Abstract
Background
Previous research with non-drug reinforcers has shown that simultaneously presenting (compounding) an extinguished cue with another cue formerly associated with the same reinforcer can increase rates of cue-controlled behavior. The present study investigated whether an extinguished cocaine cue would energize cocaine seeking when presented simultaneously with another cocaine cue. This study also investigated whether extinction could be enhanced by subjecting an extinguished cocaine cue to further extinction after administration of reinstating injections of cocaine.
Methods
Rats were first trained to self-administer cocaine in the presence of three different cues. Then, one of the cues was subjected to the standard extinction treatment. Another cue was subjected to a modified extinction treatment where additional extinction sessions were preceded by non-contingent cocaine injections. The third cue was not extinguished.
Results
The cue subjected to standard extinction ceased to control cocaine seeking when presented alone, but significantly increased cocaine seeking when compounded with the non-extinguished cocaine cue. The cocaine cue subjected to the modified extinction treatment also significantly increased cocaine seeking occasioned by the non-extinguished cocaine cue.
Conclusions
Extending results of previous studies involving non-drug stimuli, the present study showed that extinguished cocaine cues can enhance cocaine seeking when compounded with other cocaine cues. These results illustrate the persistence of drug cues in controlling behavior despite extinction and highlight the need for developing treatments that eliminate this residual energizing capacity that survives extinction.
Keywords: extinction, drug cues, cocaine, self-administration, cue exposure, stimulus compounding, rats
1. Introduction
Drug cues, such as people, places, or things (e.g., paraphernalia) associated with drug use, play an important role in driving drug abuse and addiction. For example, drug cues elicit craving for the drug (Childress et al., 1999; Volkow et al., 2006), activate the same brain reward circuitry that is activated by the drug itself (Volkow et al., 2006, 2008), and contribute to relapse after abstinence (Grusser et al., 2004; Kosten et al., 2006; Sinha and Li, 2007). An intervention that reduces the power of drug cues could help to improve treatment outcomes.
Extinction has been used in such an effort. Extinction is the presentation of a conditioned cue without the reinforcer (or unconditioned stimulus; US) with which the cue was previously paired. For example, cue-exposure therapy is an extinction-based treatment where drug users are repeatedly exposed to drug cues without the drug (Drummond et al., 1995). Experiencing the cues in the absence of the drug should theoretically break (or inhibit) the cue-drug association and thereby reduce the ability of the drug cues to drive drug-related behavioral or neurological responses. While there have been a handful of cue-exposure studies reporting promising results (Loeber et al., 2006; Rohsenow et al., 2001), the outcomes of most cue-exposure studies have been less encouraging than expected (e.g., Marissen et al., 2007; for review, see Conklin and Tiffany, 2002).
Basic learning research on extinction may suggest potential reasons for the disappointing results of cue-exposure therapy (Conklin and Tiffany, 2002). For example, experiments with rats have found that a non-drug cue (i.e., a stimulus associated with food or shock) that appeared to be behaviorally silent after extensive extinction still energized responding when presented simultaneously with another cue associated with the same reinforcer or US (Kearns and Weiss, 2005; Hendry, 1983; Reberg, 1972; Rescorla, 2006). Reberg (1972) performed the original experiment demonstrating this effect. In that study, rats were trained on a procedure where two conditioned stimuli (CSs) were each separately paired with electric shock. The CSs came to elicit a fear response that suppressed rats’ lever pressing for food. Then, one of the CSs was subjected to extensive extinction (i.e., the CS was repeatedly presented without shock). The extinguished CS no longer had any suppressive effect on lever pressing by the end of the extinction phase. But when this extinguished CS was presented simultaneously with a still-active CS (i.e., a CS not subjected to extensive extinction), there was greater suppression of lever pressing than when the still-active CS was presented alone. This suggests that extinguished cues retain residual excitatory associative properties even after extinction appears to have eliminated their control over behavior. This latent excitation is revealed when the extinguished cue is compounded with another cue that has an excitatory history. Kearns and Weiss (2005) replicated and extended Reberg’s (1972) finding by showing that compounding (i.e., simultaneously presenting) extinguished food-associated discriminative stimuli (SDs) also reactivated these cues’ control over behavior.
It is possible that drug cues extinguished as part of cue-exposure therapy are similarly reactivated when they are presented simultaneously with non-extinguished, still-active drug cues encountered outside of the treatment setting. If so, this might help explain why cue-exposure therapy has not been more effective. The primary goal of the present study was to model this situation in rats by compounding an extinguished SD that previously occasioned cocaine self-administration with another cocaine SD that was not extinguished. A second goal was to attempt to develop a treatment that would more effectively extinguish cocaine cues and eliminate potential residual excitatory properties that might survive standard extinction. This treatment was based on recent basic learning research showing that extinction can be enhanced by increasing the excitation present during additional non-reinforced exposure to previously extinguished cues (Janak and Corbit, 2011; Leung and Westbrook, 2008; Rescorla, 2006). These previous studies used either stimulus compounding (Janak and Corbit, 2011; Rescorla, 2006) or spontaneous recovery (Lueng and Westbrook, 2008) to reactivate the previously extinguished cues prior to subjecting them to additional extinction. Drug self-administration research has shown that non-contingent presentation of the drug is one of the most effective ways of reinstating extinguished cocaine seeking (de Wit and Stewart, 1981; Shaham et al., 2003; Shalev et al., 2002). Therefore, the present study used non-contingent injections of cocaine to reinstate cocaine seeking during additional non-reinforced exposures to the previously extinguished cocaine cues.
The effectiveness of this modified extinction treatment was evaluated on a stimulus compounding test, where the treated cue was presented simultaneously with a non-extinguished cocaine cue. Previous research has shown that stimulus compounding tests are very sensitive measures of the associative properties conditioned to discriminative cues (Weiss, 1978; Weiss and Schindler, 1985). If the modified treatment is effective in enhancing extinction, it would be expected that a cue subjected to it would be less likely (than a cue subjected to standard extinction) to energize responding when compounded with a non-extinguished cocaine cue. Such an outcome could suggest a way to improve extinction-based drug abuse treatments. For example, after standard extinction of cocaine cues, individuals might be given (under medical supervision) small amounts of cocaine or a similar drug with lower abuse potential (e.g., methylphenidate) prior to additional non-reinforced exposures to the cues.
2. Materials and methods
2.1. Subjects
Eight adult male Long-Evans rats served as subjects. Rats were individually housed in plastic cages with wood chip bedding and metal wire tops. Subjects were maintained at 85% of their free-feeding weights (approximately 350–450 g) throughout the experiment by feeding them approximately 15 g of rat chow following training sessions. Rats had unlimited access to water in their home cages. The colony room where the rats were housed had a 12-h light:12-h dark cycle with lights on at 08:00 h. Training sessions were conducted 5–7 days per week during the light phase of the light:dark cycle. Throughout the experiment, rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and all procedures were approved by American University’s Institutional Animal Care and Use Committee (IACUC).
2.2. Apparatus
Training took place in six operant chambers. Each chamber was 20 cm high, 23 cm long, and 18 cm wide and had aluminum front and rear walls, white translucent plastic side walls, a clear plexiglass ceiling, and a grid floor. A response lever and food trough were located on the front wall of the chamber. A tone stimulus (4000 Hz and 80 dB) was delivered through a speaker mounted 21.5 cm above the chamber and inside the sound-attenuation chest. A light stimulus was provided by two 15-cm, 25-W, 120-VAC tubular light bulbs located 10 cm outside of the chamber’s side walls. The light bulbs were operated at approximately 110 VAC. A fan (Sunon, model # KDE2412PMB3-6A) mounted on top of the chamber was used to provide a “fan” stimulus that this lab has used in previous studies (Lombas et al., 2008a, 2008b). When the fan was activated, air blew through ventilation holes in the ceiling of the chamber. Operation the fan also produced some vibration and associated running hum. The square frame that housed this fan measured approximately 12 cm × 12 cm and was positioned over the response lever. The fan was operated at 15 VDC. Each chamber was housed inside a sound attenuation chest (Weiss, 1970) that also had a continuously operating ventilation fan (separate from the stimulus fan). A shielded houselight mounted on the ceiling (and near the rear wall) of the sound attenuation chest provided a low level of continuous illumination during all sessions. The illumination produced by this houselight was just enough to make the rat barely discernible when in the chamber. Experimental procedures were controlled by Med-Associates software (Med-PC, St. Albans, VT) running on a PC located in an adjacent room.
Cocaine (National Institute on Drug Abuse, Bethesda, MD) in saline solution at a concentration of 2.56 mg/ml was infused at a rate of 3.19 ml/min by 10-ml syringes driven by Harvard Apparatus (South Natick, MA) or MED Associates (St. Albans, VT) syringe pumps located outside of the sound-attenuation chests. Tygon tubing extended from the 10-ml syringes to a 22-gauge rodent single-channel fluid swivel and tether apparatus (Alice King Chatham Medical Arts, Hawthorne, CA) that descended through the ceiling of the chamber. Cocaine was delivered to the subject through Tygon tubing that passed through the metal spring of the tether apparatus. This metal spring was attached to a plastic screw cemented to the rat’s head to reduce tension on the catheter.
2.3. Procedure
2.3.1. Lever-press acquisition
To facilitate later lever pressing for cocaine, rats were first trained for 2 sessions to lever press for food pellets. Food pellets were available for lever pressing on a fixed-ratio 1 (FR-1) schedule and were also presented non-contingently every 120 s on average (range: 90–150 s) if a lever press was not made. These two sessions lasted approximately 60 min.
2.3.2. Surgery
Rats were then surgically prepared with chronic indwelling jugular vein catheters, using a modification of the procedure originally developed by Weeks (1962). In brief, under ketamine (60 mg/kg) and xylazine (10 mg/kg) anesthesia, approximately 3 cm of Silastic tubing (0.044mm i.d., 0.814mm o.d.) was inserted into the right jugular vein. This Silastic tubing was connected to 8 cm of vinyl tubing (Dural Plastics; 0.5mm i.d., 1.0mm o.d.) that was passed under the skin around the shoulder and exited the back at the level of the shoulder blades. The vinyl tubing was threaded through a section of Tygon tubing (10 mm long, 4 mm diameter) that served as a subcutaneous anchor. Six stainless steel jeweler’s screws were implanted in the skull, to which a 20-mm plastic screw was cemented with dental acrylic. Catheters were flushed daily with 0.1 ml of a saline solution containing 1.25 U/ml heparin and 0.08 mg/ml gentamycin.
2.3.3. Cocaine self-administration
After 1 week of recovery in the homecage, rats were trained to self-administer cocaine on a 4-component multiple schedule. During light, tone, or fan SD components, 1.0 mg/kg cocaine infusions were available for lever pressing according to an FR-1 schedule. These SD components alternated with SΔ components where all stimuli were absent and lever pressing did not result in cocaine. Each of the three types of SD component (light, tone, or fan) were equally likely to follow an SΔ component, with the restriction that there were no more than two consecutive SD components of the same type. Initially, SD components lasted 900 s and SΔ components lasted 300 s. Over sessions, the FR value operative in SD components was gradually increased from 1 to 10. Once a rat displayed regular responding on the FR-10 schedule, a variable-ratio (VR) 10 schedule was introduced in place of the FR-10 schedule during light, tone, and fan components. Now, an average of 10 (range: 1–15) lever presses were required for each cocaine infusion. SD and SΔ components lasted 300 s on average (range: 240–360 s). After 2 sessions on this VR-10 procedure, the dose of self-administered cocaine was lowered to 0.5 mg/kg/infusion where it remained for the rest of the experiment.
After 2 sessions of training with the 0.5 mg/kg/infusion dose, rats were put on the terminal baseline schedule. As previously, cocaine was available on a VR-10 schedule during light, tone, and fan SD components, but was not available during SΔ components when these stimuli were absent. Now, the lengths of SD and SΔ components were reduced to 180 s on average (range: 120–240 s). In addition, a 60-s response correction contingency operated during the final 60 s of each SΔ component. According to this contingency, a 60-sec SΔ-termination clock was reset to zero by a response. Thus, at least 60 s had to pass from the time of the last lever press in an SΔ component to the onset of the next SD component. This contingency was designed to reduce response rates during SΔ components. The length of the response-correction contingency was increased as necessary for individual rats up to as long as 300 s if responding persisted during SΔ components. Rats were trained on this terminal baseline schedule for a minimum of 8 sessions and until (1) response rates in all SD components averaged over two sessions were at least 3 times faster than rates in SΔ, and (2) tone and light response rates were within 20% of each other. Catheter patency was confirmed at the end of self-administration training by aspirating blood through the catheter or by observing rapid ataxia (loss of motor control within 10 s) after infusion of 0.1 ml of a saline solution containing 0.3 mg ketamine and 0.4 mg xylazine.
2.3.4. Extinction Phase 1
All rats were then given 3 sessions of extinction of the light and the tone. The light and the tone were subsequently counterbalanced in their roles as the stimuli subjected to the modified extinction treatment or the standard extinction treatment. The light and the tone were selected for these roles because our laboratory has much experience with these stimuli and our goal was to equate the treated stimuli as much as possible in terms of salience. The fan was not subjected to extinction, because this stimulus would serve as the non-extinguished excitor on subsequent stimulus compounding tests. During the extinction Phase 1 sessions, the light and the tone were each presented 6 times. Each tone or light component lasted 180 s and was followed by a 180-s period where these stimuli were absent. Tone and light were equally likely to follow each absence component, with the restriction that there were no more than 2 consecutive SD components of the same type (light or tone). Lever pressing did not produce cocaine infusions at any time and the response correction contingency in SΔ was discontinued.
2.3.5. Extinction Phase 2
The light and the tone were then each subjected to 3 additional extinction sessions. For one of the SDs, designated Stimulus X, these extinction sessions were preceded by an i.p. injection of 15 mg/kg cocaine. This dose was chosen because previous research has shown it to be an effective reinstater of extinguished cocaine seeking (Keiflin et al., 2008a). For the other SD, designated Stimulus Y, these additional extinction sessions were preceded by an i.p. injection of saline (equivolume to 15 mg/kg cocaine). The light and the tone were counterbalanced in their roles as Stimulus X and Stimulus Y. Only one stimulus type was presented within a session. Stimulus X and Stimulus Y extinction sessions alternated. Half of the subjects received these sessions in the order X,Y,X,Y,X,Y and the other half in the order Y,X,Y,X,Y, X. I.p. injections were administered 5–10 minutes prior to placing the rat in the chamber. During an extinction session, the to-be-extinguished stimulus was presented 8 times for periods that lasted 180 s and were separated by 120-s periods where all stimuli were absent. Lever pressing was not reinforced at any time. To minimize the possibility that the i.p. cocaine injection administered prior to Stimulus X extinction sessions would also reinstate responding during Stimulus Y extinction sessions, a session of context exposure alone was administered on the day after each Stimulus X or Stimulus Y extinction session. During these context exposure sessions, the animal was placed in the chamber for 40 min without any stimulus presentations. This was designed to reduce any potential excitation conditioned to the context by the i.p. injection of cocaine, since previous research has shown that reinstatement depends on context excitation levels (Bouton and Bolles, 1979a). Context exposure sessions were not preceded by injections of cocaine or saline. Thus, during Phase 2, there were a total of 12 sessions: 3 Stimulus X extinction sessions with each followed by a session of exposure to the chamber alone and 3 Stimulus Y extinction sessions with each followed by a session of exposure to the chamber alone.
2.3.6. Stimulus Compounding Test 1
After the final extinction Phase 2 session, the first of two stimulus compounding tests was administered. This test consisted of 6 presentations each of the fan alone (F), the fan-plus-light compound (FL), and the fan-plus-tone compound (FT). These test stimuli were presented in the order F, FL, FT, FL, FT, F, FT, F, FL, F, FT, FL, FL, F, FT, FT, FL, F. Thus, each block of 3 test stimuli contained 1 presentation of each stimulus and the ordinal position of each stimulus within a block was balanced over blocks. Each test condition lasted 75 s and was followed by 75-s periods where all stimuli were absent. Lever presses were not reinforced at any time during the test.
2.3.7. Stimulus Compounding Test 2
When the first compounding test ended, rats remained in the chamber and were given a fan alone component during which they could self-administer 0.5 mg/kg cocaine infusions on an FR-1 schedule. The onset of this fan component coincided with the non-contingent administration of a single 0.5 mg/kg cocaine infusion. Once rats self-administered 3 infusions, this fan component ended. After a 75-s period where all stimuli were absent, the second compounding test began. The procedural details of this test were the same as those described above for the first stimulus compounding test. As previously, lever presses were not reinforced at any time during the test. Catheter patency was determined following this test using the methods described previously.
2.4. Data Analysis
For all statistical tests, α = 0.05. Separate repeated measures ANOVAs were performed on response rates during the final two self-administration sessions, all extinction Phase 1 sessions, all extinction Phase 2 sessions, the last extinction Phase 1 and first extinction Phase 2 sessions, and the two stimulus compounding tests. Paired-sample t-tests were performed where appropriate following significant ANOVAs. Paired-sample t-tests were also used to compare the fan-plus-X and fan-plus-Y compounds in terms of the percentage of test blocks where responding was greater in the compound as compared to the fan alone.
3. Results
3.1. Self-administration
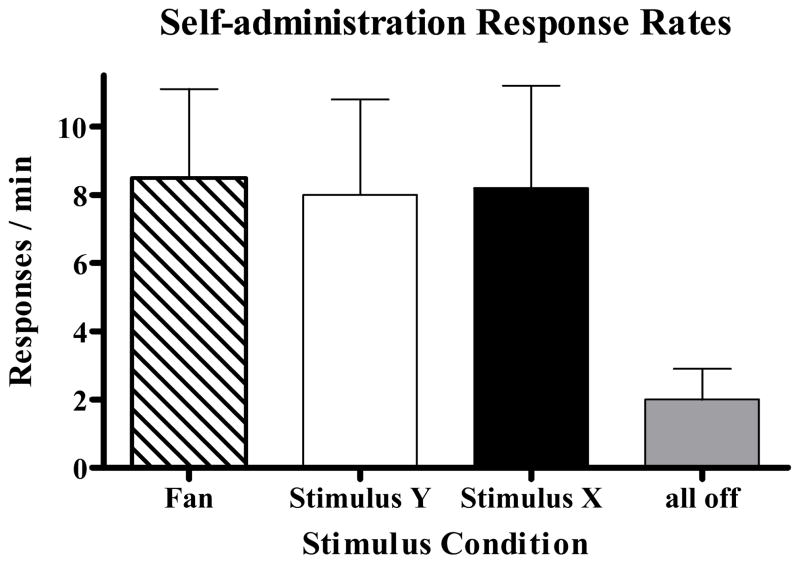
Rats had a mean of 28.5 (± 3.0 SEM) total cocaine self-administration sessions, 19.8 (± 3.2 SEM) of which were terminal baseline sessions. Response rates averaged over the final two sessions during fan, Stimulus X, and Stimulus Y were 8.5 (± 2.6 SEM), 8.2 (± 3.0 SEM), and 8.0 (± 2.8 SEM) responses per min, respectively (see Figure 1). A repeated measures ANOVA indicated that there was no significant difference in response rates controlled by these SDs (F[2,14] = 0.4, p > 0.6). The mean response rate during the absence of all stimuli (SΔ) was 2.0 (± 0.9 SEM). A paired-samples t-test confirmed that the mean SD response rate was significantly greater (t[7] = 3.5, p < 0.05) than the SΔ response rate, which was to be expected since rats were trained to a criterion that required them to respond at least 3 times faster in the SDs as compared to SΔ.
Figure 1.
Mean (±SEM) response rates in the fan, Stimulus X, Stimulus Y, and all-stimuli-off components averaged over the last two self-administration sessions.
3.2. Extinction Phase 1
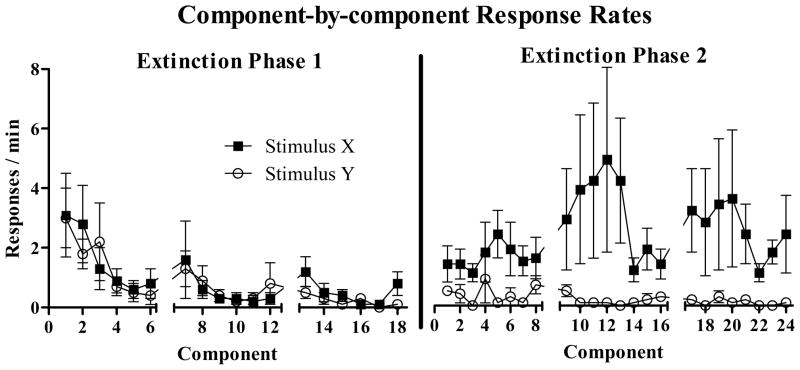
The left portion of Figure 2 presents mean (± SEM) responses per minute during Stimuli X and Y (tone or light, counterbalanced) during each component of the 3 sessions of the first extinction phase. Mean response rates in each stimulus declined over sessions. A 2 X 3 (stimulus by session) repeated measures ANOVA performed on the Stimuli X and Y response rates indicated that there was a significant effect of session (F[2,14] = 12.0, p < 0.005), but no significant effect of stimulus (F[2,7] = 0.0, p > 0.8) and no significant interaction (F[2,14] = 0.6, p > 0.5). The mean response rate during all-stimuli-off periods (not shown in figure) during extinction Phase 1 was 0.3 (± 0.2 SEM) responses per min.
Figure 2.
Mean (±SEM) response rates in Stimuli X (squares) and Y (circles) during each component of extinction Phase 1 (left of vertical line) and extinction Phase 2 (right of vertical line). There were 6 components per session during extinction Phase 1 and 8 components per session during extinction Phase 2.
3.3. Extinction Phase 2
The right portion of Figure 2 presents mean (± SEM) response rates during each component of each session during extinction Phase 2. The non-contingent cocaine injections administered prior to the start of Stimulus X sessions increased responding to Stimulus X. In contrast, responding to Stimulus Y remained low throughout this phase. As Figure 2 illustrates, mean response rates were higher during the Stimulus X component than during the corresponding Stimulus Y component in 24 out of 24 components during extinction Phase 2. On the final Stimulus Y extinction session, the mean response rate in Stimulus Y was 0.1 (± 0.1 SEM) responses per min and half of the subjects did not make a single response in Stimulus Y during the entire session. The mean response rate in all-stimuli-off periods during Phase 2 extinction sessions was 0.8 (± 0.2 SEM) responses per min.
To further test for reinstatement, an additional 3 × 2 (stimulus by session) repeated measures ANOVA was performed on Stimulus X, Stimulus Y, and SΔ response rates from the final extinction Phase 1 session and the first extinction Phase 2 session. (The SΔ rate used for the Phase 2 session was the mean of the SΔ rates from the first Stimulus X and first Stimulus Y sessions in Phase 2). There were significant main effects of stimulus (F[2,14] = 11.9, p < 0.01) and session (F[1,7] = 6.8, p < 0.05) as well as a significant stimulus by session interaction (F[2,14] = 4.2, p < 0.05). Subsequent paired-sample t-tests indicated that responding significantly increased over sessions for Stimulus X (t[7] = 2.5, p < 0.05), but not for Stimulus Y (t[7] = .8, p > 0.45) or for SΔ(t[7] = 1.3, p > 0.23).
3.4. Stimulus Compounding Test 1
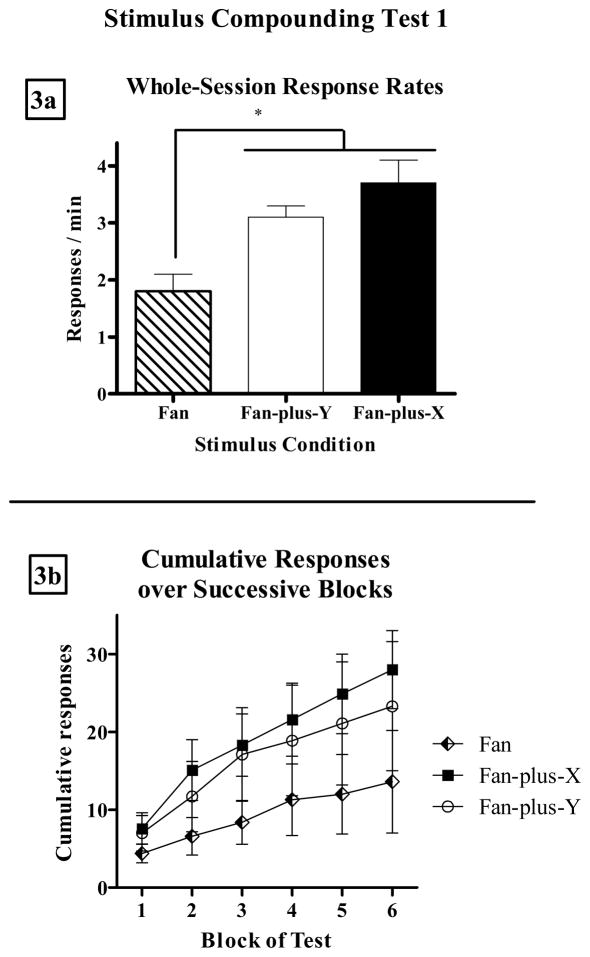
Figure 3a presents mean (± SEM) response rates over the whole test during each test stimulus condition from the first stimulus compounding test. Figure 3b presents mean (± SEM) cumulative responses over the 6 blocks of the test. One rat (j9) did not make any responses during this test and therefore was excluded from analysis. Stimulus Y, which controlled essentially no responding on its final extinction session, enhanced rates of cocaine-seeking behavior when compounded with the fan. Compounding Stimulus X with the fan similarly enhanced responding. A repeated measures ANOVA confirmed that response rates significantly differed over test conditions (F[2,12] = 5.9, p < 0.05). Subsequent paired sample t-tests indicated that response rates during fan-plus-X and fan-plus-Y compounds were both significantly higher than in fan alone (t[6]’s > 2.6, p’s < 0.05), but there was no difference between the two compound conditions (t[6] = 0.9, p > 0.3). The mean response rate during all-stimuli-off periods during the first compounding test was 0.3 (± 0.1 SEM) responses/min.
Figure 3.
(a) Mean (±SEM) whole-session response rates during fan alone (striped bar), fan-plus-Y (white bar), and fan-plus-X (black bar) on Stimulus Compounding Test 1. Asterisk indicates p < 0.05. (b) Mean (±SEM) cumulative responses in fan (diamonds), fan-plus-Y (circles), and fan-plus-X (squares) over the 6 blocks of the test.
Table 1 presents individual subject data concerning block-by-block comparisons of each compound with the fan alone. For each subject and for each compound, the table presents the number of blocks where there was at least 1 response in the compound or the fan alone and the percentage of those blocks where responding in the compound was higher than in the fan alone. As the table shows, for both compounds there was greater responding to the compound than to the fan alone on approximately 70–75% of the test blocks (excluding those blocks where no responses were made). A paired samples t-test indicated that this percentage did not differ over compounds (t[6] = 0.6, p > 0.55).
Table 1.
Stimulus Compounding Test 1.
| Fan-plus-X vs. Fan alone | Fan-plus-Y vs. Fan alone | |||
|---|---|---|---|---|
| Subject | # of blocks with at least one response in compound or fan alone | % of blocks where compound responding > fan alone responding | # of blocks with at least one response in compound or fan alone | % of blocks where compound responding > fan alone responding |
| j2 | 6 | 67 | 6 | 33 |
| j7 | 4 | 100 | 3 | 100 |
| j10 | 4 | 75 | 3 | 67 |
| j11 | 3 | 67 | 3 | 33 |
| j12 | 6 | 50 | 6 | 67 |
| q16 | 4 | 100 | 3 | 100 |
| q22 | 5 | 60 | 6 | 83 |
| Mean | 4.6 | 74.0 | 4.3 | 69.0 |
| SEM | 0.4 | 7.3 | 0.6 | 10.6 |
There were 6 total test blocks per test, with each block consisting of one presentation each of Fan alone, Fan-plus-X, and Fan-plus-Y components. Subject j9 did not make any responses on this test and therefore was excluded from data analysis.
3.5. Stimulus Compounding Test 2
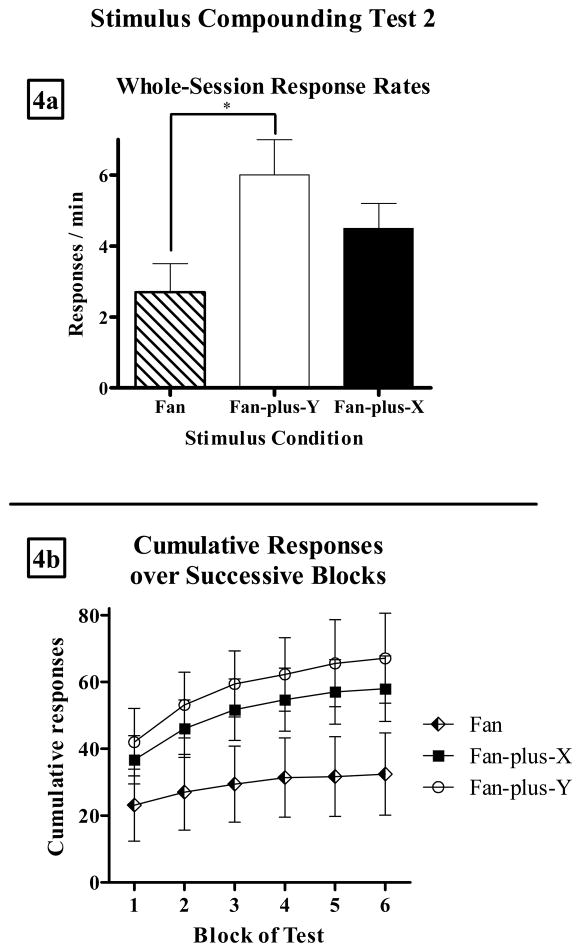
Figure 4a presents mean (± SEM) response rates over the whole test during each stimulus condition from the second compounding test, which followed a single fan component where rats self-administered 3 cocaine infusions. Figure 4b presents cumulative responses over the 6 blocks of the test. One rat (j11) was found to have a nonfunctional catheter after this test and therefore was excluded from analysis. As Figure 4 illustrates, presentation of Stimulus Y simultaneously with the fan increased rats’ rate of cocaine-seeking by 100%, while Stimulus X only enhanced fan responding by approximately 67%. A repeated measures ANOVA indicated that there was a significant effect of test condition (F[2,12] = 6.4, p < 0.05). Subsequent paired-sample t-tests revealed that responding in the fan-plus-Y compound was significantly higher than responding in fan alone (t[6] = 5.2, p < 0.005), but the difference between responding in fan-plus-X and fan alone did not quite achieve significance (t[6] = 1.9, p = 0.11). There was no significant difference between the two compound conditions (F[6] = 1.3, p > 0.2). The mean response rate during all-stimuli-off periods during the second compounding test was 0.1 (± 0.04) responses/min.
Figure 4.
(a) Mean (±SEM) whole-session response rates during fan alone (striped bar), fan-plus-Y (white bar), and fan-plus-X (black bar) on Stimulus Compounding Test 2. Asterisk indicates p < 0.05. (b) Mean (±SEM) cumulative responses in fan (diamonds), fan-plus-Y (circles), and fan-plus-X (squares) over the 6 blocks of the test.
Table 2 presents the results of block-by-block comparisons of each compound with the fan alone for individual subjects. Similar to the results from Test 1, for each compound rats responded more to the compound than to the fan alone on approximately 70–75% of the test blocks. A paired-samples t-test indicated that this percentage did not significantly differ for the two compounds (t[6] = 0.4, p > 0.65).
Table 2.
Stimulus Compounding Test 2.
| Fan-plus-X vs. Fan alone | Fan-plus-Y vs. Fan alone | |||
|---|---|---|---|---|
| Subject | # of blocks with at least one response in compound or fan alone | % of blocks where compound responding > fan alone responding | # of blocks with at least one response in compound or fan alone | % of blocks where compound responding > fan alone responding |
| j2 | 6 | 67 | 6 | 67 |
| j7 | 5 | 60 | 6 | 83 |
| j9 | 3 | 67 | 4 | 100 |
| j10 | 6 | 67 | 5 | 60 |
| j12 | 6 | 33 | 6 | 67 |
| q16 | 3 | 100 | 3 | 67 |
| q22 | 6 | 100 | 5 | 80 |
| Mean | 5.0 | 70.5 | 5.0 | 74.8 |
| SEM | 0.5 | 8.8 | 0.4 | 5.2 |
There were 6 total test blocks per test, with each block consisting of one presentation each of Fan alone, Fan-plus-X, and Fan-plus-Y components. Subject j11 was found to have a nonfunctional catheter following this test and was therefore excluded from data analysis.
4. Discussion
The present experiment demonstrated that an extinguished cocaine cue, which did not control cocaine seeking when presented alone, was able to enhance cocaine seeking when presented simultaneously with another (non-extinguished) cocaine cue. This result replicates and extends those of previous studies involving non-drug reinforcers/USs showing that compounding extinguished cues leads to a reappearance of the behavior previously controlled by those cues (Hendry, 1982; Kearns and Weiss, 2005; Reberg, 1972; Rescorla, 2006). These studies and the present one show that a cue retains residual excitation even after extinction appears to have eliminated the power of the cue to control behavior on its own. This phenomenon, along with phenomena such as spontaneous recovery (e.g., Brooks and Bouton, 1994), reinstatement (Rescorla and Heth, 1975), context renewal (Bouton and Bolles, 1979b), and rapid reacquisition (Napier et al., 1992), shows that extinction does not erase the associations originally conditioned to a cue and that given the proper circumstances a behaviorally silent cue can exhibit a reappearance of its ability to influence behavior. Such dynamics may at least partially contribute to the discouraging results of extinction-based treatments such as cue-exposure therapy. For example, a drug cue subjected to extinction may show no control over behavior after cue exposure in the clinic, but is reactivated when compounded with a non-extinguished cue encountered in the user’s normal environment. Consideration of this possibility, plus all of the other situations where extinguished drug cues might regain their control over behavior (e.g., spontaneous recovery, reinstatement, renewal), suggest that more effective extinction strategies must be developed if cue-exposure treatments are to be successful.
It is possible that the reactivation of the extinguished cues when compounded with the fan was due in part to context renewal processes. Research with non-drug reinforcers has shown that presenting an extinguished cue in a novel context different from the extinction context can lead to a reappearance of responding (AAB renewal; Bouton and Ricker, 1994). The fan-plus-X and fan-plus-Y compounds may have functioned as novel contexts different from the context in which X and Y were extinguished. It should be noted, though, that the fan in the present experiment was a discrete cue presented for relatively brief periods during the test while in context renewal studies, contextual cues are typically present for the duration of the test session. Similarly, while fan-plus-X and fan-plus-Y would have been novel compounds, the rats had much exposure to the fan alone during initial self-administration training. Finally, in a similar stimulus compounding experiment using shock-paired cues rather than cocaine-paired cues, Hendry (1982) found that compounding an extinguished shock cue with a novel cue did not result in a reactivation of the shock cue, but compounding the shock cue with another extinguished shock cue did.
The present experiment investigated a treatment designed to enhance the extinction of a cue (Stimulus X) by administering reinstating injections of cocaine prior to further non-reinforced exposure to the cue. Though rats made over 10 times as many unreinforced responses during Stimulus X as they did during Stimulus Y in extinction Phase 2, Stimulus X still significantly enhanced cocaine seeking when compounded with the fan (that had not been subjected to extinction) during the first stimulus compounding test. Results from the second compounding test were largely similar. Though the difference between mean response rates in fan-plus-X vs. fan alone only approached significance (p = 0.11), 6 out of 7 rats responded more during fan-plus-X components than during fan alone components on a majority of test blocks (see Table 2).
The Stimulus X extinction treatment employed here was based on previous non-drug experiments indicating that increasing the amount of excitation present during non-reinforced exposure to a cue facilitates extinction learning (Rescorla, 2000, 2006). The present experiment employed pre-session non-contingent cocaine injections in an effort to heighten excitation during the extinction of Stimulus X in the second extinction phase. These non-contingent cocaine injections were effective in reinstating cocaine seeking controlled by Stimulus X. But Rescorla (2006) has shown that the critical factor determining how much extinction a stimulus undergoes is not the number of responses controlled by that stimulus during extinction, but rather the amount of excitation present during non-reinforced exposure to the cue. This suggests that the non-contingent cocaine injections in the present experiment increased responding without increasing the amount of excitation experienced during subsequent non-reinforced exposure to the cue.
State-dependent extinction learning is another possible reason for the lack of effectiveness of the modified extinction treatment tested here. Delamater (2004) noted that this is a general problem for pharmacological treatments designed to enhance extinction. If drugs that are administered during extinction produce a discriminable internal cue, then any extinction learning that takes place may be specific to the drug state. Previous research has shown that a 15-mg/kg i.p. cocaine injection, like those administered during extinction Phase 2, can certainly produce a discriminable internal cue (Keiflan et al., 2008b). Thus, state-dependent extinction learning may partly explain why no enhancement of extinction was observed for Stimulus X on the first stimulus compounding test, which took place in the absence of cocaine.
On the transition from self-administration to extinction, rats’ response rates dropped from approximately 8 responses per min (see Figure 1) to approximately 3 responses per min during the first component of the first extinction session (see Figure 2). It may have been expected that a rate closer to 8 responses per min would be observed early on in extinction. However, that rate reflects responding over the course of a 3-hr session where rats self-administered many cocaine infusions. On the first extinction session, rats lever pressed for the first time in a cocaine-free state. Previous studies have shown that that cocaine, whether self-administered or non-contingently administered, can have direct operant-response-rate-increasing effects in rats (Cantin et al., 2010; Jasyna et al., 1998). For example, in a relevant recent study by Cantin et al. (2010), rats were trained on progressive ratio schedules to respond for either cocaine or saccharin reinforcers. Rats responded at a much greater (~6 times faster) rate when cocaine was the reinforcer as compared to when saccharin was the reinforcer. However, when a 10-min post-reinforcer delay period was introduced to allow time for the direct effects of cocaine to dissipate somewhat, the rate of responding for cocaine dropped so much that there was no longer any difference between rates of responding for cocaine vs. saccharin. Further, when tested under extinction conditions, where the direct effects of cocaine were completely absent, the rate of response for cocaine was significantly lower than that for saccharin. Cantin et al. (2010) concluded that the direct effects of cocaine inflated self-administration response rates in their study. Similarly, the rate-increasing effects of self-administered cocaine likely also contributed to the relatively large difference observed in the present experiment between response rates during cocaine self-administration sessions and the initial extinction session. It is worth noting that comparably large drops in response rate have been observed in previous studies upon the transition to extinction from self-administration of cocaine on FR-10 (Valles et al., 2006) or VR-10 schedules (Kearns and Weiss, 2007).
The results of the present study show that extinguished cocaine cues can still energize cocaine-seeking behavior when they are presented simultaneously with non-extinguished cocaine cues. Improved extinction treatments that deal with this phenomenon are clearly necessary, given the importance of drug cues in driving drug craving and relapse (Childress et al., 1999; Kosten et al., 2006; Volkow et al., 2006). Basic research on extinction learning has shown that increasing excitation present during non-reinforcement of cues can enhance cue extinction (Rescorla, 2000, 2006). Future research that attempts to increase this excitation through means other than non-contingent administration of cocaine could prove to be informative. By drawing on these and other findings from basic research on extinction, an effective means of neutralizing the power of drug cues might be developed (Conklin and Tiffany, 2002).
Acknowledgments
Role of Funding Source
This research was supported by Award Number R01DA008651 from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. NIDA did not play a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Brendan J. Tunstall for his helpful comments on a draft of the manuscript.
Footnotes
Contributors
Both authors designed the experiment. David Kearns performed the experiment, analyzed the data, and wrote the first draft of the manuscript. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
Both authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979a;10:445–466. [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979a;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Ricker ST. Renewal of extinguished responding in a second context. Anim Learn Behav. 1994;22:317–324. [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psychol Anim Behav Process. 1994;20:366–379. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psych. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol. 2004;57B:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Drummond DS, Tiffany ST, Glautier S, Remington B. Addictive Behaviour: Cue Exposure Theory and Practice. Wiley and Sons; West Sussex, England: 1995. [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Grusser S, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296 –302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hendry JS. Summation of undetected excitation following extinction of the CER. Anim Learn Behav. 1982;10:476–482. [Google Scholar]
- Janak PH, Corbit LH. Deepened extinction following compound stimulus presentation: noradrenergic modulation. Learn Mem. 2010;18:1–10. doi: 10.1101/lm.1923211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, Yasar S, Goldberg SR. Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology. 1998;140:257–271. doi: 10.1007/s002130050766. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Reinstatement of a food-maintained operant response by compounding discriminative stimuli. Behav Processes. 2005;70:194–202. doi: 10.1016/j.beproc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug Alcohol Depend. 2007;90:193–202. doi: 10.1016/j.drugalcdep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Vouillac C, Cador M. Level of operant training rather than cocaine intake predicts level of reinstatement. Psychopharmacology. 2008;197:247–261. doi: 10.1007/s00213-007-1026-2. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Isingrini E, Cador M. Cocaine-induced reinstatement in rats: evidence for a critical role of cocaine stimulus properties. Psychopharmacology. 2008;197:649–660. doi: 10.1007/s00213-008-1083-1. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent subjects. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Leung HT, Westbrook RF. Spontaneous recovery of extinguished fear responses deepens their extinction: a role for error-correction mechanisms. J Exp Psychol Anim Behav Process. 2008;34:461–74. doi: 10.1037/0097-7403.34.4.461. [DOI] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol. 2007;45:515–529. doi: 10.1348/014466505X82586. [DOI] [PubMed] [Google Scholar]
- Lombas AS, Kearns DN, Weiss SJ. A comparison of the effects of discriminative and Pavlovian inhibitors and excitors on instrumental responding. Behav Processes. 2008a;78:53–63. doi: 10.1016/j.beproc.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Lombas AS, Kearns DN, Weiss SJ. Differential effects of a food-based conditioned inhibitor on food- or cocaine-seeking behavior. Learn Motiv. 2008b;39:323–333. doi: 10.1016/j.lmot.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM. Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom. 2007;76:97–105. doi: 10.1159/000097968. [DOI] [PubMed] [Google Scholar]
- Napier RM, Macrae M, Kehoe EJ. Rapid reaquisition in conditioning of the rabbit’s nictitating membrane response. J Exp Psychol Anim Behav Processes. 1992;18:182–92. doi: 10.1037//0097-7403.18.2.182. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Reberg D. Compound tests for excitation in early acquisition and after prolonged extinction of conditioned suppression. Learn Motiv. 1972;3:246–258. [Google Scholar]
- Rescorla RA. Extinction can be enhanced by a concurrent excitor. J Exp Psychol Anim Behav Processes. 2000;26:251–260. doi: 10.1037//0097-7403.26.3.251. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Deepened extinction from compound stimulus presentation. J Exp Psychol Anim Behav Processes. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Valles R, Rocha A, Nation JR. The effects of acquisition training schedule on extinction and reinstatement of cocaine self-administration in male rats. Exp Clin Psychopharmacology. 2006;14:245–253. doi: 10.1037/1064-1297.14.2.245. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. An effective and economical sound attenuation chamber. J Exp Anal Behav. 1970;13:37–39. doi: 10.1901/jeab.1970.13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Discriminated response and incentive processes in operant conditioning: a two-factor model of stimulus control. J Exp Anal Behav. 1978;30:361–381. doi: 10.1901/jeab.1978.30-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Schindler CW. Conditioning history and inhibitory instrumental stimulus control: independent-groups and within-subjects measures. Anim Learn Behav. 1985;13:215–222. [Google Scholar]