Abstract
Background and Purpose
White matter injury (WMI) is the leading cause of brain injury in preterm survivors and results in myelination failure. Although axonal degeneration occurs in necrotic lesions, the role of axonopathy in myelination failure remains controversial for diffuse non-necrotic WMI, which is currently the major form of WMI. We determined the burden of axonopathy in diffuse lesions.
Methods
We analyzed WMI in a preterm fetal sheep model of global cerebral ischemia that replicates the relative burden of necrotic and non-necrotic human WMI. WMI was analyzed at 1-or 2-weeks after ischemia and identified by ex vivo high-field (11.7 Tesla) MRI of fixed brain tissue. Axonal integrity was analyzed by immunohistochemical detection of axon injury markers and by transmission electron microscopy (EM) to quantify axon loss and degeneration in MRI-defined lesions.
Results
Axonal degeneration, defined by staining for neurofilament protein and β-amyloid precursor protein, was restricted to discrete necrotic foci with robust microglial activation. Unexpectedly, axonal degeneration was not visualized in the major form of WMI, which comprised large non-necrotic lesions with diffuse reactive astrogliosis. In these major lesions, quantitative EM studies confirmed no significant differences in the density of intact and degenerating axons or in the distribution of axon diameters relative to controls.
Conclusions
The mechanism of myelination failure differs significantly in perinatal WMI dependent upon the burden of necrosis. Axonopathy is associated with focal necrotic injury but not with primary diffuse non-necrotic lesions, which supports that intact axons in the primary lesions are potential targets for myelination.
Keywords: axonal injury, white matter injury, hypoxia-ischemia, prematurity, magnetic resonance imaging, electron microscopy
Cerebral hypoxia-ischemia (H-I) is a common cause of white matter injury (WMI) in the developing brain and a leading cause of life-long neurological disability in survivors of premature birth and infants with congenital heart disease.1 WMI is now the most common lesion in children with cerebral palsy (CP),2 and manifests as non-progressive motor deficits and cognitive/learning disabilities. Advances in neuroimaging have identified a shift from predominantly large necrotic WMI (periventricular leukomalacia; PVL) to focal or diffuse nondestructive lesions.3 However, the burden of small foci of microscopic necrosis, which are poorly defined by MRI, was recently found to be low in human4 and in a preclinical model of WMI in fetal sheep.5
The propensity for myelination failure distinguishes WMI from other forms of CP that involve gray matter injury.6 In necrotic lesions, myelination failure arises from acute degeneration of axons and glia.7 Due to the pronounced reduction in necrotic WMI in contemporary cases, there has been increased study of myelination failure in non-necrotic lesions where diffuse astrogliosis predominates. One emerging mechanism of myelination failure in non-necrotic lesions involves disrupted maturation of the oligodendrocyte (OL) lineage. Although HI triggers substantial degeneration of late oligodendrocyte progenitors (preOLs) during acute WMI, preOLs mount a robust regenerative response, but fail to differentiate in chronic lesions.4,5, 8 Presently controversial, however, is the extent to which axonopathy contributes to myelination failure in these non-necrotic lesions and lesions with microscopic necrosis.9
We addressed here the role of axonopathy in diffuse WMI, because it has significant implications for therapeutic strategies to promote myelination in chronic WMI. We employed a fetal sheep model of global cerebral ischemia where we recently defined the relative burden of focal microscopic necrosis and diffuse WMI through a combination of histopathology and high-field MRI.5 Both forms of WMI involve variable degrees of astroglial and microglial activation. Since reactive glia can promote chronic inflammation and the generation of factors deleterious to axonal survival, we tested the hypothesis that the burden of axonopathy would be greater in necrotic lesions with mixed glial reaction than in non-necrotic diffuse WMI with predominantly astroglial reaction. We undertook the first quantitative ultrastructural studies to define axonal integrity in diffuse WMI identified by high field MRI. Axonal degeneration was restricted to discrete foci of microscopic necrosis with pan-cellular loss, and significant axonopathy was not detected within large lesions with diffuse WMI. Hence, perinatal WMI displays differential susceptibility to axonopathy that is related to the burden of necrosis.
Materials and Methods
Animal Surgery
Surgery was performed on time-bred sheep of mixed western breed between 88–91d of gestation (term 145d) modified from a previously described protocol.10 For detailed methods on surgical procedures, physiological monitoring and blood analyses, please see http://stroke.ahajournals.org.
Cerebral Hypoperfusion Studies
Ischemia of 25 min duration was performed on the second or third post-operative day using a model similar to that previously reported.5 Briefly, mild fetal and maternal hypoxia was induced by administering an 11% O2 air mixture to the ewe. After 10 min, sustained cerebral hypoperfusion was initiated by occlusion of the common brachiocephalic artery after inflation of the brachiocephalic occluder and reestablished by deflation of the brachiocephalic occluder. There were no significant differences in the physiological responses of the animals in the H-I group relative to controls as summarized in supplemental table S1.
Tissue Processing
The ewe and fetuses were sacrificed (barbiturate overdose, Euthasol) at 1 (control, n = 8; ischemia, n = 8) or 2 (control, n = 8; ischemia, n = 8) weeks following completion of the occlusion protocol. Heparin (1.5 mL) was administered to all fetuses via the umbilical vein. Half of the fetal brains from control or H-I groups that survived for 1 or 2 weeks (n = 4/group) were immersion-fixed at 4 °C in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for 3 d and then stored in PBS for at least 60 d. The remaining fetal brains were perfusion-fixed with 50 mL of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 followed by 1 L of 5% gluteraldehyde in 0.1 M phosphate buffer, pH 7.4 administered by cannulation of the left ventricle. Immediately upon administration of the perfusion the descending aorta and pulmonary trunk were ligated and the jugular veins clipped bilaterally.
Gluteraldehyde-fixed fetal brains were cut into five equivalent coronal blocks in proportion to the distance between the frontal and parietal poles (6–10 mm). All frontal blocks studied spanned from the genu of the corpus callosum to the optic chiasm.
Ex Vivo MRI
Tissue was embedded alongside a twin control tissue block from the same level in 0.5% agarose and immersed in PBS within a 4 cm diameter plexiglass tube. A custom single-turn solenoidal coil (5 cm diameter, 5 cm length) was utilized for radiofrequency transmission and reception. Experiments were performed using an 11.7 T magnet interfaced with a 9 cm inner diameter magnetic field gradient coil (Bruker, Rheinstetten, Germany). Procedures generally followed the previously published strategy that used diffusion tenser imaging (DTI) to characterize postmortem tissue from this and other species.11, 12 Detailed scanning and image segmentation procedures are provided at http://stroke.ahajournals.org.
Immunohistochemistry
Tissue blocks were serially sectioned at 50 μm using a Leica VTS1000 vibrating microtome (Leica Microsystems Inc., Bannockburn, IL). The detailed immunohistochemical protocols to visualize specific cell types were performed as previously described.5, 10 Detailed immunohistochemical procedures are provided at http://stroke.ahajournals.org.
Electron Microscopy (EM)
Two 2 x 1 x 1 mm blocks were dissected from regions of apparent diffuse white matter gliosis indicated by T2 hypointensities from MRI-scanned tissue blocks and corresponding controls. Tissues were processed for EM as described.13 Briefly, tissues were post-fixed in excess cacodylate-buffered 1% osmium tetroxide (pH 7.4), dehydrated, and embedded in epoxy resin. Cross sections (~900 nm thick) were stained with 1% toluidine blue and screened by bright-field microscopy. Thin sections (~90 nm) of regions of special interest were stained with 2% uranyl acetate followed by 1% lead citrate for examination by transmission EM.
Morphometric EM Studies
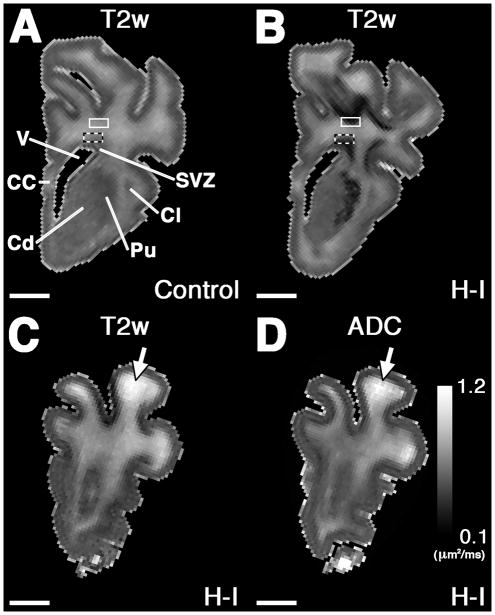
At least twenty images of each tissue block were randomly acquired at 56,400x magnification across the block. The total density of degenerating axons, intact axons and axon diameter was determined by a blinded individual in a minimum of twenty randomly acquired images for each block as previously described.14 Briefly, an unbiased counting frame (Supplemental Fig. S1), which was 2 μm in width by 2 μm in length, was used for the measurement of the number of axons per 100 μm2 and axon diameter. Axons were only counted when they were clearly identifiable. Axons were defined by cylindrical shape, a lack of ribosomes and the presence of at least one microtubule. Degenerating axons were distinguished by the presence of vacuolar bodies, disrupted plasma membrane, swollen mitochondria or dark axoplasm. Axons were counted and measured if they were partially in the counting frame and did not intersect the plane of the left side or bottom of the counting frame. The counting frame was superimposed randomly on each image (ImageJ). Axon diameter was determined by measuring the longest diameter perpendicular to the long axis of the axon in order to estimate the cross-sectional diameter (Fig. 1).
Figure 1. Tissue sampling for ultrastructural observations.
A and B, Hemisected coronal t2-weighted image (T2w) from frontal control (A) and hypoxia-ischemia (H-I, B) blocks. MRI-scanned blocks were used to identify two regions of predilection for injury in the frontal WM indicated by the dark bands in B. Superficial (solid box) and deep (checkered box) blocks of 2 x 1 x 1 mm were excised from these locations in H-I animals and corresponding controls and processed for ultrastructural analyses. C and D, T2w and ADC images, respectively, from an injured brain that had apparent necrosis in a gyrus identified by hyperintiensity on T2w and ADC images. EM images of necrosis and peri necrosis were sampled from this region. No animals had these changes in the periventricular white matter. CC, corpus callosum; Cd, caudate; Cl, claustrum; Pu, putamen; SVZ, subventricular zone; V, lateral ventricle. Scale bars: 4 mm.
Statistical Methods
Data analysis was performed using Prism 4 statistical software (GraphPad Software Inc., La Jolla, CA). Data were expressed as means ± 1 SEM unless otherwise noted. Comparisons were performed using ANOVA with post hoc inference testing done with Bonferroni multiple comparison testing. Because axon diameter data was not normally distributed, these data were analyzed with the Kruskal-Wallis non-parametric test for one-way ANOVA. A p < 0.05 was considered statistically significant.
Results
Spectrum of WMI ranges from small focal necrosis to diffuse non-necrotic lesions
In order to define the magnitude and distribution of axonopathy associated with H-I-induced WMI, we employed high field MRI to identify early chronic lesions in gluteraldehyde-fixed brain tissue from animals that survived for 1 or 2 weeks prior to ultrastructural studies by electron microscopy. As recently described for paraformaldehyde-fixed brain tissue,5 high field MRI similarly identified diffuse hypo-intense lesions in periventricular white matter that were visible on T2-weighted images (T2W) in H-I animals (Fig. 1B) but not controls (Fig. 1A). These diffuse lesions, present in all H-I animals, had FA and ADC values that did not differ from control (Supplemental Table S2). Histopathological analysis of diffuse lesions demonstrated that the non-necrotic lesions had increased reactive astrogliosis (GFAP+) and mild microglial activation (Iba1+) (Supplemental Fig. S2).
In nearly half of the animals, focal white matter lesions were identified that were hyperintense on T2 (Fig. 1C) and had increased ADC (Fig. 1D and Supplemental Table S2). Histopathological analysis (Supplemental Fig. S2) demonstrated that focal MRI abnormalities corresponded to necrotic lesions defined by low levels of reactive astrogliosis (GFAP+) and robust microglial/macrophage activation (Iba1+). Hence, consistent with recent observations,5 the spectrum of WMI generated in this study comprised large diffuse astrogliotic lesions in all animals as well as discrete foci of necrosis in a minority of the animals.
Axon injury markers identify degenerating axons in focal necrotic but not diffuse non- necrotic WMI
We next employed axon injury markers to define the burden of axonopathy in necrotic foci and diffuse lesions in 1- and 2-week survivors. Staining for neurofilament protein (NF; SMI-312) revealed that controls (Fig. 2A) and diffuse lesions (Fig. 2B) had normal-appearing axons. By contrast, at both 1 and 2 weeks, lesions with macroscopic necrosis or focal microscopic necrosis (Fig. 2C) displayed reduced NF-staining, axonal swellings and spheroids consistent with axonal degeneration. Staining for β-amyloid precursor protein (β-APP) also visualized foci of axonal degeneration (Fig. 2D). Thus, axonal degeneration was not identified in diffuse WMI, but was restricted to necrotic lesions.
Figure 2. Immunohistochemical assessment of axonal integrity in chronic white matter lesions.
A, Normal neurofilament staining (SMI-312) in control white matter. B, Neurofilament staining was intact in regions of diffuse gliosis and intact axons are visible at high magnification (B, inset). C, In a rare region of microscopic necrosis (arrows) multiple degenerating axons (arrowheads) with axonal spheroids (C, inset) are visualized with neurofilament staining in the core and immediately surrounding the necrotic focus. D, β-APP (dark brown) staining reveals injured axons (arrowheads) within and immediately adjacent to a necrotic focus (arrows), but not in the surrounding white matter. Nissl staining (blue) is used to visualize nuclei. Scale bars: A-D, 50 μm; Insets: 30 x 30 μm.
Ultrastructural analysis of axonal degeneration in focal necrotic vs. diffuse WMI
We next addressed the possibility that immunohistochemical detection of axon injury markers may be relatively insensitive to some forms of axonal degeneration in diffuse WMI. To analyze the ultra-structural integrity of axons, we processed for EM a superficial and deep region of periventricular white matter that corresponded to diffuse WMI identified by T2 hypointensities on MRI-scanned tissue blocks (insets, Fig. 1A, B).
In controls (Fig. 3A), degenerating axons were rarely visualized. Similarly, in lesions with diffuse WMI, rare degenerating axons were identified by the presence of vacuolar bodies, disrupted plasma membrane and swollen mitochondria (Fig. 3B). By contrast, in necrotic lesions, the number of axons was markedly reduced and degenerating axon profiles were frequently visualized (Fig. 3C).
Figure 3. Ultrastructural characteristics of axons within regions of diffuse white matter gliosis.
A, Low and high magnification of typical axonal ultrastructure observed within the white matter. An occasional degenerating axon was observed (arrow). B, The ultrastructure of tissue and axons appeared intact within areas of diffuse white matter gliosis (12300x). Numerous intact appearing axons with normal axoplasm and intact microtubules and occasional degenerating axons with membrane swellings, swollen mitochondria, numerous vacuoles or loss of microtubules (arrow) were visible at high power (56400x). C, A necrotic focus imaged at for comparison with evident ultrastructural damage (12300x). Large spaces appeared in the tissue with no axonal elements (*) as well as more frequent degenerating axons (arrows). Tissue adjacent to a necrotic focus contains numerous axons, but contains more profiles of degenerating axons visible at higher power (arrows, 56400x). Scale bars: A, B, C, (12300x) 2 7mu;m; A, B, C, (56400x) 500 nm.
Intact axon density is preserved in regions of diffuse WMI
We next addressed the possibility that a gradual loss of axons may occur in diffuse WMI by 1 or 2 weeks after H-I and would be detected by counting the total number of axonal profiles present in random fields sampled by EM (Supplemental Fig. S1). In a blinded analysis of ~1000 intact axons per group, there were no significant changes in the density of either intact or degenerating axons between the control and H-I groups at either 1 or 2 weeks in either the superficial or deep periventricular white matter (Table 1). In control peri-callosal deep white matter, a significant increase in axon density occurred between 1 (273 ± 24 axons/100 mm2) and 2 weeks (410 ± 33; p < 0.05) that reflected an apparent normal developmental expansion in the total population of axons and coincided with an increase in FA in this same region (Supplemental Table S2). Hence, diffuse lesions had no significant loss of axons relative to controls.
Table 1.
Ultra-structural analysis of axons in chronic WMI found no axonopathy in major lesions with diffuse astrogliosis at 1 or 2 weeks after H-I.
| Region | Axon Density (axons/100μm2) | Degenerating Axon Density (axons/100μm2) | Mean Axon Diameter (nm) | N (axons) | |
|---|---|---|---|---|---|
| SWM | |||||
| 1 wk | Control | 332 ± 58 | 9 ± 2 | 188 ± 14 | 1163 |
| H-I | 323 ± 41 | 11 ± 2 | 203 ± 15 | 1256 | |
| 2 wk | Control | 344 ± 68 | 11 ± 2 | 181 ± 11 | 958 |
| H-I | 310 ± 65 | 12 ± 1 | 191 ± 10 | 968 | |
| DWM | |||||
| 1 wk | Control | 273 ± 24 | 8 ± 3 | 206 ± 9 | 925 |
| H-I | 321 ± 17 | 11 ± 1 | 209 ± 13 | 1362 | |
| 410 ± 33 † | |||||
| 2 wk | Control | 14 ± 3 | 204 ± 6 | 1047 | |
| H-I | 364 ± 47 | 15 ± 2 | 203 ± 9 | 993 | |
Shown are the total density of axons, density of degenerating axons, mean axon diameter and total number of axons analyzed in each study (N). Data are presented as mean ± SEM, n = 4 animals per group.
p < 0.05 vs. 1 week control (ANOVA with post hoc Bonferroni between groups).
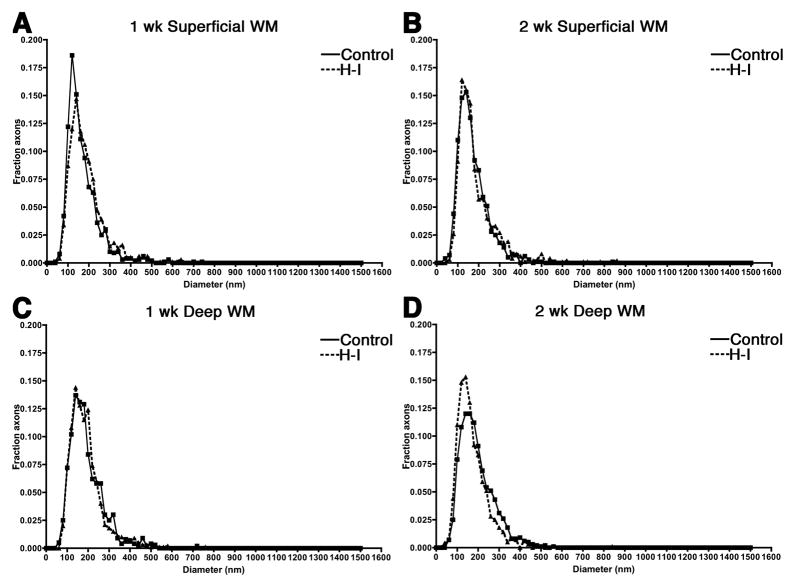
The distribution of axon diameters is unaffected by diffuse WMI
We next determined if a loss of axons in diffuse lesions would be detected as a change in the distribution of mean axonal diameters between the control and H-I groups. There was, however, no change in the mean axonal diameter in response to H-I at either 1 or 2 weeks (Table 1). Figure 4 shows the distribution of axonal diameters in the regions studied at 1 and 2 weeks. Anatomical differences in the distribution of fiber diameters and peak fiber diameters were present between the superficial (Fig 4A, B) and deep periventricular white matter WM (Fig. 4C, D), but H-I did not disrupt the normal developmental distribution of axon diameters in either region at 1 or 2 weeks after HI. Hence, diffuse WMI showed no apparent axonal loss or disruption in the normal trajectory for axon development.
Figure 4. The fraction of axons of varying caliber after hypoxia ischemia.
A through D, Histograms showing the fraction of axons of varying diameter in the white matter after hypoxia-ischemia (HI; dashed line) or sham control (Control; solid line) as determined by stereological assessment. A, One week superficial block. B, Two week superficial block. C, One week deep block. D, Two week deep block. No change in the distribution of axonal diameters was found in either region at 1 or 2 weeks.
Discussion
Failure of normal myelination is a major consequence of perinatal WMI, a common cause of CP and cognitive impairment in survivors of premature birth.6, 15 Failure to initiate myelination may be a consequence of disrupted maturation of oligodendrocyte progenitors5, 4, 8, 16 or loss of axonal integrity.9 The contribution of axonal injury to myelination failure has been resistant to study for several reasons. Several markers have been studied in developing human white matter that identify degenerating axons in early necrotic lesions.4, 17, 18 ENREF_13 However, the timing of WMI during premature brain development coincides with a period prior to the onset of myelination. Hence, it is not possible to apply markers of axonal integrity, such as nodal proteins, that are expressed later in development with the onset of myelination and are disrupted in dysmyelinated adult white matter.19, 20 Progress to define axon integrity also has been hampered by the lack of a large pre-clinical animal model that closely replicates the pathophysiology and spectrum of WMI in premature infants.21
We recently described a model of ovine chronic WMI in which global cerebral ischemia generates a similar spectrum and relative burden of pathology to human.5 The periventricular white matter of the preterm fetal sheep has few myelinated axons, consistent with its immature state. With this model, ex vivo high field MRI identified novel signal abnormalities that histologically correspond to microscopic necrosis and diffuse non-necrotic WMI.5 These lesions have been previously undetected at lower field strength.22 We found striking differences in the burden of axonal degeneration in necrotic and diffuse WMI defined by a combination of light microscopy and transmission EM.
Quantitative EM studies, guided by high field MRI, have not been applied before to study axon integrity in perinatal WMI. This approach allowed us to visualize both overt and subtle changes in the integrity of developing axons that are not feasible to detect by light microscopy. The most significant axonal degeneration was visualized in necrotic lesions that were enriched in reactive microglia and depleted in astrocytes. Lesions were confirmed to be necrotic by the presence of dystrophic axons and axonal spheroids, which degenerate during the early phase of coagulative necrosis.23–26 Reduced myelination secondary to neuro-axonal degeneration occurs in perinatal rodents after H-I27, 28 and often manifests as significant cerebral atrophy, as occurs in cystic necrotic human lesions.18, 29 By contrast, cortical neuronal loss is uncommon when only diffuse astrogliotic lesions occur in human WMI.29 In human and fetal sheep, the burden of microscopic necrosis is very low.4, 5 Here we found that most axonal degeneration was restricted to these small necrotic foci. The clinical significance of these lesions is unclear given their small size, but is likely to be influenced by the location of the lesion.
During development, small pre-myelinated axons are particularly susceptible to glutamate-mediated excitotoxicity at sites of contact with oligodendrocyte processes that involves both N-methyl-D-aspartic acid (NMDA) receptors and non-NMDA glutamate receptors.30, 31 Glutamate-mediated axonal injury is related to excessive glutamate depletion from oligodendrocyes and axons,30, 32–34 which appear to be the major sources of extracellular glutamate during energy failure from hypoxia-ischemia.13 Thus, one potential approach to prevent myelination failure in necrotic lesions may be to couple cerebral hypothermia35 with agents that block axonal degeneration via pathways that mediate excitotoxic injury.36
The paucity of axonopathy in diffuse WMI is consistent with a mechanism of myelination failure that differs substantially from necrotic WMI. Axonal degeneration was not detected in early non-necrotic diffuse WMI in human or fetal sheep .10, 37 Axonal degeneration was observed in diffuse WMI adjacent to large acute or organizing necrotic lesions,18 since axons traversed across necrotic foci to adjacent non-necrotic lesions. Large necrotic lesions are now clinically uncommon and our data supports that significant axonopathy does not occur in diffuse WMI in the absence of large necrotic lesions. Although we found no structural evidence of abnormal axons, we cannot exclude that reactive glia generate a chronic inflammatory response that results in axonal dysfunction. Electrophysiological studies would be needed to address this possibility.
An alternative mechanism for myelination failure in diffuse WMI involves disturbances in oligodendrocyte (OL) lineage maturation. Preterm neonates are susceptible to acute diffuse WMI triggered by selective vulnerability of preOLs as opposed to other glia, neurons or axons.37,38 Acute degeneration of preOLs triggers reactive astrogliosis and a compensatory increase in preOLs that fail to differentiate to mature OLs.5, 8, 16 Thus, preOL maturation arrest within diffuse WMI may contribute to chronic myelination failure in preterm survivors.4
Summary/Conclusions
In summary, our findings support that axonal degeneration occurs in association with discrete foci of microscopic necrosis with pan-cellular loss. Axonopathy was not detected within diffuse WMI where astrogliosis and preOL maturation arrest were previously observed. Hence, the primary mechanism of myelination failure in diffuse WMI appears to involve an aberrant reaction to injury in which preOLs mount a disrupted repair response with arrested maturation. Therapies directed at prevention of axonal degeneration would optimally target mechanisms related to necrotic WMI, whereas in diffuse lesions, where axons are present, alternative strategies will be required that promote arrested preOL maturation. Within adult demyelinating lesions, hyaluronan triggers the arrest of preOL maturation.39, 40 One strategy in the preterm neonate may be to target the accumulation of hyaluronan within the extracellular matrix of diffuse lesions.
Supplementary Material
Acknowledgments
We would like to thank Sue A. Aicher (PI) and Robert Kayton at the Neuroscience Imaging Core for their assistance.
Sources of Funding
Supported by the NIH (P51RR000163 (CDK); National Institutes of Neurological Diseases and Stroke: 1RO1NS054044, R37NS045737-06S1/06S2 to SAB and 1F30NS066704 to AR) the American Heart Association (Bugher Award 052705 and Grant in Aid 11GRANT7510072 to SAB) and the March of Dimes Birth Defects Foundation (SAB). High-field MRI instrumentation used in this work was purchased with support from the W.M. Keck Foundation. The Neuroscience Imaging Center at OHSU is supported by NIH grant P30 NS061800.
Footnotes
Disclosures
none
References
- 1.McQuillen P, Miller S. Congenital heart disease and brain development. Ann N Y Acad Sci. 2010;1184:68–86. doi: 10.1111/j.1749-6632.2009.05116.x. [DOI] [PubMed] [Google Scholar]
- 2.Bax M, Tydeman C, Flodmark O. Clinical and mri correlates of cerebral palsy: The european cerebral palsy study. JAMA. 2006;296:1602–1608. doi: 10.1001/jama.296.13.1602. [DOI] [PubMed] [Google Scholar]
- 3.Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 4.Buser J, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, et al. Arrested pre-oligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2011 doi: 10.1002/ana.22627. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle A, Dean J, Buser J, Gong X, Maire J, Chen K, et al. Histopathological correlates of mri-defined chronic perinatal white matter injury. Ann Neurol. 2011 doi: 10.1002/ana.22501. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpe JJ. Neurology of the newborn. Philadelphia: W.B. Saunders; 2008. [Google Scholar]
- 7.Kinney H, Back S. Human oligodendroglial development: Relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 8.Segovia K, Mcclure M, Moravec M, Luo N, Wang Y, Gong X, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:517–526. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dammann O, Hagberg H, Leviton A. Is periventricular leukomalacia an axonopathy as well as an oligopathy? Pediatr Res. 2001;49:453–457. doi: 10.1203/00006450-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Riddle A, Luo N, Manese M, Beardsley D, Green L, Rorvik D, et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. Journal of Neuroscience. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Diffusion mr imaging characteristics of the developing primate brain. Neuroimage. 2005;25:1205–1213. doi: 10.1016/j.neuroimage.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. Regional patterns of cerebral cortical differentiation determined by diffusion tensor mri. Cereb Cortex. 2009;19:2916–2929. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back SA, Craig A, Kayton R, Luo NL, Meshul C, Allcock N, et al. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007;27:334–347. doi: 10.1038/sj.jcbfm.9600344. [DOI] [PubMed] [Google Scholar]
- 14.Partadiredja G, Miller R, Oorschot D. The number, size, and type of axons in rat subcortical white matter on left and right sides: A stereological, ultrastructural study. J Neurocytol. 2003;32:1165–1179. doi: 10.1023/B:NEUR.0000021910.65920.41. [DOI] [PubMed] [Google Scholar]
- 15.Ferriero DM. Neontal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 16.Zhiheng H, Liu J, Cheung P-Y, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemia brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Arai Y, Deguchi K, Mizuguchi M, Takashima S. Expression of b-amyloid precursor protein in axons of periventricular leukomalacia brains. Pediatr Neurol. 1995;13:161–163. doi: 10.1016/0887-8994(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 18.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of ranvier. Curr Opin Neurobiol. 2006;16:508–514. doi: 10.1016/j.conb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of ranvier and improved axonal conduction. J Neurosci. 2007;27:3416–3428. doi: 10.1523/JNEUROSCI.0273-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferriero DM. Can we define the pathogenesis of human periventricular white-matter injury using animal models? J Child Neurol. 2006;21:580–581. doi: 10.1177/08830738060210071901. [DOI] [PubMed] [Google Scholar]
- 22.Ferriero DM, Miller SP. Imaging selective vulnerability in the developing nervous system. J Anat. 2010;217:429–435. doi: 10.1111/j.1469-7580.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama A, Okoshi Y, Hachiya Y, Ozawa Y, Ito M, Kida Y, et al. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol. 2001;20:87–91. [PubMed] [Google Scholar]
- 25.Marin-Padilla M. Developmental neuropathology and impact of perinatal brain damage. Ii: White matter lesions of the neocortex. J Neuropathol Exp Neurol. 1997;56:219–235. doi: 10.1097/00005072-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Banker B, Larroche J. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 27.Sizonenko SV, Sirimanne E, Mayall Y, Gluckman PD, Inder T, Williams C. Selective cortical alteration after hypoxic-ischemic injury in the very immature rat brain. Pediatr Res. 2003;54:263–269. doi: 10.1203/01.PDR.0000072517.01207.87. [DOI] [PubMed] [Google Scholar]
- 28.Sizonenko SV, Kiss JZ, Inder T, Gluckman PD, Williams CE. Distinctive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic-hypoxic injury in the p3 immature rat brain. Pediatr Res. 2005;57:865–872. doi: 10.1203/01.PDR.0000157673.36848.67. [DOI] [PubMed] [Google Scholar]
- 29.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fern R, Davis P, Waxman S, Ransom B. Axon conduction and survival in cns white matter during energy deprivation: A developmental study. J Neurophysiol. 1998;79:95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009;66:682–693. doi: 10.1002/ana.21767. [DOI] [PubMed] [Google Scholar]
- 32.Fern R, Moller T. Rapid ischemic cell death in immature oligodendrocytes: A fatal glutamate release feedback loop. J Neurosci. 2000;20:34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilke S, Thomas R, Allcock N, Fern R. Mechanism of acute ischemic injury of oligodendroglia in early myelinating white matter: The importance of astrocyte injury and glutamate release. J Neuropathol Exp Neurol. 2004;68:872–881. doi: 10.1093/jnen/63.8.872. [DOI] [PubMed] [Google Scholar]
- 34.Salter M, Fern R. Nmda receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 35.Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin Perinatol. 2008;35:735–748. vi–vii. doi: 10.1016/j.clp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Domingues AM, Taylor M, Fern R. Glia as transmitter sources and sensors in health and disease. Neurochem Int. 2010;57:359–366. doi: 10.1016/j.neuint.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Back SA, Luo NL, Mallinson RA, O'Malley JP, Wallen LD, Frei B, et al. Selective vulnerability of preterm white matter to oxidative damage defined by f2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 38.Back SA, Han BH, Luo NL, Chrichton CA, Tam J, Xanthoudakis S, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Back S, Tuohy T, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;9:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 40.Sloane J, Batt C, Ma Y, Harris Z, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through tlr2. Proc Natl Acad Sci USA. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.