Abstract
Background
Decreased diffusion (DD) consistent with acute ischemia may be detected on MRI after acute intracerebral hemorrhage (ICH), but its risk factors and impact on functional outcomes are not well defined. We tested the hypotheses that DD after ICH is related to acute blood pressure (BP) reduction and lower hemoglobin (HGB) and presages worse functional outcomes.
Methods
Patients who underwent MRI were prospectively evaluated for DD by certified neuroradiologists blinded to outcomes. HGB and BP data were obtained via electronic queries. Outcomes were obtained at 14 days and 3 months with the modified Rankin Scale (mRS), a functional scale scored from 0 (no symptoms) to 6 (dead). We used logistic regression for dependence or death (mRS 4 to 6).
Results
DD distinct from the hematoma was found on MRI in 36 of 95 patients (38%). DD was associated with greater BP reductions from baseline, and a higher risk of dependence or death at 3 months (OR 4.8, 95% CI 1.7 – 13.9, P=0.004) after correction for ICH Score (1.8 per point, 95%CI 1.2–3.1, P=0.01). Lower HGB was associated with worse ICH score, larger hematoma volume and worse outcomes, but not DD.
Conclusions
DD is common after ICH, associated with greater acute BP reductions, and associated with disability and death at 3 months in multivariate analysis. The potential benefits of acute BP reduction to reduce hematoma growth may be limited by DD. The prevention and treatment of cerebral ischemia manifested as DD is a potential method to improve outcomes.
Intracerebral hemorrhage (ICH) accounts for 8 to 15% of all strokes in high-income countries,1, 2 and a higher percentage in the developing world. ICH has a high risk of disability and death, with 20% of patients functionally independent at six months,3 and fewer than half of patients alive at one year.4
Decreased diffusion (DD) may be detected on MRI after acute ICH,5 but the underlying physiology and any potential impact on outcomes is not known. If DD is associated with outcomes, then the prevention of DD through risk factor modification might be a potential method to improve outcomes after ICH. Acute medical interventions to minimize ICH volume growth, such as aggressive blood pressure reduction6 and reversal of anticoagulant therapy, might potentially increase the risk of DD due to reduced cerebral perfusion or thrombosis.7 Greater blood pressure (BP) reduction from admission to 24 hours has been associated with more DD.5 Lower hemoglobin (HGB) has been associated with worse outcomes8 while packed red cell transfusion9 has been associated with reduced mortality in patients with ICH. Higher HGB is associated with less cerebral infarction and better outcomes after aneurysmal subarachnoid hemorrhage,10, 11 but such a relationship in patients with ICH is unclear. We tested the hypotheses that DD is associated with BP reduction and anemia, and that DD on MRI distinct from the hematoma is associated with worse functional outcomes in patients with ICH.
METHODS
Study Population
We prospectively enrolled consecutive patients with spontaneous ICH from December 2006 through March 2011. All patients were prospectively diagnosed by a board-certified vascular neurologist utilizing computed tomography (CT). Patients with ICH attributed to trauma, hemorrhagic conversion of ischemic stroke, and structural lesions were excluded. Clinical data, laboratory data, and follow-up were prospectively recorded. The likely etiology of ICH was determined by a board-certified vascular neurologist, with the diagnosis of cerebral amyloid angiopathy determined according to validated criteria.12
All patients with ICH are admitted to the Neuro/Spine ICU with a standardized order set in the electronic medical record. We prospectively recorded baseline demographic, past medical history, clinical data including the NIH-Stroke Scale (NIHSS),13 and the pre-treatment BP. We prospectively recorded the ICH Score,14 a composite of ICH volume, level of consciousness, intraventricular hemorrhage, patient age and location of ICH. ICH volume on the first scan was calculated with the abc/2 formula.15 We defined atrial fibrillation as a documented medical history or documented occurrence on telemetry during hospitalization. When an echocardiogram was obtained, we prospectively recorded the estimated ejection fraction. Blood pressure was lowered as per the most recent guidelines.16 Hemoglobin (HGB) values for all patients from admission through 14 days were electronically retrieved. We electronically retrieved hourly documented invasive and non-invasive blood pressures and administration of intravenous anti-hypertensive medications (for 37 patients admitted since December 2009, when we began electronic charting of vital signs). Medical complications (deep venous thrombosis, pulmonary embolism, pneumonia (clinical criteria from the US Centers for Disease Control) and hypotension (systolic BP < 100 mm Hg requiring vasopressors) were prospectively recorded.
We routinely obtained MRI scans in salvageable patients (those clinically unlikely to die within 48 hours from ICH symptom onset) on Siemens 1.5T MR scanners (Siemens AG, Germany). Routinely obtained MR sequences included B1000, diffusion weighted imaging (DWI), apparent diffusion coefficient (ADC) map, fluid-attenuated inversion recovery (FLAIR), T2/TSE, T1, and T2* gradient echo. The MR imaging examinations were reviewed separately by one of two certificate-of-added-qualification certified neuroradiologists (EJR and AJN), both of whom were blinded to the clinical data and outcomes. The MR imaging studies were reviewed for DD distinctly outside the hematoma, which consisted of hyperintense signal on DWI and hypointense signal on ADC maps, relative to the normal-appearing brain. The DWI and ADC maps were correlated with the conventional MR sequences, including FLAIR, T2, T2* and T1, and also with CT, to confirm that the DD was distinct from the hematoma. The hematomas demonstrated susceptibility blooming on T2* and T2, variable signal on FLAIR and T1, and were hyperdense on CT, whereas the areas corresponding to DD in the diffusion sequences were generally hyperintense on FLAIR, T2 and T2* without susceptibility blooming, hypointense on T1 and iso- or hypodense on unenhanced CT, relative to the normal-appearing brain. DD within the hematoma or immediately surrounding the hematoma was not included as a positive result, which is why it was of utmost importance to determine the exact location and extent of the hematoma with the conventional MR sequences and CT. DD immediately surrounding the hematoma was considered to be a direct compressive effect from the hematoma, and is separate from DD in the brain parenchyma that was rated as a positive result. The inter-rater reliability of the assessment of DD on MRI has previously been shown to be excellent.5, 17–19 The study was approved by the Institutional Review Board (IRB). Written informed consent to collect data and clinical outcomes was obtained from the patient or a legally authorized representative with the following exceptions: the patient died in hospital or no representative could be located for an incapacitated patient (in which case the IRB approved collection of data in a registry without consent).
Follow-Up
A certified examiner recorded the NIHSS at 14 days or discharge, whichever came first. The modified Rankin Scale (mRS) was prospectively recorded at 14 days or discharge and 3 months with a validated questionnaire.20, 21 The mRS is a validated functional outcome scale from 0 (no symptoms) to 6 (death), with 4 indicating dependence. We defined poor outcome as mRS of 4 through 6, typical for clinical studies of ICH.22
Statistical Analysis
Normally distributed data were compared with Student’s t-test or ANOVA as appropriate. Non-normally distributed data were compared with Mann-Whitney U or Kruskal-Wallis H tests as appropriate. Categorical data were compared with chi-squared. We performed correlations as appropriate. Logistic regression was used for binary outcomes (e.g. poor outcome at 3 months). Statistical calculations were performed with commercially available software (IBM SPSS Statistics v. 19, Armonk, NY).
RESULTS
Study sample
There were 95 patients in the sample. The most common ethnicities were African-American (47, 49%), Caucasian (36, 38%), and Hispanic (7, 7%). The mean age was 64.1 ± 13.5 years, and 48 (51%) were women. The most common etiologies of ICH were hypertension (59, 62%) and amyloid angiography (14, 15%). The most common hematoma location was lobar (37, 39%), followed by the thalamus (22, 23%) and lentiform nuclei (20, 21%).
Characteristics of patients who underwent MRI, stratified by the presence or absence of DD, are shown in Table 1. The incidence of DD in patients with thalamic hemorrhage was N=6 (39%), lobar hemorrhage N=14 (37%), and lentiform nuclei N=12 (60%). DD was not related to the clinical etiology of hemorrhage (P=0.3), medical complications (P≥0.2 for all), craniotomy (P=0.1), or anticoagulant reversal (P=0.5).
Table 1.
Comparison between patients that had decreased diffusion or not on MRI.
| Variable | No decreased diffusion | Decreased diffusion |
|---|---|---|
| Number of patients (N) | 56 | 39 |
|
| ||
| Glasgow Coma Scale on admit | 15 [12 – 15] | 14 [9 – 15] |
|
| ||
| NIH Stroke Scale on admit* | 5 [2 – 10] | 8 [3 – 17] |
|
| ||
| ICH Score on admit 0 | 25 (45) | 13 (33) |
| 1 | 17 (30) | 10 (26) |
| 2 | 8 (14) | 12 (31) |
| 3 | 5 (9) | 3 (8) |
| 4 | 1 (2) | 1 (3) |
|
| ||
| Days from symptom onset to MRI | 1.6 [0.8 – 2.9] | 2.3 [1.0 – 7.5] |
|
| ||
| First glucose, mg/dL | 145 ± 62 | 159 ± 69 |
|
| ||
| Systolic BP on admit, mm Hg | 203 ± 184 | 187 ± 37 |
|
| ||
| Diastolic BP on admit, mm Hg | 91 ± 25 | 102 ± 29 |
|
| ||
| Age, years | 64.6 ± 13.6 | 63.8 ± 13.5 |
|
| ||
| ICH Volume on admit, mL | 9 [4 – 20] | 12 [6–25] |
|
| ||
| Dependence prior to ICH | 4 (7) | 1 (3) |
|
| ||
| History of ischemic stroke | 8 (14) | 2 (5) |
|
| ||
| History of hypertension | 44 (78) | 31 (79) |
|
| ||
| History of diabetes | 13 (23) | 10 (25) |
|
| ||
| No Aspirin use prior to ICH | 36 (64) | 23 (59) |
|
| ||
| Statin use prior to ICH | 13 (23) | 9 (23) |
|
| ||
| History of coronary artery disease | 13 (23) | 4 (10) |
|
| ||
| Alcohol misuse | 8 (14) | 4 (10) |
|
| ||
| Cocaine use | 3 (5) | 1 (3) |
|
| ||
| Pack-years of tobacco | 0 [0 – 10] | 0 [0 – 20] |
|
| ||
| NIH-Stroke Scale at 14 days† | 4 [2 – 9] | 9 [5 – 22] |
|
| ||
| mRS at 14 days† | 4 [2 – 5] | 5 [4 – 5] |
|
| ||
| mRS at 3 months† | 1 [1 – 3] | 4 [2 – 5] |
P=0.02
P<=0.002
Data are N(%), mean ± SD or median [Q1 – Q3] as appropriate. mRS, modified Rankin Scale.
BP reduction
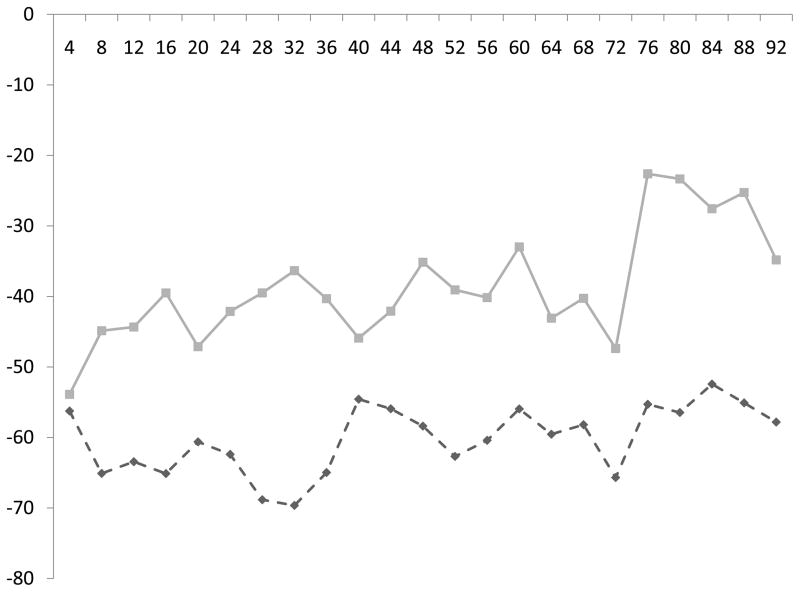
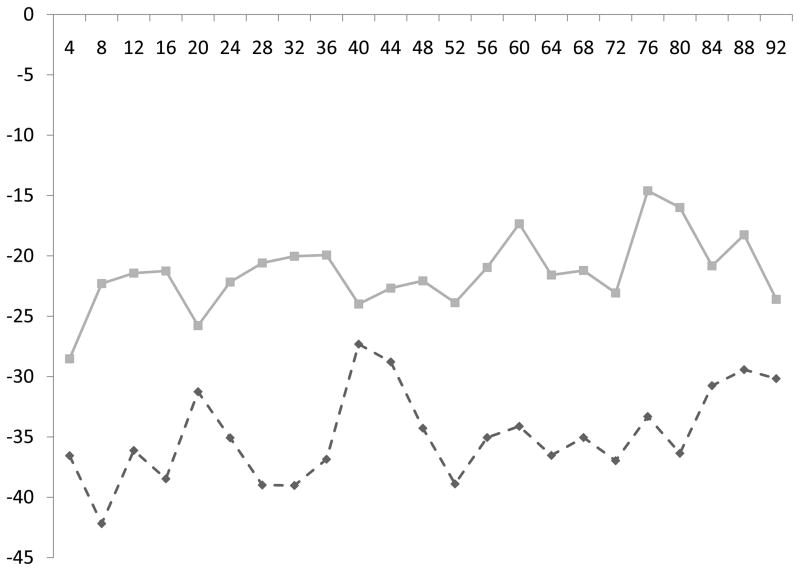
Both the absolute BP measures and change in BP from baseline (Figure) were associated with DD (P<0.001 for both). Within 96 hours of ICH symptom onset, patients with DD had a lower mean systolic BP (135 ± 16 vs. 139 ± 16 mm Hg, P=0.002) a greater mean decrease from baseline systolic BP (61 ± 41 vs. 39 ± 28 mm Hg, P<0.001), a lower mean diastolic BP (70 ± 12 vs. 75 ± 11 mm Hg, P<0.001) and a greater mean decrease from baseline diastolic BP (35 ± 34 vs. 22 ± 17 mm Hg, P<0.001). The administered dosages of nicardipine, labetalol and hydralazine were not different within 2 days of symptom onset (the mean time to MRI acquisition) or during the hospital stay (P>0.1 for all).
Figure.
Change in systolic (a) and diastolic (b) BP (vertical axis) every four hours (horizontal axis) from ICH symptom onset, stratified by the presence (dashed line) or absence (solid line) of decreased diffusion. Patients with decreased diffusion had greater reductions in BP from baseline.
HGB
Higher ICH Score was associated with lower nadir (P=0.003) and mean (P<0.001) HGB. Greater hematoma volume was associated with lower mean HGB (P=0.005) and nadir HGB (P=0.01). Poor outcome at 3 months was associated with lower mean HGB (10.7 ± 1.4 vs. 12.5 ± 1.5 g/dL, P<0.001) and lower nadir HGB (8.8 ± 1.5 vs. 10.9 ± 2.2 g/dL, P<0.001). Neither mean HGB nor nadir HGB was associated with DD (P>0.3).
Cardiac risk factors for DD
Atrial fibrillation was found in 4 (4%) of patients, 2 of whom had DD (P=0.3). Among the 81 patients who underwent echocardiography, an ejection fraction of 45–55% was found in 11 (12%), and was associated with DD in 7 (P=0.2).
Cerebral infarction and Outcomes
DD was associated with worse outcomes in univariate analysis (Table 1). DD was associated with nearly five times the odds of dependence or death at 3 months (Table 2) after controlling for previously published predictors, the ICH Score23 in one model, and admission NIHSS and age24 in another. Forcing in the delay from symptom onset to MRI acquisition did not significantly add to the model.
Table 2.
Multivariate models for dependence or death at three months. The two models are separated by the double line.
| Variable | OR (95%CI) | P | |
|---|---|---|---|
| Model 1 | Age per year | 1.05 (1.005 – 1.1) | 0.03 |
| NIH-Stroke Scale on admission, per point | 1.1 (1.05 – 1.2) | 0.003 | |
| Decreased diffusion | 4.9 (1.5 – 17.1) | 0.01 | |
| Model 2 | ICH Score, per point | 1.9 (1.2 – 3.1) | 0.01 |
| Decreased diffusion | 4.8 (1.7 – 13.9) | 0.004 |
Mechanisms of death
The mechanism of death was not associated with DD (P=0.2). Three patients with DD had withdrawal of life support, while two without DD had withdrawal of life support.
DISCUSSION
These data confirm the results of a previous report5 that DD is common after ICH. We extend those findings by documenting increased poor outcome at 3 months in multivariate models, suggesting that the prevention of DD is a potential strategy to improve outcomes after ICH. The reason for greater reductions in BP in patients with DD is not clear. Patients were all admitted to the same dedicated ICU with a standardized order set, and the use of parenteral anti-hypertensive medication was not different. While we attempted to exclude and control for residual confounding, some may still have occurred. While absolute BP differences were small (4–5 mm Hg systolic and diastolic), changes from baseline (pre-treatment) BP were larger (22 mm Hg systolic and 13 mm Hg diastolic). These findings may have implications for the acute treatment of BP in patients with acute ICH. A decrease of about 40 mm Hg systolic and 25 mm Hg diastolic may be a limit before the risk of DD becomes unacceptably large, although independent samples should be studied to confirm this. How best to determine the maximum safe BP reduction in an individual patient is not known. While INTERACT (a study of aggressive versus usual anti-hypertensive treatment)6 and ATACH (a study of progressively more stringent blood pressure goals)25 showed that strict blood pressure reduction during acute ICH is feasible, and INTERACT suggested reduced ICH volume growth, neither routinely assessed DD with MRI nor demonstrated an impact of BP reduction on functional outcomes. If either study finds that aggressive BP reduction is associated with less hematoma growth but does not improve outcomes, DD may be a potentially confounding factor. Reduced hematoma growth without an improvement in outcomes at 3 months was found in the phase III study of recombinant Factor VII,22 a drug known to be associated with thrombosis. We confirmed a previous report that found lower HGB was associated with larger hematoma volumes and worse outcomes.8 While DD was a biologically plausible intermediary factor (lower HGB might lead to DD which might lead to worse outcomes), we did not find such an association.
While DD was associated with worse outcomes, the precise mechanism is not clear and this requires further study. Data on the incidence of DD in ongoing studies of BP reduction in acute ICH might be especially valuable. It is possible that DD is a marker of vascular risk, although risk factors for ischemic stroke prior to ICH onset were not different.
The MR imaging of acute hemorrhage is not straightforward, and not all areas hyperintense on T2 and hypointense on ADC mapping are ischemic.26, 27 Imaging characteristics change as oxyhemoglobin becomes deoxygenated and over time.28 To minimize errors in interpretation, MRI scans were interpreted by certified neuroradiologists blinded to clinical data and outcomes. While we did not calculate the inter-rater reliability for this assessment, in previous studies the inter-rater reliability for ascertaining DD5, 17–19 has been excellent. We scored DD as present or absent, but a volumetric analysis might be more informative, and this is an opportunity for future research.
One might expect atrial fibrillation and a depressed ejection fraction to lead to DD. We found these risk factors in too few patients for a meaningful analysis. We did not routinely evaluate other factors for ischemic stroke (e.g. carotid stenosis or intracranial atherosclerosis) and this deserves further study.
There is likely to be some selection bias in patients who underwent MRI. We did not find, however, that patients with DD were more likely to have withdrawal of life support as a self-fulfilling prophecy as the cause of death. Strengths of this study include prospective patient identification and clinical data collection, blinded expert review of MRI scans, and prospective outcome assessment with a validated scale after discharge.
In summary, we found DD was common after ICH, associated with greater BP reduction, and was associated with greater dependence at three months in multivariate analysis. Future research should clarify how much acute BP reduction is likely to be associated with DD, and clarify other potential mechanisms. The prevention and treatment of DD may be a potential method to improve outcomes after ICH.
Acknowledgments
All those who meaningfully contributed to the manuscript are listed as an author.
Sources of Funding
This work was departmentally funded. The infrastructure for automated data retrieval was funded in part by NIH through a grant to Northwestern University’s Clinical And Translational Sciences (NUCATS) UL1RR025741.
Footnotes
AMN performed the statistical analysis.
This work was performed at Northwestern University Feinberg School of Medicine, Chicago, IL. RKG is now at Rush Medical College, Chicago, IL.
Conflict of Interest Disclosure
AMN has received research funding from Gaymar, Inc and Astellas Pharma US, unrelated to this topic, and serves as a medical safety monitor for two unrelated NIH funded studies. After the first submission, RKG was notified of an award from American Heart Association to perform prospective research on this topic.
The other authors declare they have no conflicts.
References
- 1.Feigin V, Lawes C, Bennett D, Barker-Collo S, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurology. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 3.Counsell C, Boonyakarnkul S, Dennis M, Sandercock P, Bamford J, Burn J, et al. Primary intracerebral hemorrhage in the Oxfordshire Community Stroke Project, 2: prognosis. Cerebrovascular Diseases. 1995;5:26–34. [Google Scholar]
- 4.Flaherty M, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw C, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66:1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Gupta R, Ouyang B, John S, Temes R, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010;41:89–94. doi: 10.1161/STROKEAHA.109.566257. [DOI] [PubMed] [Google Scholar]
- 6.Anderson C, Huang Y, Wang J, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 7.Diringer M, Skolnick B, Mayer S, Steiner T, Davis S, Brun N, et al. Thromboembolic events with recombinant factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41:48–53. doi: 10.1161/STROKEAHA.109.561712. [DOI] [PubMed] [Google Scholar]
- 8.Kumar MA, Rost NS, Snider RW, Chanderraj R, Greenberg SM, Smith EE, et al. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med. 2009;37:1442–1447. doi: 10.1097/CCM.0b013e31819ced3a. [DOI] [PubMed] [Google Scholar]
- 9.Sheth KN, Gilson AJ, Chang Y, Kumar MA, Rahman RM, Rost NS, et al. Packed Red Blood Cell Transfusion and Decreased Mortality in Intracerebral Hemorrhage. Neurosurgery. 2011;68:1286–1292. doi: 10.1227/NEU.0b013e31820cccb2. [DOI] [PubMed] [Google Scholar]
- 10.Naidech AM, Drescher J, Ault ML, Shaibani A, Batjer HH, Alberts MJ. Higher hemoglobin is associated with less cerebral infarction, poor outcome, and death after subarachnoid hemorrhage. Neurosurgery. 2006;59:775–779. doi: 10.1227/01.NEU.0000232662.86771.A9. discussion 779–780. [DOI] [PubMed] [Google Scholar]
- 11.Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35:2383–2389. doi: 10.1097/01.CCM.0000284516.17580.2C. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Olinger C, Marler JR, Barsan W, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill J, Bonovich D, Besmertis L, Manley G, Johnston SC, Tuhrim S. The ICH Score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 15.Kothari R, Brott T, Broderick J, Barsan W, Sauerbeck L, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 16.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the mangement of intracerebral hemorrhage in adults: 2007 update. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 17.Saur D, Kucinski T, Grzyska U, Eckert B, Eggers C, Niesen W, et al. Sensitivity and Interrater Agreement of CT and Diffusion-Weighted MR Imaging in Hyperacute Stroke. AJNR Am J Neuroradiol. 2003;24:878–885. [PMC free article] [PubMed] [Google Scholar]
- 18.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. CT and Diffusion-Weighted MR Imaging in Randomized Order: Diffusion-Weighted Imaging Results in Higher Accuracy and Lower Interrater Variability in the Diagnosis of Hyperacute Ischemic Stroke. Stroke. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 19.Fiebach J, Jansen O, Schellinger P, Knauth M, Hartmann M, Heiland S, et al. Comparison of CT with diffusion-weighted MRI in patients with hyperacute stroke. Neuroradiology. 2001;43:628–632. doi: 10.1007/s002340100542. [DOI] [PubMed] [Google Scholar]
- 20.Banks J, Marotta C. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JTL, Hareendran A, Grant M, Baird T, Schulz UGR, Muir KW, et al. Improving the Assess of Outcomes in Stroke. Use of a structured interview to assign grade on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 22.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 23.Hemphill J, III, Farrant M, Neill T., Jr Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73:1088–1094. doi: 10.1212/WNL.0b013e3181b8b332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 25.ATACH investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38:637–648. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischbein NJ, Roberts TP, Dillon WP. Bleed or stroke? Diffusion measurements in intracranial hematomas. AJNR Am J Neuroradiol. 2000;21:1179–1180. [PMC free article] [PubMed] [Google Scholar]
- 27.Atlas SW, DuBois P, Singer MB, Lu D. Diffusion measurements in intracranial hematomas: implications for MR imaging of acute stroke. AJNR Am J Neuroradiol. 2000;21:1190–1194. [PMC free article] [PubMed] [Google Scholar]
- 28.Silvera S, Oppenheim C, Touze E, Ducreux D, Page P, Domigo V, et al. Spontaneous intracerebral hematoma on diffusion-weighted images: influence of T2-shine-through and T2-blackout effects. AJNR Am J Neuroradiol. 2005;26:236–241. [PMC free article] [PubMed] [Google Scholar]