Abstract
Normal heart formation requires reiterative phases of canonical Wnt/β-catenin (Wnt) signaling. Understanding the mechanisms by which Wnt signaling directs cardiomyocyte (CM) formation in vivo is critical to being able to precisely direct differentiated CMs from stem cells in vitro. Here, we investigate the roles of Wnt signaling in zebrafish CM formation using heat-shock inducible transgenes that increase and decrease Wnt signaling. We find that there are three phases during which CM formation is sensitive to modulation of Wnt signaling through the first 24 hours of development. In addition to the previously recognized roles for Wnt signaling during mesoderm specification and in the pre-cardiac mesoderm, we find a previously unrecognized role during CM differentiation where Wnt signaling is necessary and sufficient to promote the differentiation of additional atrial cells. We also extend the previous studies of the roles of Wnt signaling during mesoderm specification and in pre-cardiac mesoderm. Importantly, in pre-cardiac mesoderm we define a new mechanism where Wnt signaling is sufficient to prevent CM differentiation, in contrast to a proposed role in inhibiting cardiac progenitor (CP) specification. The inability of the CPs to differentiate appears to lead to cell death through a p53/Caspase-3 independent mechanism. Together with a report for an even later role for Wnt signaling in restricting proliferation of differentiated ventricular CMs, our results indicate that during the first 3 days of development in zebrafish there are four distinct phases during which CMs are sensitive to Wnt signaling.
Keywords: cardiomyocyte, differentiation, Wnt signaling, zebrafish
Introduction
Proper Wnt/β-catenin (Wnt) signaling is required to direct many developmental processes and for the maintenance of tissues in adults through controlling specification, differentiation, and proliferation (Chien et al., 2009; Moon et al., 2004). Of the organs that Wnt signaling regulates during development, much attention has recently been paid to the effects of Wnt signaling during cardiomyocyte (CM) development (Cohen et al., 2008; Kwon et al., 2008; Tzahor, 2007). In vivo and in vitro, the effects of Wnt signaling have been shown to oscillate between promoting and restricting CM formation at different developmental steps (Kwon et al., 2009; Naito et al., 2006; Qyang et al., 2007; Tzahor, 2007; Ueno et al., 2007). The ability of the effects of Wnt signaling on CM formation to be translated from developmental models to embryonic stem (ES) cell culture offers the potential for rapid generation of novel stem cell based tissue engineering and regenerative therapies to combat congenital heart defects and cardiomyopathies (Domian et al., 2009). However, to ultimately direct the precise differentiation of CMs in vitro, we need to better understand the underlying mechanisms by which Wnt signaling directs CM formation during these different phases of development.
The earliest phase of development during which Wnt signaling affects CM formation is during mesoderm induction, when Wnt signaling promotes cardiac progenitor (CP) specification as a consequence of inducing ventro-lateral mesoderm (Naito et al., 2006; Ueno et al., 2007). Because Wnt signaling also promotes mesoderm and dorsal vessel formation in Drosophila, it has been proposed that this function of Wnt signaling is a conserved feature of metazoan development (Naito et al., 2006; Tzahor, 2007). After mesoderm induction, the role of Wnt signaling then changes in the pre-cardiac mesoderm, i.e. the mesoderm after the initial phase of induction from which the CPs will eventually arise but prior to the onset of CP marker gene expression, such as nkx2.5, tbx5 and gatas 4–6. Studies in vertebrate embryos and ES cells have suggested that in the pre-cardiac mesoderm Wnt signaling is necessary to inhibit CP specification (Foley et al., 2007; Foley and Mercola, 2005; Lavery et al., 2008b; Marvin et al., 2001; Naito et al., 2006; Paige et al., 2010; Schneider and Mercola, 2001; Tzahor and Lassar, 2001; Ueno et al., 2007; Wang et al., 2007). One proposed mechanism for how Wnt signaling affects CP specification in the pre-cardiac mesoderm comes from ES cells, where it has been suggested that vascular or hematopoietic fates are specified at the expense of cardiac fates (Naito et al., 2006; Wang et al., 2007). However, other comparable studies using ES cells have not found this inverse relationship, clouding the mechanism behind Wnt signaling in this phase (Abu-Issa et al., 2004; Kwon et al., 2009; Paige et al., 2010; Ueno et al., 2007; Willems et al., 2011). Therefore, while the consensus is that Wnt signaling restricts CP specification in the pre-cardiac mesoderm, the disparities between some of these studies indicate we still do not have a complete picture of the mechanism by which Wnt signaling affects the pre-cardiac mesoderm.
Most recent work has focused on the role of Wnt signaling in the second heart field (SHF) of vertebrates. During this phase when CP marker genes are expressed in the SHF, it has been proposed that Wnt signaling promotes the renewal and proliferation of CPs at the expense of differentiation (Klaus et al., 2007; Kwon et al., 2009; Qyang et al., 2007). Transgenic Wnt signaling reporters indicated that Wnt signaling occurs in the SHF, and pan-mesodermal depletion of Wnt signaling indicates that Wnt signaling is necessary and sufficient for specification of CPs in the SHF (Klaus et al., 2007; Lin et al., 2007). Modulation of Wnt signaling using drivers specific to the SHF has indicated that Wnt signaling can expand the isl1 expressing CPs in the SHF through inducing proliferation (Cohen et al., 2007; Kwon et al., 2009; Lin et al., 2007; Qyang et al., 2007). In addition, a recent study suggests that Wnt2, which is expressed in the pharyngeal mesoderm of mouse embryos, is necessary for the specification and proliferation of atrial progenitor cells in the posterior SHF (Tian et al., 2010). Therefore, in the SHF of mice and ES cells, it appears that Wnt signaling tightly regulates a balance between specification, proliferation/renewal and differentiation of CPs.
Studies of the zebrafish liebeskummer/ruvbl2 mutant have indicated an even later role for Wnt signaling in differentiated ventricular CMs, where Wnt signaling is required to autonomously restrict ventricular proliferation (Rottbauer et al., 2002). The timing of this role, where the phenotype is seen by 3 days post-fertilization, is comparatively much later than the other developmental roles of Wnt signaling in CM formation in mice. Therefore, studies suggest that there are at least 3 roles of Wnt signaling through the first 3 days of development in zebrafish: the first two roles may be conserved with mouse embryos and both human and murine ES cells (Marvin et al., 2001; Naito et al., 2006; Paige et al., 2010; Tzahor and Lassar, 2001; Ueno et al., 2007; Willems et al., 2011), while it is unclear if the later role in the differentiated ventricular cells is conserved with other vertebrates. More recently, it was demonstrated that cells are added to both poles of the zebrafish heart at time points between these stages (de Pater et al., 2009; Zhou et al., 2011), which is reminiscent of later addition of CMs by the SHF in mammals and chicks (Dyer and Kirby, 2009). However, it was not determined if Wnt signaling affects the differentiation of the SHF as in mouse (Kwon et al., 2007; Kwon et al., 2009; Qyang et al., 2007). Therefore, while there are parallels between heart development in zebrafish and other vertebrates, we still do not have a clear understanding of the number of roles for Wnt signaling or mechanisms that Wnt signaling uses in zebrafish to regulating CM development.
In this study, we examine the roles of Wnt signaling in CM development of zebrafish during three phases of development: during mesoderm induction, in the pre-cardiac mesoderm, and during CM differentiation. With respect to the role of Wnt signaling in mesoderm induction, we extend previous studies through showing that the zebrafish Wnt8a paralogs promote atrial and ventricular CM specification similarly, although with slight dorso-ventral (DV) biases. With respect to the role of Wnt signaling in pre-cardiac mesoderm, we find that the pre-cardiac mesoderm is sensitive to Wnt signaling from the initiation of gastrulation through early somitogenesis, which is longer than previously demonstrated. Importantly, we also define a new mechanism for the role of Wnt signaling in pre-cardiac mesoderm. In contrast to the prevailing model that Wnt signaling inhibits CP specification through potential fate transformations, we instead find that Wnt signaling is only sufficient to prevent atrial and ventricular CM differentiation, without significant effects on CP specification. Furthermore, the presumptive CPs that cannot differentiate appear to die through a p53/Caspase-3 independent mechanism. Finally, we also define a new role for Wnt signaling during CM differentiation in zebrafish, where Wnt signaling is necessary and sufficient to promote atrial CMs. Although this is reminiscent of the role of Wnt2 in mice, our data indicate that the mechanism used to modulate atrial cell number in zebrafish is different than in mouse. Therefore, these studies substantially extend our understanding of the genetic and cellular mechanisms by which Wnt signaling controls CM formation in vivo and illuminates a previously unrecognized mechanism controlling atrial CM differentiation in zebrafish.
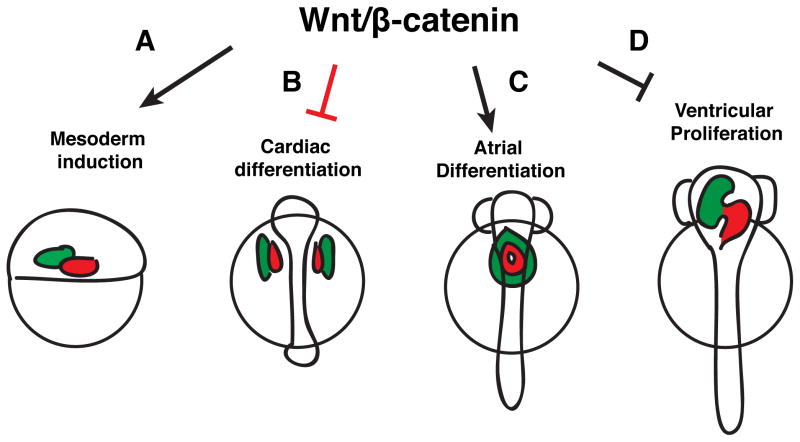
Altogether with the later role of Wnt signaling in ventricular CMs (Rottbauer et al., 2002), these data suggest a model where there are four distinct phases that CMs are sensitive to modulation of Wnt signaling through the first 3 days of zebrafish development. Specifically, the first two phases move from promoting to then restricting CM development in the pre-gastrula embryo and pre-cardiac mesoderm, respectively. Then, Wnt signaling has specific effects on atrial and ventricular cell development through promoting additional atrial progenitor cell specification and subsequent differentiation, followed by repressing proliferation in differentiated ventricular cells.
Materials and Methods
Zebrafish husbandry and transgenic lines used
Zebrafish were maintained and embryos were obtained and raised under standard conditions (Westerfield, 1993). The following transgenic lines were used: Tg(hsp70l:wnt8a-GFP)w34 (Weidinger et al., 2005), Tg(hsp70l:dkk1-GFP)w32 (Stoick-Cooper et al., 2007), Tg(hsp70l:Δtcf3-GFP)w26 (Lewis et al., 2004), Tg(−5.1myl7:nDsRed)f2 (Mably et al., 2003), Tg(−5.1myl7:EGFP)twu26 (Huang et al., 2003), Tg(kdrl:nlsEGFP)zf109 (Blum et al., 2008) and Tg(kdrl:mCherry)ci5 (Proulx et al., 2010).
Heat-shock experiments
For all experiments, unless otherwise indicated, the Tg(hsp70l:wnt8a-GFP)w34 and Tg(hsp70l:dkk1-GFP)w3 lines were used to increase and decrease Wnt signaling at the designated stages in embryos, respectively. For heat-shock experiments, hemizygous transgenic Tg(hsp70l:wnt8a-GFP)w34, Tg(hsp70l:dkk1-GFP)w3 or Tg(hsp70l:Δtcf3-GFP)w26 adults were crossed with wild-type, homozygous Tg(−5.1myl7:nDsRed)f2, Tg(−5.1myl7:EGFP)twu26, Tg(kdrl:nlsEGFP)zf109 or Tg(kdrl:mCherry)ci5 adults. Resulting embryos were then heat-shocked at the designated stage in a Biorad PCR machine by raising the temp to 37°C for 30 min, similar to what has been reported previously (Tucker et al., 2008). After ~1 h, the GFP from the transgenes was easily visible and the GFP positive (GFP+) sibling transgenic carriers were manually sorted from their heat-shocked GFP negative sibling embryos (Supplemental Fig. 1), which served as controls, using a Zeiss M2BioV12 Stereomicroscope. Heat-shocked transgenic embryos at the sphere stage produced dorsalized and ventralized phenotypes equivalent to what has been reported previously (data not shown; Stoick-Cooper et al., 2007; Weidinger et al., 2005). Similarly, heat-shocks administered at the 16 somite (s) stage affected axin2 expression, a Wnt signaling responsive gene, at the 20s stage in the predicted manner for the Tg(hsp70l:wnt8a-GFP)w34 and Tg(hsp70l:dkk1-GFP)w3 embryos (Supplemental Fig. 2). Together, these data suggest that we are effectively modulating Wnt signaling with the Tg(hsp70l:wnt8a-GFP)w34 and Tg(hsp70l:dkk1-GFP)w3 lines at all stages examined, consistent with what has been reported previously (Stoick-Cooper et al., 2007; Weidinger et al., 2005).
Zebrafish embryo injections
Zebrafish embryos were injected at the one cell stage with morpholinos (MOs) or mRNA. wnt8a.1 and wnt8a.2 MOs and wnt8a.1 mRNA were reported previously (Lekven et al., 2001). For MOs, a mixture of both MOs containing 2ng each was injected. For wnt8a.1 mRNA, 50pg was injected. Capped mRNA was made using a Message Machine Kit (Ambion). p53 MO was reported previously (Robu et al., 2007) and injected at 5ng into one cell stage Tg(hsp70l:wnt8a-GFP)w34 embryos.
In situ hybridization
In situ hybridization (ISH) was performed as reported previously (Oxtoby and Jowett, 1993). All probes were reported previously: myl7 (formerly called cmlc2; ZDB-GENE-991019-3), amhc (ZDB-GENE-031112-1), vmhc (ZDB-GENE-991123-5), nkx2.5 (ZDB-GENE-980526-321), gata4 (ZDB-GENE-980526-476), gata6 (ZDB-GENE-000622-1), aldh1a2 (ZDB-GENE-011010-3), etv2 (ZDB-GENE-050622-14), kdrl (ZDB-GENE-000705-1), fli1a (ZDB-GENE-980526-426), tbx5a (ZDB-GENE-991124-7), tbx6 (ZDB-GENE-980526-171), and flh (ZDB-GENE-990415-75).
Area measurements
Areas of myl7, vmhc and amhc expressing cells were measured as previously reported (Waxman et al., 2008). Briefly, ImageJ was used to measure the areas of the total amount of cells expressing myl7 and vmhc at the 20s stage and amhc and tbx5a at the 22s stage. Because slight variation can occur between ISH of different experiments, only samples from individual experiments were compared to each other. Measurements were made using arbitrary units. Student’s t-test was used to determine if the means from the individual experiments were statistically different (p<0.05).
Cell counting and imaging of zebrafish hearts
Cell counting was done as previously described (Waxman et al., 2008). Briefly, hemizygous transgenic Tg(hsp70l:wnt8a-GFP)w34 or Tg(hsp70l:dkk1-GFP)w3 adults were crossed to homozygous Tg(−5.1myl7:nDsRed)f2 adults. After selection of heat-shocked control sibling embryos (HCSEs) and GFP+ embryos, embryos were harvested at 48 hours post-fertilization (hpf), fixed and processed using an α-DsRed (Clontech) and S46 antibody (Stainier and Fishman, 1992). Embryos were then gently flattened underneath a cover slip. The hearts were photographed and the number of cardiac cells in each chamber was counted. Because slight variation in cell number can occur between different clutches of embryos and experiments, only counts from individual experiments were compared to each other. Student’s t-test was then used to determine if the means from the individual experiments were statistically different (p<0.05). For imaging of hearts, without the nuclear DsRed transgene, the MF20 antibody (Stainier and Fishman, 1992) was used to visualize all cardiac cells.
Phospho Histone H3 (pHH3) staining
An anti-pHH3 antibody (Ser10; Millipore) was used to identify proliferating cells. To be able to distinguish between atrial and endocardial cells, we crossed the transgenic Wnt and Dkk lines to the Tg(kdrl:nlsEGFP)zf109 line, which allows for the visualization of the nuclei in endothelial cells. After fixation and processing as above, except at 24 and 26 hpf, we used the α-AMHC (S46) antibody. To enhance the visualization of GFP after fixation, we used an α-GFP antibody (Millipore).
Acridine Orange
Embryos were placed in 10 μg/ml acridine orange (AO; Sigma) for 30 mins in embryo water (without methylene blue) (Westerfield, 1993). Embryos were then washed 3X for 10 mins with embryo water while gently shaking on a nutator. Embryos were visualized and photographed using a Zeiss M2BioV12 Stereo microscope and Zeiss Axio Imager Z1 with ApoTome.
Whole Mount Immunohistochemistry
Detection of activated Caspase-3 at 20s was performed as previously described (Sidi et al., 2008).
Quantitative Real Time PCR
Total RNA was isolated from staged embryos homogenized in TRIzol (Ambion) and collected using RNeasy mini columns (Qiagen). TURBO DNA-free kit (Applied Biosystems) was used to remove genomic contamination. 1 μg RNA was used for cDNA synthesis using the ThermoScript Reverse Transcriptase kit (Invitrogen). Quantitative real time PCR (qPCR) for myl7, amhc, vmhc, nkx2.5, tbx5, gata4, and β-actin was performed using standard PCR conditions in a Bio-Rad CFX PCR machine with Power SYBR Green PCR Master Mix (Applied Biosystems). Expression levels were standardized to β-actin expression. All experiments were performed in triplicate. Primer sequences and PCR protocol are available upon request.
Results
Zebrafish Wnt8a paralogs are necessary and sufficient to promote atrial and ventricular CM specification in the pregastrula embryo
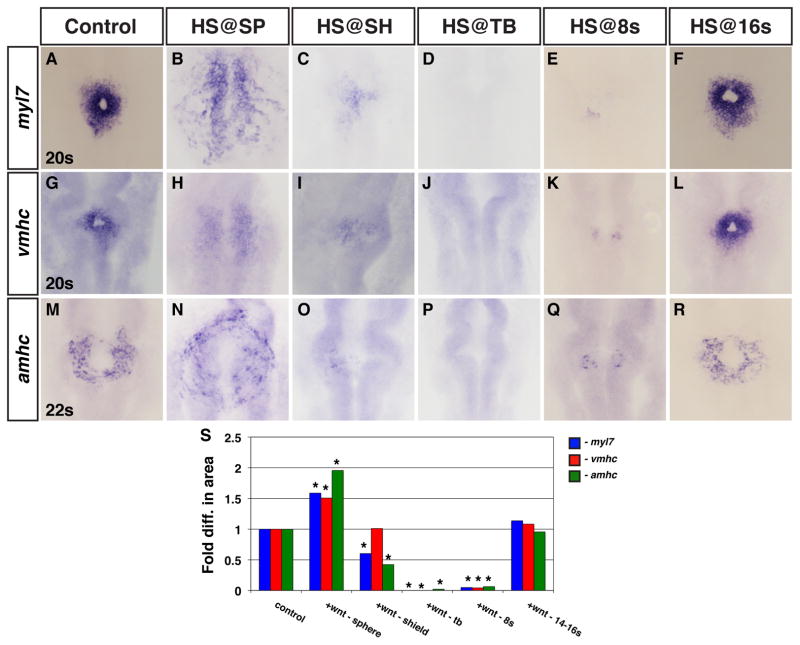
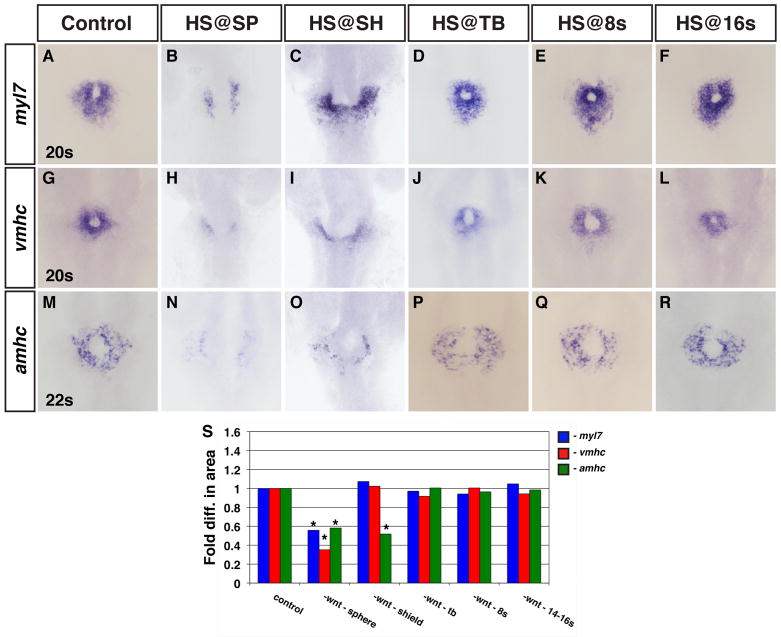
Previous studies have not determined if Wnt signaling, an effector of dorso-ventral patterning (DV) (Baker et al., 2010; Lekven et al., 2001; Ueno et al., 2007), affects ventricular and atrial cells similarly in the pregastrula embryo. BMP signaling, also an effector of DV patterning (Nguyen et al., 1998), differentially affects the formation of these CMs consistent with their relative positions within the pre-gastrula embryo (Keegan et al., 2004; Marques and Yelon, 2009). Therefore, we hypothesized that Wnt signaling may differentially affect ventricular and atrial CM formation during this early patterning event. To determine the role of Wnt signaling in ventricular and atrial CM formation prior to gastrulation, we used the heat-shock inducible Tg(hsp70l:wnt8a-GFP)w34 and Tg(hsp70l:dkk1-GFP)w3 lines to modulate Wnt signaling at the sphere stage. We then examined the earliest ventricular and atrial specific differentiation markers, ventricular myosin heavy chain (vmhc) and atrial myosin heavy chain (amhc), at the 20s and 22s stages, respectively (Berdougo et al., 2003; Yelon et al., 1999). We also examined myl7 at the 20s stage, which at this stage primarily marks ventricular cells though later it is expressed in all differentiated CMs (Berdougo et al., 2003; Yelon et al., 1999). Similar to what has been previously reported (Ueno et al., 2007), we found that increasing and decreasing Wnt signaling prior to gastrulation, using Tg(hsp70l:wnt8a-GFP)w34 and Tg(hsp70l:dkk1-GFP)w3 embryos respectively, resulted in increased and decreased myl7 (Fig. 1B, Fig. 2B and Supplemental Table 1). With respect to vmhc and amhc, we found that modulation of Wnt signaling had similar effects on both these markers (Fig. 1H, N, S; Fig. 2H, N, S and Supplemental Table 1), though quantification of the amount of cells revealed potential DV biases (Fig. 1S, Fig. 2S and Supplemental Table 1). The more ventrally located atrial cells had a greater fold increase when Wnt signaling was increased relative to the more dorsally located ventricular cells (Fig. 1S; Keegan et al., 2004). Conversely, the ventricular cells were slightly more sensitive to loss of Wnt signaling (Fig. 2S).
Figure 1. Effects of increased Wnt signaling on CM differentiation markers.
(A–F) myl7, (G–L) vmhc and (M–R) amhc expression in representative HCSEs and embryos when Wnt signaling is increased using the Tg(hsp70l:wnt8a-GFP)w34 line at the designated stages. Increased Wnt signaling promoted (B,H,N) and then inhibited (C–E,I–K,O–Q) the expression of CM differentiation markers when Wnt signaling was increased prior to gastrulation at the sphere (SP) stage and from the initiation of gastrulation [shield (SH)] through early somitogenesis. (F,L,R) Increasing Wnt signaling at 16s does not overtly affect CM differentiation markers. (S) Graph indicating the fold difference in areas of cardiac differentiation marker expression. Asterisks indicate a statistical difference in the areas from HCSEs and GFP+ embryos in Supplemental Table 1 according to Student’s t-test. Images in all figures are dorsal views with anterior up, unless otherwise indicated.
Figure 2. Wnt signaling is necessary for CM formation in the pregastrula embryo.
(A–F) myl7, (G–L) vmhc and (M–R) amhc expression in representative HCSEs and embryos when Wnt signaling is decreased using the Tg(hsp70l:dkk1-GFP)w32 line at the designated stages. (B,H,N) Wnt signaling is necessary prior to gastrulation to promote ventricular and atrial marker expression. (C, I) Decreasing Wnt signaling at the initiation of gastrulation (shield stage) causes aberrant morphology of CMs expressing myl7 and vmhc. (O) Decreasing Wnt signaling at the initiation of gastrulation reduces the amount of cells expression amhc. (D–F,J–L,P–R) The expression of CM differentiation markers is not affected when Wnt signaling is decreased from TB through 16s. (S) Graph indicating the relative difference in area of cells expressing these markers. Asterisks indicate a statistical difference in the areas from HCSEs and GFP+ embryos in Supplemental Table 1 according to Student’s t-test.
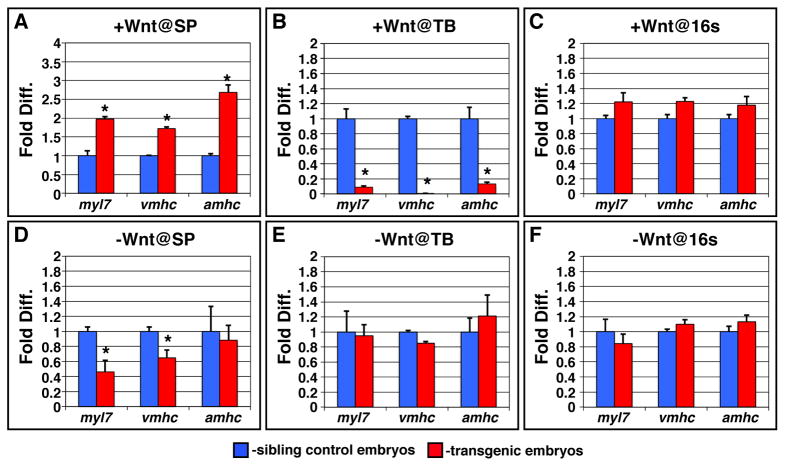
To corroborate the ISH analysis, we also used qPCR to examine the expression of the CM differentiation markers in embryos where Wnt signaling had been modulated prior to gastrulation. Similar to the trends that we observed with the ISH analysis (Fig. 1S and 2S), we found that increasing Wnt signaling promoted the expression of the cardiac differentiation markers (Fig. 3A), while decreasing Wnt signaling reduced the expression of the cardiac differentiation markers (Fig. 3D). Like with the ISH, amhc expression was more sensitive to increased Wnt signaling, while vmhc was more sensitive to decreased Wnt signaling. We note that in contrast to the ISH analysis we did not observe a significant effect on amhc expression using qRT-PCR. However, this could be due to that amhc expression was more variable in control sibling and transgenic embryos. Altogether, our data suggest that Wnt signaling is necessary and sufficient for the formation of both atrial and ventricular CMs, which exhibit slight DV biases in sensitivity to Wnt modulation.
Figure 3. qPCR analysis of myl7, vmhc and amhc expression after modulation of Wnt signaling.
(A) Increasing Wnt signaling at the sphere stage causes an increase in CM differentiation marker gene expression. (B) Increasing Wnt signaling at the TB stage causes a decrease in CM differentiation marker gene expression. (C) Increasing Wnt signaling at the 16s stage does not have a significant effect on CM differentiation marker gene expression. (D) Decreasing Wnt signaling causes a decrease in myl7 and vmhc expression. (E,F) Decreasing Wnt signaling at the TB and 16s stages does not affect CM differentiation marker gene expression. Analysis of gene expression was performed on embryos at the 20–22s stages. All trends of expression examined with qPCR, except for amhc after decreasing Wnt signaling at sphere, are similar to those observed in Figs. 1 and 2 with quantification by ISH area. The inability to detect a decrease in amhc expression could be due to the higher variability of amhc observed in both control and transgenic embryos. Although vmhc is also expressed in the somites, trends with respect to expression closely parallel those observed in the heart. However, we cannot rule out that the trends observed reflect additional effects of Wnt signaling on vmhc in the somites. Error bars for all qPCR analysis indicate the standard deviation of the triplicates used for analysis. Asterisks for all qPCR experiments indicate a statistically significant difference (p<0.05) in expression using Student’s t-test.
We next wanted to determine which endogenous Wnt(s) control CM formation through patterning the mesoderm. In zebrafish, two Wnt8a paralogs (wnt8a.1 and wnt8a.2) are required for ventro-lateral mesoderm induction (Baker et al., 2010; Lekven et al., 2001; Ramel and Lekven, 2004), yet it has not specifically been shown that these Wnt8as are necessary for CM specification and differentiation, as would be predicted. Therefore, we tested if the Wnt8as are required for CM formation through co-injection of wnt8a.1 and wnt8a.2 MOs (Lekven et al., 2001). We found that depletion of these wnt8as causes a reduction in cells expressing myl7, vmhc and amhc (Fig. 4B,E,H). While the trends on the cardiac differentiation markers were similar to what we observed using the Tg(hsp70l:dkk1-GFP)w32 line (Fig. 2B,H,N), the effects on the cardiac differentiation markers was less strong. The minor differences in effects on CM differentiation markers could be due to differences in efficacy of Wnt signaling inhibition from the hsp70l:dkk1-GFP transgene vs. the wnt8a MOs. Alternatively, another possibility is that wnt3a also plays a minor role promoting cardiac progenitor specification, consistent with its limited role in DV patterning of the mesoderm compared to wnt8a.1 and wnt8a.2 (Thorpe and Moon, 2004). Gain-of-function experiments with injected wnt8a.1 mRNA led to the opposite effect, an increase the amount of cells expressing myl7, vmhc and amhc (Fig. 4C,F,I). Therefore, these results further support that increased Wnt signaling prior to gastrulation is sufficient to promote the formation of differentiated CMs, while the wnt8a paralogs are necessary for the formation of differentiated CMs in zebrafish embryos.
Figure 4. Zebrafish Wnt8a paralogs are required to promote proper CM formation.
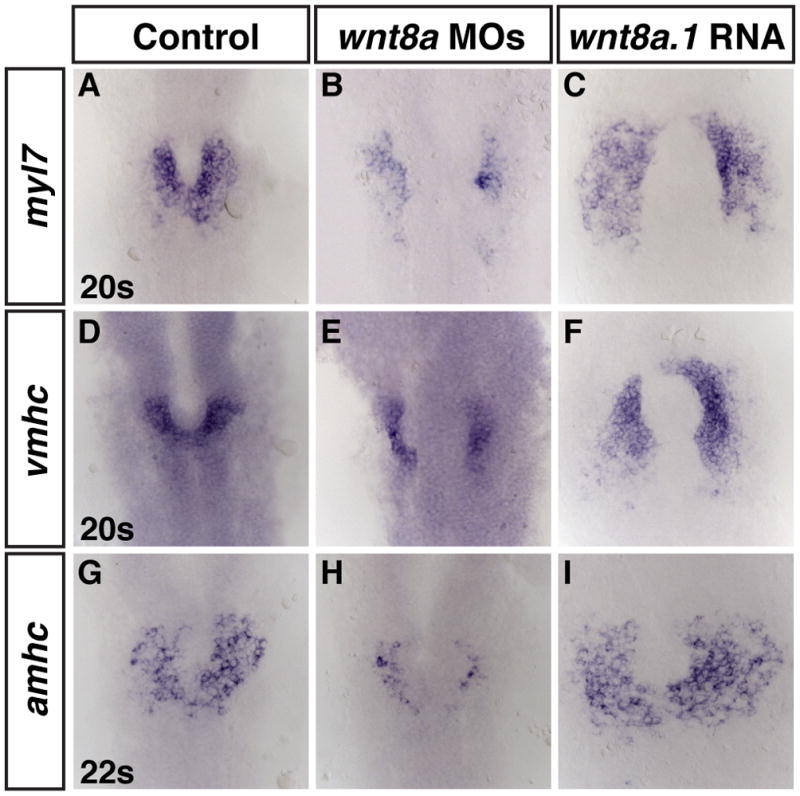
(A,D,G) Control uninjected embryos. (B,E,H) Embryos depleted of wnt8a.1 and wnt8a.2 display a reduced mount of cells expressing myl7, vmhc and amhc. (C,F,I) Embryos injected with wnt8a.1 mRNA display increased numbers of cells expressing myl7, vmhc and amhc.
Wnt8a paralogs in zebrafish are necessary for ventro-lateral mesoderm specification (Baker et al., 2010; Lekven et al., 2001; Martin and Kimelman, 2008; Ueno et al., 2007). The subtle differences in atrial and ventricular sensitivity could be consistent with effects on ventro-lateral mesoderm specification and the more ventral location of atrial progenitors compared to the ventricular progenitors in the pregastrula embryo (Keegan et al., 2004), like what has been found with BMP signaling (Marques and Yelon, 2009). To confirm that our abrogation of Wnt signaling is affecting mesoderm specification, we first examined aldh1a2, an early Wnt responsive gene that is initially expressed throughout the ventro-lateral mesoderm (Weidinger et al., 2005), in wnt8a morphants and wnt8a.1 mRNA injected embryos. Consistent with effects on ventro-lateral mesoderm specification, we found that decreasing and increasing Wnt8a signaling reduced and expanded aldh1a2 expression, respectively, at the margin in shield stage embryos (Supplemental Fig. 3).
We next examined markers of mesodermal DV patterning at ~80% epiboly in Tg(hsp70l:dkk1-GFP)w3 embryos heat-shocked at the sphere stage. Shortly after its initial expression in the ventro-lateral mesoderm, aldh1a2 is no longer directly sensitive to Wnt signaling and its expression becomes excluded from the ventral and dorsal regions of the embryo, making it an excellent marker for DV patterning events by 80% epiboly. In embryos with decreased Wnt signaling at the sphere stage, we found that shortly before the end of gastrulation aldh1a2 was now expressed on the ventral most side while the dorsal region was expanded (Supplemental Fig. 4A,B). Moreover, floating head (flh), a marker of dorsal mesoderm (Talbot et al., 1995), and tbx6, a marker of ventro-lateral mesoderm (Hug et al., 1997), were expanded and reduced in the dorsal embryo respectively (Supplemental Fig. 4E,F,I,J), consistent with what has been reported previously for loss of Wnt signaling in the early embryo (Baker et al., 2010; Lekven et al., 2001). Therefore, based on the effects upon DV markers, we conclude that zebrafish wnt8a paralogs affect atrial and ventricular specification as a consequence of their role in dorso-ventral patterning of the mesoderm.
Wnt signaling is sufficient to inhibit CM differentiation during gastrulation and early somitogenesis
We next wanted to better understand the role of Wnt signaling in the pre-cardiac mesoderm. Previously, the length of this second phase, when Wnt signaling was proposed to inhibit CM progenitor specification, was not determined (Ueno et al., 2007). Therefore, to precisely define the time period when the pre-cardiac mesoderm is sensitive to modulation of Wnt signaling, we modulated Wnt signaling using the aforementioned Wnt8 and Dkk transgenic lines at the shield and tail bud (TB; end of gastrulation) stages, as well as stages that were not examined previously: the 8s stage (initiation of robust CP gene expression) and 16s stage (initiation of cardiac differentiation gene expression). We then examined the areas of myl7, vmhc, and amhc expression, as described above. We found that increasing Wnt signaling inhibited CM formation through 8s and embryos were most sensitive to increased Wnt signaling at the TB stage (Fig. 1C–E, I–K, O–Q, S). The CM differentiation markers were no longer sensitive to increased Wnt signaling at the 16s stage (Fig. 1F, L, R, S). The loss of CM differentiation marker expression after increasing Wnt signaling at the TB stage and lack of effect on CM differentiation marker expression after increasing Wnt signaling at the 16s stage were confirmed using qPCR (Fig. 3B and C). Therefore, in zebrafish embryos, the pre-cardiac mesoderm is sensitive to increased Wnt signaling over a relatively long period of time, extending from the initiation of gastrulation through early somitogenesis.
When we examined embryos with decreased Wnt signaling, we were surprised to find that other than the shield stage, we did not observe an effect on the amount of differentiated CMs, despite aberrant morphologies of the fusing heart fields (Fig. 2C,I). Decreasing Wnt signaling at the shield stage, we found that there was decreased amhc expressing cells (Fig. 2O). This is likely due to the progressive nature of D-V patterning (Martin and Kimelman, 2008; Tucker et al., 2008) and the relatively ventral position of atrial progenitors compared to ventricular progenitors (Keegan et al., 2004). Consistent with this hypothesis, examination of aldh1a2, tbx6 and flh at 80% epiboly after decreasing Wnt signaling at the shield stage indicated a modest dorsalization (Supplemental Fig. 4C,D,G,H,K,L). Using qPCR to examine CM marker expression at the 20–22s stages and counting the number of cells in the atria and ventricles at 48 hpf, we also did not observe an increase in atrial or ventricular CM marker expression or number after decreasing Wnt signaling at the TB stage (Fig. 3E and Supplemental Table 2). Likewise, after decreasing Wnt signaling at the 16s stage, we found that there was not an effect on CM marker expression using qPCR (Fig. 3F). To corroborate these data we examined the hearts of Tg(hsp70l:Δtcf3-GFP)w26 embryos, which express a dominant negative TCF3, after heat-shocks at the TB and 16s stages. The hearts of the transgenic heat-shocked embryos were more linear than HCSEs, but were not discernibly larger at 48 hpf (Supplemental Fig. 5A,B). Therefore, these results suggest that in zebrafish increased Wnt signaling is sufficient to inhibit the formation of differentiated CMs in the pre-cardiac mesoderm from the initiation of gastrulation through early somitogenesis, but that Wnt signaling does not appear to be necessary to limit differentiated CMs over the same time period.
Because of the lack of inverse relationship observed with Wnt signaling in the pre-cardiac mesoderm, we speculated that there could be effects on the specification of CPs, which are then compensated for during differentiation leading to a normal number of CMs. To explore this possibility, we examined the CP markers nkx2.5 and gata4, which also marks more anterior mesoderm progenitors, at the 8s stage after modulating Wnt signaling at the TB stage. In contrast to what has been reported previously (Ueno et al., 2007), we did not find a discernible effect on either of the CP markers in embryos with decreased Wnt signaling (Supplemental Fig. 6A,B,E,F), suggesting that decreased Wnt signaling is not affecting CP specification. When we examined embryos with increased Wnt signaling, we were also surprised that the majority (89%; n=45) had normal nkx2.5 expression (Supplemental Fig. 6C,D) and no discernible effect on gata4 expression (Supplemental Fig. 6G,H). The lack of effects on CP markers via ISH after modulation of Wnt signaling at the TB stage was confirmed using qPCR (Supplemental Fig. 6I,J). Because we did not observe a strong effect of increased Wnt signaling at the TB stage upon nkx2.5 expression at 8s, we next examined nkx2.5 expression at the 14s stage via ISH. In this case, 100% (n=27) of embryos had nkx2.5 expression that was indistinguishable from HCSEs (Fig. 5A,B), further supporting that increased Wnt signaling in the pre-cardiac mesoderm does not affect CP specification. Altogether, these results suggest that increased Wnt signaling does not affect CP specification nor is Wnt signaling required to limit the number of CPs in the pre-cardiac mesoderm.
Figure 5. Increased Wnt signaling at the TB stage causes a loss of nkx2.5 expression after the 14 somite stage.
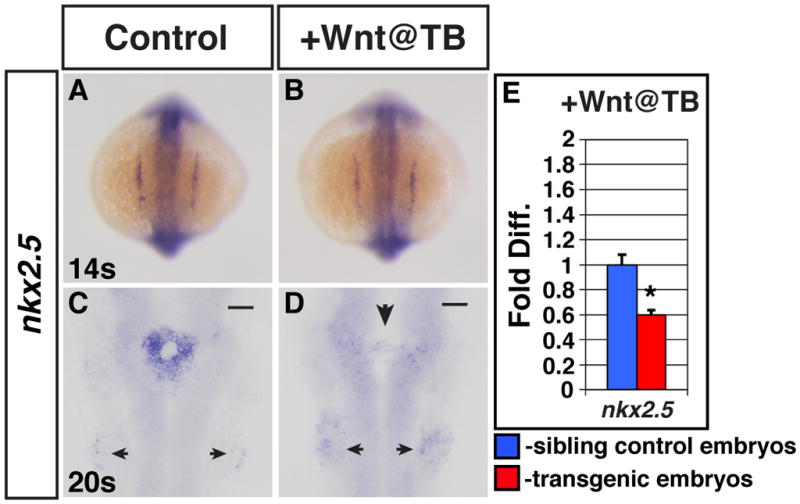
(A,C) HCSEs. (B,D) GFP+ sibling embryos. Expression of nkx2.5 is normal in embryos at the 14s stage after increasing Wnt signaling at the TB stage (B; 100%, n=27), compared to HCSEs (A; n=38). (C) nkx2.5 expression in HCSEs (n=21). (D) Expression of nkx2.5 is lost by 20s (100% had severely reduced nkx2.5 expression, n=27) in GFP+ embryos after heat-shock at the TB stage. There are diffuse nkx2.5 expressing cells at the midline (large arrowhead). Posterior pharyngeal arch staining appears increased in embryos with increased Wnt signaling at the TB stage (small arrowheads). (E) qPCR analysis of nkx2.5 expression indicates total nkx2.5 expression is reduced in 20–22s stage GFP+ embryos after increasing Wnt signaling at the TB stage.
Increased Wnt signaling in pre-cardiac mesoderm prevents CM differentiation leading to cell death
We have found that increased Wnt signaling in the pre-cardiac mesoderm eliminates expression of cardiac differentiation markers, yet we find that CP specification initially is unchanged. One potential model that could explain this is similar to what has been found for Wnt signaling and cardiac cell differentiation of the SHF, where it has been proposed that Wnt signaling promotes cell renewal/proliferation of CPs at the expense of differentiation (Qyang et al., 2007). Because we did not observe an increase in nkx2.5 expression at 14s (Fig. 5A,B), we examined nkx2.5 expression later at the 20s stage in embryos after Wnt signaling had been increased at the TB stage. In contrast to a model where CPs are maintaining a proliferative state, we found that there were a small number of cells flanking the midline that expressed nkx2.5 at a low level in these embryos (Fig. 5C,D). Interestingly, we also found that there appeared to be a modest increase in pharyngeal cells expressing nkx2.5 (Fig. 5C,D), yet overall nkx2.5 expression was decreased (Fig. 5E). The pharyngeal expression is thought to initiate later and not be derived from the cardiac population of nkx2.5 expressing cells (Schoenebeck et al., 2007). Thus, our data do not support a model where increased Wnt signaling in the pre-cardiac mesoderm causes CPs to renew/proliferate at the expense of differentiation.
It has been proposed that in pre-cardiac mesoderm of mouse embryoid bodies (EBs) Wnt signaling can drive the formation of hematopoietic/vascular lineages at the expense of cardiac lineages (Naito et al., 2006; Wang et al., 2007). To test this model, we examined etv2, which marks vascular lineages and anterior myeloid lineages (Sumanas et al., 2008; Sumanas and Lin, 2006) and the vascular markers kdrl (Liao et al., 1997) and fli1a (Thompson et al., 1998) at the 20s stage in embryos when Wnt signaling was modulated at the TB stage. However, we did not observe a discernible effect on these markers when increasing or decreasing Wnt signaling (Supplemental Fig. 7A–L). Therefore, these results suggest that increased Wnt signaling is not sufficient to promote an overtly detectable fate transformation between cardiac and hematopoietic or vascular lineages in zebrafish embryos.
Because it appeared that increased Wnt signaling in pre-cardiac mesoderm did not induce CPs to increase nor to differentiate as another cell type, we asked if the increased Wnt signaling could be causing these cells to die. To assay apoptotic cell death in embryos with increased Wnt signaling at the TB stage, we treated embryos with the vital dye Acridine Orange at the 20s stage (Furutani-Seiki et al., 1996). In contrast to HCSEs, where there were only sporadic dying cells throughout the embryo and never large clusters of apoptotic cells, embryos with increased Wnt signaling at the TB stage had large bilateral populations of dying cells (Fig. 6A,B; Fig. 7A–F). The clusters of apoptotic cells were exactly where CPs would be expected to reside in the lateral plate mesoderm (LPM) if they had not migrated to the midline and begun to differentiate (Fig. 7A–F and Supplemental Fig. 8A–F; Bussmann et al., 2007). From one representative experiment, 100% (n=24) of embryos with increased Wnt signaling at the TB stage had clusters of apoptotic cells in the LPM at 20s, while 0% (n=14) of their HCSEs had clusters of apoptotic cells. Dying cells were also observed in the anterior neural tissue where Wnt repression is also required (Kim et al., 2000). However, in other regions of the transgenic embryos, there was only minimal sporadic cell death equivalent to what was observed in HCSEs. Furthermore, the analysis of hematopoietic and vascular markers (Supplemental Fig. 7A,B,E,F,I,J) and the lack of AO positive endothelial cells (Fig. 7A–F) suggests that these other LPM fates are not significantly affected and would be unlikely to contain a significant amount of dying cells.
Figure 6. Increased Wnt signaling at the TB stage causes cell death independent of Caspase-3 activation.
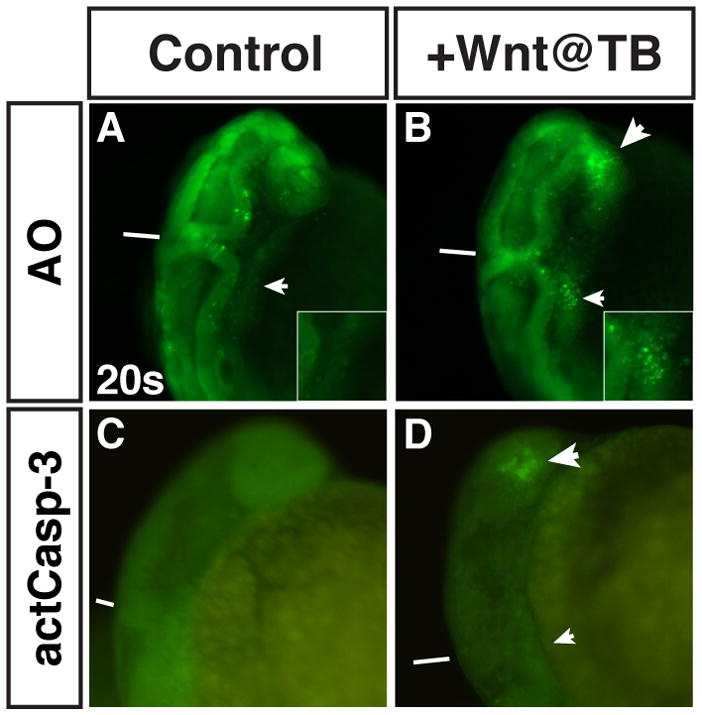
(A,C) HCSEs. (B,D) GFP+ sibling embryos. (A,B) Embryos at 20s with AO staining to detect dying cells. There is increased cell death in the LPM at 20s after increasing Wnt signaling at TB (B; small arrowhead and inset) compared to HCSEs (A; arrow and inset). Significant cell death was also observed in the anterior neural tissue (large arrowhead in B). (C,D) Embryos at 20s with whole mount immunohistochemical analysis of activated Caspase-3. (D) Activated Caspase-3 is not detected in the LPM where AO staining is observed at 20s after increasing Wnt signaling at the TB stage (small arrowhead). However, there is substantial activated Caspase-3 detected in the anterior neural tissues (large arrowhead), which is also observed with AO staining (B). Views in A and B are dorso-lateral. View C and D are lateral. Lines in A–D indicate the position of the midbrain-hindbrain boundary. CPs/CMs are located immediately posterior to this boundary in the LPM.
Figure 7. Increased Wnt signaling at the TB stage induces cell death in the anterior LPM.
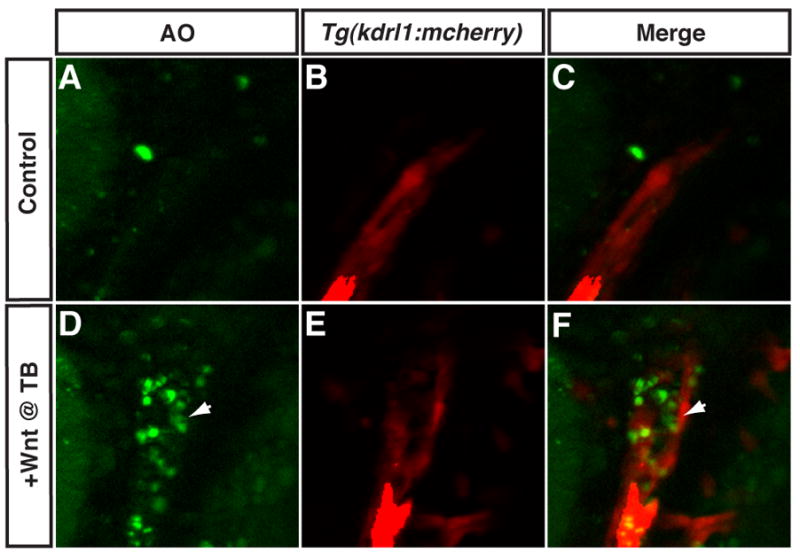
(A–C) Representative HCSE. (D–F) Representative GFP+ embryo after increasing Wnt signaling at the TB stage. Hemizygous Tg(hsp70l:wnt8a-GFP)w34 fish were crossed to hemizygous Tg(kdrl:mCherry)ci5 fish to examine the position of apoptotic cells relative to endothelial cells in the LPM. Images are from a single optical plane taken with a Zeiss Axio Imager and ApoTome. In the transgenic embryo with increased Wnt signaling at the TB stage, there are AO positive apoptotic cells adjacent to the endothelial cells (arrows in D and F), while the HCSE does not have a significant cluster of apoptotic cells in the same region of the anterior LPM (A and C). Images are dorso-lateral views with anterior to the top and dorsal to the left.
One mechanism by which these cells could be dying is through p53-mediated apoptosis (Pyati et al., 2007). To determine if the CP cells are dying through a p53-dependent mechanism and if inhibition of p53 can rescue CM differentiation, we injected Tg(hsp70l:wnt8a-GFP)w34 embryos with a p53 MO and then assayed CM differentiation markers in transgenic and HCSEs after heat-shock at the TB stage. However, depletion of p53 was not able to restore the expression of CM differentiation marker (data not shown). One potential model that the inability to rescue CM differentiation suggested is that these cells are dying through a p53-independent mechanism. To test this hypothesis, we examined activated Caspase-3 after increasing Wnt signaling at the TB stage because activated Caspase-3 in many contexts is a downstream mediator of p53-dependent apoptosis (Pyati et al., 2007; Sidi et al., 2008). In transgenic embryos with increased Wnt signaling at the TB stage, we did not observe activated Caspase-3 in the LPM where we observed dying cells after AO treatment (Fig. 6C,D). However, we did observe activated Caspase-3 in the anterior neural tissue (Fig. 6C,D), which provided a positive internal control and suggests that the Wnt induced neural cell death is via Capase-3 mediated apoptosis. Altogether, these results suggest that increased Wnt signaling in the pre-cardiac mesoderm does not affect CP specification, but instead prevents them from differentiating, which potentially results in their death through a p53/Caspase-3 independent apoptotic mechanism.
Wnt signaling is sufficient to promote the additional differentiation of atrial cells
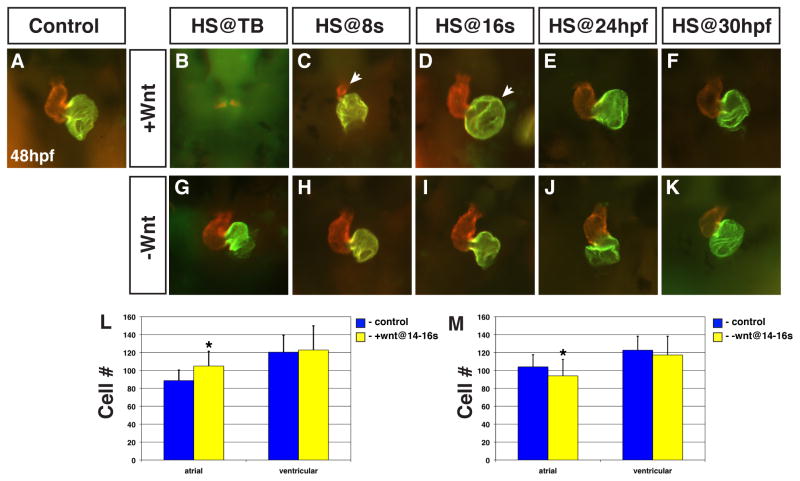
There is substantial developmental time and events between the 8s stage and 48 hpf, when Wnt signaling has been shown to modulate ventricular CM proliferation (Rottbauer et al., 2002). Therefore, we next wanted to determine if there were additional roles of Wnt signaling in CM formation between 8s and 48 hpf. To do so, we modulated Wnt signaling in the transgenic embryos at the 16s stage, 24 hpf and 30 hpf and examined the hearts at 48 hpf. Interestingly, even though we did not observe a noticeable effect of Wnt signaling on the expression of CM differentiation markers at 20s or 22s (Fig. 1F,L,R,S; Fig. 2F,L,R,S; Fig. 3C,F), by 48 hpf the hearts from embryos receiving increased Wnt signaling at 16s had significantly larger atria (Fig. 8D; Supplemental Movies 1 and 2). As would be expected from our analysis of earlier markers, hearts from embryos with increased Wnt signaling at TB and 8s were almost absent and significantly smaller, respectively (Fig. 8B,C), while decreased Wnt signaling did not discernibly disturb the hearts (Fig. 8G,H). We also found that increased Wnt signaling at 24 hpf caused hearts with slightly enlarged atria (Fig. 8E), while hearts appeared normal after increasing Wnt signaling at 30 hpf (Fig. 8F). At 48 hpf, after Wnt signaling was decreased with either the Tg(hsp70l:dkk1-GFP)w32 or Tg(hsp70l: Δtcf3-GFP)w26 lines at 16s, 24 hpf, and 30 hpf the hearts appeared relatively normal, though occasionally the atria looked smaller after decreasing Wnt signaling at the 16s stage (Fig. 8I–K; Supplemental Fig. 5C,D). Thus, we have uncovered a previously unrecognized role for Wnt signaling in zebrafish where inappropriate Wnt signaling during CM differentiation can affect atrial size.
Figure 8. Wnt signaling is necessary and sufficient to promote atrial cell development during CM differentiation.
(A) Representative HCSE. (B–F) GFP+ embryos with increased Wnt signaling. (G–K) GFP+ embryos with decreased Wnt signaling. Decreasing Wnt signaling from TB through 30 hpf had inconsistent and modest effects on morphology. (B,C) Increasing Wnt signaling at TB or 8s almost eliminates and greatly reduces the size of the hearts, respectively, at 48 hpf. Arrow in C indicates the severely smaller ventricle. (D) When Wnt signaling is increased at 16s, the atrium is significantly enlarged (arrow). Although the ventricles were dismorphic, we did not find an effect on ventricular cell number. (E) Increasing Wnt signaling at 24 hpf causes modestly enlarged atria. (F) Increasing Wnt signaling at 30 hpf no longer has a discernible effect on the heart. (L) Increasing Wnt signaling at 16s causes a specific increase in atrial cell number. (M) Decreasing Wnt signaling at 16s causes a modest reduction in atrial cell number. Images are frontal views. Red indicates ventricle and green indicates atrium. Asterisks indicate a significant difference in the cell number from HCSEs and GFP+ embryos in Supplemental Table 3 according to Student’s t-test.
To determine if the Wnt induced increase in atrial size is due to an increase in cell number, as opposed to only changes in morphology, we counted the number of cells in each chamber (Waxman et al., 2008). Indeed, we found that increasing Wnt signaling at 16s caused an ~20% increase in atrial cell number, while not affecting ventricular cell number (Fig. 8L; Supplemental Table 3). Based on these results, we expected that decreased Wnt signaling might cause a decrease in atrial cell number, so we also complemented the gain-of-function experiments by counting the number of atrial and ventricular CMs after decreasing Wnt signaling at 16s. Although only 1 out of 3 experiments indicated a statistically significant decrease in atrial cell number relative to HCSEs (Fig. 8M), all 3 experiments demonstrated a modest decrease in atrial cell number relative to HCSEs (Supplemental Table 3), suggesting a trend where Wnt signaling is necessary, although minimally, during CM differentiation for appropriate atrial CM number. Therefore, our results suggest that Wnt signaling is necessary and sufficient to specifically promote proper atrial cell number during CM differentiation.
We next wanted to explore the mechanism by which Wnt signaling affected atrial cell development at the 16s stage resulting in increase cell number. We hypothesized that Wnt signaling could be promoting additional atrial cell development through an effect on differentiation and/or proliferation. We began by exploring if Wnt signaling was affecting differentiation through modulating gata gene expression, because recently it was shown that Wnt2 in mice promotes posterior SHF/atrial specification and proliferation through positively regulating gata6 expression (Tian et al., 2010). However, when we modulated Wnt signaling at the 16s stage and examined gata6 or gata4 expression at 26 hpf, we did not find an effect on the expression of either of these markers (Fig. 9C,D; Supplemental Fig. 9C–F). The atrial region of the heart is already morphologically expanded by this stage, as easily seen by the endocardial expression of gata4 (Fig. 9E,F). By comparison, increasing Wnt signaling at 8s significantly reduced gata6 expression, consistent with an expected inhibitory effect on the unspecified pre-cardiac mesodermal cells (Fig. 9A,B). Decreasing Wnt signaling at 8s did not discernible affect gata6 expression (Supplemental Fig. 9A,B). We also examined gata6 expression at 20s after Wnt signaling was modulated at 16s and found no difference between HCSEs and embryos with modulated Wnt signaling (Supplemental Fig. 10A–D). Therefore, Wnt signaling does not appear to modulate atrial cell formation through regulation of gata6 or gata4. These results suggest that despite the similarity of the later sensitivity of atrial cell formation to Wnt signaling in zebrafish and mouse embryos, zebrafish likely use a different mechanism to regulate the addition of atrial cells.
Figure 9. Modulating Wnt signaling does not affect gata4 or gata6 expression in differentiating CMs.
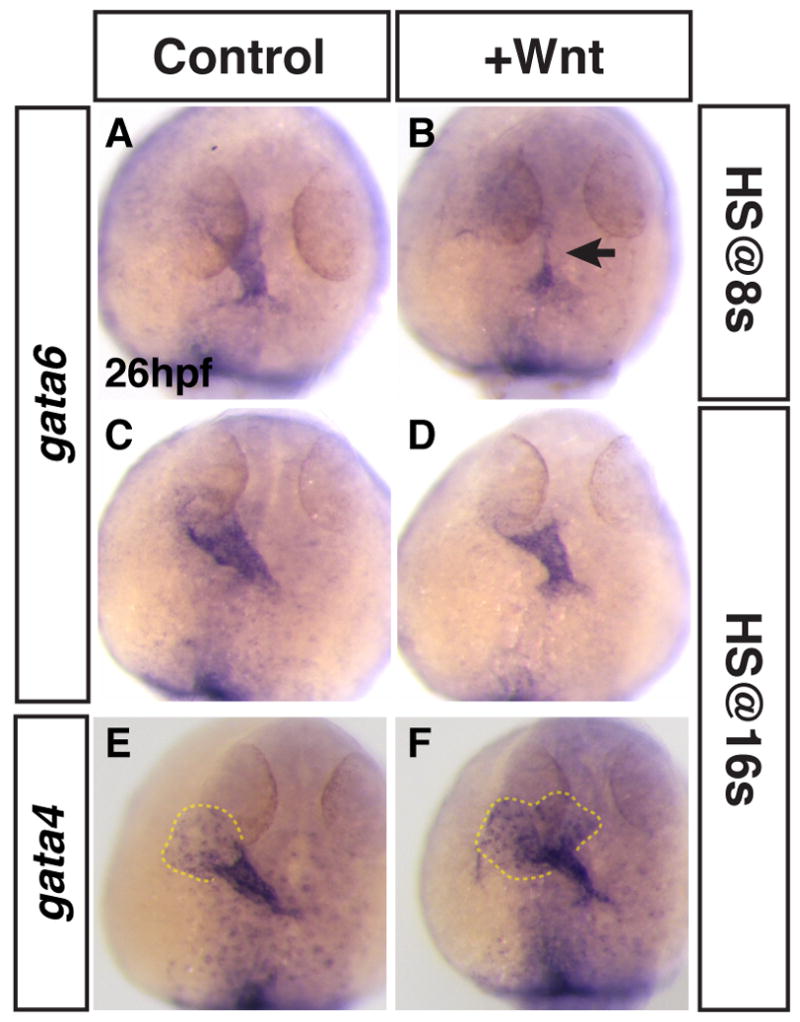
(A,C,E) HCSEs. (B,D,F) GFP+ embryos with increased Wnt signaling at the 8s and 16s stages. (B) Increasing Wnt signaling at 8s decreases the amount of gata6 expression (arrow). (D,F) Increasing Wnt signaling at 16s did not discernibly affect gata6 or gata4 expression at 26 hpf. However, the endocardial morphology of gata4 expression indicates that the venous pole is expanded by 26 hpf (E,F; dashed outline).
Our results examining myl7, gata4 and gata6 expression indicate that there is a morphological difference in the atria between HCSEs and embryos with increased Wnt signaling at 16s that arises between 20 and 26 hpf, which suggests that the increase in atrial cell number may be occurring sometime between 20s and 26 hpf. To define the earliest time point that we can see a difference in atrial size, we examined the CP markers nkx2.5 and tbx5a at 22s and amhc at 24 hpf. Although we did not find an increase in nkx2.5 expression (Fig. 10A,B,G,H), which is understandable because at this stage it primarily marks ventricular cells in zebrafish, we did find an increase in the amount of tbx5a expression via analysis of ISH and qPCR (Fig. 10C,D,G,H,I; Supplemental Table 4). Interestingly, the ~30% fold increase in area of tbx5a expressing cells is highly reminiscent of the ~20% fold increase in atrial cell number at 48 hpf. Furthermore, by 24 hpf, the atria were expanded when Wnt signaling was increased at 16s (Fig. 10E,F). similar to what we observed with gata6 (Fig. 9E,F). Therefore, our results suggest that increasing Wnt signaling during CM differentiation causes an increase in the expression of the CP marker tbx5a by 22s, which precedes the enlarged atrial morphology visible at 24 hpf.
Figure 10. Effects of increased Wnt signaling during atrial CM differentiation are evident by the 22 somite stage.
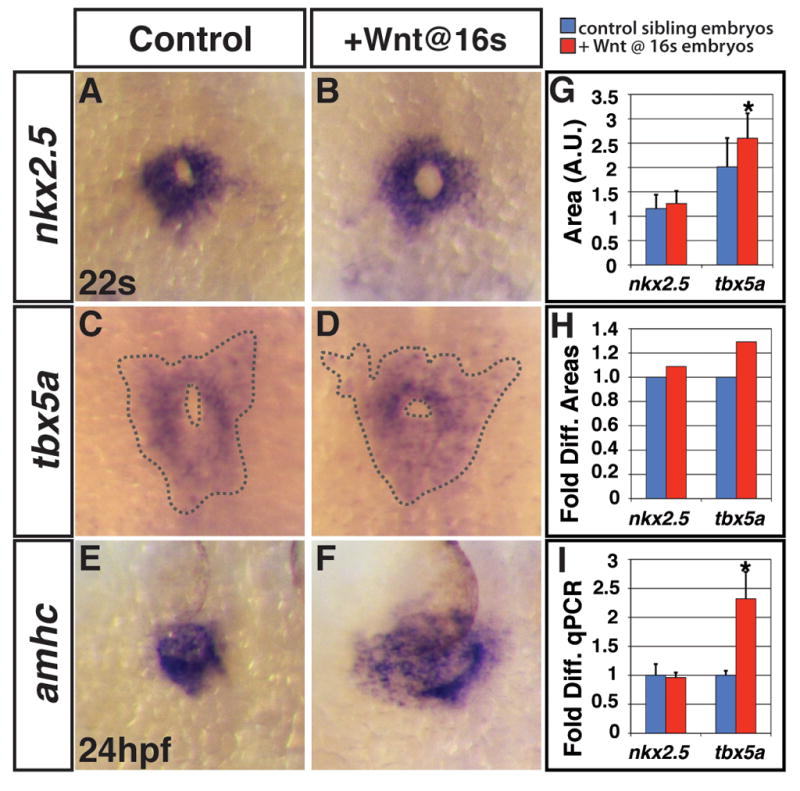
(A,C,E) HCSEs. (B,D,F) GFP+ embryos with increased Wnt signaling at the 16s stage. (B) The amount of cells expressing nkx2.5, which at 22s primarily marks ventricular cells, is not increased when Wnt signaling is increased during cardiac differentiation. (D) The amount of cells expressing tbx5a, which is expressed in both ventricular and atrial cells, is modestly increased by 22s. (F) When Wnt signaling is increased at 16s, amhc expression indicates that the atria are wider compared to controls (E) by 24 hpf. (G) Areas of the amount of cells expressing nkx2.5 and tbx5a in arbitrary units. (H) Fold difference in the areas of cells expressing nkx2.5 and tbx5a. (I) qPCR analysis of nkx2.5 and tbx5a expression at 22s. There is an increase in tbx5a greater than what is observed with the ISH analysis. However, we did observe an increase in tbx5a is expressed in the forelimb mesenchyme (data not shown). Therefore, the increase in tbx5a expression observed with qPCR likely reflects an increase in tbx5a expression from both populations. No difference in tbx5a expression in the eye was observed between HCSEs and embryos with increased Wnt signaling at the 16s stage at the 22s stage (data not shown). Asterisks indicates significant difference using Student’s t-test.
We next wanted to understand if the increase in atrial progenitors and CMs is also occurring through an effect on proliferation. To test if Wnt signaling is promoting increased proliferation during atrial CM differentiation, we increased Wnt signaling in Tg(kdrl:nlsEGFP)zf109 embryos at 16s, to be able to distinguish the endocardium from the myocardium, and used antibodies for AMHC (S46) and phospho-Histone H3 (pHH3) (Supplemental Fig. 11A–C). Unfortunately, prior to or at 24 hpf we could not visualize the GFP in these embryos after fixation (data not shown). However, the proliferation rate in HCSEs and embryos with increased Wnt signaling was not statistically different at 24 hpf (Supplemental Table 5) or at 26 hpf, when we could distinguish the difference between the endocardial and atrial cells (Supplemental Table 6; Supplemental Fig. 11A–C). Therefore, we presently conclude that increased Wnt signaling during CM differentiation is not discernibly affecting proliferation leading to more atrial cells. The lack of evidence to support an effect on proliferation makes us currently favor a model where during atrial CM differentiation Wnt signaling promotes the additional specification of CPs leading to excess atrial cells.
Discussion
In the present study, we examined the role of Wnt signaling in CM formation during 3 distinct phases of CM development in zebrafish. First, we provide evidence that zebrafish Wnt8a paralogs regulate the specification of both ventricular and atrial CMs through promoting ventro-lateral mesoderm. Second, we define a mechanism where Wnt signaling is sufficient, but is not necessary, to inhibit CM differentiation, which subsequently may lead to their death through a p53/Caspase-3 independent mechanism. Third, we define a mechanism where Wnt signaling promotes a surplus of atrial cells during CM differentiation through promoting the additional specification and differentiation of atrial progenitors. Taken together with a previous study that defined a role for Wnt signaling in preventing proliferation of differentiated ventricular cells (Rottbauer et al., 2002), we propose that Wnt signaling has 4 distinct roles in regulating CM formation in the early zebrafish embryo (Fig. 11).
Figure 11. Model depicting the roles of Wnt signaling in CM formation at the four distinct phases of development.
(A) Wnt signaling in the pregastrula embryo promotes the formation of CMs indirectly through its role in ventro-lateral mesoderm induction. (B) Wnt signaling is sufficient (red inhibitory sign), but alone not necessary, to restrict cardiac differentiation in the pre-cardiac mesoderm. (C) Wnt signaling is necessary and sufficient to promote the addition of atrial cells after the initiation of CM differentiation. (D) Wnt signaling is required to repress proliferation in differentiated ventricular cells (Rottbauer et al., 2002).
Wnt signaling effects on the pre-cardiac mesoderm of vertebrates
With respect to the pre-cardiac mesoderm, previous studies have suggested that Wnt signaling in the pre-cardiac mesoderm is necessary and sufficient to inhibit CP specification and differentiation (Foley et al., 2007; Foley and Mercola, 2005; Lavery et al., 2008b; Marvin et al., 2001; Naito et al., 2006; Schneider and Mercola, 2001; Tzahor and Lassar, 2001; Ueno et al., 2007). However, our results suggest that Wnt signaling is sufficient, but not required, to prevent cardiac differentiation and importantly that increased Wnt signaling is not sufficient to affect CP specification. Unfortunately, we presently do not understand the reasons behind the differences seen with the previous study using zebrafish (Ueno et al., 2007). Although we used the same transgenic lines as those reported in Ueno et al. (2007), it is possible that the combination of technical differences, including the PCR machine based method of heat-shock, quantitative ISH/area and qPCR analysis, which were not previously used, and examining additional stages has allowed for the different observations and conclusions.
When comparing our results to other vertebrate models, however, there is significant similarity. For instance, the loss-of function results reported here with zebrafish are consistent with chick explant experiments, which indicated that inhibition of Wnt signaling alone could not expand the amount of CMs (Tzahor and Lassar, 2001). Instead, Wnt signaling depletion needed to be coupled with BMP addition to stimulate an increase in CMs. Similarly, in mouse ES cells, both inhibition of Wnt and activation of BMP signaling downstream of Notch is required to promote cardiac cell development instead of hemangioblast development (Chen et al., 2008). In human ES cells, it was found that Wnt signaling is required to limit CM differentiation in the context of BMP addition, which is used to direct the mesodermal differentiation of these cells (Paige et al., 2010; Willems et al., 2011). In addition, in an endodermal conditional β-catenin KO mouse that leads to ectopic hearts, there is also ectopic BMP expression (Lickert et al., 2002), further implicating the connection of these two pathways in restricting CM formation. Although studies in mouse EBs and in Xenopus embryos have suggested that inhibition of Wnt signaling alone is required to increase CPs (Foley et al., 2007; Foley and Mercola, 2005; Lavery et al., 2008b; Marvin et al., 2001; Naito et al., 2006; Schneider and Mercola, 2001), these studies do not rule out that BMP signaling is concomitantly required to expand the CM population. Altogether, these disparities indicate that future studies are clearly necessary to be able to thoroughly understand and compare the individual vs. dual requirements of Wnt and BMP signaling in the pre-cardiac mesoderm of vertebrate embryos and ES cells.
Our data also suggest a new mechanism where increased Wnt signaling in the pre-cardiac mesoderm does not affect CM specification, as previously suggested (reviewed in Kwon et al., 2008; Tzahor, 2007). Instead, increased Wnt signaling in the pre-cardiac mesoderm prevents CM differentiation, which we hypothesize eventually leads to apoptotic death of the CPs via a p53/Caspase-3 independent mechanism. One possible explanation for this discrepancy is that in previous studies the CP and differentiation markers were assayed concurrently at later time points, not progressively over time points when the progenitor markers are first expressed (Afouda and Hoppler, 2009; Afouda et al., 2008; Foley et al., 2007; Foley and Mercola, 2005; Lavery et al., 2008a; Lavery et al., 2008b; Martin et al., 2010; Marvin et al., 2001; Naito et al., 2006; Tzahor and Lassar, 2001; Ueno et al., 2007). This leaves open the possibility that exploring additional time points could explain differences between this and previous studies. In addition, although cell culture studies have suggested that cardiac fates may be inhibited at the expense of vascular or hematopoietic fates in the pre-cardiac mesoderm (Chen et al., 2008; Marvin et al., 2001; Naito et al., 2006; Wang et al., 2007), we did not find an inverse relationship between these fates, in agreement with Ueno et al. (2007). Though we think it is unlikely, we cannot rule out that there is a small portion of cells that do make this fate transformation. Therefore, further evaluation is necessary to determine the extent of conservation of this previously unrecognized mechanism on the pre-cardiac mesoderm of vertebrates.
As AO labels apoptotic cells in zebrafish (Furutani-Seiki et al., 1996; Pyati et al., 2007), our results suggest a model where the inability of the CPs to differentiate appears to result in apoptosis through a p53 and Caspase-3 independent mechanism. However, we do not yet understand the mechanisms that lead to their inability to differentiate or what apoptotic pathway that is being triggered. We hypothesize that the inability of the CPs to differentiate is not a direct consequence of excess Wnt signaling at the 20s stage, because Wnt responsive genes are no longer affected at 20s after the heat-shock at the TB stage (data not shown). Rather, we propose that the inability to differentiate is a downstream consequence of the increased Wnt signaling upon the CPs in the pre-cardiac mesoderm. With respect to the apoptosis, it is surprising that a p53/Caspase-3 independent pathway is being employed. However, there are many ways to trigger apoptosis as both cell culture and zebrafish studies have uncovered p53-independent apoptotic pathways (Fujita and Ishikawa, 2011; Pyati et al., 2007; Sidi et al., 2008). Moreover, a recent study in zebrafish elucidated a conserved Chk1/Caspase-2 dependent and p53/Caspase-3 independent apoptotic pathway (Sidi et al., 2008). Therefore, it is conceivable that this Caspase-2 apoptotic pathway or another apoptotic mechanism is what is being utilized. Future studies will be aimed at elucidating the mechanisms by which Wnt signaling prevents CP differentiation and the apoptotic pathway that is being triggered.
Wnt signaling and atrial cell differentiation
A role for Wnt signaling during differentiation that expands the atrial cell population in zebrafish is immediately reminiscent of the characterization of Wnt signaling in the SHF of mice (Dyer and Kirby, 2009). Specifically, Wnt2 in mice is required to promote specification and proliferation of atrial CPs in the posterior SHF through positively regulating gata6 (Tian et al., 2010). However, our results suggest that while Wnt signaling can promote a relatively substantial increase in atrial cell number, there appears to be a minimal requirement for Wnt signaling in promoting atrial cell formation during the same time period. Furthermore, our results indicate that Wnt signaling does not discernibly regulate gata6 or gata4 expression. Therefore, these results suggest that one possibility is that despite similarities in the ability of Wnt signaling to affect atrial CM development in zebrafish and mice, these similarities do not reflect a conserved role of Wnt signaling. Alternatively, it is possible that Wnt regulation of atrial CM development is conserved in vertebrates, but a requirement for Wnt signaling and downstream components has either been lost/reduced in zebrafish or gained/expanded in mouse.
Recent studies in zebrafish have also suggested that isl1 regulates a progressive addition of atrial cells to the venous pole of the zebrafish heart (de Pater et al., 2009), naturally drawing further comparisons to the mammalian SHF. Furthermore, multiple studies in mice and stem cells have indicated that Wnt signaling controls the differentiation of isl1 cells (Klaus et al., 2007; Kwon et al., 2009; Lin et al., 2007; Qyang et al., 2007), prompting us to explore the possibility that Wnt signaling was upstream of isl1 in zebrafish. However, we found that the function of isl1 does not appear to be epistatic to Wnt signaling as depletion of isl1 does not affect the number of atrial cells when Wnt signaling is increased in differentiating CMs (data not shown). Therefore, our results currently support the notion that Wnt must be influencing atrial CM specification and differentiation using different mechanisms than described in the mammalian SHF.
If Wnt signaling is affecting atrial CM specification and not proliferation, this suggests that Wnt signaling is influencing cells to take on an atrial cell identity that would otherwise become another fate. We speculate that these additional atrial cells could be coming from two sources: the additional atrial cells could be derived from a population of cells capable of responding that resides within the differentiating atrial cell field; alternatively, Wnt signaling could be influencing a nearby cell type to take on an atrial cell fate. Future lineage studies aimed at understanding the origin of these additional cells will allow us to better understand how the size of this chamber is ultimately regulated during development, which will impact our ability to precisely derive atrial cells in culture.
Conclusions
Altogether, this is the first study to define a comprehensive picture of the distinct phases of Wnt signaling in vivo during early development in a vertebrate. The relatively clean distinctions of these four phases are likely to provide a basis to reconcile the conservation of these phases of Wnt influence in other developmental and evolutionary models and mouse and human ES cells. The novel mechanisms demonstrated here, particularly with respect to the CP apoptosis, should also provide the basis for future studies and more precise models in mouse and human stem cell culture. Ultimately, understanding the exact mechanisms through which Wnt signaling controls CM differentiation in development is needed to allow the precise direction of cell fates in vitro and the generation of novel stem cell based, tissue engineering therapies.
Supplementary Material
As previously reported (Stoick-Cooper et al., 2007; Weidinger et al., 2005), both the Dkk and Wnt8 used for the transgenic lines are tagged with GFP facilitating the easy detection of transgenic embryos. (A,C) HCSEs. (B) Representative GFP+ Tg(hsp70l:dkk1-GFP)w3 embryo 30 min after the heat-shock. For the Tg(hsp70l:dkk1-GFP)w3 embryos, fluorescence was maximal at ~1h. (D) Representative GFP+ Tg(hsp70l:wnt8a-GFP)w34 embryo 2 h after heat-shock. For the Tg(hsp70l:wnt8a-GFP)w34 embryos, fluorescence was maximal at ~2 h post-heat shock. For both transgenes, GFP expression could be detected by 30 min post-heat-shock. Overall, relative to each other, the fluorescence induced from the Dkk-GFP transgene is consistently brighter than the Wnt-GFP transgene (data not shown). Equivalent results were obtained with heat-shocks performed at the TB stage. Fluorescence in the yolk in all images is autofluorescence.
Axin2 is a Wnt responsive gene (Weidinger et al., 2005). (A,C) HCSEs at 20s. (B,D) GFP+ Tg(hsp70l:dkk1-GFP)w3 and Tg(hsp70l:wnt8a-GFP)w34 embryos at 20s, respectively. (B) After decreasing Wnt signaling at the 16s, axin2 expression is significantly downregulated relative to HCSEs (A). Arrowheads in A and B indicate the midbrain hindbrain boundary and the tip of the tail, where axin2 expression is significantly downregulated relative to HCSEs. (D) After increasing Wnt signaling at the 16s, axin2 expression is significantly upregulated compared to HCSEs (C). Arrowheads in C and D indicate the anterior brain and spinal cord, where axin2 is upregulated relative to HCSEs. View are lateral with anterior left.
(A,D) Uninjected sibling control embryo. (B,E) wnt8a.1 and wnt8a.2 MO injected embryo. aldh1a2 expression is reduced around the margin and in the animal-vegetal axis in wnt8a.1/wnt8a.2 morphants (arrowheads). (C,F) Embryo injected with wnt8a.1 mRNA. wnt8a.1 overexpression expands aldh1a2 expression in the dorsal region (arrowhead in C) and in the animal-vegetal axis (arrowheads in F). A–C are animal views. D–F are lateral views. Dorsal is to the right in all images.
Lateral view of heart of control heat-shocked Tg(−5.1myl7:EGFP)twu26 sibling embryo at 48 hpf.
Lateral view of heart at 48 hpf of Tg(−5.1myl7:EGFP)twu26; Tg(hsp70l:wnt8a-GFP)w34 embryo with increased Wnt signaling at the TB.
(A,C,E,G,I,K) HCSEs. (B,D,F,H,J,L) Embryos with decreased Wnt signaling at the designated stages. By 80% epiboly, aldh1a2 is expressed in the dorso-lateral region, while it is excluded from the dorsal and ventral regions. Tbx6 and flh expression is excluded from the dorsal region and in the dorsal region respectively at 80% epiboly. (B,F,J) Embryos with decreased Wnt signaling at the sphere stage are severely dorsalized, as indicated by the expansion of dorso-lateral aldh1a2 expression around the ventral margin and the expanded dorsal region (compare arrows in A to B, in E to F, and in I to J). (D,H,L) Embryos with decreased Wnt signaling at the shield stage are modestly dorsalized. The dorsal region is marginally expanded (upper arrows in C,D,G,H,K,L) and the ventral region is marginally reduced (lower arrows in C and D). All images are vegetal views with dorsal up.
(A,C) HCSEs. (B,D) Hearts from GFP+ Tg(hsp70l:Δtcf3-GFP)w26 embryos heat-shocked at the TB and 16s stages. (B) Although morphology was more linear when ΔTCF-GFP was induced at the TB stage, the hearts did not appear larger. However, at the TB stage overexpression of ΔTCF-GFP causes a significant amount of cell death in embryos (not shown), potentially precluding analysis. (D) Heart from transgenic embryo when ΔTCF-GFP is induced at 16s. All images are frontal views at 48 hpf. Red indicates ventricles and green indicates atria.
(A,C,E,G) HCSEs. (B,F) GFP+ embryos with decreased Wnt signaling at the TB stage. There is not a discernible difference in the expression of nkx2.5 (B) or gata4 (F) when these markers begin robust expression at 8s. (D,H) GFP+ embryos with increased Wnt signaling at the TB stage. (D) The majority of embryos with increased Wnt signaling at TB stage had nkx2.5 expression that was comparable to HCSEs. (H) There is not a discernible difference in the expression of gata4 at 8s when Wnt signaling is increased at TB stage. (I,J) qPCR analysis of nkx2.5 and gata4 expression in embryos at the 8s stage after modulating Wnt signaling at the TB stage. There is no difference in the expression levels of these CP marker genes relative to HCSEs.
(A,C,E,G,I,K) HCSEs. (B,D,F,H,J,L) GFP+ embryos. Increased Wnt signaling at the TB stage does not lead to a discernible increase in etv2 (B), a hemangioblast marker, or kdrl (F) or fli1a (J), vascular markers. Arrows in B, F, and J indicate aberrant formation of anterior cerebral veins. (D,H,L) Embryos with decreased Wnt signaling at the TB stage. Decreased Wnt signaling does not cause a discernible decrease in etv2 (D), kdrl (H) or fli1a (L). Although the specification of these lineages does not appear affected, there is a rostral shift of the mandibular arch and endocardial knot (distance between arrows in D,H,L compared to C,G,K).
(A–C) A representative HCSE. (D–F) A representative GFP+ embryo after increasing Wnt signaling at the TB stage. To examine the position of apoptotic cells relative to endothelial cells of the LPM, hemizygous Tg(hsp70l:wnt8a-GFP)w34 fish were crossed to hemizygous Tg(kdrl:mCherry)ci5 fish as in Fig. 7. In a transgenic embryo after Wnt signaling was increased at the TB stage, there are apoptotic cells (arrow in D and F) adjacent to the endothelials in the anterior LPM (arrows in B and E). The HCSE does not have a significant amount of apoptotic cells in the same region of the anterior LPM. The anatomical position of these dying cells and endothelial cells is reminiscent of the more dorsal position of CMs relative to the endothelial cells reported in Bussmann et al. (2007). Arrow in B indicates endothelial cells that are more medial endocardial precursors. Images are lateral views with anterior to the top and dorsal to the left.
(A,C,E) HCSEs. (B,D,F) GFP+ embryos with decreased Wnt signaling. There was not a difference in gata4 or gata6 expression or heart morphology at 26 hpf when Wnt signaling was decreased at 8s (B) or 16s (D,F).
(A,C) HCSEs. (B) GFP+ embryo with decreased Wnt signaling. (D) GFP+ embryo with increased Wnt signaling.
Representative heart from Tg(hsp70l:wnt8a-GFP)w34;Tg(kdrl:nlsEGFP)zf109bv embryo stained for pHH3 (red) and S46 (AMHC; green) at 26 hpf after heat-shock at 16s. (A) Nuclear GFP marking the endothelial cells (white arrow) is internal to the AMHC (yellow arrows) expressing atrial myocardial cells. (B) A single cell is stained for pHH3. (C) Overlay indicating that the single cell positive for pHH3 (red arrow) is an endothelial cell. There was not a difference in the number of embryos with pHH3 positive myocardial cells or pHH3 positive cells per embryo (see Supplemental Table 5 and Supplemental Table 6).
Highlights.
Wnt/β-catenin signaling is used reiteratively during heart development.
We examine the roles of Wnt signaling in cardiac cell formation in zebrafish.
Increased Wnt signaling in pre-cardiac mesoderm prevents cardiac cell differentiation.
Increased Wnt signaling in pre-cardiac mesoderm leads to cell death.
During cardiac differentiation Wnt signaling can promote additional atrial cell differentiation.
Acknowledgments
We are grateful to D. Yelon, in whose lab this work was initiated, for helpful discussions, input and support. We thank E. D’Aniello for technical assistance with qPCR. We thank A. Lekven, K. Yutzey, U. Pyati and J. Schumacher and members of the Waxman lab for helpful discussions. JSW is supported by NIH grant R00 HL901126, a Cincinnati Children’s Trustee Award and March of Dimes research grant 5-FY11-88.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Hoppler S. Xenopus explants as an experimental model system for studying heart development. Trends Cardiovasc Med. 2009;19:220–226. doi: 10.1016/j.tcm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Baker KD, Ramel MC, Lekven AC. A direct role for Wnt8 in ventrolateral mesoderm patterning. Dev Dyn. 2010;239:2828–2836. doi: 10.1002/dvdy.22419. [DOI] [PubMed] [Google Scholar]
- Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Bakkers J, Schulte-Merker S. Early endocardial morphogenesis requires Scl/Tal1. PLoS genetics. 2007;3:e140. doi: 10.1371/journal.pgen.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VC, Stull R, Joo D, Cheng X, Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol. 2008;26:1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AC, Korol O, Timmer AM, Mercola M. Multiple functions of Cerberus cooperate to induce heart downstream of Nodal. Dev Biol. 2007;303:57–65. doi: 10.1016/j.ydbio.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Ishikawa Y. Apoptosis in heart failure-The role of the beta-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes. Circ J. 2011;75:1811–1818. doi: 10.1253/circj.cj-11-0025. [DOI] [PubMed] [Google Scholar]
- Furutani-Seiki M, Jiang YJ, Brand M, Heisenberg CP, Houart C, Beuchle D, van Eeden FJ, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Neural degeneration mutants in the zebrafish, Danio rerio. Development. 1996;123:229–239. doi: 10.1242/dev.123.1.229. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Hug B, Walter V, Grunwald DJ. tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev Biol (NY 1985) 1997;183:61–73. doi: 10.1006/dbio.1996.8490. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Meyer D, Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004;131:3081–3091. doi: 10.1242/dev.01185. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Cordes KR, Srivastava D. Wnt/beta-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle. 2008;7:3815–3818. doi: 10.4161/cc.7.24.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery DL, Davenport IR, Turnbull YD, Wheeler GN, Hoppler S. Wnt6 expression in epidermis and epithelial tissues during Xenopus organogenesis. Dev Dyn. 2008a;237:768–779. doi: 10.1002/dvdy.21440. [DOI] [PubMed] [Google Scholar]
- Lavery DL, Martin J, Turnbull YD, Hoppler S. Wnt6 signaling regulates heart muscle development during organogenesis. Dev Biol. 2008b;323:177–188. doi: 10.1016/j.ydbio.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Marques SR, Yelon D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev Biol. 2009;328:472–482. doi: 10.1016/j.ydbio.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell. 2008;15:121–133. doi: 10.1016/j.devcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Afouda BA, Hoppler S. Wnt/beta-catenin signalling regulates cardiomyogenesis via GATA transcription factors. J Anat. 2010;216:92–107. doi: 10.1111/j.1469-7580.2009.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev Biol (NY 1985) 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Look AT, Hammerschmidt M. Zebrafish as a powerful vertebrate model system for in vivo studies of cell death. Semin Cancer Biol. 2007;17:154–165. doi: 10.1016/j.semcancer.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS genetics. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottbauer W, Saurin AJ, Lickert H, Shen X, Burns CG, Wo ZG, Kemler R, Kingston R, Wu C, Fishman M. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell. 2002;111:661–672. doi: 10.1016/s0092-8674(02)01112-1. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenebeck JJ, Keegan BR, Yelon D. Vessel and blood specification override cardiac potential in anterior mesoderm. Dev Cell. 2007;13:254–267. doi: 10.1016/j.devcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF, Kanki JP, Green DR, D’Andrea AA, Look AT. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Fishman MC. Patterning the zebrafish heart tube: acquisition of anteroposterior polarity. Dev Biol. 1992;153:91–101. doi: 10.1016/0012-1606(92)90094-w. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot WS, Trevarrow B, Halpern ME, Melby AE, Farr G, Postlethwait JH, Jowett T, Kimmel CB, Kimelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Moon RT. nemo-like kinase is an essential co-activator of Wnt signaling during early zebrafish development. Development. 2004;131:2899–2909. doi: 10.1242/dev.01171. [DOI] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gilner JB, Bautch VL, Wang DZ, Wainwright BJ, Kirby SL, Patterson C. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J Biol Chem. 2007;282:782–791. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Keegan BR, Roberts RW, Poss KD, Yelon D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev Cell. 2008;15:923–934. doi: 10.1016/j.devcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio) M. Westerfield; Eugene, OR: 1993. [Google Scholar]
- Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE, Heideman W, Burns CE, Burns CG. Latent TGF-beta binding protein 3 identifies a second heart field in zebrafish. Nature. 2011;474:645–648. doi: 10.1038/nature10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As previously reported (Stoick-Cooper et al., 2007; Weidinger et al., 2005), both the Dkk and Wnt8 used for the transgenic lines are tagged with GFP facilitating the easy detection of transgenic embryos. (A,C) HCSEs. (B) Representative GFP+ Tg(hsp70l:dkk1-GFP)w3 embryo 30 min after the heat-shock. For the Tg(hsp70l:dkk1-GFP)w3 embryos, fluorescence was maximal at ~1h. (D) Representative GFP+ Tg(hsp70l:wnt8a-GFP)w34 embryo 2 h after heat-shock. For the Tg(hsp70l:wnt8a-GFP)w34 embryos, fluorescence was maximal at ~2 h post-heat shock. For both transgenes, GFP expression could be detected by 30 min post-heat-shock. Overall, relative to each other, the fluorescence induced from the Dkk-GFP transgene is consistently brighter than the Wnt-GFP transgene (data not shown). Equivalent results were obtained with heat-shocks performed at the TB stage. Fluorescence in the yolk in all images is autofluorescence.
Axin2 is a Wnt responsive gene (Weidinger et al., 2005). (A,C) HCSEs at 20s. (B,D) GFP+ Tg(hsp70l:dkk1-GFP)w3 and Tg(hsp70l:wnt8a-GFP)w34 embryos at 20s, respectively. (B) After decreasing Wnt signaling at the 16s, axin2 expression is significantly downregulated relative to HCSEs (A). Arrowheads in A and B indicate the midbrain hindbrain boundary and the tip of the tail, where axin2 expression is significantly downregulated relative to HCSEs. (D) After increasing Wnt signaling at the 16s, axin2 expression is significantly upregulated compared to HCSEs (C). Arrowheads in C and D indicate the anterior brain and spinal cord, where axin2 is upregulated relative to HCSEs. View are lateral with anterior left.
(A,D) Uninjected sibling control embryo. (B,E) wnt8a.1 and wnt8a.2 MO injected embryo. aldh1a2 expression is reduced around the margin and in the animal-vegetal axis in wnt8a.1/wnt8a.2 morphants (arrowheads). (C,F) Embryo injected with wnt8a.1 mRNA. wnt8a.1 overexpression expands aldh1a2 expression in the dorsal region (arrowhead in C) and in the animal-vegetal axis (arrowheads in F). A–C are animal views. D–F are lateral views. Dorsal is to the right in all images.
Lateral view of heart of control heat-shocked Tg(−5.1myl7:EGFP)twu26 sibling embryo at 48 hpf.
Lateral view of heart at 48 hpf of Tg(−5.1myl7:EGFP)twu26; Tg(hsp70l:wnt8a-GFP)w34 embryo with increased Wnt signaling at the TB.
(A,C,E,G,I,K) HCSEs. (B,D,F,H,J,L) Embryos with decreased Wnt signaling at the designated stages. By 80% epiboly, aldh1a2 is expressed in the dorso-lateral region, while it is excluded from the dorsal and ventral regions. Tbx6 and flh expression is excluded from the dorsal region and in the dorsal region respectively at 80% epiboly. (B,F,J) Embryos with decreased Wnt signaling at the sphere stage are severely dorsalized, as indicated by the expansion of dorso-lateral aldh1a2 expression around the ventral margin and the expanded dorsal region (compare arrows in A to B, in E to F, and in I to J). (D,H,L) Embryos with decreased Wnt signaling at the shield stage are modestly dorsalized. The dorsal region is marginally expanded (upper arrows in C,D,G,H,K,L) and the ventral region is marginally reduced (lower arrows in C and D). All images are vegetal views with dorsal up.
(A,C) HCSEs. (B,D) Hearts from GFP+ Tg(hsp70l:Δtcf3-GFP)w26 embryos heat-shocked at the TB and 16s stages. (B) Although morphology was more linear when ΔTCF-GFP was induced at the TB stage, the hearts did not appear larger. However, at the TB stage overexpression of ΔTCF-GFP causes a significant amount of cell death in embryos (not shown), potentially precluding analysis. (D) Heart from transgenic embryo when ΔTCF-GFP is induced at 16s. All images are frontal views at 48 hpf. Red indicates ventricles and green indicates atria.
(A,C,E,G) HCSEs. (B,F) GFP+ embryos with decreased Wnt signaling at the TB stage. There is not a discernible difference in the expression of nkx2.5 (B) or gata4 (F) when these markers begin robust expression at 8s. (D,H) GFP+ embryos with increased Wnt signaling at the TB stage. (D) The majority of embryos with increased Wnt signaling at TB stage had nkx2.5 expression that was comparable to HCSEs. (H) There is not a discernible difference in the expression of gata4 at 8s when Wnt signaling is increased at TB stage. (I,J) qPCR analysis of nkx2.5 and gata4 expression in embryos at the 8s stage after modulating Wnt signaling at the TB stage. There is no difference in the expression levels of these CP marker genes relative to HCSEs.
(A,C,E,G,I,K) HCSEs. (B,D,F,H,J,L) GFP+ embryos. Increased Wnt signaling at the TB stage does not lead to a discernible increase in etv2 (B), a hemangioblast marker, or kdrl (F) or fli1a (J), vascular markers. Arrows in B, F, and J indicate aberrant formation of anterior cerebral veins. (D,H,L) Embryos with decreased Wnt signaling at the TB stage. Decreased Wnt signaling does not cause a discernible decrease in etv2 (D), kdrl (H) or fli1a (L). Although the specification of these lineages does not appear affected, there is a rostral shift of the mandibular arch and endocardial knot (distance between arrows in D,H,L compared to C,G,K).
(A–C) A representative HCSE. (D–F) A representative GFP+ embryo after increasing Wnt signaling at the TB stage. To examine the position of apoptotic cells relative to endothelial cells of the LPM, hemizygous Tg(hsp70l:wnt8a-GFP)w34 fish were crossed to hemizygous Tg(kdrl:mCherry)ci5 fish as in Fig. 7. In a transgenic embryo after Wnt signaling was increased at the TB stage, there are apoptotic cells (arrow in D and F) adjacent to the endothelials in the anterior LPM (arrows in B and E). The HCSE does not have a significant amount of apoptotic cells in the same region of the anterior LPM. The anatomical position of these dying cells and endothelial cells is reminiscent of the more dorsal position of CMs relative to the endothelial cells reported in Bussmann et al. (2007). Arrow in B indicates endothelial cells that are more medial endocardial precursors. Images are lateral views with anterior to the top and dorsal to the left.
(A,C,E) HCSEs. (B,D,F) GFP+ embryos with decreased Wnt signaling. There was not a difference in gata4 or gata6 expression or heart morphology at 26 hpf when Wnt signaling was decreased at 8s (B) or 16s (D,F).
(A,C) HCSEs. (B) GFP+ embryo with decreased Wnt signaling. (D) GFP+ embryo with increased Wnt signaling.
Representative heart from Tg(hsp70l:wnt8a-GFP)w34;Tg(kdrl:nlsEGFP)zf109bv embryo stained for pHH3 (red) and S46 (AMHC; green) at 26 hpf after heat-shock at 16s. (A) Nuclear GFP marking the endothelial cells (white arrow) is internal to the AMHC (yellow arrows) expressing atrial myocardial cells. (B) A single cell is stained for pHH3. (C) Overlay indicating that the single cell positive for pHH3 (red arrow) is an endothelial cell. There was not a difference in the number of embryos with pHH3 positive myocardial cells or pHH3 positive cells per embryo (see Supplemental Table 5 and Supplemental Table 6).