Abstract
Background and Purpose
Normobaric hyperoxia (NBO) has been shown to exert neuroprotective effects against cerebral ischemia and restore penumbral oxygenation. Inspired by recent reports on postconditioning with intermittent occlusions of cerebral artery, we tested the hypothesis that intermittent NBO (iNBO) may cause oscillation of cerebral oxygenation, and thereby elicit repetitive interruptions to reperfusion, leading to attenuated ischemia/reperfusion damage after transient focal cerebral ischemia in rats.
Methods
Rats were subjected to 90 minutes of middle cerebral artery occlusion. During ischemia, animals received either air, iNBO (four cycles of 3 minutes of NBO and 2 minutes of air), continuous NBO (cNBO, 75 minutes), short NBO (sNBO, 18 minutes), or a combination of iNBO and cNBO. Infarct volume and neurologic score were evaluated at 24 and 72 hours after ischemia. Production of superoxide was assessed by the hydroethidine method, and the expression of Akt and phosphorylated Akt was examined by Western blot.
Results
iNBO and cNBO had similar effect in reducing infarct volume and neurologic deficit at 24 hours after ischemia, while at 72 hours the neuroprotection exerted by iNBO was greater than cNBO. Combining iNBO and cNBO produced no greater protection, and sNBO failed to provide neuroprotection. Both iNBO and cNBO attenuated superoxide production. Importantly, prolonged activation of Akt was observed in the iNBO group, and neuroprotection by iNBO was partly eliminated by inhibition of Akt activation.
Conclusions
iNBO may represent a novel form of postconditioning, and this neuroprotection is likely mediated by attenuating superoxide generation and activation of the Akt pathway.
Keywords: Akt, intermittent normobaric hyperoxia, neuroprotection, postconditioning
Stroke is a leading cause of mortality and adult disability.1,2 After an ischemic stroke occurs, brain cells are deprived of the glucose and oxygen they need to function. On the other hand, during reperfusion, restoration of oxygen and glucose supply can trigger a parallel cascade of deleterious biochemical processes.3 Therefore, better understanding of reperfusion injury processes will aid the development of more effective therapy for ischemic stroke. Recently, a concept in the field of cerebral ischemia has emerged by which modified reperfusion subsequent to a prolonged ischemic episode can confer neuroprotection, a phenomenon termed ‘postconditioning’. In 2006, it was first demonstrated that ischemic postconditioning with repetitive interruptions of reperfusion reduced brain infarct size in rats.4 Although evolved from preconditioning, ischemic postconditioning represents a relatively novel approach to cerebral protection and can be applied after the onset of ischemia.
It is widely accepted that improving brain tissue oxygenation is a logical and important stroke treatment strategy.5 Animal studies from our lab and others have documented that normobaric hyperoxia (NBO) has substantial neuroprotective effects in acute cerebral ischemia.6-8 Furthermore, our previous results demonstrated that NBO rapidly restored penumbral oxygenation status to the pre-ischemic level.7,9 Inspired by the reported ‘postconditioning’ phenomenon, restoration of oxygen during ischemia with NBO can be considered as prior conditioning to reperfusion. In this way, we speculated that intermittent NBO (iNBO) treatment may cause oscillation of cerebral tissue oxygenation during ischemia, and then alter the patterns of reperfusion as a novel form of conditioning; thus, rendering the brain cells more resistant to the subsequent reperfusion insult.
Reperfusion injury is mediated, in part, by the overproduction of reactive oxygen species (ROS).10 Although postconditioning is associated with reduced generation of ROS, little is known about the protective mechanisms responsible for postconditioning. Some pilot studies have shown that the activation of phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway may mediate the protective effects of postconditioning in the brain.11 Akt activation requires PI3K dependent phosphorylation at two sites, threonine 308 (Thr308) and serine 473 (Ser473).12 It is generally agreed that Akt pathway contributes to neuronal survival after stroke and phosphorylated Akt (P-Akt) at Ser473 temporarily increases after reperfusion in focal ischemia.13 More importantly, postconditioning increases Akt phosphorylation, and Akt inhibition partially blocks the protective effects of postconditioning.11,14
In this study, a focal ischemia model in rats was used to test our hypothesis that iNBO treatment reduces reperfusion injury after ischemic stroke through activation of the Akt pathway.
Materials and Methods
Focal Cerebral Ischemia and Reperfusion
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA; n=198) weighing 290–320g were used in these experiments. The UNM Laboratory Animal Care and Use Committee approved all experimental protocols. For all surgical procedures, rats were anesthetized with isoflurane (5% for induction, 2% for maintenance) in N2O:O2 (70%:30%). Temperature was maintained at 37±0.5°C with a heating pad. Transient focal ischemia was induced by intraluminal middle cerebral artery occlusion (MCAO) as previously described.7 In brief, a 4.0 nylon monofilament suture coated with silicon rubber was inserted into the internal carotid artery to occlude MCA. After 90-minute ischemia, the suture was gently withdrawn to allow for cerebral reperfusion. Sham animals were subjected to the equivalent surgical preparation, but the suture was not advanced beyond the internal carotid bifurcation.
Experimental Protocol
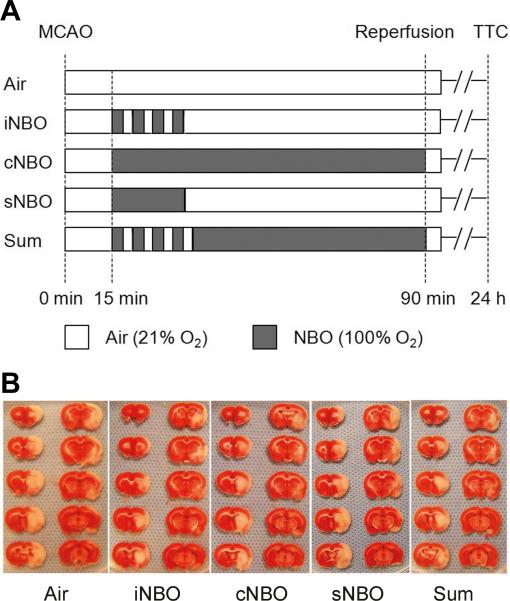
Rats were randomized into five groups, and treatment was performed according to one of the five protocols shown in Figure 1A. Air group was given air during 90 minutes of MCAO; iNBO group received four cycles of 3 minutes of NBO (100% O2) and 2 minutes of air (21% O2), followed by air until the end of MCAO; in the continuous NBO (cNBO) group, rats was continuously given NBO until reperfusion (maintained for 75 minutes). The duration of NBO/air exposure (3 min/2 min) was selected based on several considerations, including rapid response of cerebral tissue oxygenation to NBO,9 the ischemia/reperfusion duration cycles used for the postconditioning study,4 and our preliminary experimental testing with iNBO. In the short NBO (sNBO) group, rats received continuous NBO for 18 minutes (the same total duration of four cycles of NBO/Air as in iNBO), followed by air. For the iNBO + cNBO combination (Sum) group, rats received four cycles of 3 minutes of NBO and 2 minutes of air, followed by continuous NBO until reperfusion. All NBO treatment (100% O2 at ambient pressure) was initiated 15 minutes after MCAO onset. After reperfusion, these animals received no further therapy and breathed room air.
Figure 1.
Effects of various permutations of NBO treatment at 24 hr after 90 min MCAO. (A) Schematic overview of the experimental protocols; (B) Representative photographs of TTC-stained brain sections from each group of rats; (C) Quantification of infarct volume expressed as a percent of infarcted tissue to total brain. n=8; (D) Neurologic scores that were assessed immediately before the animals were sacrificed for infarct size measurement. n=5. *p<0.05, compared with Air group.
Evaluation of the Infarct Volume and Neurologic Score
2,3,5-triphenyltetrazolium chloride (TTC) staining was performed to determine the infarct volume at 24 and 72 hours after MCAO, as previously described.9 Infarction volume was calculated using Image Pro Plus software and expressed as the percent of the infarcted tissue as compared with the total brain. Neurologic deficit scores were evaluated 24 and 72 hours after MCAO based on Rogers’ eight-point scale by a blinded observer, as previously described.7 Neurologic deficient scores were analyzed by Mann–Whitney U-test.
In Situ Detection of Superoxide Anion Production
Superoxide anion was detected with the use of hydroethidine (Het) as previously described15 with some modifications. Rats were administered intravenously 0.2mg Het (Molecular Probes, Eugene, OR, USA) 10 minutes before induction of ischemia, and killed 1 hour after reperfusion and transcardially perfused with icy PBS and 4% paraformaldehyde (PFA). Some animals with sham surgery were also injected with Het solution, which served as a control for baseline of superoxide radicals. Brains were removed and fixed in 4% PFA for 24 hours, and then sectioned into 50-micron sections with a vibratome. The sections were photographed with a fluorescent microscope at excitation=510 nm and emission=580 nm. Pictures were taken with the ×40 objective and regions of interest were placed on the ischemic penumbra. The intensity of fluorescence was semiquantitatively analyzed using ImageJ software and expressed as mean value per cell.
Western Blot Analysis of Akt and Phosphorylated Akt Expression
Brain tissues from the following four groups were prepared: (1) sham surgery without ischemia; (2) ischemia plus Air; (3) ischemia plus iNBO; (4) ischemia plus cNBO. In each experimental conditions, samples corresponding to the ischemic penumbra from ipsilateral and contralateral hemispheres were harvested at 15 minutes, 1 hour, or 22.5 hours after reperfusion. Protein extraction was performed as previously described.11 Afterwards, 40µg protein was loaded per lane onto SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Blots were probed with antibodies to phosphorylated Akt (P-Akt, Ser473) and Akt (Cell Signaling, Beverly, MA, USA), and β-actin was used as the loading control. Finally, membranes were scanned with a LI-COR Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE, USA). Optical densities of protein bands were measured by Photoshop CS2 (Adobe, San Jose, CA, USA), and values were normalized to the β-actin content and expressed as relative intensity.
Protein Kinase Inhibitor Study
To assess the role of protein kinase Akt in NBO neuroprotection, 10μL of the PI3K inhibitor LY294002 (10mmol/L) or vehicle was infused into the ventricular space 1 hour before ischemia, according to procedures described previously.16,17 Infarct size was measured 24 hours after stroke as described above.
Statistical Analysis
Data are expressed as mean±SD. One-way ANOVA was used to compare the protective effects of NBO on infarct size and fluorescence intensity of Het, followed by Scheffe post hoc test. For the Akt inhibition study and optical densities of protein bands from Western blots, differences were analyzed using two-way ANOVA with Student–Newman–Keuls test. P < 0.05 was considered statistically significant.
Results
Effects of Various Permutations of NBO and Air on Infarct Volume and Neurological Score after Stroke
Subjecting animals to 90 minutes MCAO resulted in a severe infarction when measured at 24 hrs post MCAO (Figure 1B, 1C). Four short cycles of iNBO (18 minutes total) reduced infarct volume by about 34%, which is equivalent to the degree of neuroprotection afforded by 75 min continuous cNBO. However, 18 minutes continuous NBO (i.e., sNBO) failed to decrease infarct volume. These results suggest that how NBO is administered is more critical than duration in terms of neuroprotective outcome. Interestingly, combining iNBO and cNBO (Sum group) did not produce greater protection than each treatment alone (Figure 1C). Very similar results were obtained for the neurological function assessment: both iNBO and cNBO reduced the neurological scores while sNBO did not, and combining iNBO and cNBO did not afford greater neuroprotection (Figure 1D). Therefore, sNBO and Sum groups were not included in the rest of the study.
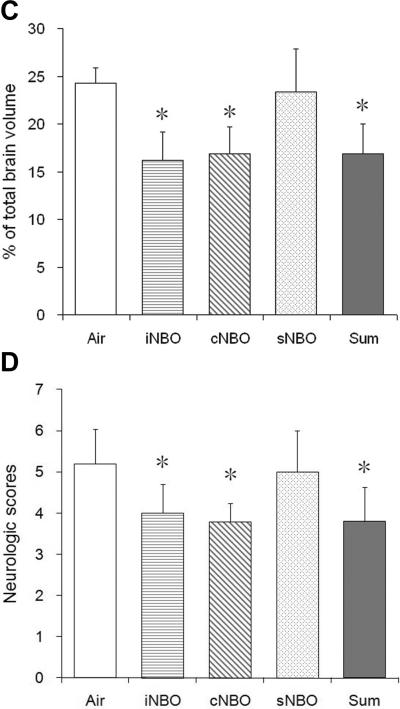
To determine whether iNBO results in extended neuroprotection, some animals were subjected to iNBO or cNBO treatment and then recovered for 72 hours. As shown in Figure 2A, more profound reduction in infarct volume was observed in the iNBO group compared with the Air group (P < 0.01). In agreement with our previous studies,7 cNBO treatment maintained the infarct reduction at 72 hours after MCAO. At this time point, the infarct size appeared to show an obvious decreasing trend in the iNBO group versus cNBO group, although it was not statistically significant (P = 0. 057). Importantly, a significant improvements (P < 0.05) in neurologic function were still found in the iNBO group, but not in the cNBO group (Figure 2B), although the neurologic scores for all groups improved considerably compared with those of 24 hours. These findings suggest that iNBO may provide better neuroprotection than cNBO at extended time.
Figure 2.
Effects of various permutations of NBO treatment at 72 hr after 90 min MCAO. (A) Quantification of infarct volume expressed as a percent of infarcted tissue to total brain. n=5; *p<0.05 and **p<0.01, respectively, compared to Air group. (B) Neurologic scores that were assessed immediately before the animals were sacrificed for infarct size measurement. n=5. *p<0.05, compared with the Air group.
To assess whether iNBO might cause any potential adverse effect, we also collected the mortality data for each treatment group. There were no differences in mortality among all groups on χ2 analysis: Air, 3/24; iNBO, 3/28; cNBO, 2/24; sNBO, 0/8; and Sum, 1/9. No rats in the sham group died. Additionally, using the concentration of thiobarbituric acid reactive substances (TBARS) in serum as an indicator of systemic oxidative stress, we found that there was no significant difference among the animal groups (data not shown). These results indicate that iNBO did not induce untoward stress to the animals.
Production of Superoxide at Early Stage of Reperfusion
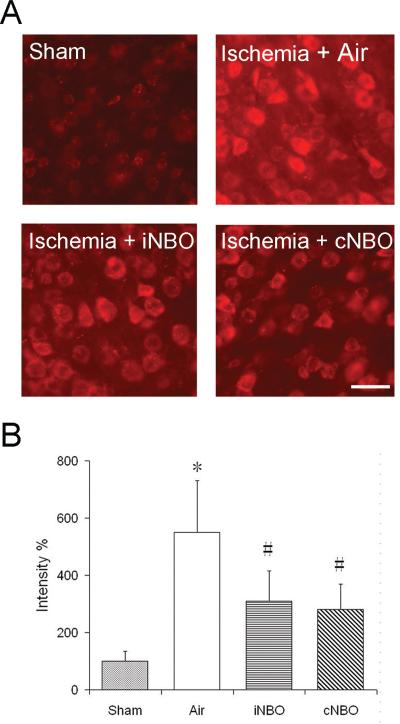
Figure 3A shows representative results of Het staining for superoxide in the ischemic penumbra. Few signals for Het staining were observed in brains subjected to sham surgery. Compared with air-treated brains with ischemia, these Het signals were markedly decreased after iNBO or cNBO treatment (Figure 3A, 3B), suggesting both patterns of NBO can inhibit superoxide production following reperfusion.
Figure 3.
iNBO treatment attenuates superoxide generation after reperfusion. (A) Representative photomicrographs of fluorescent staining of oxidized Het signals taken from ischemic penumbra tissue. Superoxide production is seen as bright red fluorescence signal. (B) Semi-quantitative analysis of red fluorescence emitted from Het. The fluorescence intensity was normalized to that of the sham brain. n=3; * p<0.01, compared to sham group; # p<0.01, compared to air-treated group; Scale bar=20 μm.
Changes in P-Akt and Akt Expression after ischemia/reperfusion
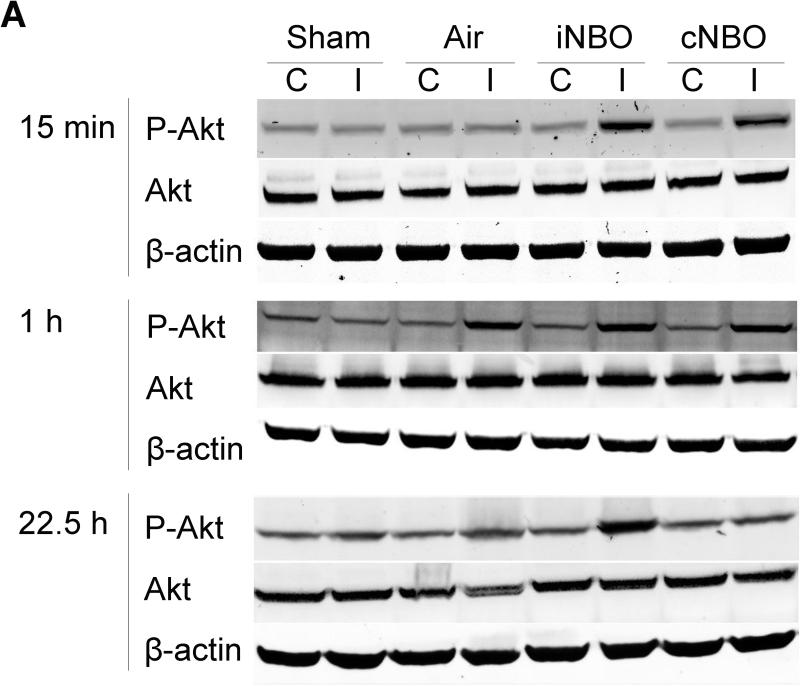
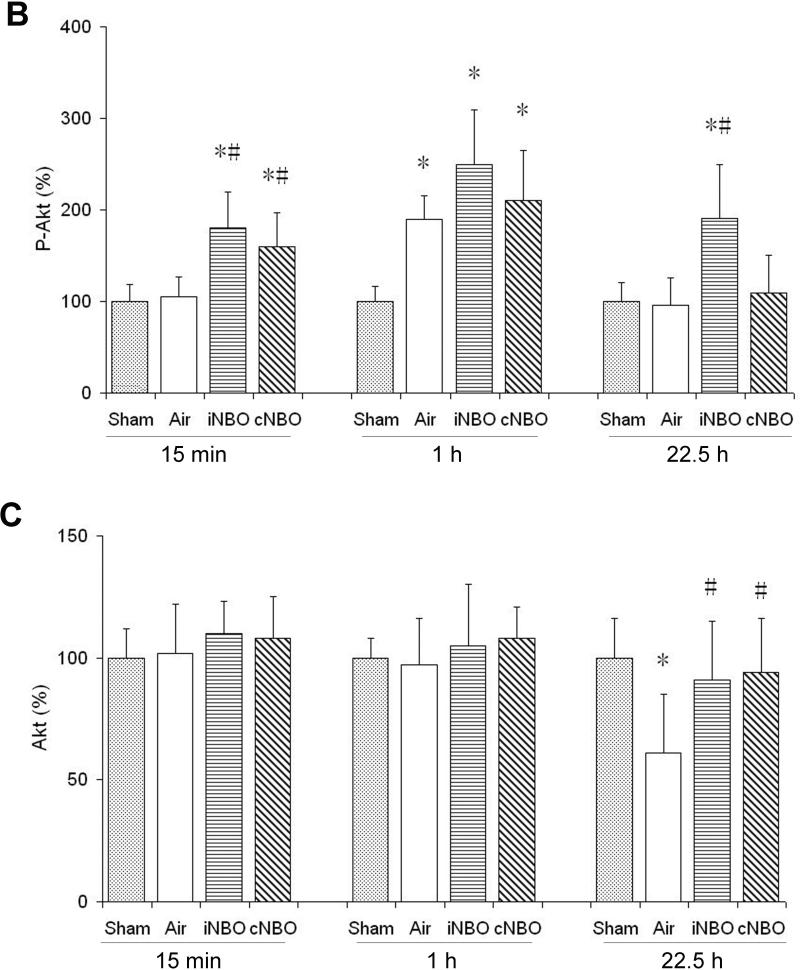
To investigate the molecular mechanism of neuroprotection by iNBO, western blot was used to analyze P-Akt and Akt expression. As shown in Figure 4A, P-Akt and Akt were constitutively expressed in the nonischemic sham brain. In the ischemic rats, P-Akt was significantly increased in the ipsilateral hemisphere at 1 hour after reperfusion, whereas it was decreased by 22.5 hours. In contrast, after iNBO or cNBO treatment, P-Akt was significantly up-regulated as early as 15 minutes after reperfusion. More importantly, P-Akt remained at an elevated level 22.5 hours after reperfusion only after iNBO treatment, but not cNBO (Figure 4A and 4B), suggesting that iNBO and cNBO differentially modulated levels of P-Akt after ischemia, and that iNBO resulted in the prolonged phosphorylation of Akt. Unlike P-Akt, Akt level was found to be unchanged at 15 minutes or 1 hour after reperfusion for all groups of animals. Interestingly, Akt level was decreased by 22.5 hours, which was restored with both iNBO and cNBO treatment (Figure 4A, 4C).
Figure 4.
Western blot analysis of P-Akt and Akt in rats. (A) Representative protein bands for P-Akt (Ser473), total Akt and β-actin from both the contralateral and ipsilateral penumbra at 15 minutes, 1 hour and 22.5 hours after reperfusion. (B) Quantitative analysis of relative abundance of P-Akt in the ipsilateral hemisphere. (C) Quantitative analysis of relative abundance of Akt in the ipsilateral hemisphere. The optical densities of all protein bands were analyzed using Photoshop. Relative optical densities of protein bands in ischemic rats were normalized to those in sham rats and calibrated with β-actin. C, Contralateral Non-ischemic Hemisphere; I, Ipsilateral Ischemic Hemisphere. n=4; * p<0.05, compared to sham group; # p<0.05, compared to air-treated group.
In contrast to the ipsilateral hemisphere, we did not observe a significant change in Akt expression or Akt phosphorylation in the contralateral side after either ischemia or NBO treatment. Additionally, no changes in levels of P-Akt and Akt were observed in the sham rats subjected to each type of NBO treatment (data not shown).
Effect of Akt Inhibition on neuroprotection exerted by iNBO and cNBO
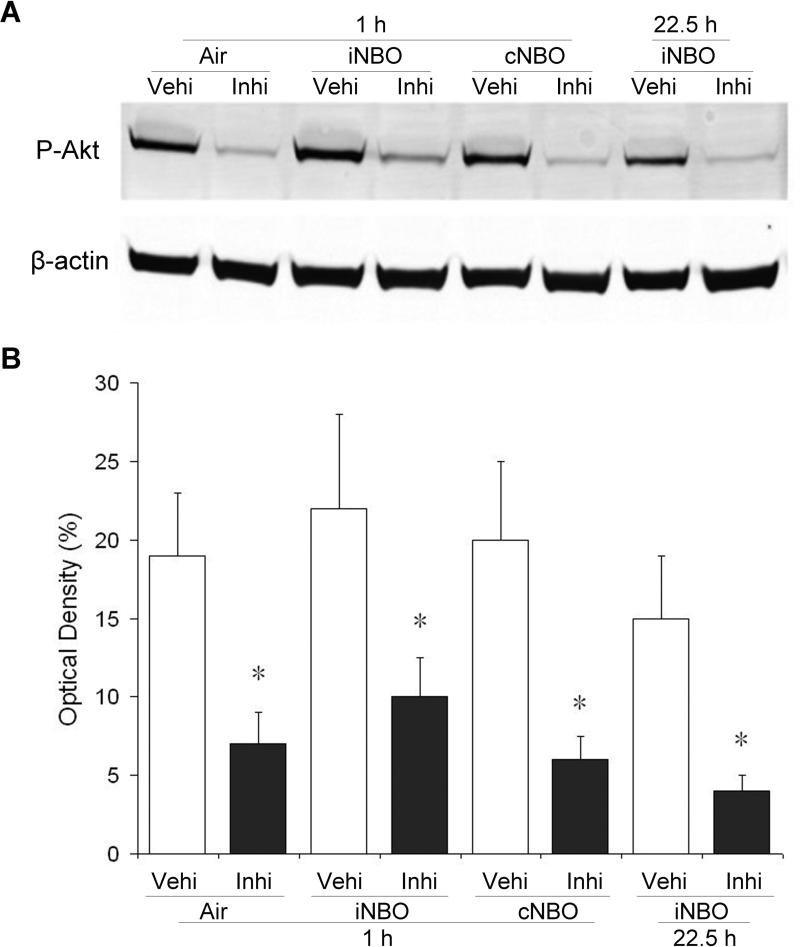
To further study the contribution of Akt activity to the protective effect by NBO, we examined the effects of inhibiting the activity of the upstream activator of Akt, PI3K, with the specific inhibitor LY294002,18 on phosphorylation levels of Akt and infarct size. As shown above in Figure 4, a significant P-Akt upregulation was observed 1 hour after reperfusion in the ipsilateral hemisphere of all animals groups following MCAO, as well as at 22.5 hours in the iNBO treatment group. Pretreatment of LY294002 abolished the elevation of Akt phosphorylation in all these animal groups (Figure 5). These findings suggest that inhibition of Akt activity partly eliminated the elevation of P-Akt induced by NBO.
Figure 5.
Pretreatment with PI3K inhibitor LY294002 inhibits phosphorylation of Akt in the ischemic penumbra. (A) Representative protein bands for P-Akt (Ser473) and β-actin from ischemic rats with intracerebroventricular injection of vehicle (Vehi) or inhibitor LY294002 (Inhi). (B) Quantitative analysis the phosphorylation of Akt. The optical density was expressed as the percentage of internal control band (β-actin). n=3; * p<0.05, compared to the vehicle-treated group.
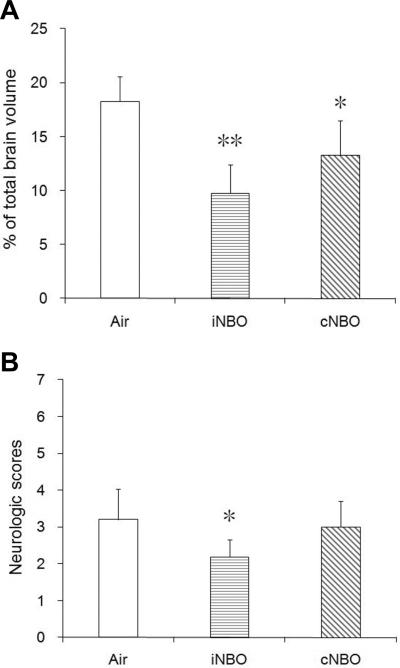
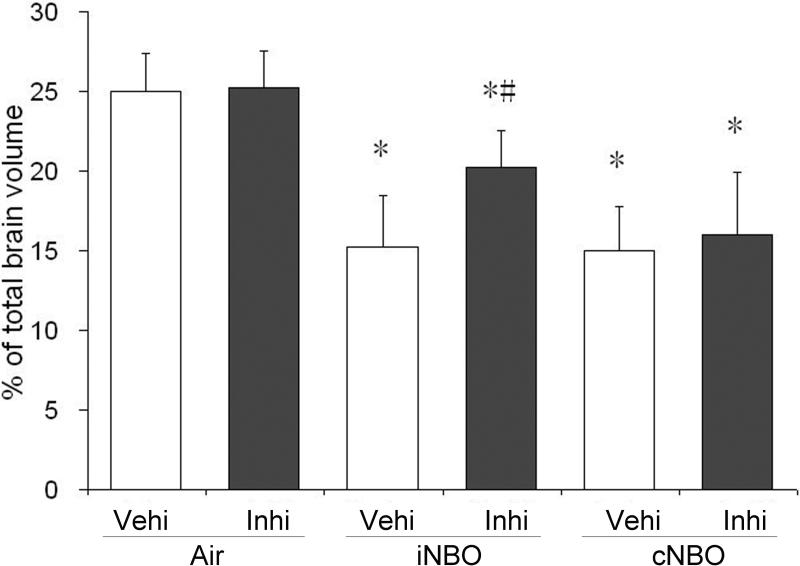
Next we evaluated the effects of Akt inhibition on infarct size after ischemia under different NBO treatment conditions. Administration of LY294002 partially abolished the protective effect of iNBO (Figure 6). However, inhibition of Akt did not affect the neuroprotective effect by cNBO. Furthermore, the use of LY294002 was not able to induce any significant change in the infarct volume of rats subjected to Air treatment. These results suggest that upregulation of P-Akt plays an important role in neuroprotective effect by iNBO, and that iNBO and cNBO may provide neuroprotection through different mechanisms.
Figure 6.
The PI3K inhibitor LY294002 partially abrogates the protective effect of iNBO. Animals were administered with inhibitor LY294002 (Inhi) or vehicle (Vehi) before ischemia. Infarct volume was determined 24 hours after ischemia. n=4; * p<0.05, compared to the air-treated group; # p<0.05, compared to iNBO vehicle group.
Discussion
It is now well established that reperfusion after ischemia can cause injury, therefore, manipulation of the reperfusion process may produce neuroprotection. Inspired by the postconditioning concept, NBO delivered during ischemia could be viewed as a prior interruption to reperfusion, which, in turn, increases the conditioning capacity to reperfusion injury. Since NBO treatment during ischemia can quickly increase penumbra tissue oxygenation,7 four short cycles of intermittent NBO/air treatment (iNBO), which is expected to cause oscillation of cerebral tissue oxygenation before reperfusion could be viewed as four episodes of post-ischemia conditioning. This hypothesis is examined in the present study. Application of iNBO (four cycles of 3 minutes of NBO and 2 minutes of air) provides effective neuroprotection against brain injury induced by transient MCAO, whereas sNBO with the same total duration (18 minutes as iNBO) fails to afford neuroprotection (Figure 1), suggesting that iNBO is not just simply providing oxygen to the oxygen-starved tissue, and that other mechanisms must be important also.
We investigated the underlying protective mechanisms of iNBO. In our experimental stroke model, hyperoxia salvaged ischemic brain tissue with a concomitant decrease in the generation of superoxide at 1 hour after reperfusion (Figure 3), which is consistent with the results reported previously.7,8 In the reported postconditioning study, a series of interruptions of reperfusion attenuated the amount of superoxide during early reperfusion after stroke.4 If the concept of postconditioning is extended, iNBO can be viewed as a process that brief episodes of interruptions are moved from after reperfusion to before reperfusion. Thus it is plausible that the protective effects of iNBO against reperfusion injury are likely to have some common mechanisms to postconditioning.
In the present study, a prolonged increase in P-Akt was observed after iNBO treatment, but not cNBO (Figure 4), which might explain why the improvement in infarct volume and neurologic function is better for iNBO than cNBO at 72 hrs after MCAO (Figure 2). Our study further demonstrated that inhibition of Akt activity with the selective PI3K inhibitor reduced the elevation of P-Akt in the groups treated with air, iNBO or cNBO (Figure 5); however, only the neuroprotective effect induced by iNBO was partly eliminated by pretreatment with the selective PI3K inhibitor (Figure 6). In this regard, iNBO is not simply a variation of NBO, but may serve as an ‘active process’ by activating prosurvival kinases such as PI3K/Akt pathway, induction of the antioxidant defenses, and elevation of various stress proteins and transcription factors, rendering the brain cells more resistant to the perturbation that occurs in the transition from ischemia to reperfusion.19-21 Alternatively, iNBO could induces intermittent hemodynamic changes that increases blood flow and volume to the ischemic region22, thus affording direct neuroprotection per se against the ongoing ischemia. Several experimental studies have reported possible mechanisms of benefit from hyperoxia, including directly increasing tissue oxygen,7 and improving aerobic metabolism and restoring mitochondrial function.23,24 Therefore, typical hyperoxia (for example cNBO) may be considered a ‘passive process’ that maintains neuronal survival and function by improving brain tissue oxygenation and regulating energy metabolism, which in turn decreases the deleterious effects caused by ischemia.
Akt activity is suggested to be upregulated by phosphorylation. Recent reports suggest that Akt is involved in the cell survival signaling pathway after transient cerebral ischemia.13 Moreover, postconditioning stimulus resulted in the prolonged activation of Akt and inhibition of Akt partly reversed the neuroprotective effect exerted by postconditioning11,14 consistent with our present results. The common activation pathways existing between iNBO and postconditioning support the concept that iNBO could be viewed as another pattern of interruption to reperfusion. Furthermore, levels of total Akt decreased 24 hours after stroke in the ischemic penumbra, and this decrease was prevented by NBO (Figure 4), but not by postconditioning14 suggesting that modulating the phosphorylation of Akt rather than its protein expression is implicated in the neuroprotection for ischemic stroke.
Results presented herein suggest that iNBO is a novel and attractive neuroprotective approach which has significant potential for clinical translation, especially since iNBO could be easily applied to stroke patients in a clinical setting. In the present study, the iNBO used here just included four cycles of 3 minutes of NBO and 2 minutes of air. It is conceivable that the pattern of iNBO could be optimized with different repetition number and duration to achieve even greater neuroprotection. On the other hand, judging by the recommendations from Stroke Therapy Academic Industry Roundtable (STAIR),25 the current study has several limitations, including lack of blinded randomization, lack of LDF monitoring to ensure similar degrees of ischemia, no physiological data obtained, and no power calculation performed. Further animal study is required to assess iNBO as a viable intervention strategy for clinical consideration.
In summary, iNBO can act as a novel form of conditioning through prior interruptions to reperfusion. The possible mechanism for the observed neuroprotection may involve reduction of superoxide and prolonged activation of Akt. Although triggered by the concept of ischemic postconditioning, iNBO may represent another form of ‘active’ conditioning, which will lead to a greater understanding of the potential of NBO and endogenous protection.
Acknowledgments
Sources of Funding
This work was supported in part by grants from NIH (P20RR15636, P30RR031156, and R01AG031725).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 2.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–32. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- 3.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–56. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–21. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 5.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–42. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–5. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–84. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 8.Singhal AB, Wang X, Sumii T, Mori T, Lo EH. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002;22:861–8. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–9. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 11.Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–41. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 12.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–4. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–70. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, et al. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–55. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noshita N, Sugawara T, Lewén A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003;34:1513–8. doi: 10.1161/01.STR.0000072986.46924.F4. [DOI] [PubMed] [Google Scholar]
- 16.Noshita N, Lewén A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–50. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 19.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 21.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–2. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 22.Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002;58:945–52. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- 23.Singhal AB, Ratai E, Benner T, Vangel M, Lee V, Koroshetz WJ, et al. Magnetic resonance spectroscopy study of oxygen therapy in ischemic stroke. Stroke. 2007;38:2851–4. doi: 10.1161/STROKEAHA.107.487280. [DOI] [PubMed] [Google Scholar]
- 24.Calvert JW, Zhang JH. Oxygen treatment restores energy status following experimental neonatal hypoxia-ischemia. Pediatr Crit Care Med. 2007;8:165–73. doi: 10.1097/01.PCC.0000257113.75488.84. [DOI] [PubMed] [Google Scholar]
- 25.Stroke Therapy Academic Industrial Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]