Ca2+ uptake occurs by a uniport mechanism driven electrophoretically by the negative-inside membrane potential without direct coupling to ATP hydrolysis or transport of other ions [19]. The activity of this mitochondrial calcium uniporter (MCU) was found 50 years ago [20,21], and the biophysical properties of this Ca2+-selective channel were extensively characterized [19,22]. However, the molecular nature of the channel was only recently identified due to progress in genome sequencing and the knowledge of the distribution of the uniporter in different eukaryotes [23,24]. Trypanosomes had a fundamental role in this discovery.
Discovery of the mitochondrial calcium uniporter (MCU) of trypanosomes
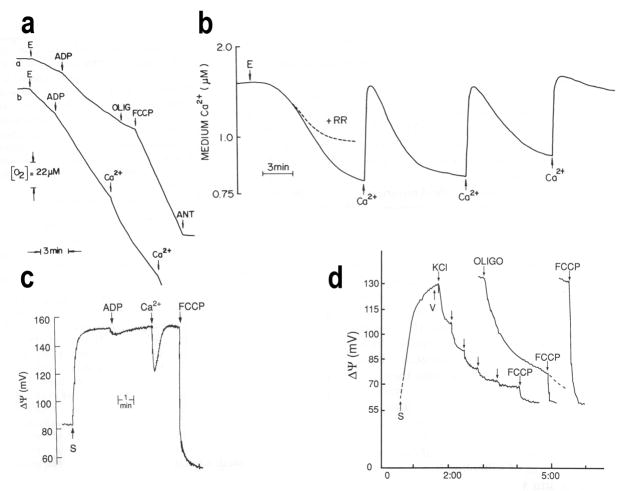
For many years after discovery of the MCU in mammalian mitochondria [20,21] it was thought that less complex life forms such as plants, insects and other invertebrates [25] or unicellular organisms, such as yeast [26], lacked a specific uptake pathway. This situation was rectified in 1989 [27,28] when it was reported that epimastigotes of Trypanosoma cruzi, the etiologic agent of Chagas disease, possesses a MCU with characteristics similar to those described in mammalian mitochondria: electrogenic transport, sensitivity to ruthenium red and low affinity for the cation. As occurs with mammalian mitochondria, addition of Ca2+ to digitonin-permeabilized T. cruzi epimastigotes in the presence of mitochondrial substrates, like succinate, and absence of ATP, stimulates respiration (Figure 1a), and this is accompanied by ruthenium red-sensitive Ca2+ uptake (Figure 1b) [28]. Successive Ca2+ addition reveals the high capacity of these mitochondria to accumulate Ca2+ (Figure 1b) [28]. Ca2+ uptake also results in a small decrease in membrane potential in agreement with its electrophoretic transfer into the mitochondria (Figure 1c) [29].
Figure 1.
Evidence for a mitochondrial calcium uniporter (MCU) in Trypanosoma cruzi. (a) Trace a shows that oxygen uptake by digitonin-permeabilized epimastigotes (E) in the presence of succinate increases after addition of ADP indicating oxidative phosphorylation. The rate of nonphosphorylating respiration was obtained by the addition of oligomycin (OLIG) and the maximal rate of respiration was induced by addition of the uncoupler carbonyl cyanide p-trifluoromethoxylhydrazone (FCCP). Antimycin A (ANT) completely abolished respiration. Trace b show that addition of CaCl2 (Ca2+) to these preparations stimulated respiration indicating its electrophoretic transport into the mitochondria. (b) Succesive Ca2+ additions to these mitochondria results in Ca2+ uptake until their capacity to take up Ca2+ is exhausted. This uptake is inhibited by ruthenium red (RR). (c) The mitochondrial membrane potential in digitonin-permeabilized epimastigotes in the presence of succinate can be measured with safranine (S). After safranine addition there is an increase in absorbance that indicates stacking of the dye to the energized mitochondrial membrane. A membrane potential value of 140–150 mV was calculated using the Nernst equation. Addition of CaCl2 to these preparations results in a decrease in membrane potential, compatible with the electrophoretic influx of Ca2+ into the mitochondria. (d) Determination of the mitochondrial membrane potential of BS trypanosomes in situ. The increase in absorbance after safranine (S) addition is reversed by the subsequent addition of oligomycin (OLIGO) or FCCP. Titration of ΔΨ was performed by the addition of known concentrations of KCl (arrows) in the presence of valinomycin (V). A membrane potential value of 130 mV was calculated. Reproduced with permission from references [28] (a,b), [29] (c) and [38] (d).
This MCU was later described in other trypanosomatids such as Leishmania brasiliensis [30], Leishmania mexicana, Leishmania agamae, Crithidia fasciculata [31], Leishmania donovani [32], in the infective stages of T. cruzi [33,34], and finally in Trypanosoma brucei [35–37]. The finding of a MCU uniporter in the bloodstream (BS) stage of T. brucei [38] was surprising because these stages lack a respiratory chain. However, Lehninger et al. had described in 1963 [39] that Ca2+ uptake into rat liver mitochondria under favorable conditions could be energized by ATP in the absence of respiration, in which case it was inhibited by oligomycin, and not by inhibitors of the respiratory chain. This is also what happens in BS trypanosomes: the mitochondrial membrane potential is dependent on hydrolysis of ATP by the ATP synthase which acts as an ATPase [38,40–42], allowing for Ca2+ to still be electrophoretically transported by the MCU [38]. Figure 1d shows that the membrane potential of BS trypanosomes is collapsed by oligomycin. Ca2+ uptake by BS trypanosomes has three characteristics: 1) It occurs until the ambient free Ca2+ concentration is lowered to 0.6–0.7 μM, 2) It is inhibited by oligomycin, and 3) It is associated with the depolarization of the inner membrane energized by ATP. These results indicate that Ca2+ uptake is mediated by the ATPase-dependent energization of the inner mitochondrial membrane [38].
Discovery of the MCU Protein
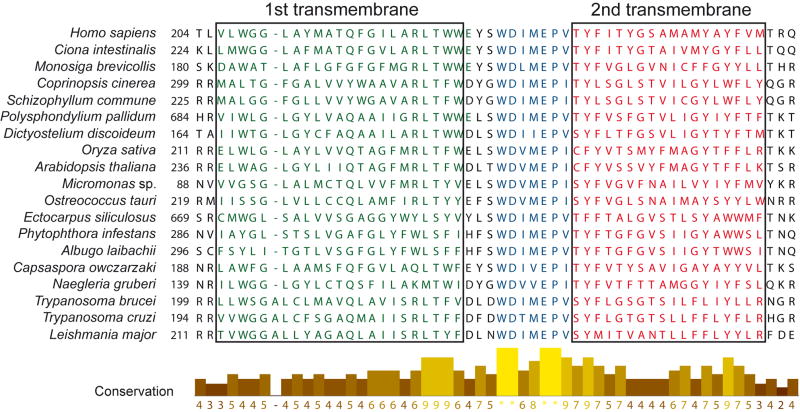
The evolutionary conservation of a MCU in vertebrates and kinetoplastids, and its absence in yeast, was utilized to identify proteins required for Ca2+ uptake [43]. From an inventory of 1 098 mouse mitochondrial proteins from 14 tissues, 1 013 of which mapped to human genes (MitoCarta, [44]), 18 fit the following criteria: (i) localization in the inner mitochondrial membrane, (ii) expression in the majority of mammalian tissues, and (iii) having homologues in vertebrates and kinetoplastids but not in the yeast Saccharomyces cerevisiae [43]. An RNAi screen of the top 13 candidates allowed identification of the mitochondrial calcium uptake 1 (MICU1) protein, an MCU regulator. Use of a similar exclusion method and examining proteins with at least two transmembrane domains that are not expressed in yeast but conserved in kinetoplastids, one protein (NP_001028431 in Mus musculus) was identified and named MCU [23]. Figure 2 shows that MCU has two highly conserved transmembrane domains present in several eukaryotes including trypanosomatids. Real time PCR demonstrated a universal tissue expression of the MCU protein and co-expression with MICU1 in mice [23]. Working with HeLa cells, silencing MCU by RNAi revealed a role of this protein in mitochondrial Ca2+ uptake independent of changes in the mitochondrial membrane potential. Overexpression of the gene increased the speed of Ca2+ uptake and mitochondrial Ca2+ concentration, and sensitized the cells to cell death following H2O2 or ceramide treatment due to Ca2+ overload. The recombinant protein was purified and showed channel activity in lipid bilayers, whereas mutagenesis of charged amino acids (glutamines) in the presumed pore-forming region of MCU abolished its channel activity. In parallel, another study performed complementary computational analyses to predict proteins functionally related to MICU1 and essential for mitochondrial Ca2+ uptake and spotlighted the same protein CCDC109A (NM_138357.1 in Homo sapiens) which was also named MCU [24]. RNAi experiments were also performed in HeLa and HEK-293 cells, as well as in mice liver to investigate the role of MCU in mitochondrial Ca2+ uptake. In contrast to the results of De Stefani et al. [23], overexpression of MCU by Baughman et al. [24] failed to stimulate Ca2+ uptake; their topology experiments suggested that the N- and C terminus of MCU face the matrix rather than the intermembrane space, and a large complex was needed to induce Ca2+ transport rather than MCU alone. These discrepancies will need to be worked out in the future.
Figure 2.
The mitochondrial calcium uniporter includes two highly conserved transmembrane domains. The alignment is of the putative transmembrane domain and pore region of MCU proteins from 19 eukaryotes including several trypanosomatids. The graph indicates the sequence conservation.
Roles of mitochondrial Ca2+ in trypanosomes
The roles of mitochondrial Ca2+ in trypanosomes are apparently more limited than in mammalian cells. None of the dehydrogenases stimulated by Ca2+ in vertebrates [45] has been studied in detail in trypanosomatids. There is no evidence that the pyruvate dehydrogenase E1 subunit, whose gene was identified in T. cruzi [46], is activated by dephosphorylation, as is the mammalian orthologous enzyme, although it seems to possess phosphorylation sites with similarity to those of the mammalian enzyme [46]. The mitochondrial isocitrate dehydrogenase present in trypanosomatids is nicotinamide adenine dinucleotide phosphate (NADP)-dependent [47], in contrast to the Ca2+-regulated mammalian nicotinamide adenine dinucleotide (NAD)-dependent isocitrate dehydrogenase. The flavin adenine dinucleotide (FAD)-glycerol phosphate dehydrogenase, which is activated by Ca2+ in vertebrates and invertebrates but apparently not in yeast and plants [45] is, as in these latter organisms, devoid of the Ca2+-binding EF-hands domains and presumably insensitive to Ca2+. In addition, BS T. brucei probably do not express these dehydrogenases, although they possess a MCU [38]. Although there are sequences with homology to the aspartate-glutamate carrier (AGC) and ATP-Mg-Pi carriers (SCaMCs), which in mammalian cells are known to be regulated by Ca2+ [17], the orthologs in trypanosomes lack EF-hand domains that are present even in the S. cerevisiae homologue [48], and are therefore presumably Ca2+ insensitive.
Experiments using aequorin targeted to the mitochondria of T. brucei revealed that intramitochondrial Ca2+ concentrations in T. brucei can reach values much higher than cytosolic Ca2+ rises when Ca2+ influx through the plasma membrane or Ca2+ release from acidic calcium stores (acidocalcisomes) are stimulated [37], just as in mammalian cells [1,2]. In fact, membrane potential-dependent Ca2+ uptake into the mitochondrion of T. brucei can be induced, as occurs in the human organelle, at both nano- and micromolar concentrations [49]. These results suggest a very close proximity of these organelles and the presence of microdomains of high Ca2+ concentration in the vicinity of the plasma membrane or acidocalcisomes [37]. Because the sarcoplasmic-endoplasmic reticulum Ca2+-ATPase [SERCA] of T. brucei has low sensitivity to thapsigargin, a microdomain of high Ca2+ concentration between the endoplasmic reticulum (ER) and the mitochondria could not be established in these studies [37]. However, these results suggest that one of the main functions of the MCU in trypanosomes would be, as in mammalian mitochondria [7,8,9], to shape the amplitude and spacio-temporal patterns of cytosolic Ca2+ increases. In mammalian cells, clustering of the outer mitochondrial membrane voltage-dependent anion channels (VDACs) at the ER/mitochondrial contact sites and in close contact with the inositol 1,4,5-trisphosphate receptor (IP3R) appear limiting for the Ca2+ uptake capacity of the organelle when Ca2+ is released from the ER [50]. Trypanosomes possess a single VDAC orthologue, porin, which is required for mitochondrial metabolite transport and is essential under growth conditions that depend on oxidative phosphorylation [51,52], yet the localization of their IP3R-like proteins is unknown [53].
Mitochondrial Ca2+ is a recognized contributor to programmed cell death (PCD), or apoptosis, in trypanosomatids. Morphological features that can be attributed to PCD, such as shrinking, membrane blebbing, mitochondrial alterations and chromatin condensation were described in T. cruzi as early as 1977 [54]. Trypanosomatids, however, lack some of the key regulatory or effector molecules involved in apoptosis in mammalian cells, such as the tumor necrosis factor (TNF)-related family of receptors, Bcl-2 family members and caspases [55,56]. Mitochondrial Ca2+ overload with changes in mitochondrial membrane potential, reactive oxygen species (ROS) generation and release of cytochrome c have been observed upon different triggers of cell death in trypanosomatids [57]. In T. brucei, the production of ROS impairs mitochondrial Ca2+ transport, leading to its accumulation in the nucleus, causing cell death [58]. In Leishmania, a mitochondrial endonuclease G is released and translocated to the nucleus [59] leading to stimulation of a caspase-independent, apoptosis-like cell death (reviewed in [57]). T. cruzi appears to be highly resistant to mitochondrial permeability transition [27], and apoptosis-like death upon mitochondrial Ca2+ overload is dependent on superoxide anion generation [60].
In summary, mitochondrial Ca2+ uptake in trypanosomatids appears to have a role in shaping the amplitude of cytosolic Ca2+ increases after influx through the plasma membrane or release from acidocalcisomes, and in apoptosis-like death, but apparently not in the regulation of ATP production.
How mitochondrial Ca2+ is released in trypanosomes
The mitochondrial Ca2+ efflux pathway in mammalian cells appears to promote the exchange of matrix Ca2+ by external Na+ (in excitable cells) or H+ (in non-excitable cells) [61]. A gene encoding the Na+/Ca2+ exchanger (NCLX) was recently identified [62] and the encoded protein was shown to possess all of the characteristics of the Na+/Ca2+ exchange activity described years ago [61]. The exchanger is located in the inner mitochondrial membrane and is inhibited by CGP-37157, which was originally discovered as an inhibitor of this activity in 1988 [63]; its overexpression enhances Na+/Ca2+ exchange activity, and its silencing reduces it. However, there are no orthologs to this gene in trypanosomatids. Evidence for a Ca2+ efflux pathway in T. cruzi has been presented [27], and in agreement with those results, trypanosomatids possess an ortholog to the Letm1 protein, which has recently been described as encoding a mitochondrial Ca2+/H+ exchanger [64]. Surprisingly, the mammalian exchanger is blocked by ruthenium 360, and partially inhibited by CGP-37157. This finding is puzzling because the insensitivity of mitochondrial Ca2+ exchangers to ruthenium red had been established before [61], and further work is necessary to confirm, or exclude, the direct role of Letm1 in mitochondrial Ca2+ handling [50].
Uniqueness of the trypanosome mitochondrion
Trypanosomes harbor peculiar mitochondria. As members of Excavata, recently viewed as the most basal eukaryotic supergroup [65], they retain some putatively very primitive features, in particular the unusual biogenesis of cytochrome c [66] and highly simplified protein-import machinery [67]. This machinery likely evolved immediately subsequent to endosymbiosis, qualifying kinetoplastids as strong candidates for one of the earliest extant eukaryotic lineages [68].
The existence of a single mitochondrion per cell in either active or repressed form (see below), along with the availability of high quality mitoproteome of procyclic form (PF) T. brucei [69], and in combination with our rather advanced knowledge of the kinetoplastid organelle qualify it as a very suitable model mitochondrion, already successfully explored in several ways.
The trypanosome mitochondrion as a model organelle
So far, we have presented an elegant use of trypanosomes in elucidating the molecular basis of mitochondrial Ca2+ influx. Similarly, dissection of the replication and maintenance of the kDNA network, the first extranuclear DNA ever observed, was very instrumental for studies of less abundant organellar DNAs in other eukaryotes, and provided one of the key insights into the topology of circular DNA molecules (for recent reviews see [70,71]). Another landmark, achieved by studying this organelle in T. brucei, Leishmania tarentolae and Crithidia fasciculata, was the discovery of RNA editing (for recent reviews see [72,73]). More recently, it was the conspicuous absence of several genes in the genomes of trypanosomatids and a few other eukaryotes that was instrumental for the identification, through phylogenetic profiling, of novel subunits of human NADH dehydrogenase (respiratory complex I) [44].
T. brucei is particularly suitable for studies of processes that control the activity of its single mitochondrion. While the organelle in the PC stage is metabolically and physiologically similar to the conventional eukaryotic mitochondrion, it transforms into a highly suppressed form in the BS stage [74]. Proteins involved in kDNA replication, mitochondrial RNA editing and processing, tRNA import and translation are present and essential throughout the life cycle [75–79], however, the morphology and metabolism of the organelle undergo extensive remodeling [74]. The ability to obtain fully functional PC mitochondria, as well as the down-regulated vesicles from the BS stage, makes them very attractive for studies of differential expression and/or import of mitochondrial proteins.
As mentioned above, another major difference between the PC and BS mitochondria is that FoF1-ATP synthase produces ATP in the former, but consumes it in the latter organelle, being essential in both [41]. The dramatic switch between the antagonistic activities of FoF1-ATP synthase during the trypanosome life cycle strikingly resembles the frequently lethal switch of orthologous synthase in the mitochondria of human heart during myocardial ischemia. This is not the only peculiar and unexpected similarity between the human and T. brucei mitochondria. Despite its uniquely simple protein import machinery [67,68], the T. brucei organelle readily accepts complex human mitochondrial import signals, making functional analyses of human proteins quite straightforward in this background [79,80]. Moreover, it is worth noting that mitoribosomes in humans and trypanosomes are the most protein-rich and rRNA-poor ribosomes known [69,81], thus it is possible that they are subject to similar, yet presently unknown, selective pressures.
Another interesting phenomenon observed in the African trypanosomes is that some lineages are prone, in nature or in the laboratory, to lose parts of their kDNA, with some mitochondria being totally devoid of kDNA [82,83]. Their host strains, T. brucei evansi, are in fact ‘petite ’ mutants [83], which spread out of Africa due to their acquired independence from the tse-tse fly as a vector [84]. These trypanosomes are particularly suitable for analyses of the interactions between the mitochondrion and cell nucleus, as organellar transcription and translation are absent without the requisite mitochondrial-encoded genes. It is rather counterintuitive that proteins responsible for kDNA replication and RNA metabolism continue to be imported [83,85], and the same was recently shown for import of nuclear-encoded tRNAs into the mitochondrion [76, 77]. It will be exciting to further examine the extent of this apparent lack of communication between the autonomous mitochondrion and the nucleus.
Concluding remarks
The inner mitochondrial membrane of trypanosomatids possesses a uniport carrier for calcium (MCU). This carrier allows the electrogenic entry of the cation driven by the electrochemical gradient generated by respiration in most trypanosomes, or by ATP hydrolysis in T. brucei BS forms (Figure 3). Calcium efflux, however, takes place by a different pathway, which appears to catalyze the electroneutral exchange of internal calcium by external protons, probably undertaken by an ortholog of Letm1. Biochemical evidence for Ca2+ uptake and for Ca2+-release channels is available for several trypanosomatids. The discovery of a functional MCU in trypanosomes, as well as knowledge of its wide distribution in other eukaryotes and absence in yeast, not only led to finding the molecular nature of this channel in mammalian mitochondria, but also demonstrates the valuable contribution of an organelle of a unicellular parasite in dissecting functions of mitochondrial proteins in general.
Figure 3.
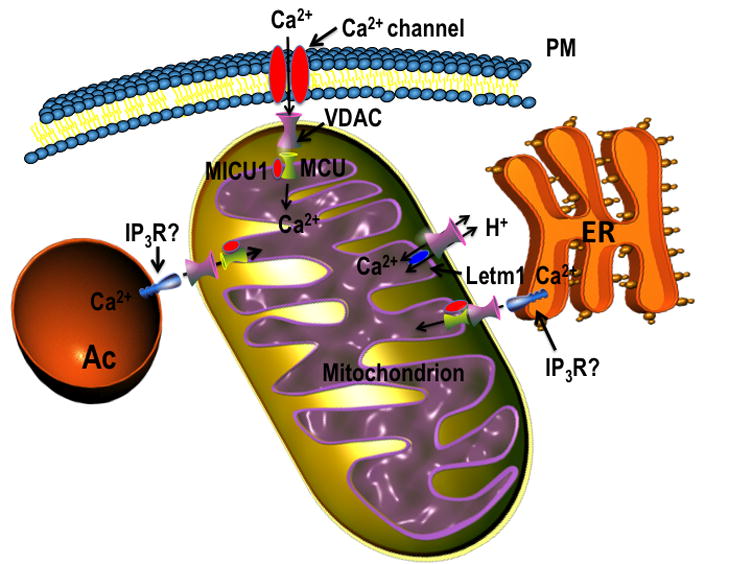
Mitochondrial Ca2+ transport in trypanosomes. The scheme depicts the molecules mediating Ca2+ influx and efflux (MICU1, MCU, Letm1) across the mitochondrial membrane at areas of the plasma membrane-, acidocalcisome (Ac)- or ER-mitochondrial association in trypanosomes. Abbreviations: MICU1, mitochondrial calcium uptake 1; MCU, mitochondrial calcium uniporter; PM, plasma membrane; ER, endoplasmic reticulum; VDAC, voltage-dependent anion-selective channel; IP3R, inositol 1,4,5-trisphosphate receptor (location unknown); Ca2+ channel (unidentified).
Acknowledgments
We thank Hassan Hashimi for comments on the manuscript, Ludék Korený for designing Figure 2, and SABioscience (QIAGEN) for a modified map version for Figure 3. R.D. is supported by the U.S. Public Health Service (NIH grants AI068647 and AI077538), and J.L. by the Grant Agency of the Czech Republic 204/09/1667, the Ministry of Education of the Czech Republic 6007665801 and the Praemium Academiae award.
Glossary
- Acidocalcisomes
acidic calcium stores rich in polyphosphate present in different organisms from bacteria to humans
- Aequorin
fluorescent protein from the jellyfish Aquora victoria used to detect calcium in vivo
- Antimycin A
potent inhibitor of the respiratory chain at the level of cytochrome b-c1
- Aspartate-glutamate carrier
transporter that exchanges aspartate for glutamate located at the mitochondrial outer membrane
- ATP-Mg-Pi carrier
transporter that exchanges ATP-Mg for Pi located at the mitochondrial outer membrane
- Bcl-2 (B cell lymphoma 2) family
is a family of apoptosis regulator proteins
- Caspases
proteases involved in cell death
- Excavata
a supergroup of unicellular eukaryotes that include many human parasites
- Isocitrate dehydrogenase
enzyme that catalyzes the conversion of isocitrate to succinate in the mitochondrial matrix
- Mitochondria
membrane-enclosed organelles found in most eukaryotic cells. Only one mitochondrion per cell is present in trypanosomes. As occurs in other eukaryotes its compartments include the outer membrane, the intermembrane space, the inner membrane, and the matrix
- Oligomycin
inhibitor of the mitochondrial ATP synthase
- Petite
yeasts and trypanosomes that have lost most or all of their mitochondrial DNA
- Pyruvate dehydrogenase
enzyme that catalyzes the conversion of pyruvate into acetyl-CoA
- Ruthenium red
potent inhibitor of the mitochondrial calcium uniporter
- Ruthenium 360
potent inhibitor of the mitochondrial calcium uniporter related to ruthenium red
- Thapsigargin
potent inhibitor of sarcoplasmic-endoplasmic reticulum (SERCA) calcium ATPase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 2.Montero M, et al. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 3.Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 4.Csordas G, et al. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csordas G, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomello M, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Hajnoczky G, et al. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 8.Boitier E, et al. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinel H, et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- 11.McCormack JG, et al. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 12.Jouaville LS, et al. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajnoczky G, et al. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 14.Voronina SG, et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology. 2010;138:1976–1987. doi: 10.1053/j.gastro.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasorsa FM, et al. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 17.Satrustegui J, et al. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, et al. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 19.Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biom. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- 20.De Luca HF, Engstrom GW. Ca2+ uptake by rat kidney mitochondria. Proc Natl Acad Sci USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasington FD, Murphy JV. Ca2+ uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 22.Kirichok Y, et al. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 23.De Stefani D, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormack JG, Denton RM. Ca2+ as a second messenger within mitochondria. Trends Biochem Sci. 1986;11:258–262. [Google Scholar]
- 26.Carafoli E, Lehninger AL. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J. 1971;122:681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docampo R, Vercesi AE. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch Biochem Biophys. 1989;272:122–129. doi: 10.1016/0003-9861(89)90202-6. [DOI] [PubMed] [Google Scholar]
- 28.Docampo R, Vercesi AE. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem. 1989;264:108–111. [PubMed] [Google Scholar]
- 29.Vercesi AE, et al. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem. 1991;266:14431–14434. [PubMed] [Google Scholar]
- 30.Benaim G, et al. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol. 1990;39:61–68. doi: 10.1016/0166-6851(90)90008-a. [DOI] [PubMed] [Google Scholar]
- 31.Vercesi AE, et al. Ca2+ transport in digitonin-permeabilized trypanosomatids. Mol Biochem Parasitol. 1990;42:119–124. doi: 10.1016/0166-6851(90)90119-7. [DOI] [PubMed] [Google Scholar]
- 32.Vercesi AE, Docampo R. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem J. 1992;284:463–467. doi: 10.1042/bj2840463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno SNJ, et al. Calcium homeostasis in Trypanosoma cruzi amastigotes: presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool. Mol Biochem Parasitol. 1992;52:251–261. doi: 10.1016/0166-6851(92)90057-q. [DOI] [PubMed] [Google Scholar]
- 34.Docampo R, et al. Effect of thapsigargin on calcium homeostasis in Trypanosoma cruzi trypomastigotes and epimastigotes. Mol Biochem Parasitol. 1993;59:305–313. doi: 10.1016/0166-6851(93)90228-p. [DOI] [PubMed] [Google Scholar]
- 35.Moreno SNJ, et al. Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release. J Biol Chem. 1992;267:6020–6026. [PubMed] [Google Scholar]
- 36.Vercesi AE, et al. Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. J Biol Chem. 1993;268:8564–8568. [PubMed] [Google Scholar]
- 37.Xiong ZH, et al. Selective transfer of calcium from an acidic compartment to the mitochondrion of Trypanosoma brucei. Measurements with targeted aequorins. J Biol Chem. 1997;272:31022–31028. doi: 10.1074/jbc.272.49.31022. [DOI] [PubMed] [Google Scholar]
- 38.Vercesi AE, et al. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol Biochem Parasitol. 1992;56:251–257. doi: 10.1016/0166-6851(92)90174-i. [DOI] [PubMed] [Google Scholar]
- 39.Lehninger AL, et al. Respiration-dependent accumulation of inorganic phosphate and Ca ions by rat liver mitochondria. Biochem Biophys Res Commun. 1963;10:444–448. doi: 10.1016/0006-291x(63)90377-2. [DOI] [PubMed] [Google Scholar]
- 40.Nolan DP, Voorheis HP. The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur J Biochem. 1992;209:207–216. doi: 10.1111/j.1432-1033.1992.tb17278.x. [DOI] [PubMed] [Google Scholar]
- 41.Schnaufer A, et al. The F1-ATP synthase complex in bloodstream stage trypanosomes has an unusual and essential function. EMBO J. 2005;24:4029–4040. doi: 10.1038/sj.emboj.7600862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown SV, et al. ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot Cell. 2006;5:45–53. doi: 10.1128/EC.5.1.45-53.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perocchi F, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagliarini DJ, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Buscaglia CA, et al. A putative pyruvate dehydrogenase alpha subunit gene from Trypanosoma cruzi. Biochim Biophys Acta. 1996;1309:53–57. doi: 10.1016/s0167-4781(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 47.Leroux AE, et al. Functional characterization of NADP-dependent isocitrate dehydrogenase isozymes from Trypanosoma cruzi. Mol Biochem Parasitol. 2011;177:61–64. doi: 10.1016/j.molbiopara.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Cavero S, et al. Identification and metabolic role of the mitochondrial aspartate-glutamate transporter in Saccharomyces cerevisiae. Mol Microbiol. 2003;50:1257–1269. doi: 10.1046/j.1365-2958.2003.03742.x. [DOI] [PubMed] [Google Scholar]
- 49.Xiong ZH, Ruben L. Trypanosoma brucei: the dynamics of calcium movement between the cytosol, nucleus, and mitochondrion of intact cells. Exp Parasitol. 1998;88:231–239. doi: 10.1006/expr.1998.4249. [DOI] [PubMed] [Google Scholar]
- 50.Mammucari C, et al. Molecules and roles of mitochondrial calcium signaling. BioFactors. 2011;37:219–227. doi: 10.1002/biof.160. [DOI] [PubMed] [Google Scholar]
- 51.Pusnik M, et al. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol. 2009;26:671–680. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- 52.Singha UK, et al. Downregulation of mitochondrial porin inhibits cell growth and alters respiratory phenotype in Trypanosoma brucei. Eukaryot Cell. 2009;8:1418–1428. doi: 10.1128/EC.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich PN, et al. Identification of contractile vacuole proteins in Trypanosoma cruzi. PloS On. 2011;e6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Docampo R, et al. Trypanosoma cruzi: ultrastructural and metabolic alterations of epimastigotes by beta-lapachone. Exp Parasitol. 1977;42:142–149. doi: 10.1016/0014-4894(77)90071-6. [DOI] [PubMed] [Google Scholar]
- 55.Smirlis D, et al. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasit Vectors. 2010;3:107. doi: 10.1186/1756-3305-3-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaczanowski S, et al. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit Vectors. 2011;4:44. doi: 10.1186/1756-3305-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smirlis D, Soteriadou K. Trypanosomatid apoptosis: ‘Apoptosis’ without the canonical regulators. Virulence. 2011;2:253–256. doi: 10.4161/viru.2.3.16278. [DOI] [PubMed] [Google Scholar]
- 58.Ridgley EL, et al. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem J. 1999;340:33–40. [PMC free article] [PubMed] [Google Scholar]
- 59.Gannavaram S, et al. Conservation of the pro-apoptotic nuclease activity of endonuclease G in unicellular trypanosomatid parasites. J Cell Sci. 2008;121:99–109. doi: 10.1242/jcs.014050. [DOI] [PubMed] [Google Scholar]
- 60.Irigoin F, et al. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem J. 2009;1418:595–604. doi: 10.1042/BJ20081981. [DOI] [PubMed] [Google Scholar]
- 61.Carafoli E. The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta. 2010;1797:595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Palty R, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiesi M, et al. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem Pharmacol. 1988;37:4399–4403. doi: 10.1016/0006-2952(88)90623-5. [DOI] [PubMed] [Google Scholar]
- 64.Jiang D, et al. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hampl V, et al. Phylogenomic analyses support the monophyly of Excavata and resolve relationships among eukaryotic “supergroups”. Proc Natl Acad Sci USA. 2009;106:3859–3864. doi: 10.1073/pnas.0807880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen JW, et al. Order within a mosaic distribution of mitochondrial c-type cytochrome biogenesis systems? FEBS J. 2008;275:2385–2402. doi: 10.1111/j.1742-4658.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 67.Lithgow T, Schneider A. Evolution of macromolecular import pathways in mitochondria, hydrogenosomes and mitosomes. Phil Trans R Soc Lond B, Biol Sci. 2010;365:799–817. doi: 10.1098/rstb.2009.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavalier-Smith T. Kingdoms protozoa and chromista and the eozoan root of the eukaryotic tree. Biol Lett. 2010;6:342–345. doi: 10.1098/rsbl.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panigrahi AK, et al. A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics. 2009;9:434–450. doi: 10.1002/pmic.200800477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shlomai J. The structure and replication of kinetoplast DNA. Curr Mol Med. 2004;4:623–647. doi: 10.2174/1566524043360096. [DOI] [PubMed] [Google Scholar]
- 71.Liu B, et al. Fellowship of the rings: the replication of kinetoplast DNA. Trends Parasitol. 2005;21:363–369. doi: 10.1016/j.pt.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Stuart KD, et al. Complex management: RNA editing in trypanosomes. Trends Biochem Sci. 2005;30:97–105. doi: 10.1016/j.tibs.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Lukeš J, et al. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr Genet. 2005;48:277–299. doi: 10.1007/s00294-005-0027-0. [DOI] [PubMed] [Google Scholar]
- 74.Hannaert V, et al. Evolution of energy metabolism and its compartmentation in Kinetoplastida. Kinetoplastid Biol Dis. 2003;2:11. doi: 10.1186/1475-9292-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimi H, et al. The assembly of F1F0-ATP synthase is disrupted upon interference of RNA editing in Trypanosoma brucei. Int J Parasitol. 2010;40:45–54. doi: 10.1016/j.ijpara.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Cristodero M, et al. Mitochondrial translation is essential in bloodstream form of Trypanosoma brucei. Mol Microbiol. 2010;78:757–769. doi: 10.1111/j.1365-2958.2010.07368.x. [DOI] [PubMed] [Google Scholar]
- 77.Paris Z, et al. Futile import of tRNAs and proteins into the mitochondrion of Trypanosoma brucei evansi. Mol Biochem Parasitol. 2011;176:116–120. doi: 10.1016/j.molbiopara.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niemann M, et al. Mitochondrial translation in trypanosomatids: a novel target for chemotherapy? Trends Parasitol. 2011;27:429–433. doi: 10.1016/j.pt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Long S, et al. Stage-specific requirement for Isa1 and Isa2 proteins in the mitochondrion of Trypanosoma brucei and heterologous rescue by human and Blastocystis orthologues. Mol Microbiol. 2011;81:1403–1418. doi: 10.1111/j.1365-2958.2011.07769.x. [DOI] [PubMed] [Google Scholar]
- 80.Long S, et al. Mitochondrial localization of human frataxin is necessary but processing is not for rescuing frataxin deficiency in Trypanosoma brucei. Proc Natl Acad Sci USA. 2008;105:13468–13473. doi: 10.1073/pnas.0806762105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zíková A, et al. Trypanosoma brucei mitochondrial ribosomes: affinity purification and component identification by mass spectrometry. Mol Cell Proteom. 2008;7:1286–1296. doi: 10.1074/mcp.M700490-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schnaufer A, et al. Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. Int J Parasitol. 2002;32:1071–1084. doi: 10.1016/s0020-7519(02)00020-6. [DOI] [PubMed] [Google Scholar]
- 83.Lai DH, et al. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc Natl Acad Sci USA. 2008;105:1999–2004. doi: 10.1073/pnas.0711799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lun ZR, et al. Trypanosoma brucei: two steps to spread out from Africa. Trends Parasitol. 2010;26:424–427. doi: 10.1016/j.pt.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Domingo GJ, et al. Dyskinetoplastic Trypanosoma brucei contains functional editing complexes. Eukaryot Cell. 2003;2:569–577. doi: 10.1128/EC.2.3.569-577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]