Abstract
Objectives
Response patterns may differ between patients with first episode and multi-episode schizophrenia. This analysis explored trial duration with first episode patients and whether early limited improvement predicts ultimate lack of treatment response with first episode patients as it does with multi-episode patients.
Methods
112 subjects (mean age=23.3 years [SD=5.1]) who presented between November 1998 and October 2004 with a first episode of psychosis and had a DSM-IV diagnosis of schizophrenia, schizophreniform or schizoaffective disorder, were randomly assigned to treatment with olanzapine or risperidone for 16 weeks. Treatment response, the primary outcome measure, was defined as a rating of mild or better on all of the positive symptom items on the SADS-C + PD. Response rates were calculated for each study week. A logistic regression analysis examined the association between percent reduction in symptom severity scores from baseline values at weeks 2, 4 or 8 and response by week 16.
Results
The estimated cumulative response rate by week 8 was 39.59% (95% CI: 29.77% – 49.41%) and 65.19% (95% CI: 55.11% – 75.27%) by week 16. The confidence intervals for estimated response at weeks 10, 12, 14 and 16 were not distinct. Response rates increased approximately 5 to 6 percentage points each 2 week interval between week 10 and 16. Percent reduction in symptom severity score at week 4 (but not 2 or 8) was associated (Chi-square = 3.96; df = 1, p<0.05) with responder status at week 16 (odds ratio: 1.03; 95% CI: 1.00;1.05). However, receiver operating characteristic curves did not suggest any level of percent symptom reduction that would be clinically useful as a predictor of response by week 16.
Conclusions
Many first episode patients respond between weeks 8 and 16 of treatment with a single antipsychotic medication. Limited early symptom improvement does not identify with enough accuracy to be clinically useful those first episode patients who will not improve with a full 16 week trial.
Keywords: risperidone, olanzapine, early improvement, atypical antipsychotic, prediction, response
Introduction
One crucial decision for treatment of any patient is the length of time a particular therapy is tried. First episode studies have consistently found high rates of response compared with the response rates found in studies with multi-episode patients1. If first episode patients are more responsive overall to antipsychotic treatment, does optimum duration of treatment also differ between first episode and multi-episode patients? With a highly responsive patient group, could treatment trials be short and still capture all the patients who will respond? Alternately, if many first episode patients ultimately respond to treatment, should treatment trials be lengthy in order to capture subjects who might be late responders to treatment with a single agent?
Currently available data are limited but suggest that long trials may be warranted. In a trial comparing haloperidol and risperidone, Emsley and colleagues 2 found that 11.5 % of patients who responded did so after week 8 of treatment. This study had the advantage of following subjects long-term, but the study response criteria of a =/> 20% reduction in total Positive And Negative Syndrome Scale (PANSS) 3 scores from baseline differs from the more stringent response criteria used in most first episode studies for assessing outcome with young patients first starting treatment 4–6. Our opportunity to examine first episode trial duration occurred in the context of a previously reported comparison of olanzapine and risperidone 7 for first episode schizophrenia. Among first episode trials employing stringent response criteria, this study had the advantage of examining response at 16 weeks of treatment as opposed to other studies that often examined response at 12 weeks of treatment 5, 6, 8–10. We also wished to address a related clinical question arising from treatment trials lasting several months. Studies with multi-episode patients 11–17 have suggested that response patterns early in treatment can identify patients who are not likely to respond to a treatment with a particular medication. If similar methods are also valid with first episode patients, then those patients who will not respond to a treatment could be spared from being exposed to an ineffective treatment for several months.
Patients and Methods
The study methods and subjects have been previously presented 7 in detail. Data were collected from November 1998 to October 2004. Inclusion criteria included: age 16 to 40 years; current Diagnostic Statistical Manual-IV (DSM-IV)18-defined diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder; lifetime history of less than 12 weeks of antipsychotic medication treatment; a rating of 4 or more on the positive symptoms items of the Schedule for Affective Disorders and Schizophrenia Change Version with psychosis and disorganization items (SADS-C +PD) 19 or current negative symptoms demonstrated by a rating of 4 or more on the affective flattening, alogia, avolition, or anhedonia global items of the Hillside clinical trials version of the Scale for the Assessment of Negative Symptoms (SANS)20. Women were required to have a negative pregnancy test and to agree to use a medically accepted birth control method. Subjects were not included in the study if they had: a) a diagnosis of psychosis due to general medical condition, substance-induced psychotic disorder or mental retardation by DSM-IV criteria b) a condition/treatment know to affect the brain c) the need to use a medication with psychotropic effects for any medical condition d) the presence of significant suicidal or homicidal risk. e) any medical contraindications to treatment with risperidone or olanzapine. All subjects provided written informed consent (for subjects younger than 18 years old, written parental consent and written subject assent were obtained).
Subjects were randomly assigned to treatment with either olanzapine or risperidone for 16 weeks. The initial daily dose was 2.5 mg for olanzapine and 1 mg for risperidone. The study had a variety of assessments. The SADS-C + PD19 was used to assess psychopathology. Intraclass correlation coefficients with three psychopathology raters for the items comprising the positive symptoms response criteria were: severity of delusions=0.79, severity of hallucinations=0.90, impaired understandability=0.66, derailment=0.67, illogical thinking=0.82, bizarre behavior=0.97, and Clinical Global impression (CGI)21 severity=0.63. Assessments were done weekly for the first four weeks and then every two weeks. Subjects who did not achieve CGI ratings of at least minimal improvement by 10 weeks were terminated from controlled treatment.
Treatment response: definition and statistical analysis
With young patients first beginning treatment, treatment goals are high so substantial resolution of all positive symptoms is the goal. Thus, our definition of treatment response required a rating of mild or better on all the following SADS-C+PD items: severity of delusions, severity of hallucinations, impaired understandability, derailment, illogical thinking and bizarre behavior. Cumulative response rates were computed using standard survival methods for all study weeks with psychopathology assessments (weeks 1, 2, 3, 4, 6, 8, 10, 12, 14, and 16).
Prediction of treatment response
Studies with multi-episode patients have successfully used percent reduction in BPRS or PANSS scores from baseline after a few weeks of treatment to predict treatment response or non-response at study completion11–17. The SADS-C+PD has detailed assessments of psychotic symptoms, but also of several other symptom domains not included in the BPRS or PANSS. To examine whether the prediction methods based upon change in symptoms assessed by the BPRS or PANSS instruments that are useful with multi-episode patients are applicable with first episode patients, we derived a total symptom severity score by adding the scores on the subset of SADS-C+PD items that correspond to items on the BPRS. These items were: concern with bodily functions, self-reproach, depression, severity of hallucinations, elevated mood, psychic anxiety, agitation, subjective anger, psychomotor retardation, impaired understandability, grandiosity, distrustfulness, severity of delusions. Most SADS-C+PD items are rated on a severity scale from 1 (not at all) to 6 (extreme); the depression and distrustfulness items have an extra scale point (7) for particularly prominent symptoms.
For the prediction analyses, we focused upon three time points: 2, 4 and 8 weeks of treatment. Week 2 was chosen based upon findings from studies with multi-episode subjects13, 14, 16 that have found lack of improvement after two weeks of treatment to predict lack of response to prolonged treatment. Week 4 and week 8 were chosen to mirror clinical practice since clinicians often evaluate patients monthly. In making decisions about continuing treatment at a particular week in treatment, clinicians focus upon those patients who remain symptomatic (as the proper course of action for patients who have improved adequately is clear). Therefore, we decided to include in the prediction of response analysis, including the receiver operating characteristic (ROC) curve analysis, only patients who did not fulfill our stringent response criteria at the weeks of interest. Thus the sample for the prediction analysis at week 2 consisted of subjects who remained symptomatic after 2 weeks of treatment; subjects who met response criteria before 2 weeks of treatment were not included in this prediction analysis. Samples for the 4 and 8 week analyses were similarly selected.
For the three time points of interest, we performed a logistic regression analysis using response status at week 16 as the dependent variable and the percent reduction in symptom severity score at the appropriate study week (2, 4, or 8) from baseline values as the independent variable. If a significant effect of percent symptom reduction was found, ROC curves were derived to aid in detecting levels of percent symptom reduction that would be clinically useful as a predictor of response by 16 weeks of treatment.
Studies with multi-episode subjects often have reported sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for 20% reduction of symptoms as an early predictor of ultimate response to treatment. To aid readers in comparing our results with earlier investigations, we calculated these variables based upon a 20% reduction in our symptom scores. Sensitivity was defined as the percentage of subjects who met response criteria by week 16 who achieved a 20% reduction or more on the symptom severity score compared with baseline at the specified week. Specificity was defined as the percentage of subjects who did not meet response criteria by week 16 who had less than a 20% reduction on the symptom severity score compared with baseline at the specified week. PPV was defined as the percentage of subjects who achieved a 20% reduction or more on the symptom severity score compared with baseline at the specified week who met response criteria by week 16. NPV was defined as the percentage of subjects who had less than a 20% reduction on the symptom severity score compared with baseline at the specified week who did not meet response criteria by week 16.
Results
Subjects and protocol implementation
As reported previously 7 the 112 subjects were young (mean age 23 years, SD=5), mostly male (70%), of diverse ethnic backgrounds (54% African-American; 20% Caucasian; 13% Hispanic; 6% Asian and 7% Other groups) and usually from lower class to low middle class socioeconomic backgrounds. Subjects had had psychotic symptoms for an average of slightly over two years before study entry (mean: 113.1 weeks, SD=158.8). The mean SADS-C+PD severity score for hallucinations was 4.6 (SD=1.5) and 5.3 (SD=0.8) for delusions. These scores indicate that subjects had prominent psychotic symptoms at study entry. For example, the scale anchor for a 5 severity rating for the severity of delusions item is “delusion has a significant effect on his actions; e.g. often asks family to forgive his sins, preoccupied with belief that he is a new Messiah”. Also reflecting severe symptoms at study entry, the mean score on the Global Assessment Scale (GAS)22 was 24.3 (SD=6.9). At entry, 87 subjects (78%) were antipsychotic medication naïve, and 15 (13%) had only 1 to 7 days of lifetime antipsychotic medication use.
Eighty-one (72%) of the 112 subjects completed 4 months of study participation. Olanzapine and risperidone subjects had similar lengths of study participation during the trial (log-rank test, chi-square = 0.10, df=1, p<0.75); mean length of participation was 11.5 (95% CI: 10.21, 12.76) weeks with olanzapine and 12.05 (95% CI: 11.53, 12.57) weeks with risperidone. The mean modal daily dose was 11.8 (SD=5.4) mg for olanzapine-treated subjects and 3.9 (SD=1.5) mg for risperidone-treated subjects.
Cumulative response rates by week
Cumulative response rates for olanzapine and risperidone treated subjects did not differ. Cumulative response for these analyses was therefore calculated for the entire sample (table 1) and not for individual medication groups. Confidence intervals for estimated cumulative response at week 8 and week 16 were distinct. If the trial had stopped at week 8, one would have obtained an estimated response rate of 39.59% (95% CI: 29.77% – 49.41%) compared with an estimated cumulative response rate of 65.19% (95% CI: 55.11% – 75.27%) at week 16. The confidence intervals for estimated cumulative response overlapped between weeks 10, 12, 14 and 16. The estimated response did increase approximately 5 to 6 percentage points between each of 2 week interval between week 10 and 16 resulting in an estimated cumulative response rate at week 10 of 48.4% and 65.2% at week 16.
Table 1.
Cumulative response rate by study week. (N=112)
| time to first response Week | Response % | 95% CI |
|---|---|---|
| 1 | 2.8% | 0%–5.9% |
| 2 | 12.1% | 5.8%–18.4% |
| 3 | 17.8% | 10.4%–25.3% |
| 4 | 21.8% | 13.7%–29.8% |
| 6 | 32.0% | 22.8%–41.3% |
| 8 | 39.6% | 29.8%–49.4% |
| 10 | 48.4% | 38.2%–58.6% |
| 12 | 54.4% | 44.1%–64.7% |
| 14 | 60.4% | 50.1%–70.7% |
| 16 | 65.2% | 55.1%–75.3% |
Computed using standard survival methods
Prediction of response
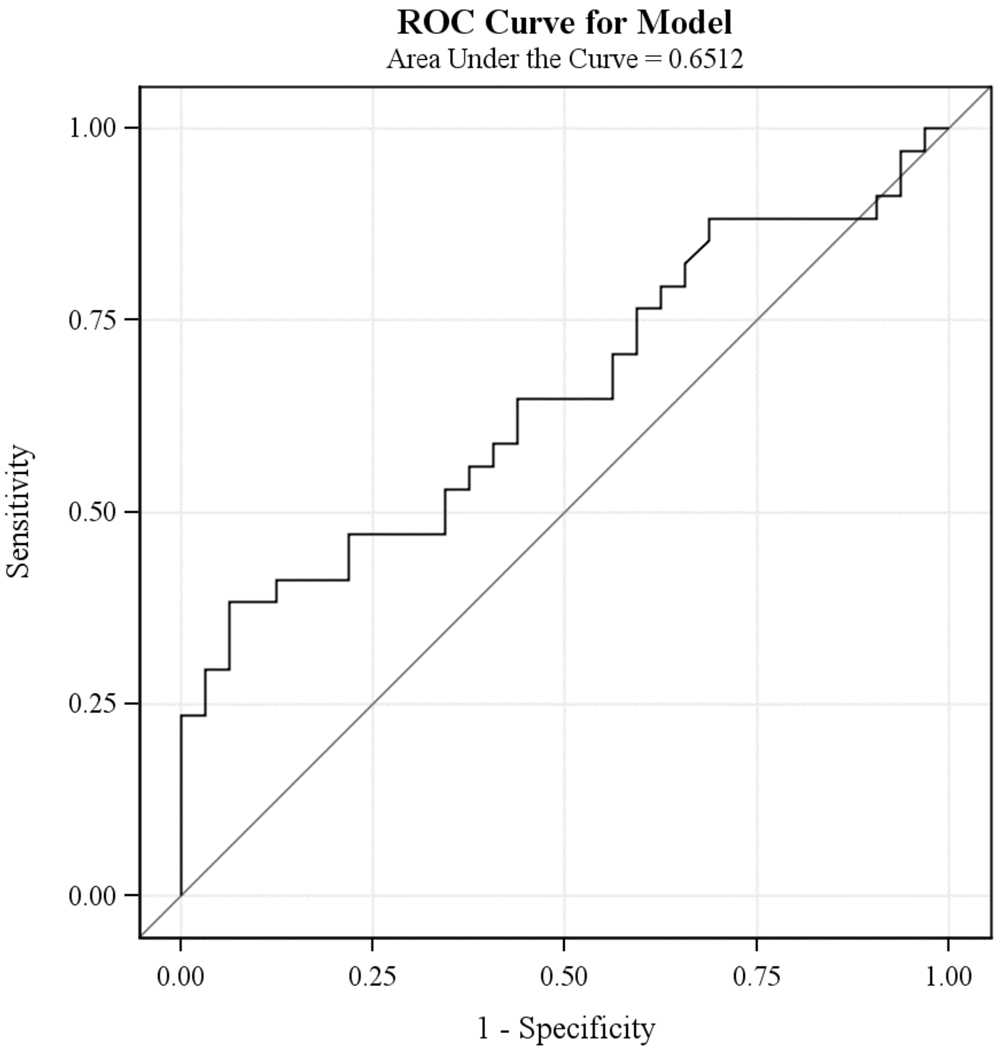
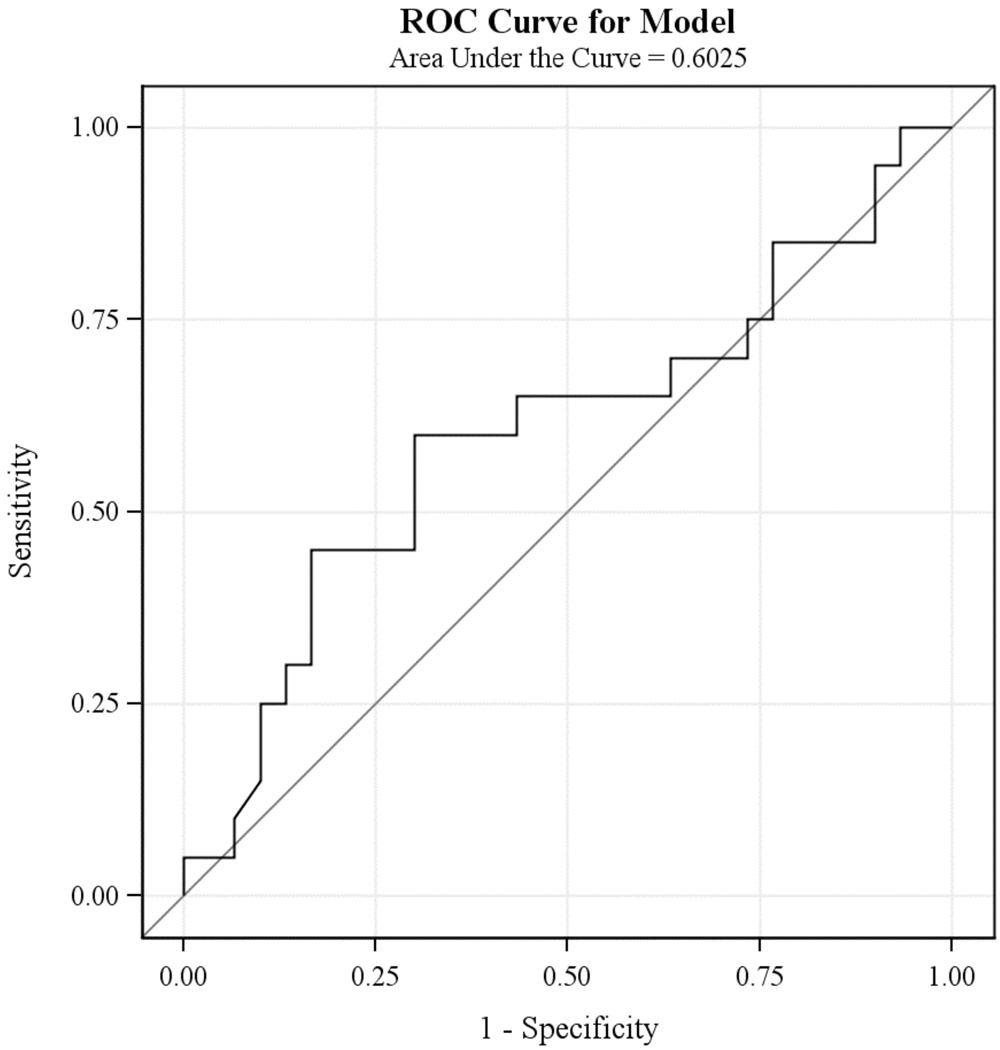
The logistic regression analyses revealed that the percent reduction in symptom severity score from baseline values at study week 4 (Chi-square = 3.96; df = 1, p<0.05), but not at study week 2 (Chi-square = 1.95; df = 1, p < 0.16) or week 8 (Chi-square = 1.97; df = 1, p < 0.16), was associated with responder status at week 16. However, the odds ratio for the estimated effect of percent reduction in symptom severity at week 4 was only 1.03, with the 95% confidence interval (1.00; 1.05) containing 1.00. Consistent with these results, inspection of ROC curves did not suggest any level of percent reduction of symptoms at week 4 (figure 1) or week 8 (figure 2) that would be clinically useful as a predictor of response at week 16. As an example, using the cutoff of 20% reduction in symptoms that is widely used in studies of multi-episode patients as a predictor of week 16 response status results in a sensitivity of 61.8% (43.6%–77.8%); specificity of 56.3% (37.7%–73.6%); PPV of 60% (42.1%–76.1%) and NPV of 58.1% (39.1%–75.5%) (Table 2).
Figure 1.
Receiver Operating Characteristics Curves: Percent Symptom Reduction from Baseline at Week 4 and Response at Week 16
Figure 2.
Receiver Operating Characteristics Curves: Percent Symptom Reduction from Baseline at Week 8 and Response at Week 16
Table 2.
Twenty percent reduction in symptom severity from baseline at study weeks 2, 4 and 8 as a predictor of response at week 16
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|
| Study Week | ||||
| Week 2 (n=85) | 51.1 (35.8%–66.3%) | 65 (48.3%–79.4%) | 62.2 (44.8%–77.5%) | 54.2 (39.2%–68.6%) |
| Week 4 (n=66) | 61.8 (43.6%–77.8%) | 56.3 (37.7%–73.6%) | 60 (42.1%–76.1%) | 58.1 (39.1%–75.5%) |
| Week 8 (n=50) | 65 (40.8%–84.6%) | 50 (31.3%–68.7%) | 46.4 (27.5%–66.1%) | 68.2 (45.1%–86.1%) |
Discussion
A variety of factors must be considered in choosing the optimum length for an antipsychotic trial for either clinical or research purposes. Clinicians and researchers must balance the negative consequences of prematurely terminating a trial (e.g. patients being incorrectly assessed as treatment resistant, exposing patients to potential difficulties related to switching treatments unnecessarily) with the negative consequences of keeping patients on a treatment that will ultimately be ineffective. For clinicians and researchers making these decisions, our data are important as they suggest that longer treatment trials should be considered as a substantial percent of first episode patients will respond after prolonged treatment with a single antipsychotic agent. Emsley and colleagues 2 found that 11.5% percent of responders (defined as ≥ 20% reduction from baseline in the PANSS total score) in their trial responded after week 8. Our results, based upon more stringent response criteria requiring absence of substantial positive symptoms, confirm the finding that a substantial number of patients achieve response only after 8 weeks of treatment. In our study, cumulative response increased from 39.6% (29.8%–49.4%) at week 8 to 65.2% (55.1%–75.3%) at week 16. Of note, our results suggest that some patients respond during the period from week 12 to week 16, a period which was not examined in other first episode studies with stringent response criteria. In our study, there was an increase of almost 11 percentage points in the estimated cumulative response rates between week 12 and 16.
A crucial question for assessing trial length is the desired outcome of treatment. For research purposes this is determined by the response criteria selected. Studies of schizophrenia have often defined response as percent reduction (often 20%) from baseline values in total scores of scales such as the BPRS or PANSS. Studies specific to early phase subjects such as ours have often employed more stringent response criteria as substantial resolution of symptoms is the goal with young subjects first starting treatment. Recently, there has been increased interest in the field in more stringent response criteria in studies with subjects at all illness phases. The recently proposed criteria for remission (Andreasen’s et al 23) require a similar substantial level in absolute improvement in positive symptoms as our response criteria (although it should be noted that the remission criteria also require improvement in negative symptoms and also require improvement to be sustained over a longer period).
Studies with multi-episode patients have demonstrated the utility of using limited improvement early in treatment to identify subjects who will not respond to a longer trial of antipsychotics 11–17. Unfortunately, our data suggest that with a first episode population these methods do not classify subjects with enough accuracy to be clinically useful. We examined percent change from baseline at three time points (weeks 2, 4 and 8) as a predictor of response at week 16 and found the best prediction at week 4. Even at the week 4 time point, examination of the ROC curves did not suggest any level of percent reduction of symptoms at week 4 that would be clinically useful as a predictor of later response. For example, a clinician might assume that a subject who had not achieved a 20% reduction in symptoms from baseline values by week 4 would not improve if continued on the same treatment for a full 16 weeks. However, our data suggest that this assumption would be incorrect with many patients as approximately 40% of subjects who had less than a 20% reduction in symptoms by week 4 meet stringent response criteria by week 16.
The methodology of our early prediction analyses differed from those of prior studies with multi-episode patients. Studies with multi-episode patients have examined all subjects (including those with substantial improvement) at a particular study week early in treatment in relation to response at the trial end13–17. Our methods instead examined only those subjects who had not met response criteria by a particular week early in treatment in relation to ultimate treatment outcome. We chose this analytic strategy because it models clinical practice. Clinicians do not usually consider new treatment options for patients who have responded to a particular medication. The clinical question instead is what to do for patients who have not responded by a particular week of treatment. Our analyses therefore focused on this patient group.
The underlying mechanism for the differences between first episode and multi-episode patients in suggested trial length and the utility of clinical prediction of response is unknown. The fact that there are differences is consistent with accumulated data that first episode and multi-episode patients differ in some aspects of response to antipsychotic treatment. Although direct comparisons are lacking, the response rates reported in treatment studies with first episode patients are markedly better than the usual response rates in studies of multi-episode patients 1, 24–26.
Our study has limitations. First, our study provided treatment with olanzapine or risperidone; it is unknown how generalizable our findings are to first episode patients being treated with other antipsychotics. Second, despite the use of survival analysis, our study results may under estimate the 16-week response rate due to the 28% subjects who left the study prior to Week 16 (e.g. subjects who withdraw from study or treatment, subjects for whom treatment was changed). Since these subjects had a shorter observation period, they had less time to achieve response. Third, our study found that a substantial percentage of subjects who had very limited improvement during the first weeks of treatment meet full response criteria if treated with the same agent for 16 weeks. Our study did not address whether a larger percentage of these subjects would have met response criteria if their medication had been switched after the first few weeks of treatment with the initial agent. Kinon and colleagues 27 compared 12 week outcomes of multi-episode subjects who had limited improvement after 2 weeks of treatment with risperidone. These subjects were randomly assigned to continue on risperidone or be switched to olanzapine. Subjects switched to olanzapine had a small improvement in PANSS scores by study end compared with subjects who remained on risperidone. Categorical response rates based upon either a 20%, 30% or 40% reduction in PANSS total score at end point did not differ between groups although a higher proportion of subjects switched to olanzapine attained a 50% reduction in symptoms. The generalizability of these findings to first episode patients is unknown, especially given the overall differences in response patterns between first episode and multi-episode patients. We are not aware of any prospective study with a first episode sample that has compared continuation versus early switching strategies. Finally, our study only examined controlled treatment over 16 weeks. We found a substantial number of subjects who responded between weeks 12 and 16. This raises the currently unanswered question of whether additional subjects who had not responded by 16 weeks of treatment would have responded if treated for longer than 16 weeks with their original agent.
Early prediction of response with first episode patients remains an important question. Our results suggest that clinical variables alone may not provide clinically useful levels of prediction. Studies with genetic 28, imaging 29 and physiological 30 assessments have demonstrated specific predictors of treatment response with first episode samples. Whether biological and clinical predictors can be combined into useful predictor models that can be adapted to clinical settings is an important question for future study.
Acknowledgements
We wish to thank Margaret G. Woerner, Ph.D (no current institutional affiliation), Nina R. Schooler Ph.D (The Zucker Hillside Hospital, Glen Oaks, NY) Handan Gunduz-Bruce M.D (Yale school of Medicine, New Haven, CT), Rachel Miller (NIMH, Bethesda, MD), L.C.S.W, Raman C. Patel, M.D. (Bronx-Lebanon Hospital Center, Bronx, NY) for their contributions to the study.
Funding of this study was provided by National Institute of Health grants R01MH 60004 (Dr. Robinson), P30MH074543 (Dr. Kane), M01RR018535 (Feinstein Institute for Medical Research General Clinical Research Center) and NIDA K23 DA015541 (Dr. Sevy).
Footnotes
This work was presented as a poster at the ACNP meeting, Hollywood. Fl. December 9th, 2009.
Disclosures:
Dr. Robinson has received research support from Lilly, Janssen, and Bristol-Myers Squibb. Dr. John Kane serves as a Consultant and/or Advisory Board member for BMS, Otsuka, Lilly, Janssen, Pfizer, Wyeth, Vanda, GSK, Lundbeck, J & J, PGX Healthcare, Proteus, a shareholder of MedAvante, and serves on Speaker’s Bureau for BMS, Janssen, Astra Zeneca, and Lilly. Dr. Sevy has served as a consultant for Abbott laboratories. Dr. Gallego, Ms. McCormack, and Dr. Lesser report no competing interests. Ms. Napolitano had no competing interests when she performed her work reported in the manuscript.
Dr. Woerner, Dr. Gunduz-Bruce, Dr. Patel and Ms. Miller report no competing interest. Dr. Schooler has been a consultant for Hoffman La Roche and Lundbeck and has received grant support from Astra-Zeneca, Janssen, Pfizer, Lilly, Novartis and BMS. She has been a speaker or has been an advisory board member for Abbot, Lilly, Roche, Janssen, Lundbeck and Merck.
ClinicalTrials.gov identifier: Preventing Morbidity in First-Episode Schizophrenia NCT00000374. http://clinicaltrials.gov/ct2/show/NCT00000374.
Reference List
- 1.Lieberman J, Jody D, Geisler S, et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry. 1993 May;50(5):369–376. doi: 10.1001/archpsyc.1993.01820170047006. [DOI] [PubMed] [Google Scholar]
- 2.Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006 Apr;163(4):743–745. doi: 10.1176/ajp.2006.163.4.743. [DOI] [PubMed] [Google Scholar]
- 3.Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Perkins D, Lieberman J, Gu H, et al. Predictors of antipsychotic treatment response in patients with first-episode schizophrenia, schizoaffective and schizophreniform disorders. Br J Psychiatry. 2004 Jul;185:18–24. doi: 10.1192/bjp.185.1.18. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007 Jul;164(7):1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003 May;28(5):995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006 Dec;163(12):2096–2102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003 Aug;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 9.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008 Mar 29;371(9618):1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 10.Emsley R, Oosthuizen PP, Kidd M, Koen L, Niehaus DJ, Turner HJ. Remission in first-episode psychosis: predictor variables and symptom improvement patterns. J Clin Psychiatry. 2006 Nov;67(11):1707–1712. doi: 10.4088/jcp.v67n1106. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Chou LS, Hsu CY, Chen YS, Lane HY. Early prediction of clinical response in schizophrenia patients receiving the atypical antipsychotic zotepine. J Clin Psychiatry. 2007 Oct;68(10):1522–1527. doi: 10.4088/jcp.v68n1008. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Ascher-Svanum H, Stauffer V, Kinon BJ, Kollack-Walker S, Ruberg S. Optimal thresholds of early response to atypical antipsychotics: application of signal detection methods. Schizophr Res. 2009 Aug;113(1):34–40. doi: 10.1016/j.schres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Ascher-Svanum H, Nyhuis AW, Faries DE, Kinon BJ, Baker RW, Shekhar A. Clinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr Bull. 2008 Nov;34(6):1163–1171. doi: 10.1093/schbul/sbm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinon BJ, Chen L, Ascher-Svanum H, et al. Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res. 2008 Jul;102(1–3):230–240. doi: 10.1016/j.schres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Correll CU, Malhotra AK, Kaushik S, McMeniman M, Kane JM. Early prediction of antipsychotic response in schizophrenia. Am J Psychiatry. 2003 Nov;160(11):2063–2065. doi: 10.1176/appi.ajp.160.11.2063. [DOI] [PubMed] [Google Scholar]
- 16.Leucht S, Shamsi SA, Busch R, Kissling W, Kane JM. Predicting antipsychotic drug response - replication and extension to six weeks in an international olanzapine study. Schizophr Res. 2008 Apr;101(1–3):312–319. doi: 10.1016/j.schres.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Leucht S, Busch R, Kissling W, Kane JM. Early prediction of antipsychotic nonresponse among patients with schizophrenia. J Clin Psychiatry. 2007 Mar;68(3):352–360. doi: 10.4088/jcp.v68n0301. [DOI] [PubMed] [Google Scholar]
- 18.DSM-IV APATFo. DSM-IV: diagnostic and statistical manual of mental disorders. 1994 [Google Scholar]
- 19.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978 Jul;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 20.Robinson D, Woerner M, Schooler N. Intervention research in psychosis: issues related to clinical assessment. Schizophr Bull. 2000;26(3):551–556. doi: 10.1093/oxfordjournals.schbul.a033476. [DOI] [PubMed] [Google Scholar]
- 21.Guy W, Bonato R. CGI: Clinical global impressions. ECDEU Assessment Manual for Psychopharmacology. 1976:217–222. Revised. [Google Scholar]
- 22.Endicott J, Spitzer R, Fleiss J, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33(6):766. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005 Mar;162(3):441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 24.Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009 Oct 1;23(10):837–855. doi: 10.2165/11314280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE. The natural course of schizophrenia: a review of first-admission studies. Schizophr Bull. 1992;18(2):185–207. doi: 10.1093/schbul/18.2.185. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman JA, Alvir JM, Woerner M, et al. Prospective study of psychobiology in first-episode schizophrenia at Hillside Hospital. Schizophr Bull. 1992;18(3):351–371. doi: 10.1093/schbul/18.3.351. [DOI] [PubMed] [Google Scholar]
- 27.Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. Jan;35(2):581–590. doi: 10.1038/npp.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lencz T, Robinson DG, Xu K, et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006 Mar;163(3):529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 29.Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naive first-episode psychosis patients. Schizophr Res. 2009 Aug;113(1):65–71. doi: 10.1016/j.schres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Merlo MC, Kleinlogel H, Koukkou M. Differences in the EEG profiles of early and late responders to antipsychotic treatment in first-episode, drug-naive psychotic patients. Schizophr Res. 1998 Apr 10;30(3):221–228. doi: 10.1016/s0920-9964(97)00156-4. [DOI] [PubMed] [Google Scholar]