Abstract
Objective
Increased cardiovascular (CV) risk has been reported in adults who are childhood cancer survivors (CCS). We sought to determine the emergence of CV risk factors in CCS while still children.
Study design
CCS in remission ≥5 years from cancer diagnosis (n=319, age=14.5yrs), and their siblings (controls, n=208, age=13.6yrs) participated in this cross-sectional study of CV risk, which included physiologic assessment of insulin sensitivity/resistance (hyperinsulinemic euglycemic clamp). Adjusted comparisons between CCS major diagnoses (leukemia [n=110], central nervous system tumors [n=82], solid tumors [n=127]) and controls were performed using linear regression for CV risk factors and insulin sensitivity.
Results
Despite no significant differences in weight and body mass index, CCS had greater adiposity (waist [73.1 vs. 71.1cm, p=0.02]; percent fat [28.1vs.25.9%, p=0.007]), lower lean body mass (38.4vs.39.9 kg, p=0.01) than controls. After adjustment for adiposity, CCS had higher total cholesterol (154.7vs.148.3mg/dl, p=0.004), LDL-cholesterol (89.4vs.83.7mg/dl, p=0.002), triglycerides (91.8 vs. 84mg/dl, p=0.03) and were less insulin sensitive (Mlbm 12.1vs.13.4mg/kg/min, p=0.002) than controls.
Conclusions
CCS have greater CV risk than healthy children. Because CV risk factors track from childhood into adulthood, early development of altered body composition and decreased insulin sensitivity in CCS may contribute significantly to their risk of early CV morbidity and mortality.
Keywords: cardiometabolic risk, metabolic syndrome, children, cholesterol, adiposity
Effective therapies of childhood cancer have led to dramatic improvements in survival rates1,2. As childhood cancer survivors (CCS) progress into adulthood, clinical and epidemiological research is focusing on long-term adverse medical effects from cancer treatment to characterize and understand the “consequences of cure”3. Among these are the metabolic syndrome (MetS), or combinations of cardiovascular (CV) risk factors, e.g., obesity, dyslipidemia, hypertension, insulin resistance, all known to be potent risk factors for premature CV disease in adults4, and among leading causes of non-relapse deaths in survivors of childhood cancer5–7.
Although most studies relating CCS to CV risk have been performed in adults8–10, studies in smaller cohorts of CCS have shown increased prevalence of CV risk factors in late adolescence and young adulthood11,12, and an increased incidence of obesity and hypertension were reported in a sample of children who survived leukemia13. These studies were limited by small numbers of subjects, lack of concurrent controls and use of surrogate markers for insulin resistance, which are less sensitive estimates of insulin resistance in youth14.
The present study is focused on the early development of CV risk and its relation to insulin sensitivity/resistance. We undertook a comprehensive assessment in a large population of CCS while they are still children and compared the CV risk profile and euglycemic hyperinsulinemic clamp, the “gold standard” for measurement of insulin sensitivity/resistance, between a cohort of children who survived a variety of childhood cancers and a control group of healthy sibling children.
Methods
This study was approved by the Institutional Review Board: Human Subjects Committee at the University of Minnesota Medical Center and Children’s Hospitals and Clinics of Minnesota. Consent (and assent as appropriate) was obtained from children and their parent/guardian(s). The participants were CCS, age 9–18 years at examination, who were in remission at least five years from cancer diagnosis and who had received treatment at the University of Minnesota Medical Center or the Children’s Hospitals and Clinics of Minnesota. Recipients of hematopoietic cell transplant were excluded from the study. A control group consisted of eligible healthy siblings who were 9–18 years old and had never had cancer.
Of 723 eligible CCS, 66 could not be located. The remaining 657 were contacted, and consent for participation was obtained from 319 (49%) CCS and 208 of their siblings (controls). There were no significant differences in age, sex, race, diagnosis, age at diagnosis and length of follow-up (time from diagnosis to study evaluation) between the 319 CCS participants and the 338 CCS non-participants.
Participants underwent a two-day examination at the University of Minnesota Clinical Research Center. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Waist circumference was measured in duplicate midway between the anterior superior iliac spine and the lower rib margin directly over the skin, the method used in all prior studies from our group designed to measure the site of natural waist and the level of minimal abdominal width, considered the level of the smallest circumference around the waist. Tanner stage was assigned according to pubic hair development in boys and breast and pubic hair development in girls. Dual-energy X-ray absorptiometry (DXA) measurements were obtained with a Lunar Prodigy scanner (software version 9.3; General Electric Medical Systems, Madison, WI, USA). Fat mass was expressed as a percent of body mass (PFM) and lean body mass (LBM) was expressed in kilograms. Visceral fat and subcutaneous fat were determined using volumetrics from a limited abdominal CT scan without contrast, using a Siemens Somaton Sensation 40 slice. The average of two blood pressure measurements from the right arm of rested, seated subjects was used in analyses.
Hyperinsulinemic euglycemic clamps were conducted after a 10–12 hour overnight fast as previously described15. Intravenous catheters were inserted into an arm vein for infusion of potassium phosphate, insulin, and glucose and into a contralateral vein for blood sampling. Baseline insulin and glucose levels were determined from samples drawn at −5 and 0 min before beginning the insulin and glucose infusions. Insulin infusion was started at time 0 at a rate of 1 mU/kg/min for 3 hours. An infusion of 20% glucose was given and adjusted to maintain euglycemia [serum glucose level of 100 mg/dl (5.6 mmol/L)] with plasma glucose determined every 10 min. Insulin sensitivity (M) was determined by the amount of glucose required to maintain euglycemia over the final 40 minutes of the clamp study and expressed as mg/kg/min of glucose with adjustment for lean body mass (Mlbm) obtained from LBM. Lower Mlbm values are indicative of lower insulin sensitivity, i.e., greater insulin resistance.
Plasma glucose was analyzed at the bedside with a Beckman Glucose Analyzer II (Beckman Instruments Inc, Fullerton, CA). Serum insulin was determined using a chemoluminescence immunoassay (Immulite Insulin DPC, Los Angeles, CA, USA). Homeostasis model assessment insulin resistance (HOMA-IR) was calculated using fasting insulin and glucose values based on the equation HOMA-IR= [(fasting glucose units of mmol/L * insulin units in µU/mL)/22.5]16. Serum lipids were analyzed from fasting blood samples obtained at the time catheters were placed for the clamp, using a Vitros 5600. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation.
The criteria for MetS were based on pediatric modification17,18 of the adult MetS criteria (ATPIII)19 and consisted of 3 or more of the following criteria: 1) waist circumference >90th percentile for age and sex; 2) triglycerides >110 mg/dL (1.24 mmol/L); 3) high density lipoprotein cholesterol ≤40 mg/mL (1.03 mmol/L); 4) blood pressure ≥90th percentile for age and sex and 5) fasting glucose ≥100 mg/dL (5.6 mmol/L).
Descriptive statistics are expressed as frequencies and percents or mean ± standard error (SE), as appropriate. Multivariable linear regression models were used to compare mean outcome measures between groups, with adjustments for age, sex, race and Tanner stage (unless noted otherwise). All analyses including data from both CCS and sibling controls utilized robust variance estimates from generalized estimating equations to account for intra-family correlation. Adjusted means were evaluated at the mean levels of covariates included in the models. Additional models were fit adjusting for adiposity using BMI and PFM. Duration of time elapsed since diagnosis was examined as a risk factor in a linear and non-linear fashion on Mlbm levels. Logistic regression models were fit to evaluate differences between groups in prevalence of MetS and its components (as defined above). Odds Ratios (OR) and associated 95% confidence intervals (CI) are reported. Partial correlation coefficients, also adjusted for the same factors were calculated to evaluate the associations between CV risk factors among CCS and controls separately. A two-sided p-value ≤ 0.05 was considered to be statistically significant, although due to the high number of statistical tests carried out, those between 0.01 and 0.05 should be viewed with caution.
Results
The characteristics of the study population are described in Table I. There were no significant differences in sex distribution between CCS and controls. The length of follow-up (time from diagnosis to study evaluation) was not different between the cancer groups (p=0.50). CCS were slightly older, but similar to controls in degree of sexual maturation. A higher prevalence of white, non-Hispanic participants was present in the controls compared with CCS. Further analyses were adjusted for age at study, sex, race and Tanner stage. Based upon overall similarities in therapeutic exposures, CCS were grouped into three major diagnostic groups: leukemia (n=110), central nervous system (CNS) tumors (n=82) and solid tumors (n=127).
Table 1.
Characteristics of Study Population
| Variable | Category | CCS(n=319) | Controls (n=208) | p-value |
|---|---|---|---|---|
| Age | Years (mean ± SEa) | 14.5 ± 0.1 | 13.6 ± 0.2 | <0.001 |
| Sexb | Male | 171 (54%) | 112(54%) | 0.93 |
| Female | 148 (46%) | 96 (46%) | ||
| Race/Ethnicityb,c | White Non-Hispanic | 274 (86%) | 194(93%) | <0.001 |
| Others | 45 (14%) | 14 (7%) | ||
| White Hispanic | 4(1%) | 4 (2%) | ||
| Black | 14(4%) | 3(1%) | ||
| Others | 27 (9%) | 7 (4%) | ||
| Tanner | Tanner Stage (mean ± SEa) | 3.6 ± 0.1 | 3.3 ± 0.1 | 0.07 |
| 1b | 33(10%) | 33(17%) | ||
| 2 | 54(17%) | 32 (15%) | ||
| 3 | 39 (12%) | 36(17%) | ||
| 4 | 88 (28%) | 45 (22%) | ||
| 5 | 105(33%) | 60 (29%) | ||
| Diagnosis b | Leukemia | NA | ||
| Acute Lymphoblastic | 102 (32%) | |||
| Acute Myeloid | 8 (3%) | |||
| CNS | NA | |||
| Glial tumors | 38 (12%) | |||
| Retinoblastoma | 16(5%) | |||
| Other tumorsd | 15 (5%) | |||
| Neuroectodermal tumors | 13(4%) | |||
| Solid Tumors | NA | |||
| Sarcoma | 32 (10%) | |||
| Renal | 30 (9%) | |||
| Neuroblastoma | 23 (7%) | |||
| Other tumorse | 22 (7%) | |||
| Non-Hodgkin's Lymphoma | 20 (6%) | |||
| Time from diagnosis to study | Years (mean ± SEa) (range) | |||
| All CCS | 10.1 ± 0.2(5.0–17.8) | NA | ||
| Leukemia | 10.2 ± 0.3 (5.1–16.0) | |||
| CNS | 9.7 ± 0.4 (4.3–17.1) | |||
| Solid Tumors | 10.2 ± 0.3 (5.5–17.8) |
SE: Standard Error
Data displayed as n (%)
Per low cell counts, White Hispanic, Black, and Other categories collapsed for the comparison between CCS and controls.
The anthropometric and body composition characteristics of the study population are described in Table II. Despite no significant differences in weight, BMI or BMI percentile between CCS and controls, CCS were significantly shorter, had a significantly higher degree of body fatness, expressed as waist circumference, waist to height ratio and PFMDXA, and had significantly lower LBM. This pattern was seen in CCS as a whole and in survivors of leukemia; in addition, CNS tumor survivors had greater abdominal visceral and subcutaneous fat, but the lower LBM did not reach statistical significance. These patterns were not present in solid tumor survivors. Based on age and sex specific criteria for overweight/obesity17,18 BMI ≥85th percentile was present in similar proportion in CCS (31.4%) and controls (32.2%) (p=0.9), and waist circumference ≥75th percentile was more prevalent in CCS (11%) than controls (6.7%) (OR:2.1, 95% CI: 1.4–3.2, p<0.001); in addition waist to height ratio ≥0.5, an indicator of increased CV risk20 was present in 24% of CCS and 11.2% (OR 2.5, 95% CI 1.5–4.1, p <0.001) of controls. Systolic blood pressure >90th percentile was present in 10.7% CCS vs. 7.2% controls, but this was not statistically significant (p=0.2).
Table 2.
Comparison of Body Composition between CCS and Controls
| CCS (n=319) |
Leukemia (n=110) |
CNS (n=82) |
Solid Tumors (n=127) |
Controls (n=208) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| mean ± SE | p | mean ± SE | p | mean ± SE | p | mean ± SE | p | mean ± SE | |
| Height (cm) | 158.2 ± 0.6 | 0.01 | 156.4 ± 1.0 | <0.001 | 156.2 ± 1.0 | 0.04 | 158.7 ± 0.9 | 0.31 | 159.9 ± 0.7 |
| Weight (kg) | 57.2 ± 1.1 | 0.85 | 55.4 ± 1.7 | 0.48 | 58.1 ± 2.0 | 0.39 | 55.4 ± 1.4 | 0.92 | 57.0 ± 1.2 |
| Body Mass Index (kg/m2) | 22.4 ± 0.3 | 0.08 | 22.2 ± 0.5 | 0.15 | 23.1 ± 0.6 | 0.08 | 21.8 ± 0.5 | 0.68 | 21.8 ± 0.4 |
| Body Mass Index Percentile | 67.5 ± 2.0 | 0.51 | 68.0 ± 2.8 | 0.25 | 71.0 ± 3.2 | 0.71 | 64.6 ± 3.6 | 0.66 | 66.1 ± 2.4 |
| Waist (cm) | 73.1 ± 0.9 | 0.02 | 72.8 ± 1.2 | 0.03 | 74.7 ± 1.6 | 0.01 | 70.7 ± 1.1 | 0.67 | 71.1 ± 1.0 |
| Waist (cm) to Height ratio (cm) | 0.5 ± 0.005 | 0.001 | 0.46 ± 0.007 | 0.06 | 0.48 ± 0.009 | 0.07 | 0.45 ± 0.008 | 0.36 | 0.4 ± 0.006 |
| Percent Fat Mass (DXA) | 28.1 ± 0.8 | 0.007 | 28.6 ± 1.1 | 0.004 | 29.9 ± 1.2 | 0.002 | 26.3 ± 1.4 | 0.52 | 25.9 ± 0.9 |
| Lean Body Mass (DXA) (kg) | 38.4 ± 0.5 | 0.01 | 37.0 ± 0.9 | <0.001 | 37.3 ± 0.9 | 0.06 | 38.6 ± 0.8 | 0.37 | 39.9 ± 0.6 |
| Abdominal Visceral Fat (CT) (cm3) | 22.3 ± 1.1 | 0.17 | 22.5 ± 1.3 | 0.09 | 25.5 ± 2.3 | 0.01 | 18.4 ± 1.2 | 0.51 | 21.0 ± 1.2 |
| Abdominal Subcutaneous Fat (CT) (cm3) | 85.2 ± 4.5 | 0.07 | 83.5 ± 6.2 | 0.09 | 97.6 ± 8.5 | 0.01 | 73.1 ± 6.4 | 0.81 | 77.0 ± 4.9 |
All measures adjusted for age-at-study, gender, race, and Tanner score
Among the CV risk factors, total cholesterol, non HDL-C, LDL-C, triglycerides, and triglyceride- to HDL-C ratio were significantly higher in CCS than controls (Table III). In subgroup analyses compared with controls, these differences were present in survivors of CNS but not in survivors of solid tumors; total cholesterol and LDL-C were significantly higher in leukemia survivors. Based on age specific criteria for lipids21 elevated total cholesterol, LDL-cholesterol and triglycerides were more prevalent in CCS (21.9%, 16.4% and 22.9 % respectively), than in controls (14.9%, 11.5% and 17.8% respectively), but these differences were not statistically significant (p=0.4–0.9). CCS as a whole and all diagnostic groups were significantly less insulin sensitive (expressed as lower Mlbm) than controls (Table III and Figure). HOMA-IR and fasting insulin did not differ between CCS and controls. The prevalence of the MetS was not significantly different between CCS (n=25, 8%) and controls (n=11, 5%), OR 1.6 (95% CI: 0.7–3.4; p=0.20).
Table 3.
Comparisons between CCS and Controls for CV Risk Factors and Insulin Resistance
| All CCS (n=319) |
Leukemia (n=110) |
CNS (n=82) |
Solid Tumors (n=127) |
Controls (n=208) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjustment | mean ± SE | pb | mean ± SE | pb | mean ± SE | pb | mean ± SE | pb | mean ± SE | |
| Systolic Blood Pressure (mmHg) | 110.9 ± 0.8 | 0.59 | 110.8 ± 1.3 | 0.63 | 109.8 ± 1.3 | 0.84 | 110.7 ± 1.4 | 0.53 | 110.5 ± 1.0 | |
| PFMa | 110.6 ± 0.8 | 0.95 | 110.3 ± 1.3 | 0.93 | 109.0 ± 1.2 | 0.41 | 110.4 ± 1.4 | 0.59 | 110.6 ± 0.9 | |
| Diastolic Blood Pressure (mmHg) | 58.3 ± 0.7 | 0.22 | 58.6 ± 0.9 | 0.10 | 57.8 ± 0.9 | 0.35 | 58.0 ± 1.1 | 0.76 | 57.5 ± 0.7 | |
| PFMa | 58.1 ± 0.7 | 0.35 | 58.3 ± 0.9 | 0.18 | 57.6 ± 0.9 | 0.55 | 57.9 ± 1.1 | 0.81 | 57.6 ± 0.7 | |
| Total Cholesterol (mg/dL) | 154.7 ± 2.1 | 0.004 | 149.8 ± 2.8 | 0.03 | 159.3 ± 4.2 | 0.008 | 152.3 ± 3.4 | 0.23 | 148.3 ± 2.5 | |
| PFMa | 153.2 ± 2.0 | 0.04 | 148.2 ± 2.8 | 0.13 | 155.7 ± 3.7 | 0.05 | 151.3 ± 3.1 | 0.28 | 148.7 ± 2.5 | |
| LDL-Cholesterol (mg/dL) | 89.4 ± 1.9 | 0.002 | 86.7 ± 2.6 | 0.01 | 91.6 ± 3.7 | 0.02 | 88.8 ± 3.3 | 0.10 | 83.7 ± 2.2 | |
| PFMa | 88.0 ± 1.7 | 0.03 | 85.0 ± 2.5 | 0.06 | 88.2 ± 3.1 | 0.11 | 87.8 ± 2.9 | 0.12 | 84.1 ± 2.1 | |
| HDL-Cholesterol (mg/dL) | 47.3 ± 0.8 | 0.21 | 46.1 ± 1.0 | 0.33 | 46.6 ± 1.4 | 0.29 | 47.6 ± 1.3 | 0.29 | 48.4 ± 1.0 | |
| PFMa | 47.8 ± 0.7 | 0.58 | 46.9 ± 0.9 | 0.91 | 47.7 ± 1.3 | 0.77 | 48.1 ± 1.1 | 0.35 | 48.3 ± 0.9 | |
| Triglycerides (mg/dL) | 91.8 ± 5.5 | 0.03 | 85.9 ± 5.3 | 0.38 | 109.1 ± 11.1 | 0.004 | 80.5 ± 3.7 | 0.48 | 84.0 ± 5.9 | |
| PFMa | 89.1 ± 5.4 | 0.20 | 82.4 ± 5.4 | 0.99 | 103.4 ± 10.8 | 0.02 | 78.6 ± 3.2 | 0.61 | 84.8 ± 5.8 | |
| Triglycerides to HDL ratio (mg/dl) | 2.2 ± 0.15 | 0.006 | 2.0 ± 0.15 | 0.19 | 2.7 ± 0.33 | 0.007 | 1.9 ± 0.13 | 0.13 | 1.8 ± 0.16 | |
| PFMa | 2.1 ± 0.14 | 0.05 | 1.9 ± 0.15 | 0.78 | 2.5 ± 0.30 | 0.03 | 1.8 ± 0.11 | 0.16 | 1.9 ± 0.15 | |
| Non-HDL Cholesterol (mg/dl) | 107.4 ± 2.2 | 0.001 | 103.8 ± 2.8 | 0.02 | 112.6 ± 4.1 | 0.002 | 104.6 ± 3.4 | 0.10 | 99.9 ± 2.5 | |
| PFMa | 105.4 ± 1.9 | 0.02 | 101.3 ± 2.7 | 0.12 | 108 ± 3.4 | 0.02 | 103.2 ± 2.9 | 0.11 | 100.0 ± 2.3 | |
| Glucose (mg/dL) | 87.4 ± 1.8 | 0.2 | 85.9 ± 1.1 | 0.58 | 85.1 ± 1.2 | 0.09 | 88.1 ± 4.1 | 0.49 | 89.3 ± 2.3 | |
| PFMa | 87.2 ± 1.8 | 0.14 | 85.6 ± 1.0 | 0.34 | 85.0 ± 1.3 | 0.03 | 87.9 ± 3.9 | 0.43 | 89.3 ± 2.2 | |
| Insulin (mU/L) | 10.6 ± 0.6 | 0.7 | 10.9 ± 0.8 | 0.54 | 12.2 ± 1.4 | 0.20 | 9.1 ± 0.7 | 0.49 | 10.3 ± 0.7 | |
| PFMa | 9.9 ± 0.5 | 0.36 | 9.9 ± 0.8 | 0.46 | 10.6 ± 1.1 | 0.78 | 8.6 ± 0.7 | 0.26 | 10.4 ± 0.6 | |
| HOMA-IR | 2.3 ± 0.1 | 0.8 | 2.3 ± 0.2 | 0.58 | 2.6 ± 0.3 | 0.25 | 2.0 ± 0.1 | 0.42 | 2.2 ± 0.2 | |
| PFMa | 2.1 ± 0.1 | 0.25 | 2.1 ± 0.2 | 0.39 | 2.3 ± 0.3 | 0.94 | 1.8 ± 0.1 | 0.20 | 2.3 ± 0.1 | |
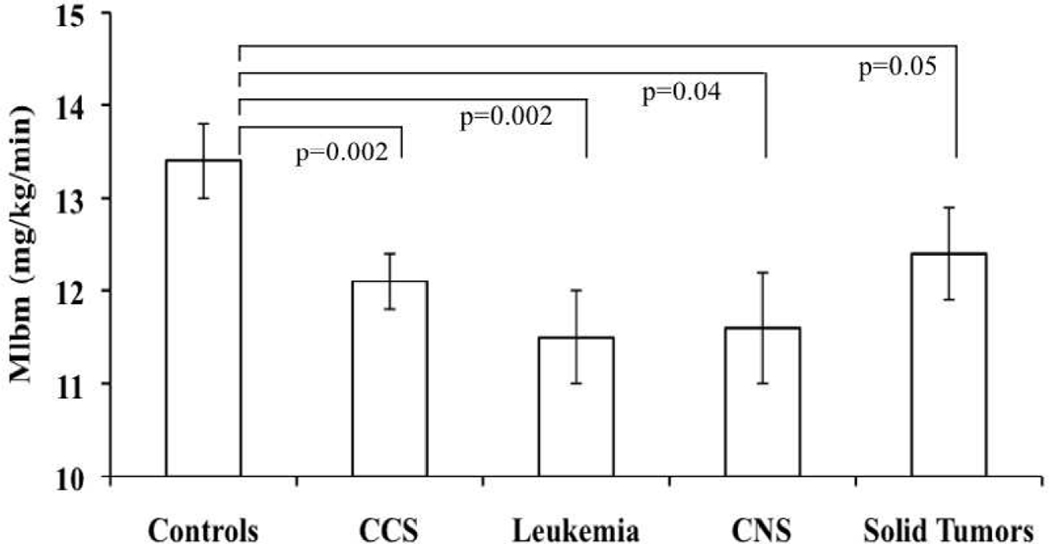
| Mlbm (mg/kg/min) | 12.1 ± 0.3 | 0.002 | 11.5 ± 0.5 | 0.002 | 11.6 ± 0.6 | 0.04 | 12.4 ± 0.5 | 0.05 | 13.4 ± 0.4 | |
| PFMa | 12.2 ± 0.3 | 0.006 | 11.6 ± 0.5 | 0.006 | 11.8 ± 0.6 | 0.11 | 12.5 ± 0.5 | 0.06 | 13.3 ± 0.4 | |
All measures adjusted for age-at-study, sex, race and Tanner score
Additional adjustment for percent fat mass (PFM)
p-value for comparison between CCS (or subgroup) with controls, based on adjusted regression models
Euglycemic hyperinsulinemic clamps not performed on 3 cases (2 CNS, 1 Solid Tumor) and 1 control, due to pre-existing diagnosis of diabetes
Figure.
Adjusted mean Mlbm (mg/kg/min) by CCS, controls and by diagnosis group. P-values comparing means from linear regression models adjusted for age-at-study, Tanner score, sex and race. ↓Mlbm represents ↓Insulin Sensitivity or ↑Insulin Resistance.
Additional analyses were carried out to determine the influence of body fatness on these relations. After adjustment for PFM, CCS as a whole continued to have significantly higher total cholesterol, LDL-C, and lower insulin sensitivity than controls (Table III). Adjustment for adiposity explained some of the differences between the individual diagnostic groups and controls. However, higher triglycerides in CNS tumor survivors and lower insulin sensitivity in leukemia survivors remained significant compared with controls. Adjustment for BMI yielded similar results (data not shown).
Insulin sensitivity in CCS was modestly but significantly inversely correlated with measures of body fatness (BMI, waist circumference, PFM, subcutaneous fat, visceral fat (r= −0.26 to −0.17, p≤0.003) and CV risk factors (LDL-C, HDL-C, triglycerides, systolic and diastolic blood pressure, r= −0.28 to 0.19, p≤0.05). Similar correlations were present in controls for waist circumference (r= −0.21, p=0.005), systolic blood pressure, and HDL-C and triglycerides (r= −0.23 to 0.17, p<0.02). After adjustment for BMI and PFM, in both CCS and controls, the correlations remained significant for waist (r= −0.13, p=0.02 and r=−0.19, p=0.008, respectively), systolic blood pressure (r= −0.16, p=0.005 and r= −0.15, p=0.05, respectively) and triglycerides (r= −0.23, p<0.001 and r= −0.20, p=0.007, respectively), and only for HDL-C in CCS (r= 0.12, p=0.04). There were few differences in body composition and the CV risk factors among the individual diagnostic groups. PFM was higher in survivors of CNS tumors and leukemia than in survivors of solid tumors (p=0.01 and p=0.02, respectively); survivors of leukemia had lower LBM than solid tumors (p =0.04); survivors of CNS tumors had higher triglycerides than survivors of leukemia and solid tumors (all p=0.01). Insulin sensitivity was not significantly different among survivors of leukemia, CNS tumors and solid tumors (p=0.10–1.0).
There was no significant association between time since diagnosis and the CV risk factors or Mlbm, after accounting for age at study, sex, race, Tanner score, and BMI (p=0.30 and p=0.66, respectively).
Discussion
We show that CCS are more insulin resistant and more likely to have adverse levels of CV risk factors in comparison with their healthy sibling controls prior to reaching adulthood. In particular, significantly higher waist circumference and percent body fat and significantly lower lean body mass were found in CCS than controls without a significant difference in BMI. Adiposity, usually expressed as an increased BMI, is associated with adverse levels of the CV risk factors and insulin resistance in pediatric populations22–24. However, in this population BMI provided a less accurate representation of adiposity, which was identified only after DXA screening.
Previous studies in adults showed greater insulin resistance in CCS than healthy controls, as expressed by HOMA-IR or fasting insulin9,10. In the current study, HOMA-IR and fasting insulin showed weak correlations with Mlbm (r = −0.35), similar to results from prior studies in large cohorts14, and were not different between CCS and controls. By using a direct measure of insulin resistance (the hyperinsulinemic euglycemic clamp), it was possible to show a lesser degree of insulin sensitivity in CCS than controls, even after adjustment for adiposity, suggesting that insulin sensitivity in CCS may be related to cancer and/or its therapy. Insulin sensitivity was significantly related to CV risk factors in both CCS and controls, but the relation was stronger in CCS, independent of adiposity and pubertal stage. Insulin resistance per se is not a disease in childhood, but a condition related to obesity and elevated CV risk factors22 and valuable in identifying children at greatest risk for future CV disease. Prior studies in children have shown that low insulin sensitivity is a significant predictor of future increased CV risk25. Although it has been associated with the metabolic syndrome, insulin resistance is not synonymous with obesity and may be an initiating factor in CV risk26, even in non-obese populations27. Thus, the lower level of insulin sensitivity in CCS may influence an early elevation of the CV risk factors and suggests that the difference in risk factor levels between CCS and the control group will become more apparent with ongoing maturity and aging.
In the current study, there was no difference in the prevalence of MetS between CCS and controls, despite significant differences in insulin sensitivity and other CV risk factors. A study in young adult survivors of childhood leukemia also found no difference in the prevalence of MetS, but showed significant differences in some of the individual component CV risk factors compared with controls10. These data support the importance of focusing on CV risk factors as continuous variables in youth, as opposed to relying on the dichotomous classification used to diagnose the MetS for the assessment of CV risk.28
The study has some limitations. With a participation rate of 49%, selection bias cannot be completely excluded. The population was predominantly white non-Hispanic, therefore the findings may not be generalizable to other racial/ethnic groups. Despite the adjustment for age and puberty, the fact that CCS were older than controls may contribute to some of the differences noted. Sex differences in outcomes were not addressed, due to lack of power for statistical analyses by sex within each diagnostic category. Modern cancer therapy relies on multi-modal treatments including surgery, radiation therapy and/or multi-agent chemotherapy. Determining the impact of any individual therapy becomes very complex and requires very large numbers of subjects. Therefore, the current analysis incorporates the combined impact of the therapeutic exposures on different diagnostic groups of survivors. Survivors of CNS tumors and leukemia were found to have a higher burden of CV risk factors than survivors of solid tumors. Likely, this may be related to differences in cancer therapies or differences in lifestyle factors on the observed outcomes. Further studies will be required in order to identify specific exposures that may convey a higher risk of insulin resistance and an adverse CV risk profile. Although measures of fitness were not obtained it is conceivable that similar to other populations, promotion of physical activity may improve on the adverse body composition and progressive cardiometabolic risk in CCS.
The results from this study have important implications for pediatric care. Several studies have described adverse CV events among adult CCS6, with CV deaths accounting for 26% of the absolute excess risk of death (second only to second primary cancers 51%) by 45 or more years from diagnosis7. However, most studies have concentrated on the overt clinical presentation rather than investigating the sub-clinical development of CV disease. Although the measured values of the CV risk factors in this study and in children, in general, are lower than those associated with adult CV disease, there is a widely documented strong tracking effect for the risk factors (adiposity, lipids and blood pressure) from childhood into adulthood.29,30 Thus, it is reasonable to suggest that in the presence of decreased insulin sensitivity and adverse levels of CV risk factors, children who are CCS have a higher potential for the development of early CV disease than controls, as they progress into adulthood. The lack of effect of time since diagnosis on all outcome variables further suggests that the risk profile is likely established early in the course of survivorship, indicating the importance in monitoring CV risk factors in CCS of all ages.
Acknowledgments
Funded by the National Institutes of Health NCI/NIDDK (RO1CA113930-01A1 to J.S.), the GCRC (M01-RR00400), and General Clinical Research Center Program, NCRR/NIH.
We are grateful to Mrs. Jill Lee, Anne Norris, and Dr. Brigitte Frohnert (University of Minnesota) for their contributions to the study.
List of abbreviations
- DXA
Measured by dual-energy X-ray absorptiometry
- CT
limited abdominal computed tomography scan without contrast
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- HOMA-IR
Homeostatic Model Assessment-Insulin Resistance
- Mlbm
insulin stimulated glucose uptake, measure of insulin resistance, adjusted for lean body mass
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Gurney J, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Fifth Edition. Philadelphia, P.A.: Lippincott Williams & Wilkins; 2006. pp. 1–13. [Google Scholar]
- 2.Meadows AT. Pediatric cancer survivors: past history and future challenges. Curr Probl Cancer. 2003;27:112–126. doi: 10.1016/s0147-0272(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 3.Diller L, Chow EJ, Gurney J, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–2355. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy S, Cleeman J, Daniels S, Donato Ka, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 5.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007;48:723–726. doi: 10.1002/pbc.21114. [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 8.van Waas M, Neggers SJ, Pieters R, van den Heuvel-Eibrink MM. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol. 2010;21:1121–1126. doi: 10.1093/annonc/mdp414. [DOI] [PubMed] [Google Scholar]
- 9.Oeffinger K, Adams-Huet B, Victor R, Church TS, Snell PG, Dunn AL, et al. Insulin Resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurney J, Ness K, Sibley S, O'Leary M, Dengel DR, Lee JM, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 11.Trimis G, Moschovi M, Papassotiriou I, Chrousos G, Tzortzatou-Stathopoulou F. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29:309–314. doi: 10.1097/MPH.0b013e318059c249. [DOI] [PubMed] [Google Scholar]
- 12.Pietilä S, Mäkipernaa A, Sievänen H, Kiovisto AM, Wigren T, Lenko HL. Obesity and metabolic changes are common in young childhood brain tumor survivors. Pediatr Blood Cancer. 2009;52:853–859. doi: 10.1002/pbc.21936. [DOI] [PubMed] [Google Scholar]
- 13.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz E, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–788. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 15.Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from insulin clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: finding from the third National Health and Nutrition Examination Survey, 1998–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 19.Bethesda, Md: National Institutes of Health; 2002. National Institutes of Health: Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report) [Google Scholar]
- 20.McCarthy HD, Ashwell M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message--'keep your waist circumference to less than half your height'. Int J Obes. 2006;30:988–992. doi: 10.1038/sj.ijo.0803226. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. National Cholesterol Education Program: report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:525–584. [PubMed] [Google Scholar]
- 22.Sinaiko A, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 23.Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr. 1995;126:690–695. doi: 10.1016/s0022-3476(95)70394-2. [DOI] [PubMed] [Google Scholar]
- 24.Caprio S, Bronson M, Sherwin RS, Rife F, Tamborlane WV. Co-existence of severe insulin resistance and hyperinsulinemia in preadolescent obese children. Diabetologia. 1996;39:1489–1497. doi: 10.1007/s001250050603. [DOI] [PubMed] [Google Scholar]
- 25.Sinaiko AR, Steinberger J, Moran A, Hong CP, Prineas RJ, Jacobs DR., Jr Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides and high-density lipoprotein cholesterol at age 19. Hypertension. 2006;48:730–736. doi: 10.1161/01.HYP.0000237863.24000.50. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 27.Hollenbeck C, Reaven GM. Variations in insulin-stimulated glucose uptake in healthy individuals with normal glucose tolerance. J Clin Endocrinol Metab. 1987;64:1169–1173. doi: 10.1210/jcem-64-6-1169. [DOI] [PubMed] [Google Scholar]
- 28.Kelly AS, Steinberger J, Jacobs DR, Jr, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2010 doi: 10.3109/17477166.2010.528765. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinaiko AR, Donahue RP, Jacobs DR, Jr, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults: the Minneapolis Children’s Blood Pressure Study. Circulation. 1999;99:1471–1476. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am J Epidemiol. 2007;166:527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]