Abstract
Dexamethasone loaded poly(lactic-co-glycolic acid) (PLGA) microsphere/PVA hydrogel composites have been investigated as an outer drug-eluting coating for implantable devices such as glucose sensors to counter negative tissue responses to implants. The objective of this study was to develop a discriminatory, accelerated in vitro release testing method for this drug-eluting coating using United States Pharmacopeia (USP) apparatus 4. Polymer degradation and drug release kinetics were investigated under “real-time” and accelerated conditions (i.e. extreme pH, hydro-alcoholic solutions and elevated temperatures). Compared to “real-time” conditions, the initial burst and lag phases were similar using hydro-alcoholic solutions and extreme pH conditions, while the secondary apparent zero-order release phase was slightly accelerated. Elevated temperatures resulted in a significant acceleration of dexamethasone release. The accelerated release data were able to predict “real-time” release when applying the Arrhenius equation. Microsphere batches with faster and slower release profiles were investigated under “real-time” and elevated temperature (60°C) conditions to determine the discriminatory ability of the method. The results demonstrated both the feasibility and the discriminatory ability of this USP apparatus 4 method for in vitro release testing of drug loaded PLGA microsphere/PVA hydrogel composites. This method may be appropriate for similar drug/device combination products and drug delivery systems.
Keywords: Accelerated in vitro release, Drug-eluting coating, USP apparatus 4, PLGA microspheres, PVA hydrogel, Dexamethasone
1. Introduction
During the past several decades, implantable biosensors have gained increasing attention due to their potential for continuous monitoring of metabolites and biochemical markers (Gilligan et al., 2004; Poscia et al., 2005; Vaddiraju et al., 2010b; Wang, 2001). However, tissue damage during implantation and the continuous presence of the foreign device in the body can trigger a cascade of events, which lead to negative foreign body responses such as inflammatory reaction and fibrous encapsulation (Anderson et al., 2008; Gifford et al., 2006; Morais et al., 2010). These foreign body responses can result in erroneous data and eventually lead to loss of biosensor functionality (Vaddiraju et al., 2010a; Vaddiraju et al., 2010b). Accordingly, suppression of inflammation and fibrous encapsulation at the implant vicinity over an extended period is crucial to ensure the long-term functionality of these biosensors.
Recently, an outer drug-eluting biosensor coating composed of PLGA microsphere/PVA hydrogel composites has been developed to counter negative tissue responses associated with the implantation of biosensors and other implantable medical devices (Bhardwaj et al., 2008; Morais et al., 2010; Onuki et al., 2008; Patil et al., 2004). This drug-eluting coating can achieve long-term delivery of various tissue response modifiers (TRMs) such as the anti-inflammatory agent (dexamethasone), growth factors and combinations thereof (Bhardwaj et al., 2007, 2010; Galeska et al., 2005; Hickey et al., 2002; Norton et al., 2005; Patil et al., 2007) from slow releasing PLGA microspheres, while also allowing the rapid influx of small molecule analytes through the PVA hydrogel matrix (Galeska et al., 2005; Vaddiraju et al., 2009). TRM release from this drug eluting biosensor coating occurs via diffusion, PLGA polymer erosion or a combination thereof (Faisant et al., 2002) and is dependent on polymer properties (e.g. molecular weight, copolymer composition and crystallinity) (Park, 1995; Tracy et al., 1999), drug properties (Miyajima et al., 1999b; Sandor et al., 2001) as well as dissolution conditions (Dunne et al., 2000; Klose et al., 2010).
“Real-time” in vitro drug release from this biosensor coating under simulated physiological conditions can range from days to months and consequently, a discriminatory, accelerated in vitro release testing method is essential to assure the performance of such systems as well as assist in product development and quality control of implantable biosensors and other medical devices. Accelerated drug release from PLGA microspheres has been achieved by increasing the polymer degradation rate via extreme pH conditions (Faisant et al., 2002; Zolnik and Burgess, 2007), elevated temperature (Zolnik et al., 2006) as well as the addition of hydro-alcoholic solvents to the release medium (Kamberi et al., 2009). Other conditions such as radiation (used for sterilization purposes) and the addition of surfactants can also accelerate drug release from PLGA microspheres (Faisant et al., 2006). Since accelerated release testing requires stress conditions (e.g. extreme pH and elevated temperature), it is possible that the drug release mechanism may change during accelerated release testing compared to that in “real-time” studies. Nevertheless, accelerated release tests should be predictive of “real-time” release tests and should be able to differentiate different formulations (Burgess et al., 2004; Burgess et al., 2002).
At present, there is no standard pharmacopeial or regulatory in vitro release method for implants and drug/device combination products. A variety of methods have been used for in vitro release testing of drug-eluting stents and implants such as different vial methods (Schliecker et al., 2004), capillary system (Iyer et al., 2007), USP apparatus 7 (Kamberi et al., 2009) and the flow-through cell method (Neubert et al., 2008; Seidlitz et al., 2011). In addition, the procedures and apparatus used vary among laboratories. Therefore, it is important to develop a compendial method to allow comparison of results from different laboratories, as well as to facilitate regulatory approval of these products.
USP apparatus 4, which was originally developed for controlled release oral solid dosage forms, has been shown to be suitable for other controlled release parenteral systems such as microspheres (Rawat and Burgess, 2011b; Voisine et al., 2008), liposomes (Bhardwaj and Burgess, 2010) and implants (Browne and Kieselmann, 2010). USP apparatus 4 can be operated at low volume and its hydrodynamic flow conditions can be modified (Iyer et al., 2006). Accordingly, it may be possible to mimic the in vivo conditions at the implantation sites (e.g. subcutaneous tissue) using flow through cells. In addition, the media volume used with the USP apparatus 4 can be modified to allow testing of various formulations, and this is of particular importance for many low dose parenteral formulations.
In the present study, USP apparatus 4 was used in a closed-loop configuration to develop a discriminatory, accelerated in vitro drug release testing method for dexamethasone loaded PLGA microsphere/PVA hydrogel composite coatings. The effect of different accelerated conditions on the in vitro drug release kinetics was investigated. A relationship between “real-time” release and accelerated release was developed for different composite coating formulations.
2. Materials and methods
2.1. Materials
Dexamethasone, poly(vinyl alcohol) (PVA, MW 30–70 kDa), sodium chloride (NaCl, ACS grade) and sodium azide (NaN3) were purchased from Sigma-Aldrich (St. Louis, MO). PVA (99 % hydrolyzed, MW 133 kDa) was purchased from Polysciences, Inc. (Warrington, PA). PLGA Resomer® RG503H (inherent viscosity 0.32–0.44 dl/g) was a gift from Boehringer-Ingelheim. PLGA DLG2A (inherent viscosity 0.15–0.25 dl/g), PLGA DLG3A (inherent viscosity 0.25–0.35 dl/g) and PLGA DLG4A (inherent viscosity 0.35–0.45 dl/g) were kindly provided by SurModics Pharmaceuticals (Birmingham, AL). Methylene chloride, sodium mono-hydrogen phosphate (NaH2PO4, ACS grade), acetonitrile (ACN, HPLC grade), dimethyl sulfoxide (DMSO, ACS grade) and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburghm, PA). Disodium hydrogen phosphate (Na2HPO4, ACS grade) was purchased from VWR International. Nanopure™ quality water (Barnstead, Dubuque, IA) was used for all studies.
2.2. Methods
2.2.1. Preparation of PLGA microspheres
Dexamethasone loaded PLGA microspheres were prepared using an oil-in-water (o/w) emulsion-solvent extraction method reported previously (Galeska et al., 2005; Zolnik et al., 2006). Briefly, 2 g PLGA was dissolved in 8 ml methylene chloride. 200 mg dexamethasone was dispersed in this PLGA solution using a homogenizer (PowerGen 700D, Fisher Scientific) at 10,000 rpm for 30 s. This organic phase was then emulsified in 40 ml of a 1% (w/w) PVA (MW 30–70 kDa) solution and homogenized at 10,000 rpm for 2.5 min. The resultant emulsion was poured into 500 ml of a 0.1% (w/w) PVA (MW 30–70 kDa) solution and stirred at 600 rpm under vacuum to achieve rapid evaporation of methylene chloride. The hardened microspheres were washed three times with de-ionized water and collected by filtration (Durapore® membrane filter, 0.45 µm, Fisher Scientific). The prepared microspheres were vacuum-dried and stored at 4°C until further use.
2.2.2. Preparation of PLGA microsphere/PVA hydrogel composite coatings
PLGA microsphere/PVA hydrogel composite coatings were prepared using a mold fabrication process. Briefly, 75 mg PLGA microspheres were homogenously dispersed in a 5 % (w/w) PVA solution. A grooved mold was filled with this PLGA microsphere containing dispersion and dummy sensors (silicon chips) were placed into the mold. These were then subjected to three freeze-thaw cycles according to the method established previously (Galeska et al., 2005).
2.2.3. High performance liquid chromatography (HPLC)
The quantification of dexamethasone was conducted using a Perkin Elmer HPLC system (series 200) with a UV absorbance detector (Perkin Elmer, Shelton, CT) set at 242 nm. The mobile phase consisted of acetonitrile/water/phosphoric acid (30/70/0.5%, v/v/v). A Zorbax® C18 (4.6 mm×15 cm) analytical column was used with the flow rate set at 1 ml/min. The chromatographs were analyzed by PeakSimple™ Chromatography System (SRI instruments, Torrance, CA). This method is a stability indicating HPLC assay.
2.2.4. Particle size analysis
An AccuSizer 780A autodiluter particle sizing system (Santa Barbara, CA) was used to determine the mean particle size of PLGA microspheres. About 25 mg of microspheres were dispersed in 1 ml of 0.1% (w/v) PVA (MW 30–70 kDa) solution. 100 µl of the dispersion was used for particle size analysis. All measurements were conducted in triplicate and the results are reported as the mean ± SD.
2.2.5. Drug loading
5 mg of dexamethasone loaded PLGA microspheres were dissolved in 10 ml tetrahydrofuran (THF). This solution was filtered (Millex® HV, PVDF 0.45 µm syringe filter) and the dexamethasone concentration was determined via HPLC as described before using an injection volume of 5 µl. To determine the drug loading in the composite coatings, 10 mg of these coatings were dissolved in 1 ml DMSO and the volume was made up to 10 ml with THF. The solution was then filtered and the dexamethasone concentration was determined as described above.
Drug loading was determined as: percent drug loading = (weight of drug loaded/weight of microspheres or composite coatings used) × 100. All measurements were conducted in triplicate and the results are reported as the mean ± SD.
2.2.6. Glass transition temperature
The glass transition temperature of the prepared composite coatings was analyzed using a TA instrument Q1000 differential scanning calorimeter (New Castle, DE). Samples were heated from −40°C to 80°C, and cooled to −40°C at a rate of 10°C/min. The first cycle of the thermograms was used to determine the glass transition temperature (Tg) using Universal Analysis software (TA Instruments). All measurements were conducted in triplicate and the results are reported as the mean ± SD.
2.2.7. In vitro release studies
“Real-time” in vitro release testing of the composite coatings were conducted using a USP apparatus 4 (Sotax CE7 smart, Sotax, Horsham, PA) equipped with flow-through implant cells in a closed system mode at 37 °C, in which the composite coatings were placed into the implant cells and 40 ml of 0.1 M phosphate-buffered saline (PBS, pH 7.4) with 0.01 % sodium azide was circulated at a flow rate of 8 ml/min. At pre-determined intervals, 1 ml samples were withdrawn and replenished with 1 ml of fresh media. The samples were analyzed via HPLC as described before using an injection volume of 20 µl. For accelerated release testing, the following accelerated test conditions were investigated: (i) extreme pH conditions (pH 2.4 and pH 10.0); (ii) PBS (0.1 M, pH 7.4) containing 10 % (v/v) ethanol; and (iii) elevated temperatures (45, 50, 53 and 60°C). All the measurements were conducted in triplicate and the mean values and standard deviations were reported.
2.2.8. In vitro degradation studies
PLGA microspheres and composite coatings were incubated in the different “real-time” and accelerated release conditions in flat bottomed vials and placed in a shaker water bath (C76, New Brunswick Scientific, NJ) at 100 rpm. At known intervals, samples were decanted, vacuum dried for 24 h and analyzed by SEM. All measurements were conducted in triplicate.
2.2.9. Statistical data analysis
Statistical analysis to evaluate significant differences between different microsphere and composite coating formulations were performed using the student t-test. The level of significance was accepted at p < 0.05.
3. Results and discussion
3.1. Characterization of PLGA microspheres and composite coatings
Model formulation 503H microspheres were prepared with PLGA 503H. Other PLGA polymers (PLGA DLG2A, DLG3A and DLG4A) were used to prepare microsphere formulations DLG2A, DLG3A and DLG4A, respectively. These formulations were selected since the inherent viscosities of these polymers are close to that of PLGA 503H and therefore, could be used to investigate the discriminatory ability of the accelerated in vitro release testing method as well as to determine any relationship between “real-time” and accelerated release. The PLGA microspheres were prepared with similar drug loading (between 7.63 ± 0.16 % and 8.10 ± 0.18 %) to minimize the number of variables that can affect drug release (Liggins and Burt, 2004). The average size of these microspheres was around 7 µm, and there were no significant differences among different formulations (p>0.05). Table 1 shows the characteristics of different composite coating formulations containing the microspheres. The dexamethasone loading in these formulations was similar (around 4.4 %). As expected, embedding the PLGA microspheres into PVA hydrogels did not change the Tg of these microspheres. The Tg of the microspheres increased with the increase in the inherent viscosity of the polymers.
Table 1.
Characteristics of PLGA microsphere/PVA hydrogel composite coatings.
| Formulation | Ratio of LA/GA | Inherent viscosity (dl/g) | Drug loading (%) | Glass transition temperature (°C) |
|---|---|---|---|---|
| DLG2A | 50:50 | 0.15–0.25 | 4.54 ± 0.14 | 38.7 ± 0.4 |
| DLG3A | 50:50 | 0.25–0.35 | 4.48 ± 0.17 | 40.5 ± 0.6 |
| 503H | 50:50 | 0.32–0.44 | 4.41 ± 0.24 | 41.4 ± 0.3 |
| DLG4A | 50:50 | 0.35–0.45 | 4.32 ± 0.04 | 42.7 ± 0.2 |
3.2. Effect of extreme pH conditions on the in vitro drug release of 503H composite coatings
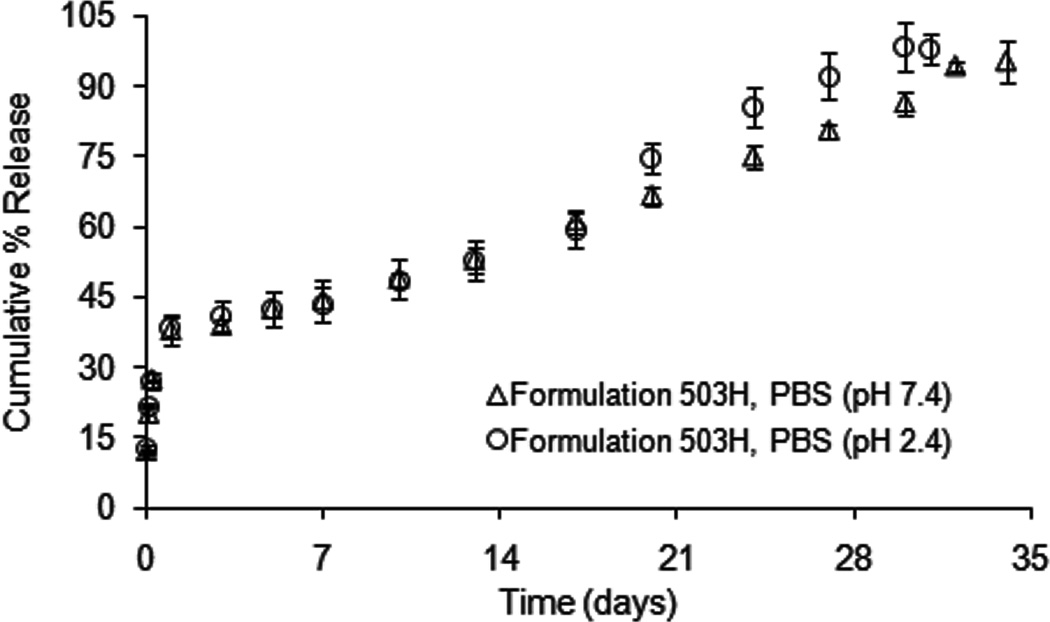
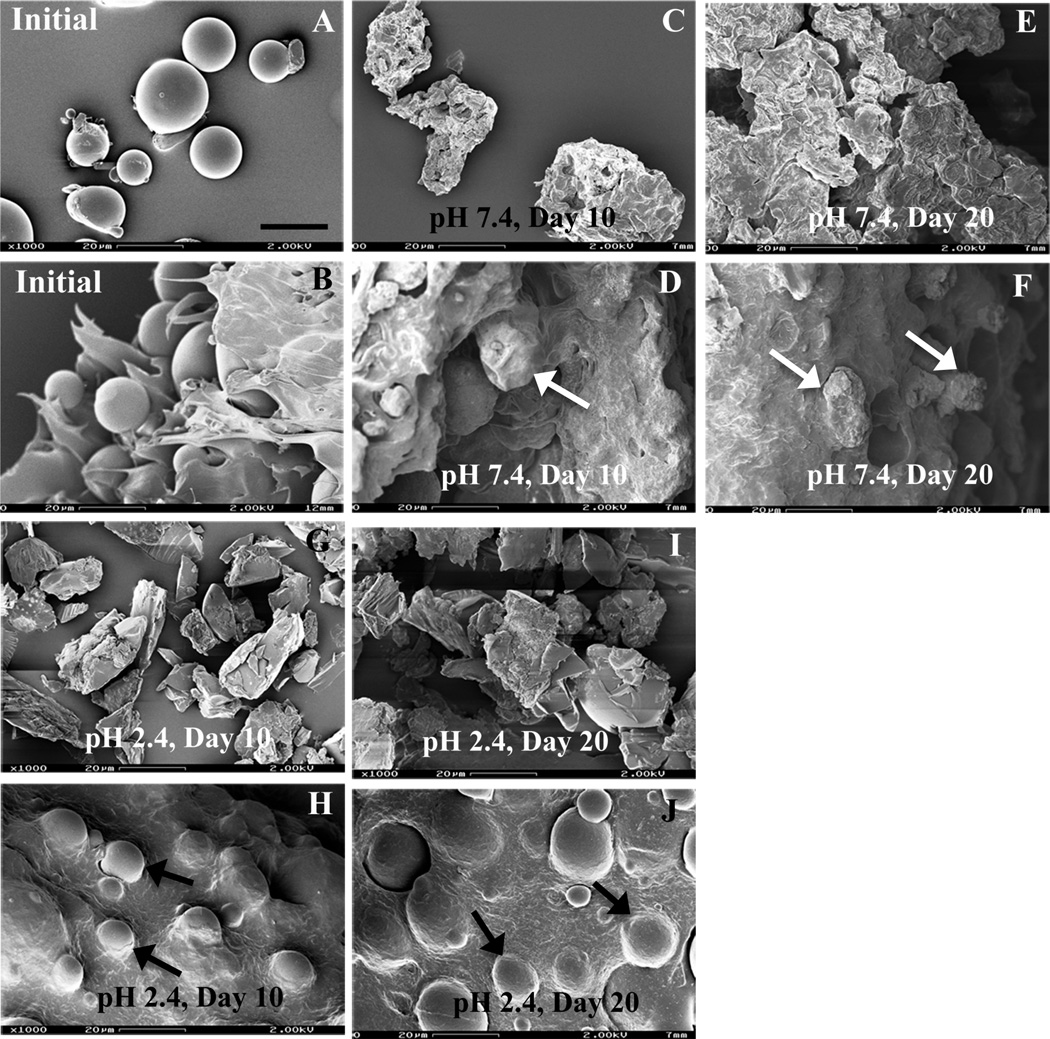
“Real-time” drug release kinetics of formulation 503H composite coatings exhibited a typical triphasic release profile with an initial burst release (ca. 39 % in 24 h), followed by a lag phase and then a secondary apparent zero-order phase (2.06 day−1) (Fig. 1). The morphology of the PLGA microspheres alone and embedded in the PVA hydrogel, revealed a spherical geometry with smooth surfaces (Fig. 2A and B, respectively). As degradation proceeded at pH 7.4, microsphere erosion became evident by day 10 (Fig. 2C) and the microspheres alone appeared to agglomerate to form a large block of polymer by day 20 (Fig. 2E). Like the PLGA microspheres alone, those microspheres embedded in the composite coating followed an “inside-out” degradation profile as evident from their wrinkled appearance (indicated by white arrows in Fig. 2D and F) and no agglomerate was observed. The PLGA microsphere/PVA hydrogel composites showed a slightly lower initial burst release compared to the PLGA microspheres alone reported previously (Rawat and Burgess, 2011a). It is known that the initial burst release is controlled by diffusion, while the secondary zero-order release phase is governed by polymer erosion as well as diffusion (Faisant et al., 2002; Zolnik et al., 2006). Therefore, it is rationalized that the presence of the PVA hydrogel matrix creates a barrier to diffusion of surface-associated drug into the bulk media resulting in a lower initial burst release (Bhardwaj et al., 2010). In addition, the PVA hydrogel prevents agglomeration of the microspheres and the formation of a large polymer block. Drug release from eroding microspheres inside the composite coating was faster than that from the aggregated polymer block which has much larger dimensions (von Burkersroda et al., 2002).
Fig. 1.
Dexamethasone release from formulation 503H composite coatings at 37°C in PBS (pH 7.4 and pH 2.4), using USP apparatus 4: (△) pH 7.4, and (○) pH 2.4. (Mean±std dev; n=3).
Fig. 2.
SEM micrographs of formulation 503H (PLGA microspheres and composites) after exposure to different release conditions (PBS pH 7.4 and pH 2.4) at 37°C over time: (A, B) initial; (C, D) pH 7.4, day 10; (E, F) pH 7.4, day 20; (G, H) pH 2.4, day 10; (I, J) pH 2.4, day 20. Symbols: white arrows - “insideout” degrading microspheres; and black arrows - “outside-in” degrading PLGA microspheres. Top row, microspheres alone, pH 7.4. Second row, composites, pH 7.4. Third row, microspheres alone, pH 2.4. Fourth row, composites, pH 2.4. Scale bar: 20 µm.
As shown in Fig.1, acidic conditions (PBS, pH 2.4) slightly accelerated drug release from the composite coating. A similar initial burst release (ca. 40 % in 24 h) and a faster secondary zero-order release phase (2.68 day−1) was observed compared to the “real-time” release profile. Morphological studies showed an “outside-in” degradation pattern at pH 2.4 compared to the “inside-out” degradation at pH 7.4 (Fig. 2), which was consistent with microsphere degradation behavior reported previously (Zolnik and Burgess, 2007). It was evident that the microspheres alone were fragmenting by day 10 (Fig. 2G) and fusion of fragmented microspheres was observed by day 20 at pH 2.4 (Fig. 2I). However, PLGA microspheres within the PVA hydrogel had smooth surfaces throughout the entire study period with no evidence of microsphere fragment fusion (indicated by black arrows in Fig. 1H and J). Low pH is known to catalyze PLGA degradation and accelerate drug release from PLGA microspheres (Faisant et al., 2002; Zolnik and Burgess, 2007). However, acidic pH conditions (pH 2.4) failed to significantly accelerate drug release from the composite coatings. This may also be a result of the presence of the PVA hydrogel. As previously reported, the PLGA microspheres became progressively brittle with time at pH 2.4 (Zolnik and Burgess, 2007), and these fragmented microspheres experienced faster degradation than the intact microspheres. It appears that the PVA hydrogel protected the PLGA microspheres from breaking into fragments, and it is speculated that this may be the reason why the microspheres embedded within the PVA hydrogel do not degrade at a significantly faster rate at pH 2.4.
Alkaline pH has been utilized to enhance degradation of the PLGA polymers (Alexis, 2005; de Jong et al., 2001). However, dexamethasone degraded rapidly under alkaline condition (pH 10.0) (data not shown) and so alkaline pH was not appropriate for in vitro release testing of this delivery system. Accordingly, these results show that extreme pH (acidic and alkaline) conditions do not appear to be suitable for accelerated in vitro release testing of dexamethasone from the composite coatings.
3.3. Effect of hydro-alcoholic medium on the in vitro drug release of 503H composite coatings
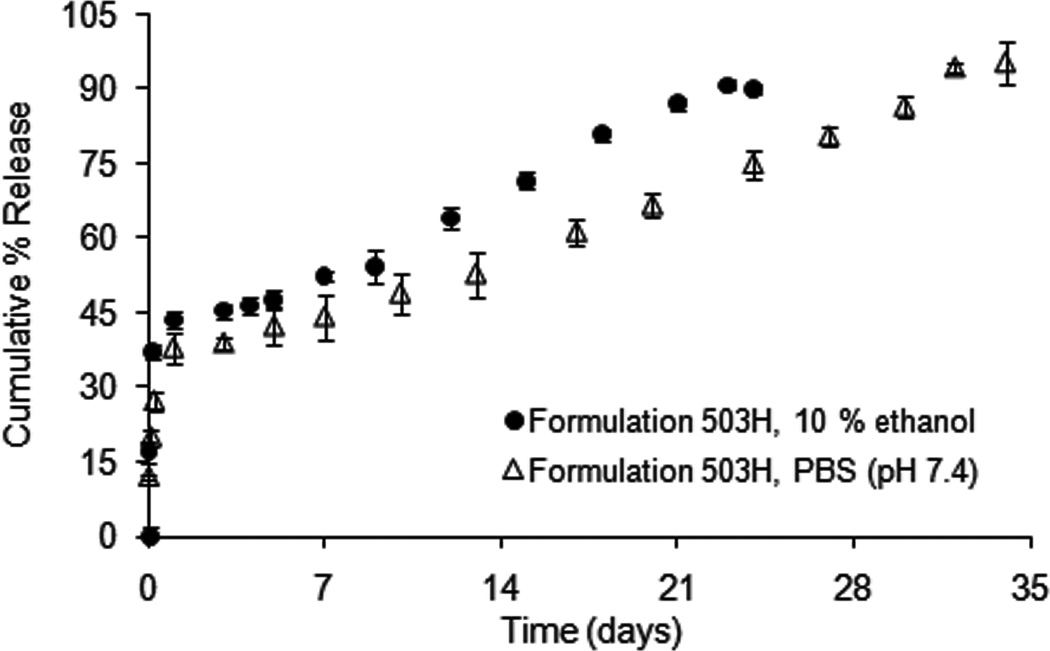
The dexamethasone release profiles from formulation 503H composite coatings in PBS (pH 7.4) with and without addition of hydro-alcoholic solvent are shown in Fig. 3. Introducing ethanol (10 %, v/v) into the release medium did not change the typical triphasic release profile of the PLGA microsphere/PVA hydrogel composite coating. A higher initial burst release (ca. 44 % in 24 h) and a faster zero-order release phase (2.5 day−1) was observed compared to the “real-time” release profile. The time to complete release was reduced from 34 days to 23 days.
Fig. 3.
Dexamethasone release from formulation 503H composite coatings in PBS (pH 7.4) and PBS (pH 7.4) containing 10 % (v/v) ethanol at 37°C, using USP apparatus 4: (△) PBS (pH 7.4), and (●) 10 % ethanol (v/v). (Mean±std dev; n=3).
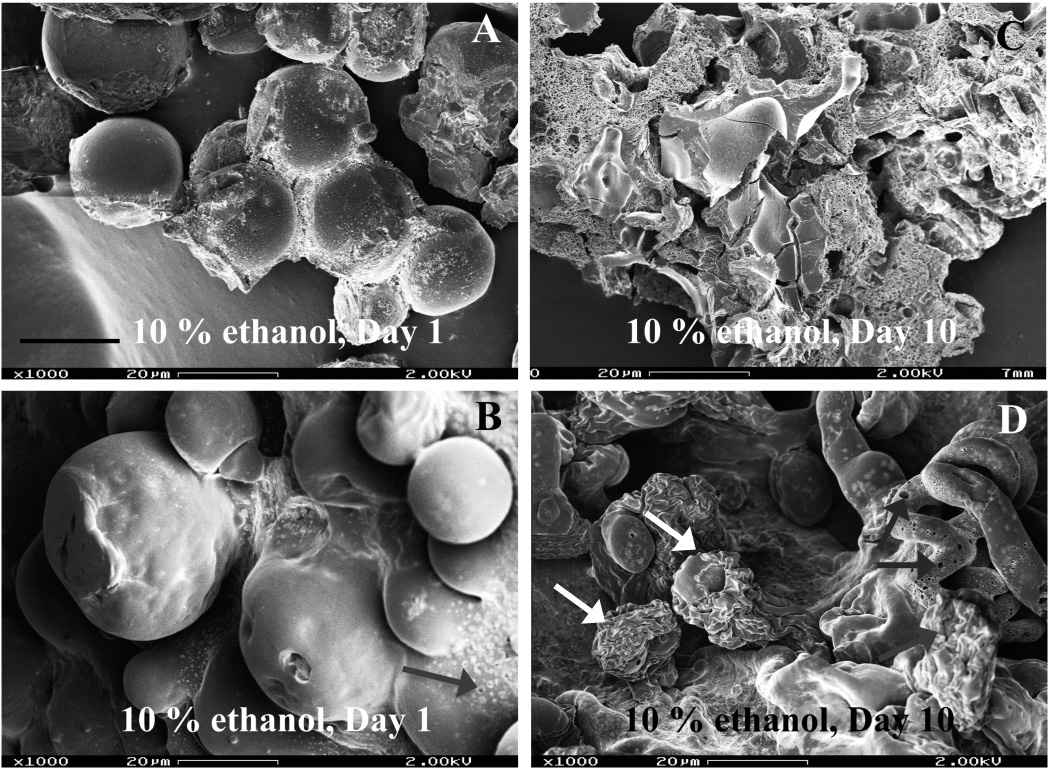
Fig. 4 shows the morphology studies of PLGA microspheres with and without PVA hydrogel under hydro-alcoholic conditions. PLGA microspheres alone showed the usual morphological changes (i.e. surface pitting and pore formation) after exposure to hydro- alcoholic solutions (Fig. 4A) and formed a large block of polymer by day 10 (Fig. 4C). The PLGA microspheres inside the PVA hydrogel matrix followed the “inside-out” degradation mechanism (indicated by white arrows in Fig. 4D). In addition, the PVA hydrogel exhibited an incrementally porous structure (indicated by grey arrows in Fig. 4B and D). The increased porosity of the PVA hydrogel might be due to the swelling property of the hydro-alcoholic solvent (Kamberi et al., 2009). Since drug diffusion governs the initial burst release and contributes to the secondary zero-order release phase as well, the formation of a porous structure in the PVA hydrogel is expected to facilitate drug diffusion through the PVA hydrogel matrix, thus accelerating dexmethasone release from the composite coating. These factors will increase drug release from PLGA microsphere/PVA hydrogel composite coatings. However, the use of organic solvents in the USP apparatus 4 is limited due to extractables from plastics in the tubings of the apparatus 4. It should be noted that as necessary different plastic tubings can be utilized with the USP apparatus 4. However, it was concluded that hydro-alcoholic medium is not a good accelerated release condition for the PLGA microsphere/PVA hydrogel composite coating.
Fig. 4.
SEM micrographs of formulation 503H (PLGA microspheres and composites) after exposure to PBS (pH 7.4) containing 10 % (v/v) ethanol at 37°C over time: (A, B) day 1 and (C, D) day 10; Symbols: white arrows - “inside-out” degrading PLGA microspheres, grey arrows - porous hydrogel matrix. Top row, microspheres alone. Second row, composites. Scale bar: 20 µm.
3.4. Effect of elevated temperature on the in vitro drug release of 503H composite coatings
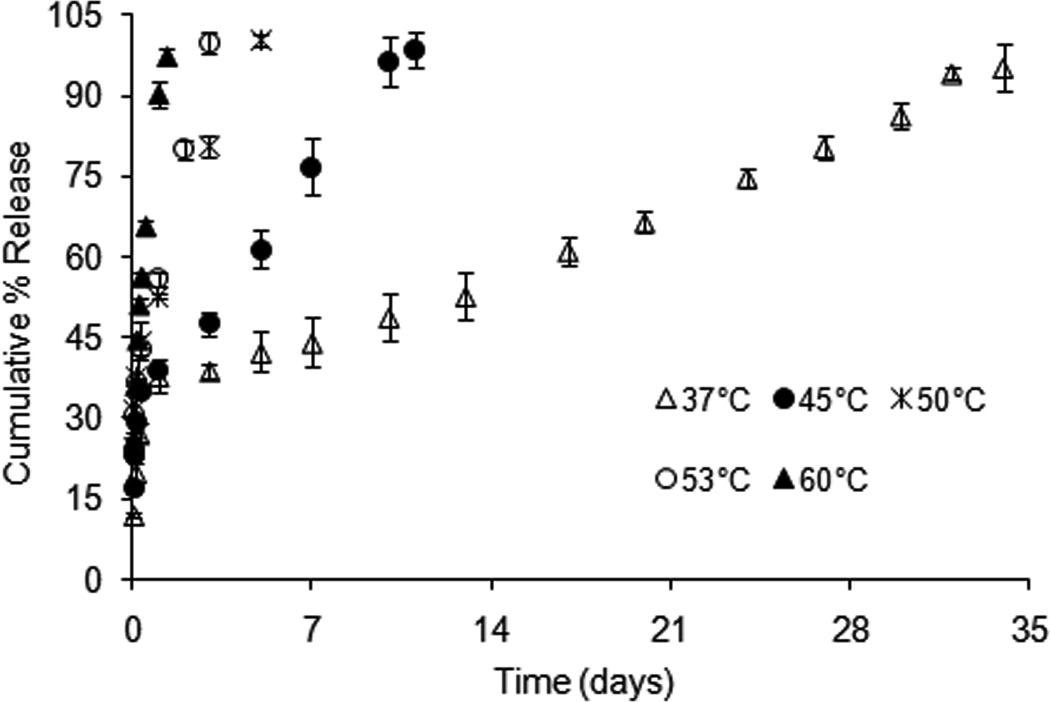
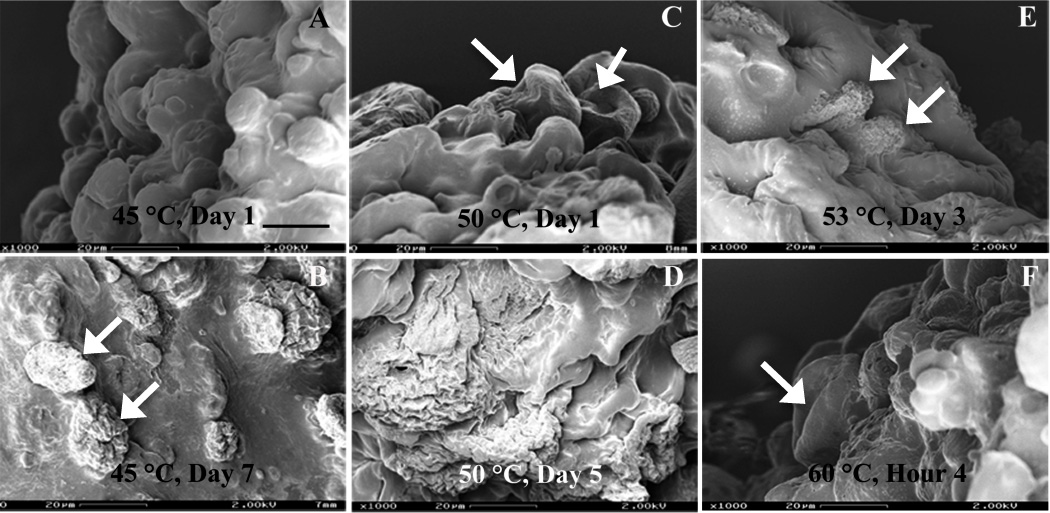
Elevated temperatures (45, 50, 53 and 60°C) were used to accelerate dexamethasone release from formulation 503H composite coatings. As shown in Fig. 5, the overall drug release was significantly accelerated at elevated temperatures. The time to complete release was reduced from 34 days (37°C) to 10, 5, 3 and 1.3 days at 45, 50, 53 and 60°C, respectively. Moreover, no lag release phase was observed. Drug release characteristics changed from the typical triphasic profile at 37°C to a biphasic profile: with the initial burst release, followed by a zero-order release phase at elevated temperatures. The calculated zero-order rate constants were 6.58, 12.13, 21.68 and 45.87 day−1 at 45, 50, 53 and 60°C, respectively. The morphological tests of the composite coatings at elevated temperatures are shown in Fig. 6. The PLGA microspheres inside the PVA hydrogel followed an “inside-out” degradation pattern at elevated temperatures (indicated by white arrows in Fig. 6) and their degradation accelerated with increasing temperature. Based on this, the drug release mechanism of the post-burst release phase appeared unaltered under elevated temperature accelerated tests.
Fig. 5.
Dexamethasone release from formulation 503H composite coatings in PBS (pH 7.4) at elevated temperatures, using USP apparatus 4: (△) 37°C, (●) 45°C, (×) 50°C, (○) 53°C and (▲) 60°C. (Mean±std dev; n=3).
Fig. 6.
SEM micrographs of formulation 503H composites after exposure to PBS (pH 7.4): (A) 1 day, at 45°C; (B) 7 days, at 45°C; (C) 1 day, at 50°C; (D) 5 days, at 50°C; (E) 3 days, at 53°C; (F) 4 hours, at 60°C; Symbol: white arrow - “inside-out” degrading PLGA microspheres. Scale bar: 20 µm.
To determine whether drug release rates at elevated temperatures can be used to predict “real-time” release, these data were plotted according to the Arrhenius equation.
| (1) |
where k is the zero-order release rate, A is a constant, Ea is the energy of activation, R is the gas constant (cal/deg mol) and T is the absolute temperature.
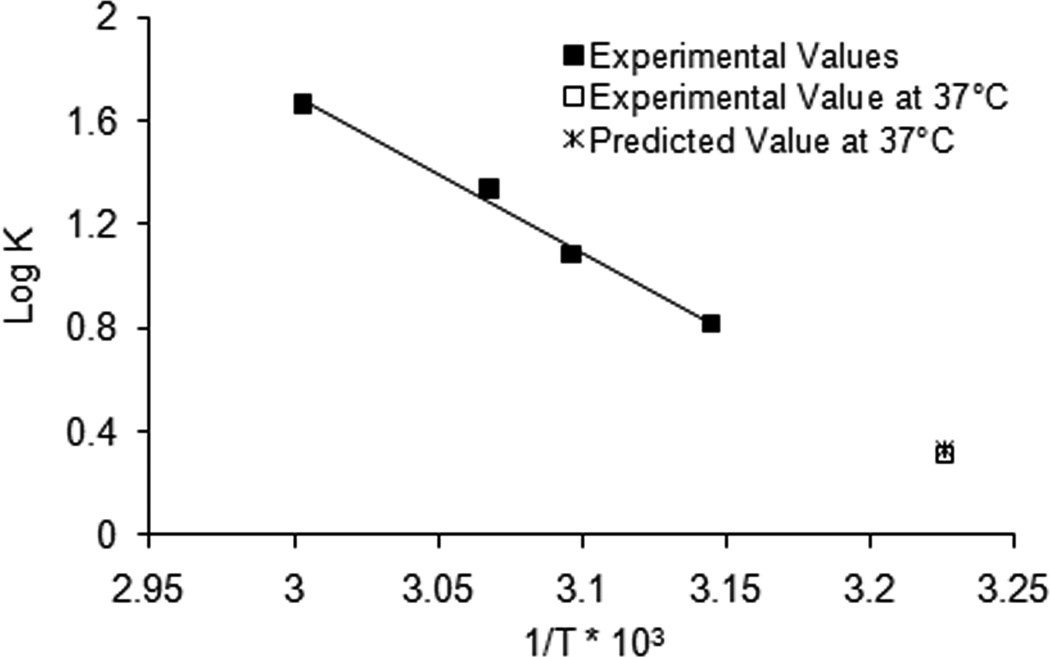
Log k versus 1/T was plotted and the slope was −Ea/2.303R. The calculated activation energy of dexamethasone release from formulation 503H was 27.68 kcal/mol based on the experimental values at 45, 50, 53 and 60°C (shown as solid squares in Fig. 7). This calculated activation energy closely matches the energy of activation for PLGA polymer degradation (23.6 kcal/mol) (Miyajima et al., 1999a), further confirming that the apparent zero-order release phase was governed by PLGA polymer erosion. Ea was used to predict the apparent zero-order rate constant at 37°C (2.13 day−1) (shown as cross in Fig. 7), which was in agreement with the experimental value (2.05 day−1) (shown as open square in Fig. 7). Accordingly, elevated temperature accelerated in vitro release tests showed good prediction of the “real-time” release using the Arrhenius equation (Fig. 7). Since the drug release rate was fastest at 60°C, this temperature was selected to further investigate the discriminatory ability of elevated temperature accelerated in vitro release testing.
Fig. 7.
Arrhenius plot of the calculated zero-order rate constants of dexamethasone release from formulation 503H composite coatings as a function of temperature at 45°C, 50°C, 55°C and 60°C (■). Rate constants at 37°C for predicted and experimental values are shown as a cross (×) and an open square (□), respectively.
3.5. Discriminatory ability study of “real-time” and elevated temperature accelerated tests
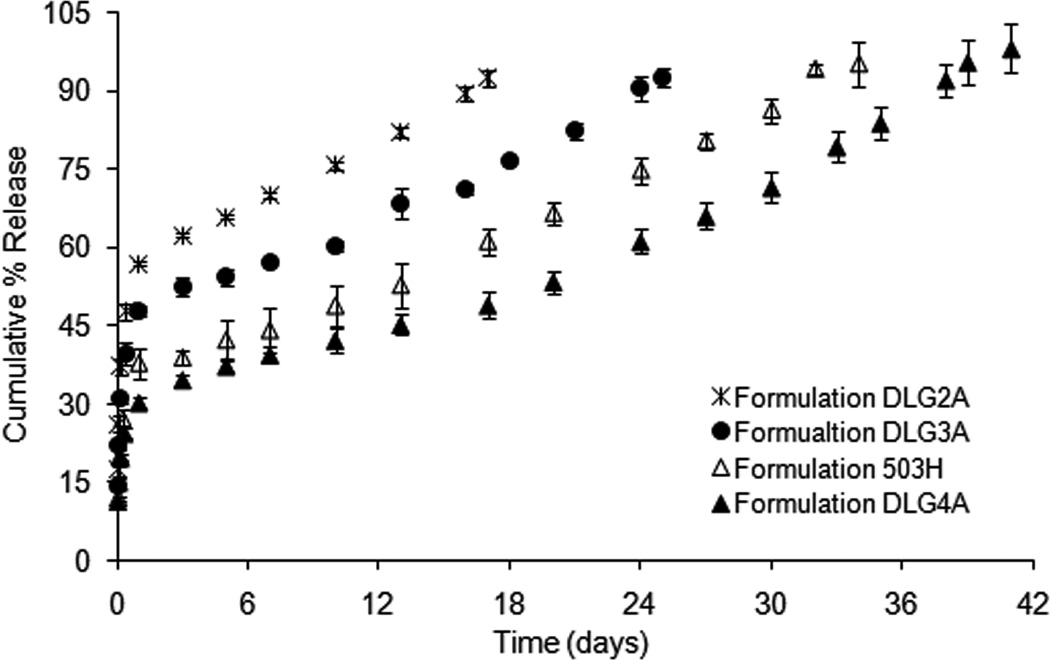
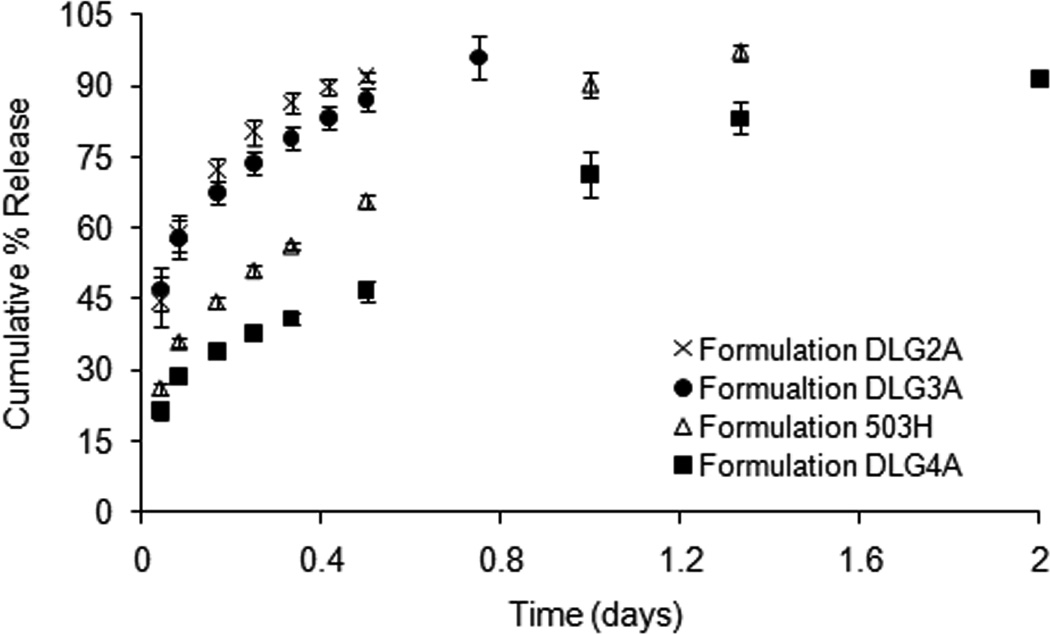
Fig. 8 shows the “real-time” in vitro release profiles of composite coating formulations 503H, DLG2A, DLG3A and DLG4A. The initial burst release decreased with increasing inherent viscosity of the PLGA polymers (approximately 57 %, 48 %, 39 % and 30 % in 24 h for DLG2A, 503H, DLG3A and DLG4A, respectively). PLGA polymers with low inherent viscosities have better hydration and swelling properties, thus resulting in faster diffusion of surface-associated drug into the release medium (Messaritaki et al., 2005). It was noted that dexamethasone release mechanisms appeared different among these formulations. Composite coatings DLG2A and DLG3A exhibited biphasic release profiles (an initial burst release and an apparent zero-order release phase), while composite coatings 503H and DLG4A followed the typical triphasic release profile. The time to reach complete drug release was 18, 27, 34 and 41 days for formulations DLG2A, DLG3A, 503H and DLG4A at 37°C, respectively. These results showed a good “real-time” discriminatory ability using USP apparatus 4 for in vitro release testing of the composite coatings.
Fig. 8.
Dexamethasone release from composite coatings (formulations DLG2A, DLG3A 503H and DLG4A) in PBS (pH 7.4) at 37°C, using USP apparatus 4: (×) DLG2A, (●) DLG3A, (△) 503H and (▲) DLG4A. (Mean±std dev; n=3).
As shown in Fig. 9, the time to reach complete drug release at 60°C was 0.5, 1, 1.33 and 2 days for composite coating formulations DLG2A, DLG3A, 503H and DLG4A, respectively. The apparent zero-order release rate constants at 37°C and 60°C were calculated for these formulations. The ratios of the rate constants at 60°C and 37°C were 36.8, 31.5 for formulations DLG 2A and DLG 3A, and 22.2 and 21.7 for formulations 503H and DLG 4A, respectively, indicating good correlation between the “real-time” and accelerated tests for formulations with similar release characteristics. The accelerated release tests using USP apparatus 4 were able to discriminate between formulations with similar release characteristics (formulation 503H and DLG4A) as well as formulations with dissimilar release characteristics (formulations 503H and DLG3A). Moreover, it should be noted that the burst release phase for formulations with dissimilar release characteristics (formulations 503H and DLG3A) can be distinguished. However, that was not the case for formulations with similar release characteristics (formulations 503H and DLG4A). Therefore, it is recommended that “real-time” studies should be used to determine the burst release phase as suggested in a previous workshop report (Burgess et al., 2004).
Fig. 9.
Dexamethasone release from composite coatings (formulations 503H, DLG2A, DLG3A and DLG4A) at 60°C in PBS (pH 7.4), using USP apparatus 4: (×) DLG2A, (●) DLG3A, (△) 503H and (■) DLG4A. (Mean±std dev; n=3).
4. Conclusions
This study demonstrated the feasibility of USP apparatus 4 for in vitro release testing of drug loaded PLGA microsphere/PVA hydrogel composite coatings. Accelerated release tests at elevated temperature showed good discriminatory ability for the different formulations investigated. Furthermore, the accelerated data of formulations with similar release characteristics correlated well with the "real-time" release at 37°C. Accordingly, elevated temperature accelerated in vitro release testing using USP apparatus 4 can be employed as a rapid quality control test for PLGA based drug-eluting coatings as well as other implants. This method is based on an official USP dissolution/release apparatus and consequently, is a promising method for compendial adaptation for in vitro release testing of implantable drug device combination products and drug delivery systems.
Acknowledgements
This work was supported by the National Institutes of Health Grant (#RFA-DK-09) and the US Army Medical Research (#W81XWH-07-1-0688). The authors would also like to thank Dr. Archana Rawat for her help with in vitro release studies. Support from Sotax Corporation in providing USP dissolution apparatus 4 is highly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jie Shen, Email: jie.shen@uconn.edu.
Diane J. Burgess, Email: d.burgess@uconn.edu.
References
- Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym. Int. 2005;54:36–46. [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Burgess DJ. A novel USP apparatus 4 based release testing method for dispersed systems. Int. J. Pharm. 2010;388:287–294. doi: 10.1016/j.ijpharm.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors. J. Diabetes Sci. Technol. 2008;2:1016–1029. doi: 10.1177/193229680800200611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices. J. Diabetes Sci. Technol. 2007;1:8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int. J. Pharm. 2010;384:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Browne DC, Kieselmann K. Low-level drug release-rate testing of ocular implants using USP Apparatus 4 dissolution and HPLC end analysis. Dissolution Technol. 2010;17:12–14. [Google Scholar]
- Burgess DJ, Crommelin DJ, Hussain AS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: EUFEPS workshop report. AAPS J. 2004;6:100–111. doi: 10.1208/ps060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DJ, Hussain AS, Ingallinera TS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: workshop report. AAPS PharmSci. 2002;4:E7. doi: 10.1208/ps040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong SJ, Arias ER, Rijkers DTS, van Nostrum CF, Kettenes-van den Bosch JJ, Hennink WE. New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer. 2001;42:2795–2802. [Google Scholar]
- Dunne M, Corrigan I, Ramtoola Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials. 2000;21:1659–1668. doi: 10.1016/s0142-9612(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int. J. Pharm. 2006;314:189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Faisant N, Siepmann J, Benoit JP. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur. J. Pharm. Sci. 2002;15:355–366. doi: 10.1016/s0928-0987(02)00023-4. [DOI] [PubMed] [Google Scholar]
- Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7:E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R, Kehoe JJ, Barnes SL, Kornilayev BA, Alterman MA, Wilson GS. Protein interactions with subcutaneously implanted biosensors. Biomaterials. 2006;27:2587–2598. doi: 10.1016/j.biomaterials.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Gilligan BC, Shults M, Rhodes RK, Jacobs PG, Brauker JH, Pintar TJ, Updike SJ. Feasibility of continuous long-term glucose monitoring from a subcutaneous glucose sensor in humans. Diabetes Technol. Ther. 2004;6:378–386. doi: 10.1089/152091504774198089. [DOI] [PubMed] [Google Scholar]
- Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23:1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Barr WH, Karnes HT. Profiling in vitro drug release from subcutaneous implants: a review of current status and potential implications on drug product development. Biopharm. Drug Dispos. 2006;27:157–170. doi: 10.1002/bdd.493. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Barr WH, Karnes HT. A 'biorelevant' approach to accelerated in vitro drug release testing of a biodegradable, naltrexone implant. Int. J. Pharm. 2007;340:119–125. doi: 10.1016/j.ijpharm.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Kamberi M, Nayak S, Myo-Min K, Carter TP, Hancock L, Feder D. A novel accelerated in vitro release method for biodegradable coating of drug eluting stents: Insight to the drug release mechanisms. Eur. J. Pharm. Sci. 2009;37:217–222. doi: 10.1016/j.ejps.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Klose D, Siepmann F, Willart JF, Descamps M, Siepmann J. Drug release from PLGA-based microparticles: effects of the "microparticle:bulk fluid" ratio. Int. J. Pharm. 2010;383:123–131. doi: 10.1016/j.ijpharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Liggins RT, Burt HM. Paclitaxel loaded poly(L-lactic acid) (PLLA) microspheres. II. The effect of processing parameters on microsphere morphology and drug release kinetics. Int. J. Pharm. 2004;281:103–106. doi: 10.1016/j.ijpharm.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Messaritaki A, Black SJ, van der Walle CF, Rigby SP. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. J. Control. Release. 2005;108:271–281. doi: 10.1016/j.jconrel.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Koshika A, Okada J, Ikeda M. Effect of polymer/basic drug interactions on the two-stage diffusion-controlled release from a poly(L-lactic acid) matrix. J. Control. Release. 1999a;61:295–304. doi: 10.1016/s0168-3659(99)00149-2. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Koshika A, Okada J, Ikeda M. Mechanism of drug release from poly(L-lactic acid) matrix containing acidic or neutral drugs. J. Control. Release. 1999b;60:199–209. doi: 10.1016/s0168-3659(99)00083-8. [DOI] [PubMed] [Google Scholar]
- Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert A, Sternberg K, Nagel S, Harder C, Schmitz KP, Kroemer HK, Weitschies W. Development of a vessel-simulating flow-through cell method for the in vitro evaluation of release and distribution from drug-eluting stents. J. Control. Release. 2008;130:2–8. doi: 10.1016/j.jconrel.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Norton LW, Tegnell E, Toporek SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26:3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J. Diabetes Sci. Technol. 2008;2:1003–1015. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol. Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J. Control. Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Poscia A, Messeri D, Moscone D, Ricci F, Valgimigli F. A novel continuous subcutaneous lactate monitoring system. Biosens. Bioelectron. 2005;20:2244–2250. doi: 10.1016/j.bios.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Rawat A, Burgess DJ. Effect of physical ageing on the performance of dexamethasone loaded PLGA microspheres. Int. J. Pharm. 2011a;415:164–168. doi: 10.1016/j.ijpharm.2011.05.067. [DOI] [PubMed] [Google Scholar]
- Rawat A, Burgess DJ. USP apparatus 4 method for in vitro release testing of protein loaded microspheres. Int. J. Pharm. 2011b;409:178–184. doi: 10.1016/j.ijpharm.2011.02.057. [DOI] [PubMed] [Google Scholar]
- Sandor M, Enscore D, Weston P, Mathiowitz E. Effect of protein molecular weight on release from micron-sized PLGA microspheres. J. Control. Release. 2001;76:297–311. doi: 10.1016/s0168-3659(01)00446-1. [DOI] [PubMed] [Google Scholar]
- Schliecker G, Schmidt C, Fuchs S, Ehinger A, Sandow J, Kissel T. In vitro and in vivo correlation of buserelin release from biodegradable implants using statistical moment analysis. J. Control. Release. 2004;94:25–37. doi: 10.1016/j.jconrel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Seidlitz A, Nagel S, Semmling B, Grabow N, Martin H, Senz V, Harder C, Sternberg K, Schmitz KP, Kroemer HK, Weitschies W. Examination of drug release and distribution from drug-eluting stents with a vessel-simulating flow-through cell. Eur. J. Pharm. Biopharm. 2011;78:36–48. doi: 10.1016/j.ejpb.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Tracy MA, Ward KL, Firouzabadian L, Wang Y, Dong N, Qian R, Zhang Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057–1062. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 2010a;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Singh H, Burgess DJ, Jain FC, Papadimitrakopoulos F. Enhanced glucose sensor linearity using poly(vinyl alcohol) hydrogels. J. Diabetes Sci. Technol. 2009;3:863–874. doi: 10.1177/193229680900300434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens. Bioelectron. 2010b;25:1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisine JM, Zolnik BS, Burgess DJ. In situ fiber optic method for long-term in vitro release testing of microspheres. Int. J. Pharm. 2008;356:206–211. doi: 10.1016/j.ijpharm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- von Burkersroda F, Schedl L, Gopferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- Wang J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis. 2001;13:983–988. [Google Scholar]
- Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release. 2007;122:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. J. Control. Release. 2006;112:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]