Abstract
Indian Hedgehog (Ihh) is a key component of the regulatory apparatus governing chondrocyte proliferation and differentiation in the growth plate. Recent studies have demonstrated that the primary cilium is the site of Ihh signaling within the cell, and that primary cilia are essential for bone and cartilage formation. Primary cilia are also postulated to act as mechanosensory organelles that transduce mechanical forces acting on the cell into biological signals.
In this study, we used a hydrostatic compression system to examine Ihh signal transduction under the influence of mechanical load. Our results demonstrate that hydrostatic compression increased both Ihh gene expression and Ihh-responsive Gli-luciferase activity. These increases were aborted by disrupting the primary cilia structure with chloral hydrate.
These results suggest that growth plate chondrocytes respond to hydrostatic loading by increasing Ihh signaling, and that the primary cilium is required for this mechano-biological signal transduction to occur.
Keywords: Growth plate chondrocyte, hydrostatic compression, primary cilium, Indian hedgehog
Introduction
Many studies of mechanical loading of cartilage and bone tissue have demonstrated clear effects at the level of gene expression, protein translation, extracellular matrix production, and in membrane transport processes [1–5]. These studies also suggest that there are multiple cellular pathways capable of responding to the physical stimulation resulting from mechanical forces.
It has been well-established that the hedgehog (Hh) family plays an important role not only during chondrogenesis and limb formation, but also during longitudinal growth at the growth plate [6–7]. Loss of Indian hedgehog in skeletal tissues results in severe dwarfism due to reduced proliferation and abnormal maturation of growth plate chondrocytes. When Ihh is over-expressed in mice, chondrocyte proliferation is enhanced [8–9]. Smoothened (Smo) is one of the two hedgehog receptor proteins involved in the Ihh signal transduction pathway. Binding of Ihh to its receptor Patched (Ptc) allows Smo to initiate the signaling cascade that leads to activation of the Cubitus interrupts (Ci) transcription factor family members Gli1, Gli2, and Gli3 [10–11].
Primary cilia have a unique hair-like structure, and act as a cellular sensory organelle. The intraflagellar transport (IFT) complex is required for ciliary function, which occurs through a complex of multi-subunit proteins resulting in transfer of precursors back and forth from the flagellar tip to the cell body. Recent studies have shown that a key step in Ihh activation occurs when Smo moves to the tip of the primary cilium [12–14], a translocation process that can be disrupted by the Smo antagonist cyclopamine [13–14]. Studies in mice have shown that mutations that cause IFT dysfunction result in the loss of the primary cilia, abnormal Ihh signaling, defects in limb growth and bone formation [15–19].
Primary cilia were originally linked to mechano-transduction in kidney epithelial cells, in which the cilia detect urine flow and transduce this fluid-flow signal into a transient intracellular calcium signal, resulting in increased cell proliferation. Other studies have shown that chloral hydrate treatment can abolish this cilium-dependent flow-induced Ca++ signaling event in different cell types [20–21]. Furthermore, studies of a core component of the IFT, the Tg737 gene, reveal that mice with a mutated Tg737 gene have shorter primary cilia, are unable to mount a fluid flow response, and develop unregulated cell proliferation and cyst formation (polycystic kidney disease) [22–23].
It has long been known that cartilage is sensitive to mechanical forces; however, no specific cellular mechano-transduction signaling pathway has been discovered in chondrocytes. In this study, we used our previously described [24] chondrocyte cell pellet model subjected to a hydrostatic compression loading system to determine if primary cilia transduce mechanical forces into biological signals in growth plate chondrocytes.
Materials and Methods
Epiphyseal chondrocyte culture
Primary epiphyseal chondrocytes were isolated from two day-old Sprague Dawley rats (Harlan, Indianpolis, IN) as previously described [24]. Animal use was according to a protocol approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. The primary cells were cultured either as high-density monolayers (6.6 × 105 cells/cm2) or as three-dimensional cell pellets (3 × 105 cells/pellet) in DMEM/F12 medium (Invitrogen, Carlsbad, CA) supplemented with 50 µg/ml L-ascorbic acid phosphate, 100 µg/ml sodium pyruvate, 1% penicillin-streptomycin, and ITS+ premix (Sigma Chemical, St. Louis, MO) to a final concentration of 0.625 µg/ml bovine insulin, 6.25 µg/ml transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml BSA, and 5.35 µg/ml linoleic acid. Primary chondrocyte pellets were cultured for five days before being subjected to hydrostatic compression, with or without treatment with recombinant sonic hedgehog (Shh 5 nM, R & D System, Minneapolis, MN) or chloral hydrate (4 mM, Sigma) as described below. The control samples were cultured unloaded and without any pharmacological treatment.
Hydrostatic compression
A custom-designed mechanical loading system (Figure 1) was used to apply an intermittent 1 MPa hydrostatic compression force (one hour on, one hour off). The hydrostatic loading system consists of two separate compartments, a sterile bioreactor chamber which houses the chondrocyte pellets (up to 9 pellets at a time) and a non-sterile hydraulic section of the system. The two sections were separated by a gas-permeable flexible fluoro-ethylene-propylene membrane (McMaster-Carr, Cleveland OH). Computer-controlled tandem high-speed micro-gear pumps (Micropump, IDEX Health & Science, Oak Harbor, WA) force water (maintained at 37 °C and equilibrated with 7.5% CO2 in air) through the hydraulic section thereby generating hydrostatic pressures inside the bioreactor. Heat- and gas-exchange occurs through the membrane. The pressure in the chamber is monitored by a compensated transducer, and controlled in real time by modulating pump speed and a water flow outlet restrictor. Automatic control was achieved by using custom-written C software running on an IBM PC. Half the culture medium was replaced every 12 hours during the unloaded interval. At the end of the compression period, the pellets (loaded and unloaded control) were collected and terminal assays performed.
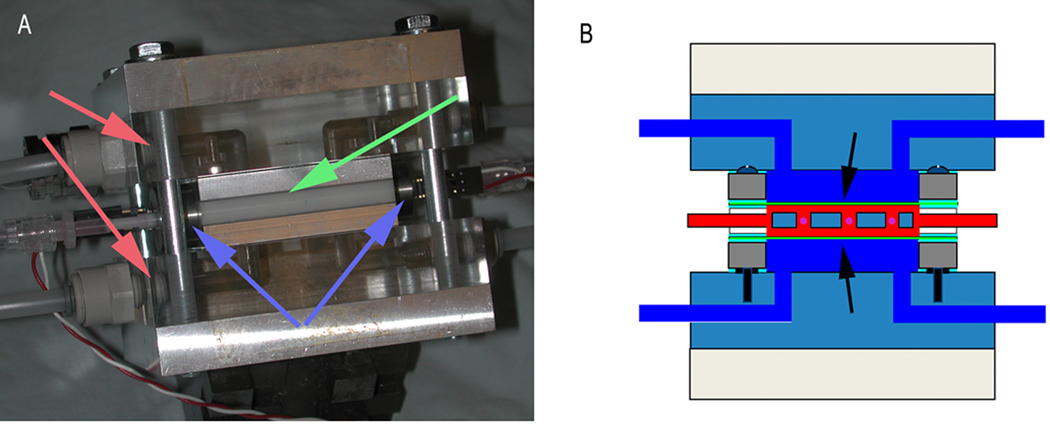
Figure 1. Custom-designed hydrostatic loading system.
The module consists of a PMMA/aluminum (A, red arrows) frame surrounding the bioreactor chamber (A, green arrow) with the intervening space filled with water (B, dark blue). When pressuring the bioreactor, the media ports are closed using valves (A, blue arrows), and pressure is applied to the sample across the flexible FEP membrane (B, arrows). A perforated insert holds up to nine aggregates. Automatic control was achieved by using custom-written C software running on an IBM-PC. This module allows the application of arbitrary hydrostatic pressure waveforms on the construct in the bioreactor.
Cell proliferation
The cell proliferation assay was carried out immediately after the end of the compression period. Cell viability was evaluated using the CellTiter 96 Aqueous One assay kit following the manufacturer’s protocol (Promega, Madison, WI). The reagent contains both a tetrazolium compound (MTS) and phenazine ethosulfate (PES), an electron coupling reagent. In metabolically active cells, reduced MTS is a soluble and colored formazan product, which is quantified by measuring the absorbance at 490 nm, and is directly proportional to the number of living cells.
Gene expression
To investigate the effect of hydrostatic loading on chondrocyte gene expression, total RNA was isolated from the cells at designed time points using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA was reverse transcribed using a SuperScript III Kit (Invitrogen, Carlsbad, CA), and quantitative real-time PCR was performed to measure Ihh and Smo gene expression. The expression of 18s RNA was used for normalization. An ABI prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) program was used for all measurements of minimum threshold cycle (Ct), and the differences in gene expression between control and treated sample were calculated using the manufacturer’s protocol. The PCR primers were designed using the Primer Express Software (Applied Biosystems):
| Gene | Primer pair |
|---|---|
| Indian Hedgehog | 5’-TGCCGACCGCCTCATG 5’-CATGACAGAGATGGCCAGTGA |
| Smoothened | 5’-TTCTCTAAGCGGCGTGAACTG 5’-AAACCGGCAACAGGTCCAT |
Gli-luciferase reporter assay
Ihh signaling was examined by measuring the activity of Gli, a downstream transcription factor in the Ihh pathway. Approximately 2 × 106 cells per 60 mm plate were transiently co-transfected overnight with an Ihh-responsive Gli-luciferase reporter plasmid and a Renilla luciferase control reporter plasmid (Promega) using Fugene 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. The Gli-luciferase reporter was kindly provided by Dr. Jeremy Reiter, University of California [19] and contains an enhancer with 8 repeating sequences which include a Shh response element and a Gli binding site. The day after transfection, the cells were collected, counted, and then cultured as a three-dimensional cell pellet (3 × 105 cells/pellet) for four days. The chondrocyte cell pellets were then subjected to hydrostatic compression loading as described above for two days prior to measurement of luciferase activity (Dual luciferase assay, Promega). Luciferase reporter activity (n = 9, repeated twice) was normalized for transfection efficiency using the Renilla luciferase activity. Gli-reporter activity was also measured following hydrostatic compression, but in the presence of either the hedgehog antagonist cyclopamine [13–14] (10 µM, Logan Natural Products, Plano, TX), or the primary cilia destabilizing chloral hydrate [20–21] (4 mM) during the compression period.
Immunostaining
Primary chondrocytes were seeded on cover slips at 1.5 × 105 cells/200 µl/cover slip in 200 µl of DMEM/F12, and cultured as monolayer for 4 days prior to a treatment with or without chloral hydrate (4 mM) for another two days. The cells were then fixed in 4% paraformaldehyde at room temperature for 10 minutes. After washing with PBS, the cells were incubated overnight at 4 °C with an antibody against acetylated-α-tubulin (Invitrogen 32–2700, Carlsbad, CA) diluted 1:20 in PBS with 1% normal donkey serum and 0.1% saponin (Sigma Chemical Co, St. Louis, MO). On the following day the cells were washed, and incubated with a tetramethyl rhodamine-coupled goat anti-mouse secondary antibody (715-026-151, Jackson ImmunoResearch, West Gove, PA), diluted 1:100 in the same dilution buffer as the primary antibody for 1 hour at room temperature. The sections were then mounted in Vectashield® mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were captured using a fluorescence microscope with a 63 × oil lens (Spectral Laser Scanning confocal microscope, Leica Microsystems, GmbH, Wetzlar, Germany). The length of cilia in control and chloral hydrate treated cells was measured using the Image J software package [25].
Immunoblotting
Monolayer cells were cultured for five days and with or without chloral hydrate treatment; the protein lysates were collected in RIPA buffer with Mini complete protease inhibitor (Roche) and the protein concentration determined using the Dc protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (60 µg) were separated on 9% SDS-PAGE gels, and transferred onto nitrocellulose membranes (n = 4, repeated twice). The membranes were probed with antibodies to Ihh (1:50 dilution, Santa Cruz Biotechnology sc-1196, Santa Cruz, CA) or Smo (1:100 dilution, MBL International Corporation, Woburn, MA, LS-A2666) in 5% non-fat dry milk in TBS-T overnight, and subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:500 dilution, Santa Cruz) for one hour prior to detection using a chemiluminescent detection system (Santa Cruz). The protein loading was normalized to the immunoreactivity level of actin (1:400, sc-8432, Santa Cruz). The Kodak 1D image analysis software (Eastman Kodak Company, Rochester, NY) was used for densitometric quantitation.
Statistical analysis
All data are presented as mean ± SD. Statistical analyses were performed using either the Student’s t-test, or analysis of variance, as appropriate. Results were considered statistically different at p < 0.05.
Results
Effects of hydrostatic compression on cell proliferation and Ihh gene signaling
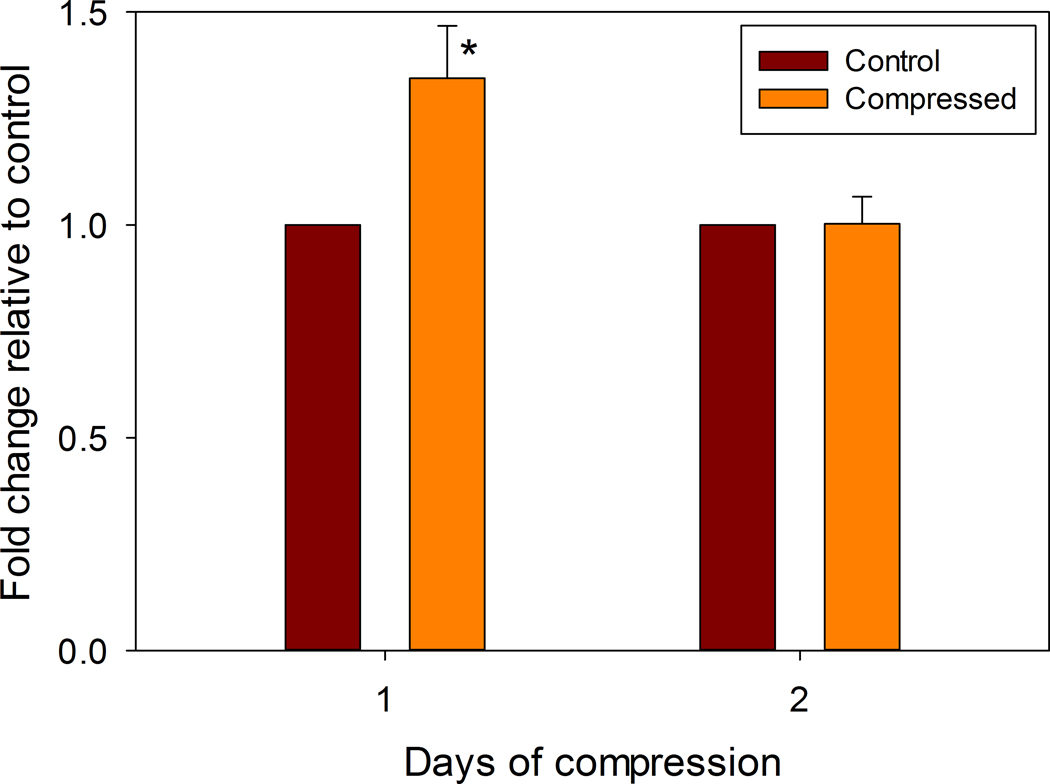
Growth plate chondrocyte pellets subjected to hydrostatic compression forces showed a transient increase in cell proliferation after 24 hours of compression before returning to control levels by 48 hours (Figure 2), but there was no change in Caspase activity on both day (data not shown).
Figure 2. Proliferation Assay.
Aggregates either were left unloaded (Control) or were hydrostatically loaded for 24 or 48 hours as described (Compressed). Immediately after compression, the cells were incubated at 37 °C in 200 µl of medium with 40 µl of the MTS/PMS solution (from the Titer AQ kit) for 1 hour. Absorbance was read at 490 nm in a 96-well plate reader. Data are shown normalized to the control group (* indicates p = 0.0005).
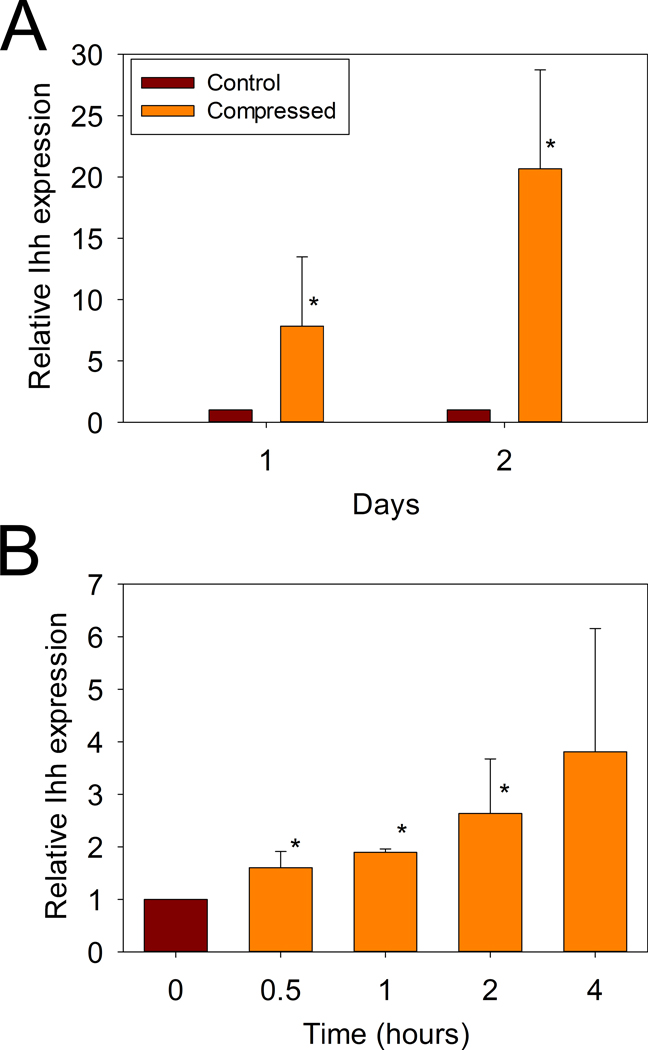
Quantitative RT-PCR analysis demonstrated that the expression of Ihh was increased more than 7-fold over the unloaded control after one day of compression, and then further increased to 20-fold over control after two days of compression.
To further investigate the time-course of this marked increase in Ihh gene expression in response to hydrostatic compression, the experiments were repeated at shorter intervals of 0.5, 1, 2, and 4 hours after the initiation of loading. Ihh expression was found to increase significantly as early as 30 minutes after the onset of hydrostatic compression (Figure 3B).
Figure 3. Effects of hydrostatic compression loading on Ihh gene expression in growth plate chondrocytes.
Unloaded (Control), and compressed samples were collected after either A: 1 (*p = 0.014), or 2 days (*p = 0.0002) or B: After 0, 0.5, 1, 2, and 4 hours of intermittent hydrostatic compression (p =0.017 by ANOVA, * p = 0.017, 0.03, 0.02 respectively).
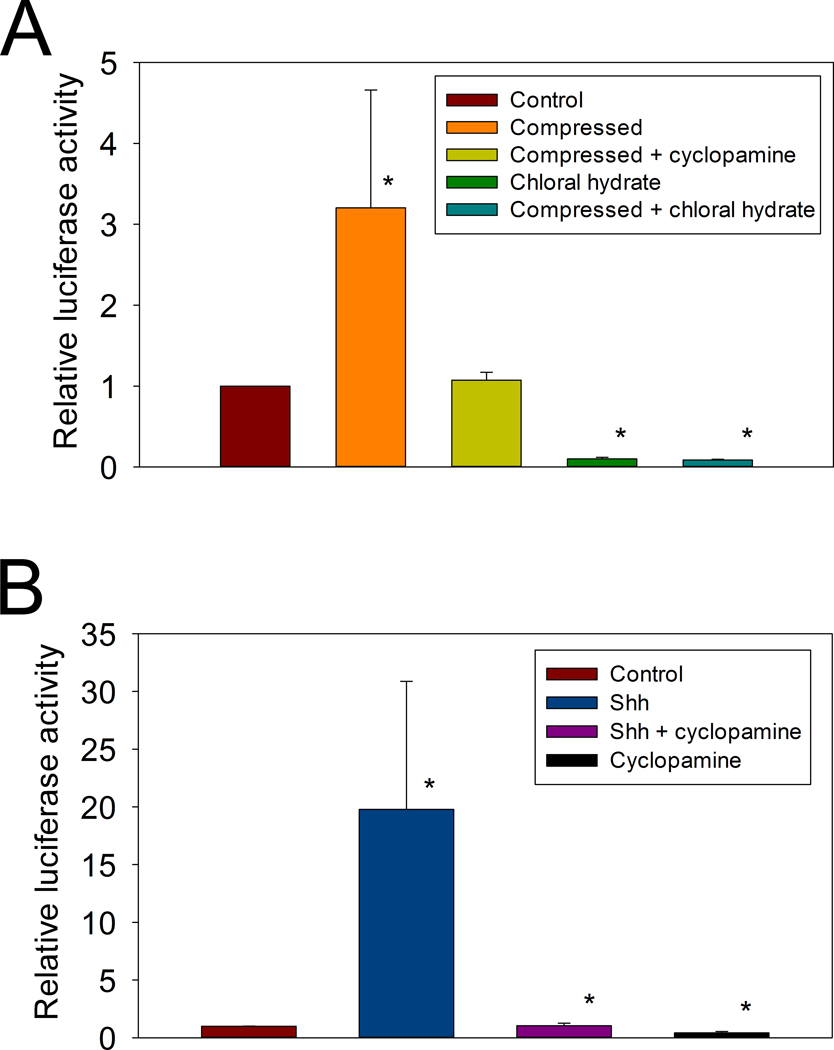
To study the effect of hydrostatic loading on downstream activity of the Ihh signaling pathway, we measured Gli-luciferase activity after two days of hydrostatic compression. This resulted in a more than 3-fold increase Gli reporter activity (Figure 4A). This activity was blocked by the addition of either 10 nM cyclopamine or 4 mM chloral hydrate during the hydrostatic loading.
Figure 4. Gli-dependent luciferase activity.
Gli-luciferase reporter construct was transiently transfected into primary growth plate chondroctyes, which were then cultured as cell pellets. A: Luciferase activity was measured after 48 hours of treatment without (Control) or with compression (Compression, * p = 0.04). The compression induced increase in activity was abolished by the addition of 10 nM cyclopamine (Compressed + cyclopamine). Four mM chloral hydrate essentially eliminated Gli-luciferase activity either without (Chloral hydrate, * p = 0.00002); or with hydrostatic compression (Compressed + chloral hydrate, * p = 0.00002); B: Cyclopamine abolishes the Shh-induced increase in Gli expression. Gli-Luciferase activity was measured after 48 hours of treatment with or without 5nM sonic hedgehog (Shh, * p = 0.006 compared to control), Shh + Cyclopamine, or Cyclopamine alone (* p < 0.05, compare to Shh).
A similar pattern was observed when cell pellets were treated with 5 nM sonic hedgehog (Shh). In this case, the Gli-luciferase activity increased 19-fold (Figure 4B), and this induced activity was also completely inhibited by the addition of 10 nM cyclopamine. This suggests that the same pathway was involved in both two experiments.
Disrupting primary cilia structure inhibits compression-induced Ihh signaling
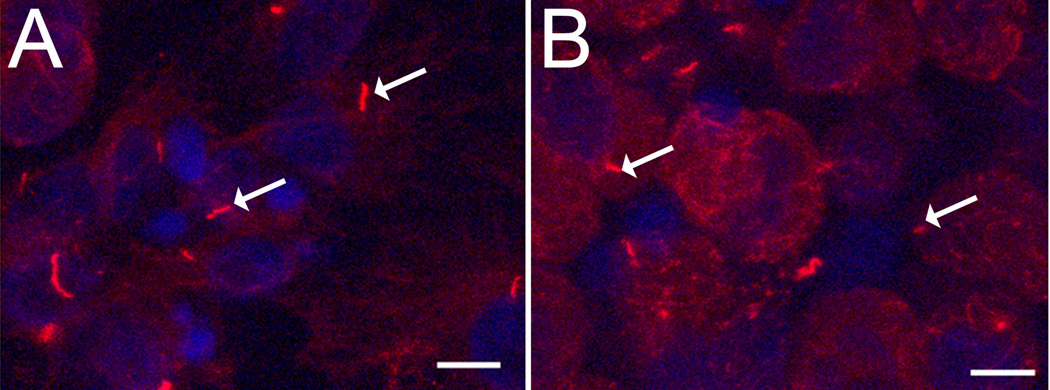
To confirm the disruptive effects of chloral hydrate on cilia structure in epiphyseal chondrocytes, immunohistochemical staining against acetylated-α-tubulin was preformed. Untreated primary chondrocytes grown in monolayer had long, smoothly curved ciliary structures (Figure 5A), while the cilia in chloral hydrate-treated cells appeared shorter, curled, and deformed (Figure 5B). The length of cilia from normal cells was 5.05 ± 2.08 µm, and after 2 days of treated with chloral hydrate cilia treatment, cilia were 2.28 ± 0.30 µm in monolayer culture system.
Figure 5. Cilia structure in growth plate chondrocytes with or without chloral hydrate treatment.
Confocal image of primary cilia (arrows) immunostained with an anti-acetylated α-tubulin antibody. A: Control; B: After 2 days of chloral hydrate (4 mM) treatment. Scale bar = 10µm.
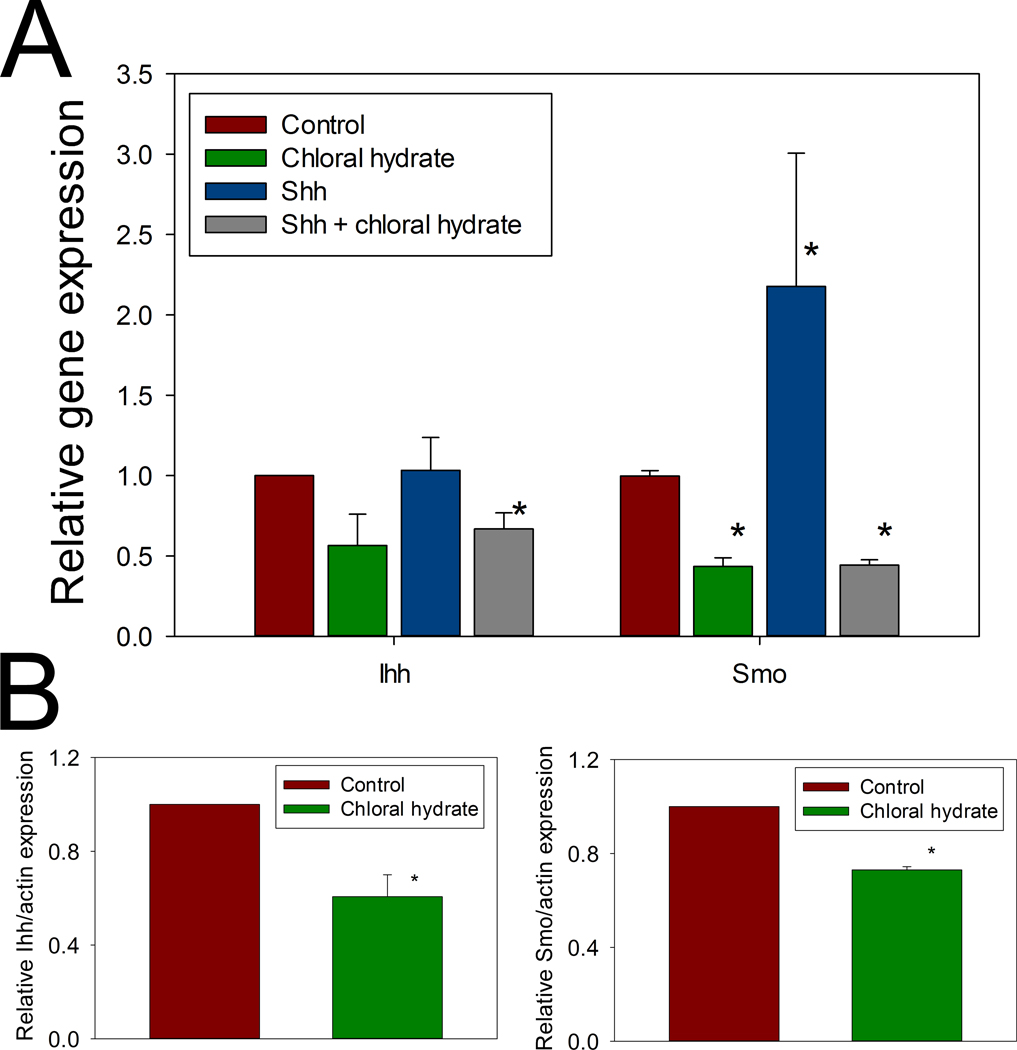
In addition to examining the effects of this disturbed ciliary structure on Ihh and Smo expression in the absence of loading, quantitative RT-PCR showed that chloral hydrate treatment reduced Ihh expression to 57% of control and decreased Smo expression to 44% of control (Figure 6A). A similar effect was shown at the protein level by immunoblotting. Immunoreactive Ihh protein decreased to 61% of control levels in the chloral hydrate-treated cells, while Smo levels were reduced to 73% of control (Figure 6B).
Figure 6. Ihh and Smo expressions were affected by chloral hydrate.
A: Chloral hydrate treatment significantly reduced mRNA level (measured by real time PCR) of Ihh (* p = 0.012) and Smo (* p = 4 × 10−6), Shh treatment significantly (* p = 10−2) increased Smo, but not Ihh expression, and the increase was abolished by chloral hydrate treatment (* p = 10−6). B: Protein levels of Ihh and Smo were also significantly reduced (* p = 0.027 and 0.001, respectively) as measured by Western blotting.
Discussion
The Hueter-Volkmann principle of physeal growth holds that compressive forces across the growth plate inhibit physeal growth, while tensile forces (or release of compressive forces) accelerate physeal growth. Although this principle was first articulated more than 150 years ago [26], the cellular and molecular mechanisms underlying this phenomenon have not yet been elucidated. Mechanical compression can reduce longitudinal growth; experimental data have shown that in extreme cases, for example if the growth plate was stapled, it became irreversible disrupted [27–28]. It should be noted that the Hueter-Volkmann principle was formulated based on a continuous compressive or tensile loading environment, as opposed to the intermittent loading found in nature or even the hydrostatic loading system used in these experiment. Thus, the relationship between loading and growing is likely much more complex. A theoretical cartilage growth force response curve was proposed by Frost [29], it suggests that with no mechanical stimulus, growth stay at basal rate. Mild tension and compression increase growth, while large compression forces can stop the growth quickly. In this study, proliferation was increased transiently but statistically significantly by compression on the first day and returned to normal on the second day (Figure 2); however the nature of this change still remains unclear. This differs from other studies of compression [30]. It should however be noted that the MTS assay we used to measure this response is sensitive to cellular metabolic activity, thus the increase in the AQ96 assay could be related to a transient increase in metabolic activity.
Many studies have investigated the relationship between mechanical loading and biological signal-transduction in growth plate chondrocytes. Animal models in which growth was restricted using a wire loop [31–32], physeal stapling [33], or overloading the growth plate using weighted backpacks [34] all demonstrate that the growth plate is biologically responsive to changes in physical forces. In vitro loading experiments using chondrocytes seeded in scaffolds, such as agarose, alginate, poly (L-lactide-co-ε-caprolactone (PLCL), and gelfoam all indicate that compression-induced changes in the chondrocyte’s environment modulates the synthesis of extracellular matrix components at the mRNA and protein levels [3–4, 35–38].
In this in vitro study, primary growth plate chondrocytes were cultured as an aggregated cell pellet with maximum cell density in all three dimensions, which is how growth plate chondrocytes are organized in vivo [24]. We have previously shown that this pellet culture system allows cells to terminally differentiate into hypertrophic chondrocytes that express high levels of alkaline phosphatase and type X collagen [39]. We used our custom-designed loading system to apply an intermittent hydrostatic compression force on these cell pellets, which resulted a markedly increased Ihh expression. As a consequence of this stimulation, Gli-luciferase reporter activity downstream in the Ihh signaling pathway was also increased. This effect was abolished by cyclopamine treatment, indicating that hydrostatic compression stimulates the Ihh pathway directly. Cyclopamine inhibits Ihh signaling by direct binding to Smoothened at its heptahelical domain [14]. Therefore it is not surprising that we observed that 2 days cyclopamine treatment completely inhibited Gli-luciferase activity, but only partially reduced Ihh expression (data no shown). We also observed that despite in presence of Shh, chloral hydrate treatment reduced Ihh expression; this partial loss of Ihh expression implies that variables other than Smo, cyclopamine, chloral hydrate treatment and mechanical loading can alter Ihh expression.
Ihh serves an important role in cartilage development. In experimental studies of Ihh as a mechnotransduction mediator in the mandibular condylar cartilage, Tang et al. showed that Ihh expression, and the number of proliferating cells were significantly increased on day 3 and day 7 of mandibular advancement [40]. Wu and his colleagues have reported that mechanical stress stimulated chondrocyte proliferation and Ihh expression [41] in a three-dimensional collagen sponge scaffold system. However, the nature of this loading model makes it difficult to resolve the type of mechanical load experienced by cell within the sponge.
There is increasing evidence that the primary cilium is involved and acts as a signaling mediator in the hedgehog family pathway [42–44]. Hh pathway activation is mediated by the receptors Patched (Ptc) and Smoothened (Smo). Singla and Reiter have proposed a dynamic model for Hh signal transduction [45], that suggests that in the presence of Hh, Smo was released by Ptc, then moves to the ciliary tip where Gli activator forms and moves down the cilium to turn on Hh dependent genes. Experimental data have shown that Ptc [10], Smo [19], and Gli [46] expression in cilia were affected by Shh treatment. Furthermore failure to form a functional primary cilium inhibits chondrocyte differentiation, and results in delayed chondrocyte hypertrophy in the growth plate. Thus, Koyama et al. [47] reported that mice deficient in the kif3a gene, which encodes an essential component of the primary cilium, had rare detectable cilia and a disorganized growth-plate pattern. In their study, the expression of genes encoding Ihh, collagen type X, VEGFA, MMP-13 and osterix-7 were barely detectable. Orpk mice containing mutations in the Tg737 gene, which encodes a protein required for cilium assembly, also have skeletal patterning and growth defects associated with significantly shorter primary cilia in chondrocytes [17]. More recent work has shown that Ellis-van Creveld (Evc and Evc2) gene products co-localized at the basal body of primary cilia and on the ciliary membrane, and are essential for Ihh signaling in the growth plate cartilage [48–49]. Mice lacking Evc had short limbs, ribs, and dental abnormalities. Although, the in situ data in this study showed normal expression of Ihh, the mRNA levels of downstream targets patch1, Gli1, and Pthrp were diminished. As the ciliary structure did not reveal any abnormalities, the authors suggested that Evc is a specific modifier of Hh signal transduction.
In chondrocytes, two major bending patterns of the primary cilia have been described by Jensen et al. in a shear stress associated model [50]. Normal primary cilia structure appeared either straight or smoothly curved, while deformed cilia were short or showed acute bends. The author suggested that, in chondrocytes, the primary cilia acted as a potential mechanosensor in skeletal patterning and growth. Our immunofluorescent staining for acetylated α-tubulin showed that chloral hydrate disturbed chondrocyte ciliary structure. The cilia were short and sharply bent or curled in the chloral hydrate-treated cells. Following withdrawal of chloral hydrate for 2 days, the appearance of the cilia recovered, approximating that of the untreated control cells. Our data indicate that primary cilia structures are functionally linked to the Ihh pathway in growth plate chondrocytes and that primary cilium function can be disrupted by chloral hydrate in these cells. Although chloral hydrate exposure affected the structure of the cilia, it otherwise did not have a notable effect on cellular morphology, which has been observed in other studies [21, 51]. We have also noticed that cilia length in cell differ between monolayer vs pellet cells, but those cells had similar response to chloral hydrate treatment (data not shown), and further, more detailed studies of cilia structure are needed. In a chondrocyte/agarose compression model, McGlashan et al [52] have observed that mechanical loading reduced cilia incidence and length, and believed that primary cilium length was an adaptive mechanism in response to mechanical loads, since cilia length and incidence are varied with location in cartilage [53]. In addition, a 48 hour structure study by Kennedy [54] showed that chloral hydrate treatment caused ciliary filament breakdown at junction of the cilium with the basal body. The electron microscope revealed 5mM chloral hydrate caused the filament breakdown while cells and all the basic component of the kinetosome appeared to be normal in structure; but after 44–48 hours cell death began to increase. Perhaps, in our study chloral hydrate disrupted Ihh signaling pathway was due to loss of Smo,Gli in those broken cilary filaments.
Conclusions
Previous studies have shown that the primary cilium is a mechanosensory organelle which transduces mechanical forces into biological signals, and that it plays an important role in the Ihh-PThrP feedback loop. Our present study demonstrates that growth plate chondrocytes respond to hydrostatic loading by increasing Ihh signaling, and that the primary cilium is required for this mechano-biological signal transduction to occur.
Highlights.
>High density primary chondrocyte pellets were examined under a hydrostatic compression> Hedgehog’s signal pathway was affected by this compression>it was through primary cilia.
Acknowledgements
We thank Dr. Jeremy Reiter for providing the Gli-Luciferase plasmid. These studies were funded in part by grants from the NIH (AR050208, JFW) and the Ohio BRTT (CTEC JFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilkins RJ, Browning JA, Urban JP. Chondrocyte regulation by mechanical load. Biorheology. 2000;37:67–74. [PubMed] [Google Scholar]
- 2.Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9:1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 3.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 4.Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48:2865–2872. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 5.Radomisli TE, Moore DC, Barrach HJ, Keeping HS, Ehrlich MG. Weight-bearing alters the expression of collagen types I and II, BMP 2/4 and osteocalcin in the early stages of distraction osteogenesis. J Orthop Res. 2001;19:1049–1056. doi: 10.1016/S0736-0266(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 6.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 8.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 10.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 11.Matise MP, Joyner AL. Gli genes in development and cancer. Oncogene. 1999;18:7852–7859. doi: 10.1038/sj.onc.1203243. [DOI] [PubMed] [Google Scholar]
- 12.Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol. 2007;9:1005–1009. doi: 10.1038/ncb435. [DOI] [PubMed] [Google Scholar]
- 13.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 14.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 16.Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol. 2007;305:202–216. doi: 10.1016/j.ydbio.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 19.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 20.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 21.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- 24.Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- 25.Rasband W. Image J. Bethesda, MD: U.S. National Institutes of Healthh; 1997–2011. [Google Scholar]
- 26.Hueter C. Anatomische Studien an den Extremitätengelenken Neugeborener und Erwachsener. Virchows Archiv fur Pathologische Anatomie und Physioloie und fur Klinische Meizin. 1862;26:484–519. [Google Scholar]
- 27.Ehrlich MG, Mankin HJ, Treadwell BV. Biochemical and physiological events during closure of the stapled distal femoral epiphyseal plate in rats. J Bone Joint Surg Am. 1972;54:309–322. [PubMed] [Google Scholar]
- 28.Herwig J, Schmidt A, Matthiab HH, Kleemann H, Buddecke E. Biochemical events during stapling of the proximal tibial epiphyseal plate in pigs. Clin Orthop Relat Res. 1987:283–289. [PubMed] [Google Scholar]
- 29.Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 3. The hyaline cartilage modeling problem. Anat Rec. 1990;226:423–432. doi: 10.1002/ar.1092260404. [DOI] [PubMed] [Google Scholar]
- 30.Mankin KP, Zaleske DJ. Response of physeal cartilage to low-level compression and tension in organ culture. J Pediatr Orthop. 1998;18:145–148. [PubMed] [Google Scholar]
- 31.Wilson-MacDonald J, Houghton GR, Bradley J, Morscher E. The relationship between periosteal division and compression or distraction of the growth plate. An experimental study in the rabbit. J Bone Joint Surg Br. 1990;72:303–308. doi: 10.1302/0301-620X.72B2.2312573. [DOI] [PubMed] [Google Scholar]
- 32.Alberty A, Peltonen J. Proliferation of the hypertrophic chondrocytes of the growth plate after physeal distraction. An experimental study in rabbits. Clin Orthop Relat Res. 1993:7–11. [PubMed] [Google Scholar]
- 33.Farnum CE, Nixon A, Lee AO, Kwan DT, Belanger L, Wilsman NJ. Quantitative three-dimensional analysis of chondrocytic kinetic responses to short-term stapling of the rat proximal tibial growth plate. Cells Tissues Organs. 2000;167:247–258. doi: 10.1159/000016787. [DOI] [PubMed] [Google Scholar]
- 34.Reich A, Jaffe N, Tong A, Lavelin I, Genina O, Pines M, Sklan D, Nussinovitch A, Monsonego-Ornan E. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol. 2005;98:2381–2389. doi: 10.1152/japplphysiol.01073.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Vezeridis PS, Nicholas B, Crisco JJ, Moore DC, Chen Q. Differential expression of type X collagen in a mechanically active 3-D chondrocyte culture system: a quantitative study. J Orthop Surg Res. 2006;1:15. doi: 10.1186/1749-799X-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassino TR, Anderson R, Love BJ, Huckle WR, Seamans DK, Forsten-Williams K. Design and application of an oscillatory compression device for cell constructs. Biotechnol Bioeng. 2007;98:211–220. doi: 10.1002/bit.21422. [DOI] [PubMed] [Google Scholar]
- 37.Hauselmann HJ, Masuda K, Hunziker EB, Neidhart M, Mok SS, Michel BA, Thonar EJ. Adult human chondrocytes cultured in alginate form a matrix similar to native human articular cartilage. Am J Physiol. 1996;271:C742–C752. doi: 10.1152/ajpcell.1996.271.3.C742. [DOI] [PubMed] [Google Scholar]
- 38.Toyoda T, Seedhom BB, Kirkham J, Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40:79–85. [PubMed] [Google Scholar]
- 39.Ballock RT, Zhou X, Mink LM, Chen DH, Mita BC. Both retinoic acid and 1,25(OH)2 vitamin D3 inhibit thyroid hormone-induced terminal differentiaton of growth plate chondrocytes. J Orthop Res. 2001;19:43–49. doi: 10.1016/S0736-0266(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 40.Tang GH, Rabie AB, Hagg U. Indian hedgehog: a mechanotransduction mediator in condylar cartilage. J Dent Res. 2004;83:434–438. doi: 10.1177/154405910408300516. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 42.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 44.Oro AE. The primary cilia, a 'Rab-id' transit system for hedgehog signaling. Curr Opin Cell Biol. 2007;19:691–696. doi: 10.1016/j.ceb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 46.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 49.Blair HJ, Tompson S, Liu YN, Campbell J, MacArthur K, Ponting CP, Ruiz-Perez VL, Goodship JA. Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC Biol. 2011;9:14. doi: 10.1186/1741-7007-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- 53.McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237:2013–2020. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy JR, Jr, Brittingham E. Fine structure changes during chloral hydrate deciliation of Paramecium caudatum. J Ultrastruct Res. 1968;22:530–545. doi: 10.1016/s0022-5320(68)90039-7. [DOI] [PubMed] [Google Scholar]