Abstract
Mechanical stimulation is necessary for maximization of geometrical properties of bone mineralization contributing to long-term strength. The amount of mineralization in bones has been reciprocally related to volume of bone marrow adipose tissue and this relationship is suggested to be an independent predictor of fracture. Physical activity represents an extrinsic factor that impacts both mineralization and marrow volume exerting permissive capacity of the growing skeleton to achieve its full genetic potential. Because geometry- and shape-determining processes primarily manifest during the linear growth period, the accelerated structural changes accompanying early childhood (ages 3 to 6 y) may have profound impact on lifelong bone health. The objective of this pilot study was to determine if a short-term physical activity intervention in young children would result in augmentation of geometric properties of bone. Three days per week the intervention group (n=10) participated in 30 minutes of moderate intensity physical activity, such as jumping, hopping and running, and stretching activities, whereas controls (n=10) underwent usual activities during the 10-week intervention period. Femoral bone marrow adipose tissue volume and total body composition were assessed by magnetic resonance imaging and dual-energy X-ray absorptiometry, respectively, at baseline and after ten weeks. Although after 10-weeks, intergroup differences were not observed, a significant decrease in femoral marrow adipose tissue volume was observed in those participating in physical activity intervention. Our findings suggest physical activity may improve bone quality via antagonistic effects on femoral bone marrow adipose tissue and possibly long-term agonistic effects on bone mineralization.
Keywords: Bone marrow adipose tissue, physical activity, pediatric bone health
1. INTRODUCTION
Long-term overall bone health is dependent upon cycles of site-specific bone morphology during maturation. Deposition of mineral on the periosteal surface influences the bone’s external bone dimension and mass distribution underlying strength, whereas the extent of net mineral removal from its endocortical surface establishes the size of the marrow cavity, influencing stiffness (ability to withstand fracture) [1–3]. While net bone mineral deposition is critical for mass, resorption is not necessarily antagonistic, particularly during growth and development. The resorptive phase of the remodeling cycle at various sites enables replacement by new bone serving to optimize material composition, micro- and macro-architecture. However, as development progresses, bone remodeling begins to be “uncoupled” in that resorbed mineral is not completely replaced, resulting in a net bone loss and an increased size of the marrow compartment. Bone marrow, or more specifically, resident adipose tissue volume (BMAT), is reciprocally related to the amount of mineral in the long bones [4, 5] in adults and has been suggested to be an independent predictor of fracture [1, 4]. Assuming a constant age-associated rate of bone resorption, individuals with lower peak bone mass (and greater bone marrow adipose tissue volume) suffer greater fracture risk and reach an osteoporotic bone density more often and earlier than their counterparts.
Attributing 60–75% of the variance in peak bone mass to genetic heritability allows for the possibility that the remaining proportion may be amenable to “external factors” aimed at optimization of inherent capacity [1–3]. Mechanical stimulation (e.g., through weight-bearing physical activity) is necessary for maintaining a normal balance between selective mineral deposition and resorption from existing surfaces with the goal of maximization of geometrical properties contributing to long-term bone strength [6]. Mechanical stress which exceeds typical day-to-day threshold (in terms of intensity and strain) and/or conveys an unusual loading pattern (e.g. jumping) has been shown to be most beneficial for anabolic bone maturation. Physical activity also increases blood supply to surrounding muscle, thereby promoting delivery of nutrients and chemical messengers. Because the geometry- and shape-determining processes primarily manifest during the linear growth period, the accelerated structural changes accompanying early childhood (~age 3 to 6 y) may have profound impact on lifelong bone health.
Interestingly, an estimated 20% increase in fracture prevalence among the pediatric population has been observed over the past decade [1–3]. Perturbation in the balance between deposition and resorption and consequential geometry, particularly during critical periods of growth, may compromise bone strength and stiffness. Accelerated infiltration of adipose tissue into the marrow cavity during this time may also lead to concessions in bone integrity. To our knowledge, no study has investigated the effect of BMAT changes in children in response to a physical activity program. Given the biomechanical advantage of optimizing cortical bone strength in early childhood, the objective of this study was to evaluate whether preschoolers participating in moderate-to-vigorous physical activity would have greater cross-sectional cortical bone volume and less bone marrow adipose tissue volume assessed via MRI segmentation in the femur compared to control counterparts.
2. MATERIALS AND METHODS
Ten children (40% European American, 60% African American) participated in a school-based physical activity intervention and an additional ten children (40% European American, 40% African American, 20% Hispanic American) were recruited to serve as controls. All measures were performed at the UAB/Nutrition Obesity Research Center Core Metabolism Laboratory and except the MRI was obtained at the UAB Division of Cardiology. Baseline measurements were obtained between February and March 2010, and follow-up measurements were obtained within 2 weeks of the 10-wk intervention period from March to May 2010.
2.1. Intervention
Since the intervention was conducted during typical school hours, all children in the participating classrooms engaged in the physical activity program. However, data were only collected from the first 10 children whose parent or guardian agreed to attend pre- and post- body composition assessment at UAB. In addition, parents of participating classrooms were notified of their child’s participation and allowed for the opportunity to opt-out. Up to two children per family were eligible to participate.
The research-based SPARK-Early Childhood curriculum was used to guide the supervised physical activity program [7–9]. Three days per week over a ten-week period a certified exercise physiologist engaged the children in 20 minutes of moderate intensity physical activity. The activities primarily focused on locomotive skills such as jumping, hopping and running. The general format for each exercise session included a five-minute warm-up activity, two 10-minute jumping activities, and a five-minute cool down activity. Children were introduced to the program and progressively trained to be active for the full 20-minute locomotive activity portion. All activities required minimal equipment (e.g. cones) and could be performed in the classroom. To monitor compliance, attendance logs were completed each day by the teacher. From records, the average student attendance was >90% across all weeks of the study.
2.2. Anthropometric Assessment
Height and weight were measured using a portable stadiometer and digital scale. Waist circumference was measured at the narrowest apparent part of the torso between the ribs and iliac crest as described by Lohman et al [10]. Waist circumference measures were obtained using a flexible tape measure (Gulick II; Country Technology, Inc., Gays Mills, WI) and recorded to the nearest 0.1 cm.
2.3. Body Composition
Adipose tissue distribution in the femur and whole-body body composition were assessed by magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry (DXA), respectively. Whereas DXA measures total tissue content based upon an areal estimate, magnetic resonance through delineation and simple signal thresholding allows contrast between tissue compartments, thus providing a more sensitive quantification of fat and bone.
MRI Imaging and Analysis
Measures for cross-sectional cortical bone volume and bone marrow adipose tissue volume assessed in the femur were acquired using MRI. MRI which involves no ionizing radiation is emerging as a comprehensive tool for fat quantification. For MRI, children were scanned using a Philips 3T system in the UAB Division of Cardiology [11]. A series of T1-weighted slices (allowing for rapid scans with strong fat-water tissue contrast) were acquired at the upper-leg regions with data acquisition beginning at the iliac crest and continuing to the knee (superior border of the patella). These slices included the ilium, sacrum, ischium, pubis, coccyx, femoral heads, and femur; however, to ensure consistency, only the volumes of tissue in the femur were included in the analysis. A standard commercial 3D inversion-prepared gradient-echo pulse sequence was used (e.g. MP-RAGE). Imaging parameters included repetition time (TR) = 6.4 msec, echo time (TE) = 3.2 msec, inversion time (TI) = 801 msec, 5 mm slices with 0 mm gap, bandwidth (BW) = 241 kHz, and one signal average. A 6 receiver array was used. The participant rested in either a prone position during the procedure with the iliac crest as the point of origin.
Following acquisition, the bone marrow and cortical bone compartments were segmented and analyzed off-site in the laboratory of Houchun Hu at the University of Southern California using SliceOMatic (Tomovision, Inc.) software. The technique used for analysis has been described elsewhere [12, 13]. Briefly, the procedure involves the transfer of images to an offline workstation, followed by the use of SliceOMatic. All tissue segmentation was performed using the “Region Growing” toolbox and the “Paint” function. As the DICOM images from each MRI exam were not normalized, the threshold for each subject’s data was determined individually based on histogram, in a manual fashion. Since BMAT exhibited bright signal intensities due to its large fat content and is surround by bone with dark signal intensity, SliceOmatic segmentation was straightforward. Performance of the chosen threshold in segmenting the BMAT was visually inspected by the segmenter. BMAT in pelvis was not included because of the difficulty in differentiating this component from adjacent intramuscular or subcutaneous adipose tissue.
DXA Imaging
Total body composition (body fat mass and bone mineral content) was measured by DXA using a GE Lunar Prodigy densitometer (GE LUNAR Radiation Corp., Madison, WI). Participants were scanned in light clothing, while lying flat on their backs with arms at their sides. DXA scans were performed and analyzed using pediatric software (enCORE 2002 Version 6.10.029). In our laboratory, the coefficient of variation (CV) for repeated measures of total body fat mass is 6.55%.
2.4. Statistical Analysis
Measures were conducted at baseline and at eleven weeks. All data were analyzed using SAS 9.1 software (SAS institute Inc. Cary, NC). Student’s t-tests for paired data were used to compare mean values between groups at baseline. General linear ANCOVA models were used to evaluate difference between intervention and control group. Analyses were conducted using raw data, percent change and adjusted data. Adjusted models included age, sex, race and change in height and weight (over intervention period) as covariates. Values are expressed as mean + SE. Statistical significance was set at p<0.05.
3. THEORY/CALCULATION
To our knowledge, no intervention study has been conducted in young children directly examining the effects of physical activity on femoral BMAT. The mechanism driving preferential partitioning of energy towards bone marrow rather than bone matrix has not been elucidated. It is known that adipocytes and osteocytes originate from the same mesenchymal stem cells (MSCs) in the bone marrow cavity [14], and an inverse relationship between osteogenesis and adipogenesis in the bone marrow cavity has been consistently demonstrated [15, 16]. Although the factors directing the cells along a particular lineage and commitment are not well established, (un)balanced osteogenic and adipogenic potential of MSCs influences the differentiation process. It was previously believed that cell differentiation involved a linear and irreversible process, but more contemporary thought highlights the plasticity of MSCs such that an intermediary/transient stage during differentiation characterized by promiscuous gene expression places the stem cell in a stand-by state. Importantly, evidence is accumulating which suggests that the differentiation towards osteocyte or adipocyte can be influenced by mechanical factors [17–20] while in this “stand-by state”. In vitro studies have demonstrated transdifferentiation under the influence of specific external stimuli [15, 21]. Stem cells cultured in an adipogenic medium (e.g. PPARγ) generally show an increase in expression of genes reflecting adipogenesis, and a decrease in those reflecting osteogenesis (e.g. Runx2); however, simultaneous exposure to mechanical strain has also been shown to prevent the increase in adipogenic gene expression (e.g. PPARγ) [15]. Stem cells from mice exposed to low magnitude mechanical signals for 14 weeks showed a 27% decrease in expression of PPARγ, and a 72% increase in expression of Runx2. Thus, it is possible that physical activity in early childhood may attenuate infiltration of adipose tissue in the femoral marrow cavity due to greater differentiation of adipocytes at the expense of osteocytes.
4. RESULTS AND DISCUSSION
Steep temporal trends in pediatric fracture [1, 2, 22, 23] cannot be explained by genotypic models, thereby suggesting potential contribution of modifications in the contemporary environment. Physical activity represents an extrinsic factor that impacts both mineral accretion and BMAT, exerting permissive capacity of the growing skeleton to achieve its full genetic potential [24]. In accordance with the consistent reports of the beneficial effects of physical activity on bone deposition in children [25–30], we hypothesized that in this moderate-intensity weight-bearing physical activity intervention during a critical period of growth and development would result in augmentation of material properties of bone in terms of mineral deposition and femoral marrow cavity volume. There were no significant differences in baseline sample characteristics (Table 1). The sample characteristics after the ten week intervention period are presented in Table 2. After 10 weeks, there were no changes or group differences in either femoral cortical bone volume (p=0.11) or total-body bone mineral content (p=0.35). However, there was a significant decrease in femoral BMAT (p=0.04) observed among children in the intervention group (Figure 1). A marginal, albeit not significant (p=0.20) increase in femoral BMAT was observed in the control group. No other statistically significant changes were observed between the groups.
Table 1.
Baseline Descriptive Sample Characteristics.
| Intervention | Control | |
|---|---|---|
| Age (yrs) | 4.8±0.2 | 5.1±0.1 |
| Sex (% male) | 56% | 47% |
| Race | 40% EA; 60% AA | 40% EA; 40% AA; 20% HA |
| Height (in) | 41.3±1.2 | 42.1±0.9 |
| Weight (lbs.) | 40.9±2.6 | 47.4±3.7 |
| Total Fat (kg) | 5.1±0.6 | 6.7±0.9 |
| Total %Fat | 26.8±1.4 | 29.6±2.1 |
| Total Lean (kg) | 12.7±0.7 | 14.0±0.8 |
| BMC (g)‡ | 601.4±32.9 | 689±41.5 |
| CBV (cm3)† | 40.0±6.8 | 41.8±3.4 |
| BMAT (cm3)† | 48.7±6.8 | 49.8±4.2 |
assessed by DXA;
assessed by MRI
EA= European American; AA= African American; HA= Hispanic American; BMC= Bone Mineral Content; CBV=Cross-sectional Bone Volume; BMAT= Bone Marrow Adipose Tissue Volume;
Table 2.
Comparison between treatment (10-week physical activity intervention program) and control group.
| Intervention | Control | p-value | |
|---|---|---|---|
| Height (cm) | 43.0±0.6 | 43.0±0.6 | 0.92 |
| Weight (lbs) | 44.0±4.1 | 52.3±3.9 | 0.18 |
| Total Fat (kg) | 5.5±0.9 | 7.4±0.9 | 0.20 |
| Total % Fat | 26.7±1.8 | 29.7±1.7 | 0.28 |
| Total Lean (kg) | 13.6±0.9 | 15.2±0.9 | 0.24 |
| BMC (g)‡ | 702.8±34 | 739.0±2.3 | 0.47 |
| CBV (cm3)† | 43.7±3.0 | 48.1±3.6 | 0.42 |
| BMAT (cm3)† | 47.4±5.1 | 50.5±4.3 | 0.80 |
adjusted for age, sex, race.
assessed by DXA;
assessed by MRI
BMC= Bone Mineral Content; CBV= Cross-sectional Bone Volume; BMAT= Bone Marrow Adipose Tissue Volume
Figure 1.
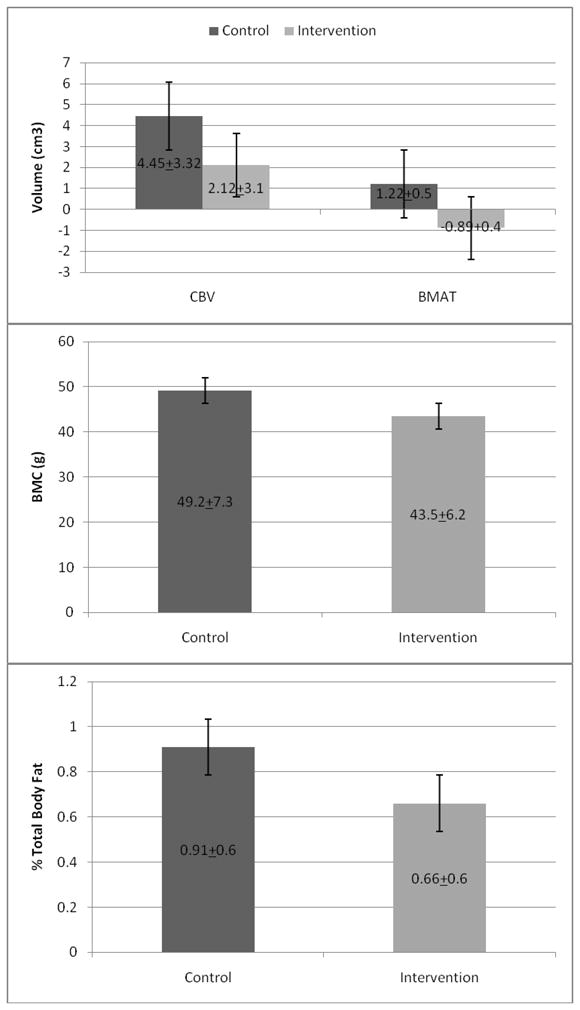
Dark gray bars represent changes in intervention group. Light gray bars represent changes in control group. CBV = cross-sectional bone volume across entire femur using MRI; BMAT = bone marrow adipose tissue volume assessed in the femur using MRI; BMC = total body bone mineral content assessed by DXA. Error bars represent standard error of the mean.
Bone is a dynamic organ that undergoes continual remodelling. Integral to bone competence is the remodelling process which takes place throughout life, yet is most active during growth and development. Impairments in the micro-architecture, even in the presence of adequate mineralization, can lead to increased fracture risk. Mathematical models suggest that optimizing bone integrity in childhood will have biologically relevant effects on skeletal competence in advanced age [1]. Whereas physical activity has been consistently documented to be beneficial to BMC, our findings suggest physical activity also may improve bone quality via antagonistic effects on BMAT thereby promoting long-term agonistic effects on bone geometry and maintenance of skeletal competence. In adults, a reciprocal relationship between bone mineral preservation and BMAT accumulation has been reported [4, 5, 31]. Further, observations of varying amounts of bone marrow adipose tissue with progression from normal to osteopenic to osteoporotic phenotypes highlight the importance of both optimization of processes involved in both mineralization as well as resorption [31]. Though BMAT is associated with bone anabolism, particularly during growth with proximity to puberty, the accelerated infiltration into long bones in particular may compromise strength. Importantly, age-related changes in marrow adiposity are associated with bone balance during a critical period in bone development in which periosteal apposition should exceed increased endocortical resorption. Plausibly, progressive increases in BMAT would lead to up-regulated receptor activity of the NF-κB ligands, affecting osteoblast apoptosis and osteoclast proliferation. Since the capacity for accretion of bone mineral is limited by chronology and development, the stage at which interventions are initiated is pivotal in terms of effectiveness. Interventions during critical periods of growth and development may contribute to “programming” osteoblast and adipocyte lineages driving physiologic processes; i.e., these cell populations become endowed with a memory in which their metabolic machinery driving the decisions towards fat or bone remains active after the stimulus has subsided [27].
The observed changes in BMAT yet absence of an association on overall bone content or thickness in children both undergoing physical activity intervention and controls were somewhat surprising. Whereas studies in adult and those in older children corroborate these findings [22, 24, 28, 32–34], previous investigations have observed that processes underlying skeletal strength may be most sensitive to mechanical strain at this critical stage [3, 6, 28]. It is estimated that the first three weeks of bone remodelling involve resorption processes, while an additional 12 weeks is necessary to complete deposition of mineral matrix. Our inability to detect significant increases in bone strength may have been related to the short duration of the intervention.
As a pilot study, the interesting findings relating to BMAT lay the groundwork for future studies of longer duration and larger sample size. Additionally, it terms of bone parameters, it must be realized that a statistically significant difference is not necessarily of biological significance; i.e., a statistically significant group difference in bone mass may not be transferred to a difference in fracture risk. However, finding an exercise-induced bone mass effect in early pubertal children can be regarded as promising. Although we examined only the appendicular skeleton, specifically the femur, that of the axial skeleton may also be relevant and warrants investigation.
5. CONCLUSIONS
Mass, composition and geometry together determine whole bone mechanical competence. Beyond the relation between physical activity and bone that has already been recognized, early physical activity interventions may present a propitious opportunity for having a beneficial effect on bone geometry and harnessing bone’s sensitivity to mechanical signals, key in regulating attenuation of femoral BMAT accrual. The capacity of stimuli to influence bone geometry is confined to a relatively short period during growth and development, with a significant contribution in early childhood. Although there were no intergroup differences following intervention, the decrease in femoral BMAT only in the exercise group suggest a potential physical activity induced benefit in lower limb skeletal integrity. Investigations of the effects of physical activity in early childhood when the skeleton may be most responsive to external stimuli may aid in deciphering the mechanisms underlying mechanical competence.
HIGHLIGHTS.
Early physical activity interventions may present a timely opportunity in which bone’s sensitivity to mechanical signals can be harnessed.
Attenuation of femoral bone marrow adipose tissue accrual may be relevant for long-term bone quality optimization.
Femoral bone marrow adipose tissue was significantly decreased in children participating in 10-week physical activity intervention whereas a significant difference was not observed in control-group
Bone mineral content was not statistically different following a 10-week physical activity intervention
Acknowledgments
The authors wish to thank Jan den Hollander and the Family Care Center staff for their invaluable contribution and assistance in providing support to participants. This research was supported by K99DK083333 (KC); K25DK087931(HHH); CA-47888 (LJH;BH); P30 DK056336 UAB Nutrition Obesity Research Center Pilot/Feasibility Grant; UAB Diabetes Research Center Human Physiology Core P60DK079626.
ABBREVIATIONS
- BMAT
Bone marrow adipose tissue BMC, Bone mineral content
- CBV
Cross-sectional bone volume measured in femur
- CV
Coefficient of variation
- DXA
Dual-energy x-ray absorptiometry
- MR
Magnetic resonance
- MRI
Magnetic resonance imaging
- MSC
Mesenchymal stem cells
- UAB
University of Alabama at Birmingham
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17:337–47. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E. An exercise in geometry. J Bone Miner Res. 2002;17:373–80. doi: 10.1359/jbmr.2002.17.3.373. [DOI] [PubMed] [Google Scholar]
- 4.Di IN, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95:2977–82. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103–8. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowda M, James F, Sallis JF, McKenzie TL, Rosengard P, Kohl HW., III Evaluating the sustainability of SPARK physical education: a case study of translating research into practice. Res Q Exerc Sport. 2005;76:11–9. doi: 10.1080/02701367.2005.10599257. [DOI] [PubMed] [Google Scholar]
- 8.Sallis JF, McKenzie TL, Alcaraz JE, Kolody B, Hovell MF, Nader PR. Project SPARK. Effects of physical education on adiposity in children. Ann N Y Acad Sci. 1993;699:127–36. doi: 10.1111/j.1749-6632.1993.tb18844.x. [DOI] [PubMed] [Google Scholar]
- 9.Sallis JF, McKenzie TL, Alcaraz JE, Kolody B, Faucette N, Hovell MF. The effects of a 2-year physical education program (SPARK) on physical activity and fitness in elementary school students. Sports, Play and Active Recreation for Kids. Am J Public Health. 1997;87:1328–34. doi: 10.2105/ajph.87.8.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohman TG, Going SB. Body composition assessment for development of an international growth standard for preadolescent and adolescent children. Food Nutr Bull. 2006;27:S314–S325. doi: 10.1177/15648265060274S512. [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 2010;18:841–7. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–e515. doi: 10.1111/j.1467-789X.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–24. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 16.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 17.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–62. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 18.Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22:1283–9. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–75. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 22.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, III, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeman E, Tsalamandris C, Formica C. Peak bone mass, a growing problem? Int J Fertil Menopausal Stud. 1993;38 (Suppl 2):77–82. [PubMed] [Google Scholar]
- 24.Greene DA, Naughton GA. Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: a randomised controlled trial. Osteoporos Int. 2011;22:489–98. doi: 10.1007/s00198-010-1317-z. [DOI] [PubMed] [Google Scholar]
- 25.Burt LA, Naughton GA, Greene DA, Courteix D, Ducher G. Non-elite gymnastics participation is associated with greater bone strength, muscle size, and function in pre- and early pubertal girls. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1677-z. [DOI] [PubMed] [Google Scholar]
- 26.Janz KF, Gilmore JM, Burns TL, Levy SM, Torner JC, Willing MC, Marshall TA. Physical activity augments bone mineral accrual in young children: The Iowa Bone Development study. J Pediatr. 2006;148:793–9. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Torner JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc. 2010;42:1072–8. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald HM, Kontulainen SA, Petit MA, Beck TJ, Khan KM, McKay HA. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos Int. 2008;19:1445–56. doi: 10.1007/s00198-008-0589-z. [DOI] [PubMed] [Google Scholar]
- 29.Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Rizzoli R, Kriemler S. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48:792–7. doi: 10.1016/j.bone.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Petit MA, Hughes JM, Wetzsteon RJ, Novotny SA, Warren M. Re: weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;41:903–5. doi: 10.1016/j.bone.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DM, Ahamed Y, Macdonald HM, McKay HA. Characterising cortical density in the mid-tibia: intra-individual variation in adolescent girls and boys. Br J Sports Med. 2008;42:690–5. doi: 10.1136/bjsm.2008.049528. [DOI] [PubMed] [Google Scholar]
- 33.Di IN, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93:2281–6. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22:434–46. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]


