Abstract
Arenaviruses are rodent-borne RNA viruses, and some have the capacity to cause hemorrhagic fever and death in infected individuals and thus have been identified as a potential bioterrorism threat. Ribavirin and supportive care are currently the approved therapeutic options for individuals suffering from arenavirus-induced hemorrhagic fever. However, new research has suggested that immune plasma treatment or kinase inhibitors may provide a therapeutic option for treating arenavirus infections in humans. This article puts forth a perspective as to the potential use of kinase inhibitors as an antiviral therapeutic for arenavirus infections.
Keywords: antiviral therapeutics, arenavirus, hemorrhagic fever, kinase inhibitors, virology
In 1933, the first recognized arenavirus, Lymphocytic choriomeningitis virus (LCMV), was isolated from samples harvested during a St. Louis encephalitis epidemic. LCMV was found to be a causative agent of aseptic meningitis in humans and identical to a virus isolated from chronically infected mice. Since then, at least 22 arenaviruses that are either pathogenic or nonpathogenic in humans have been identified [1]. Arenaviruses are rodent borne, enveloped, single-stranded bipartite RNA viruses that are grouped into two categories: the New World or Tacaribe complex viruses and the Old World or Lymphocytic choriomeningitis complex viruses. These groupings are based on phylogenetic viral RNA analysis and serology divide. Some of these arenaviruses have been shown to be pathogenic in humans with the potential to cause hemorrhagic fever. Hemorrhagic fever signs and symptoms vary between viruses, but generally include fever, fatigue, dizziness, muscle aches, loss of strength, exhaustion, capillary leak syndrome, and abnormalities of coagulation and platelet function. Severe cases of hemorrhagic fever can result in bleeding under the skin, in internal organs, from body orifices, and result in case-fatality rates of 30% of cases depending on the arenaviral infection. Of the New World arenaviruses discovered, Junin virus (JUNV), isolated in 1958 and found to be the causative agent of Argentine hemorrhagic fever, Machupo virus (MACV), isolated in 1963 and found to cause Bolivian hemorrhagic fever, Guanarito virus (GTOV), the etiologic agent of Venezuelan hemorrhagic fever, and Sabia virus, isolated in Brazil in 1990 and found to cause Brazilian hemorrhagic fever, have all been shown to be pathogenic for humans, causing hemorrhagic fevers in humans. Of the Old World arenaviruses, Lassa virus (LASV), isolated in 1969 in West Africa, causes Lassa hemorrhagic fever. While some Old and New World arenaviruses cause severe hemorrhagic fever in humans, others appear to be nonpathogenic: Pichindé virus (PICV), isolated in Colombia in 1967, is nonpathogenic in humans but produces pathology in guinea pigs similar to that observed in human Lassa fever. Because of this and since working with LASV is difficult due to the biosafety level-4 laboratory restrictions, the PICV-guinea pig model provides a more tractable model to study hemorrhagic fever pathogenesis. Since some arenaviruses cause severe hemorrhagic fever in humans, have the capacity for human-to-human spread and have limited therapeutic options, these viruses are categorized as CDC biothreat agents and National Institute of Allergy and Infectious Diseases (NIAID) category A priority pathogens. Currently, ribavirin and supportive care remain the only approved therapeutic options for arenavirus hemorrhagic fever; however, there are various efforts underway to develop vaccines. Results from recent studies suggest that more potential therapeutic options should be explored [2–8]. Of these therapeutic options, general and specific kinase inhibitors may prove to be effective in treating people infected with arenaviruses.
Reversible protein phosphorylation regulates many aspects of cell signaling in eukaryotic organisms. Kinases are enzymes that phosphorylate or add a phosphate group to a molecule or protein, while phosphatases dephosphorylate or remove phosphate groups. The addition or removal of a phosphate group usually triggers an ‘on/off’ switch within the molecule or protein, and is the trigger of phosphorylation regulation and cell signaling. It has previously been demonstrated that a differential global kinase/phosphorylation response occurs in response to PICV infection in guinea pigs [9]. This study compared the activity of the macrophage kinome, which is the complement of kinases encoded by the genome or expressed in a specific cell, following PICV infection in guinea pigs and led to the demonstration of various phosphorylation states of cell surface receptors and downstream transcription factors. Specifically, this kinomics study demonstrated that differential kinase activity occurs in response to infection in guinea pigs infected with mild and severe variants of PICV. Kinomics is the study of the kinome, for example, understanding which kinases are expressed in a particular cell type, at a particular time, and whether they are activated and capable of phosphorylating target proteins.
Another study demonstrated that pretreating cells with the general kinase inhibitor genistein resulted in inhibition of arenavirus entry and suggests that postentry events may also be inhibited, resulting in an inhibition of viral infection [8]. Additionally, a recent study demonstrated that kinase inhibitors can be utilized to prevent intracellular bacterial growth [10]. The authors of this study used an RNA interference screen of the human kinome to show that specific host kinases are activated during infection with intracellular bacteria. Inhibition of these kinases resulted in inhibited bacterial growth, in vitro. The results from these studies pose many interesting potential avenues of research with regard to cellular kinase activity in response to arenaviral infection. First, it is important to identify the cellular kinases and proteins that are activated and phosphorylated in response to arenaviral infection and whether these proteins are directly involved in the arenavirus replication cycle within the host cell. Research aimed at demonstrating whether different pathogenic and nonpathogenic arenaviruses induce similar kinase activity necessary for a productive viral infection would be of value, since it is easier and less restrictive to study nonpathogenic arenaviruses.
The identification of specific kinase inhibitors that result in infection inhibition in vitro should be considered for in vivo animal studies. Studies need to be directed to demonstrate that inhibiting kinase activity to ameliorate arenaviral infection in humans does not cause additional cell damage due to inhibition of cellular phosphorylation events. These are issues that this report will explore to determine whether utilization of a single/multiple kinase inhibitor(s) is a realistic antiviral therapy for arenavirus infections.
Kinases are involved in arenavirus trafficking within the host cell
To determine whether specific kinases are involved in arenavirus entry and trafficking within the host cell, one must first identify the mechanism by which arenaviruses enter host cells and understand the mechanism by which cellular proteins aid viral entry. Entry of Old World arenaviruses appears to be initiated when the arenavirus glycoprotein (GP) binds to the α-dystroglycan cellular receptor [11]. Once bound, it appears that Old World arenaviruses enter cells through smooth pits by an endocytic, microfilament-independent process [12,13]. However, New World arenaviruses appear to enter host cells by a different mechanism. The New World pathogenic arenaviruses JUNV, MACV and GTOV have been shown to utilize the cellular transferrin 1 receptor (TfR1) during viral entry [14]; however, the receptor utilized by other New World arenaviruses remains a mystery. Once the New World arenaviruses have bound to the cellular receptor, the viruses seem to enter cells by a clathrin-mediated endocytic mechanism that is dependent on cellular cholesterol [15,16]. This appears to be the case for both pathogenic and nonpathogenic New World arenaviruses. After endocytosis, both Old and New World arenaviruses appear to traffic through endosomes and acidification of the endosomes allows for the GP to undergo conformational changes, which includes the release of GP 1 and the exposure of the concealed GP2 fusion peptides, resulting in fusion of the virus with the endosomal membrane [17–19]. The arenavirus endosomal entry process is complex and a myriad of cellular proteins are involved, some of which must be phosphorylated for proper endosomal activity.
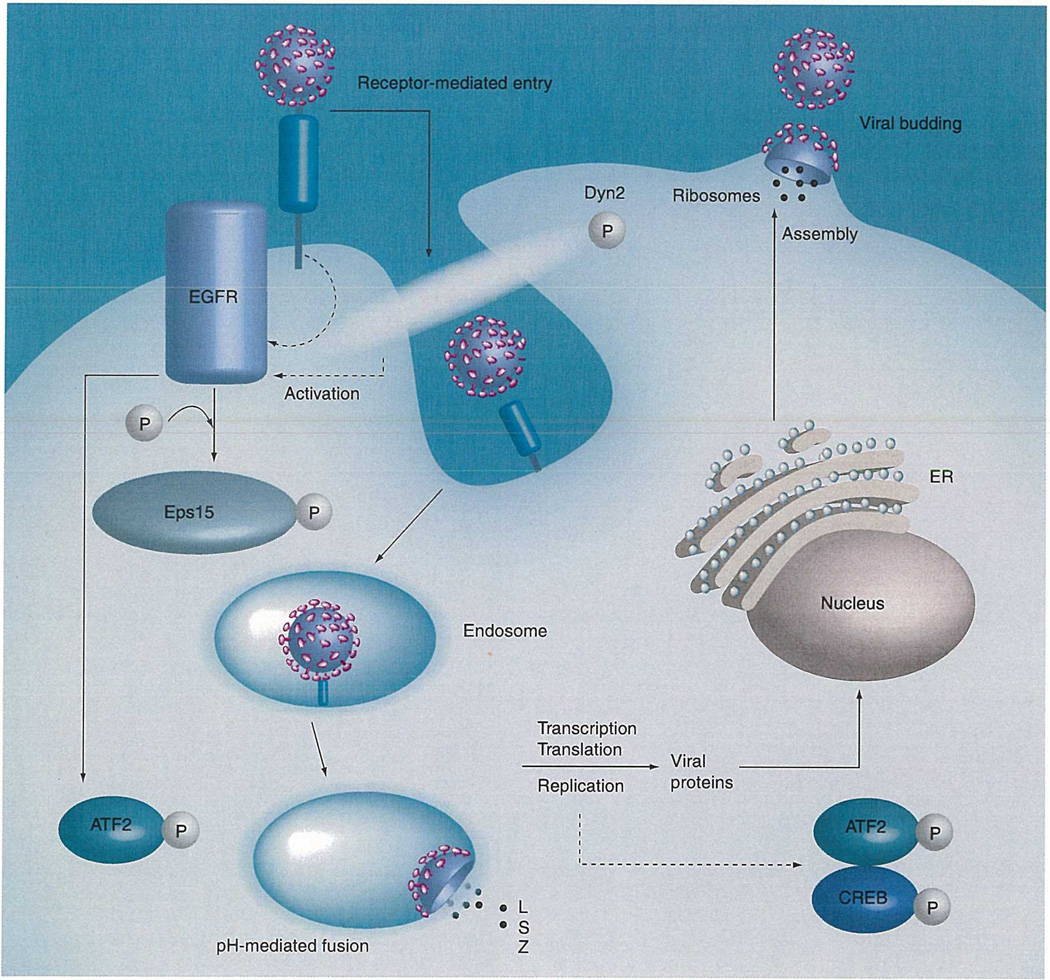
Clathrin-mediated endocytosis involves the interaction of many cellular proteins that appear to be recruited to the plasma membrane through internal signals. The EGF receptor (EGFR) substrate clone (Eps15) is required for clathrin-coated pit assembly and function (Figure 1) [20] and interacts with clathrin though the adaptor protein, AP-2 [21]. It has been demonstrated that transfecting host cells with a dominant-negative mutant of Eps15 results in the inhibition of PICV entry [16]. However, the exact mechanism of Eps15 involvement in PICV entry is not known. Eps15 is phosphorylated by EGFR [22] and EGFR has been implicated in PICV infection [9]. Therefore, it is possible that proper Eps15 function in PICV entry may depend on the phosphorylation of Eps15 through EGFR, and utilizing a kinase inhibitor to target Eps15 phosphorylation through EGFR may allow for a marked inhibition of arenavirus entry and productive infection into host cells. Additionally, clathrin-mediated endocytosis is mediated by the cellular GTPase Dynamin 2 (Dyn2) [23]. Dyn2 is a protein that when phosphorylated interacts directly or indirectly with F-Actin and cortactin, and functions to ‘pinch’ clathrin-coated pits from the membrane during the endocytic process (Figure 1)[23]. Many of these protein–protein interactions are mediated by phosphorylation events and are necessary for clathrin-mediated endocytosis. Endocytosis of PICV and JUNV is mediated by clathrin [15,16]; therefore, inhibiting the phosphorylation of Dyn2 will likely result in the inhibition of PICV and JUNV entry into host cells. The initiation of entry through clathrin-mediated endocytosis can be potentially exploited by specific kinase inhibitors to inhibit arenaviral endocytosis through clathrin-coated pits.
Figure 1. Arenavirus entry/replication cycle.
This figure represents the arenavirus entry/replication cycle and illustrates possible involvements of proteins and phosphorylation events. EGFR may be activated through receptor binding or by the endosomal entry process (the hypothesized activation is represented by dashed lines). Activation of EGFR likely leads to the phosphorylation of Eps15, which is required for arenavirus endosomal entry, as well as downstream phosphorylation events of ATF2. The phosphorylation of Dyn2 is also likely required for clathrin-mediated endosomal entry of arenaviruses. Additionally, the phosphorylation of ATF2 and CREB may be caused by viral replication, which eventually leads to cell stress. The dotted lines represent hypothesized activation or the involvement of proteins, while the solid lines represent the involvement of proteins or the trafficking of arenaviruses through the replication cycle.
EGFR: EGF receptor; ER: Endoplasmic reticulum; L: Large protein; S: Small protein; Z; RING finger Z protein.
Inhibiting arenavirus entry is not the only means by which kinase inhibitors can be used to inhibit productive infection. In fact, a true therapeutic would have to be administered to an individual postinfection, so for kinase inhibitors to effectively work it would be advantageous to inhibit viral replication and the host cell response, as well as viral entry. When considering host kinases and signaling pathways as drug targets, it is important to understand the role of each signaling event in the context of the disease caused by the virus. It is difficult to know which signaling events lead to pathogenesis when comparing an infected cell to an uninfected cell. For example, knowing which signaling events result due to the host attempting to mount an effective response to infection, and knowing which pathways to inhibit, and at what time postinfection, is of paramount importance in successful modulation of the host response as a therapeutic strategy.
Our laboratory has compared the host responses induced by two PICV variants: one variant that causes a mild, self-limiting infection (PICV P2) and one that causes a lethal disease in guinea pigs (PICV P18) [24,9,25]. The upregulated signaling pathways common to severe infection induced by PICV P18 can be targeted for further investigation. Peritoneal cells from mock, mild and virulent virus-infected animals at early and intermediate times postinfection were harvested and cell extracts were prepared. These extracts were used to phosphorylate more than 1100 peptides from known phosphoproteins in an array-based format. The data were then applied in the context of functional signaling networks using pathway analysis [9]. These networks can then be interrogated as targets for existing pharmacological inhibitors. A complication with this type of therapeutic strategy is that these types of drugs may not directly inhibit viral replication, and may act by changing the development of the immune responses. This complicates the testing of these compounds in vitro as virus titer may not be reduced. Experiments must be designed to test these effects, such as investigating cytokine production or cell-surface marker expression, rather than solely focusing on virus titer.
If phosphorylation profiling and pathway analysis reveal candidate pathways, the downstream effector molecules must also be considered. For example, if the signaling pathway regulates a family of transcription factors, it is important to understand which family members are being activated. Are the activated transcription factors transcriptional activators or repressors? As an example, it has been demonstrated that AP-1 family members and upstream activating pathways are implicated in the host response to arenavirus infection [24,9]. However, the AP-1 family of transcription factors contains members that are activators and repressors of transcription. It was then demonstrated that modulation of this pathway, using ‘thioaptamers’ to target AP-1, partially protected guinea pigs from lethal infection [25]. This shows that host-response modulation is an effective target for antiviral therapy and that proteomic and kinomic techniques can be used to identify targets. However, the less than 100% survival rate in treated animals illustrates the point that cross-talk and redundancy in cell signaling networks means that several proteins may have to be targeted simultaneously to achieve greater recovery rates. However, targeting of multiple proteins, perhaps at a lower level of inhibition, may be one means of reducing cytotoxicity.
Kinase inhibitors as potential antivirals
Since arenaviral entry, replication and productive infection appear to be dependent on different cellular kinases, kinase inhibitors have been explored to determine whether arenaviral infection can be inhibited in host cells [8]. Genistein is a general tyrosine kinase inhibitor that has been shown to inhibit entry and productive infection of various viruses [8,26–28]. Additionally, inhibition of arenaviral entry and replication has been observed in host cells pretreated with genistein [8]. Moreover, the phosphorylation of ATF2 and CREB, induced by PICV infection, is inhibited in cells treated with genistein. The results from this study suggest that genistein treatment postinfection also affects viral replication. In all, this data suggests that a general kinase inhibitor can be utilized to inhibit arenaviral infection. One caveat with utilizing genistein is that the drug is a general kinase inhibitor and affects multiple kinases. Therefore, identifying and utilizing specific kinase inhibitors may be advantageous. However, genistein has already been used in a guinea pig-asthma model [29], so future research should be conducted to demonstrate whether genistein offers protection in guinea pigs infected with various arenaviruses.
New and Old World arenaviruses may utilize different mechanisms to gain entry into host cells. However, it is likely that endocytosis and replication of all arenaviruses is dependent on cellular kinases. Therefore, it is possible to utilize either general or specific kinase inhibitors to inhibit infection of both New and Old World arenaviruses. More research needs to be performed to identify the entry mechanism of the Old World arenaviruses and to determine whether the pathogenic New World arenaviruses also utilize clathrin-mediated endocytosis. It should be noted that TfR1 utilized by the New World pathogenic arenaviruses is endocytosed through clathrin-mediated endocytosis, so it is likely that these viruses also endocytose through clathrin-coated pits. Therefore, kinase inhibitors are likely to inhibit viral infection.
Plasma leakage and abnormalities of coagulation and platelet function are symptoms of hemorrhagic fever. However, the mechanistic initiations of these symptoms in arenavirus-induced hemorrhagic fever remain to be delineated. VEGF-A is a potent permeability enhancing cytokine that has been found to elevate during dengue hemorrhagic fever [30]. The binding of VEGF receptors (VEGFRs), such as VEGFR-1 and -2, which are transmembrane receptor tyrosine kinases found on endothelial cells and progenitor cells [31], to VEGF results in receptor phosphorylation, which initiates changes in endothelial cell morphology resulting in increased permeability and proliferation [32]. Arenavirus infection in humans may result in elevated levels of VEGF, which may induce vascular permeability after binding to VEGFR-2, through tyrosine kinase phosphorylation of the transmembrane domain of the receptor. In this situation, kinase inhibitors may inhibit tyrosine phosphorylation initiated by VEGF binding to VEGFR-2 resulting in an inhibition of permeability. This may aid in preventing plasma leakage that is induced by extreme cases of arenavirus-induced hemorrhagic fever. Therefore, kinase inhibitors not only have the ability to inhibit arenavirus entry and replication, but also have the potential to ameliorate arenavirus- induced hemorrhagic fever signs and symptoms such as vascular leakage.
Future perspective
Many kinase inhibitors have been used in clinical trials in various fields of medicine. For example, cancer researchers have identified different kinase inhibitors to inhibit kinases activated in cancerous cells, which can result in constitutive growth factor signaling. Specifically, the tyrosine kinase inhibitor imatinib is used in treating chronic myelogenous leukemia (CML) and other malignancies [33], while dasatinib is a drug that inhibits Src family tyrosine kinases and is approved for use in patients with CML [34]. ABT-869 is another multitarget kinase inhibitor that has shown promising results by inhibiting tumor growth by decreasing phosphorylated forms of VEGF [35]. Additionally, erlotinib and gefitinib are drugs that specifically inhibit the EGFR tyrosine kinase domain and have been utilized in various phases of lung cancer trials [36,37].
MAPK p38 is a serine/threonine kinase that is involved in the biosynthesis of TNF-α and IL-1β and impacts signaling processes important for inflammation. SCIO-469 is a chemical compound that specifically inhibits p38 and has progressed to various Phase II cancer clinical trials, and represents another drug that specifically inhibits kinase activity [38]. Thus, other fields of medicine, specifically the cancer biology field, have demonstrated that specific kinase inhibitors can be used in patients without causing detrimental affects to natural cellular functions. Therefore, utilizing kinase inhibitors as an antiviral to treat arenavirus-induced hemorrhagic fever in humans appears to be a realistic treatment option. Additionally, identification of a kinase inhibitor that inhibits arenavirus entry and/or replication may prove to be a broad-spectrum drug that affects entry and replication of a myriad of viruses since it is likely that other viruses also require cellular kinase activity during the entry/replication cycle. However, it should be mentioned that some studies have indicated that drug-induced kinase inhibition can be associated with cytotoxicity and a loss of cell viability [39,40]. It appears that the measured cytotoxicity is dependent on the drug concentration and the specific kinases that the drug targets. Additionally, a recent study demonstrated that the kinase inhibitor gefitinib led to a reduction in cytomegalovirus viral load in the blood and lung without inhibiting viral load in the liver, spleen and kidneys of infected guinea pigs [41]. These data suggest that some kinase inhibitor drugs can potentially lead to cytotoxicity, which could ultimately endanger the host, and may function differently depending on the type of tissue. Furthermore, it is important to distinguish between cellular kinases required for viral entry and replication and cellular kinases that are only involved in cellular activities. Therefore, utilizing kinase inhibitors with specific activity would be advantageous.
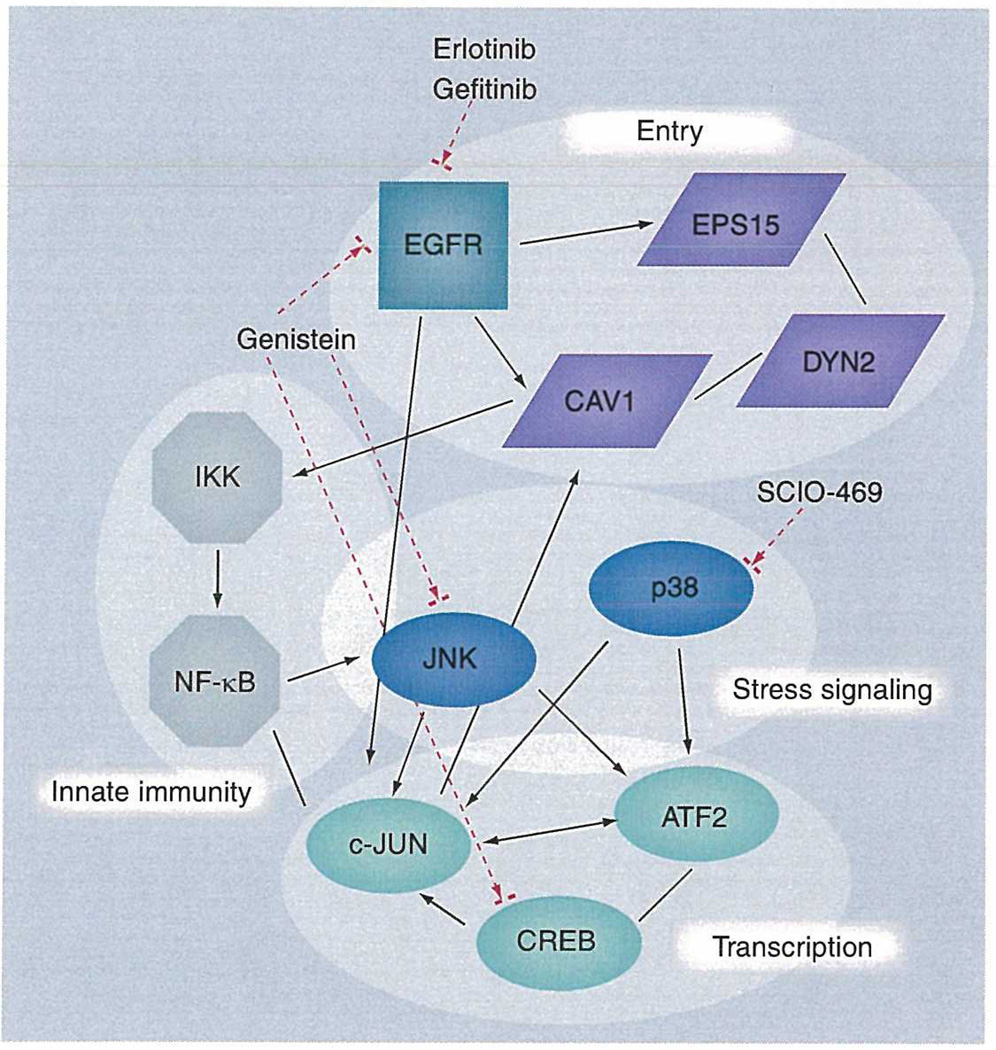
PICV infection in guinea pigs has been shown to induce involvement of EGFRs and serum response factor, which lies downstream of the Ras/Raf/meiosis specific serine/threonine-protein kinase (MEK) pathway. Targeting EGFRs with kinase inhibitors may represent another means by which to inhibit arenaviral infection (Figure 2). EGFRs may be involved in both virus entry and replication through phosphorylation events important to arenavirus entry by either direct or indirect viral triggers (Figure 1). The receptors required for entry of some arenaviruses have been characterized; however, it has not been determined whether coreceptors are required for viral infection. It is possible for arenaviruses to activate EGFRs by directly interacting or binding with the protein during viral entry, which has been reported for human cytomegalovirus (HCMV) [42]. This interaction may result in the activation of EGFRs and may trigger downstream phosphorylation events. Alternatively, the arenavirus endosomal entry process may indirectly trigger EGFR activation owing to initiating internal cellular signals that occur during viral binding and early entry events (Figure 1). These signals may activate EGFRs to trigger downstream phosphorylation events required for viral entry. Moreover, viral replication in host cells may also activate EGFRs. In any case, the EGFR appears to be critical to arenavirus infection and may be a good candidate for a kinase inhibitor since EGFRs may be involved in different phases of the arenavirus infection process.
Figure 2. Cellular proteins implicated in arenavirus infection.
The figure shows the interactions between proteins that are implicated in various stages of arenavirus infection. Black arrows and lines indicate known interactions mined from the literature. The dashed arrows indicate points of inhibition using available drugs to act on kinases and phosphorylation events which control activity. Due to the extensive interaction and crosstalk in eukaryotic signaling pathways, targeting individual proteins may result in a number of further downstream effects. Some of these may be of benefit to the host (either by directly inhibiting virus replication or by alleviating inappropriate host responses), whereas some may be detrimental. A further understanding of host signaling at a systems level, and response to perturbation with chemical drugs will be of great value in understanding the mechanism of kinase inhibitors as antiviral drugs. Additionally, these types of studies may identify further proteins of central interaction, so-called ‘hubs’, which may be of key importance in targeting specific cell signaling networks. As the number of kinase inhibitors in clinical use grows, a thorough knowledge of the role of cell signaling pathways in viral infection will afford the potential to rapidly identify novel uses of these drugs as antivirals.
EGER: EGF receptor; NF: Nuclear factor.
One particular method to identify the role of kinases in viral infection is to assay their ability to phosphorylate peptides in vitro. These targets may be consensus sequences, or sequences taken from known phosphoproteins. Cell extracts prepared from infected cells can be assayed and the active kinase complement of the cells may be used to phosphorylate the peptide with radiolabeled ATP. The activity of the upstream kinases can then be elucidated. Use of known phosphoprotein sequences may allow for more subtle effects of signaling activation to be elucidated. However, the numbers of peptides that need to be investigated, particularly as many proteins contain multiple phosphorylation sites, makes these studies more technically challenging than those involving consensus sequence-based peptides.
As it becomes clear that targeting host cell proteins is likely to prove to be an effective strategy for the treatment of certain viral infections, we are now in a position to effectively use a wide range of techniques and reagents developed for the study of host signaling. While investigation of specific pathways may identify potential targets for which existing drugs can be repositioned, high-throughput methods are ideally suited to these studies as a large number of proteins can be investigated providing candidates for further research. While high-throughput methods to investigate proteins have lagged behind genomic array technologies, techniques are available for the high-throughput investigation of the kinome and phosphoproteome. 2D-polyacrylamide gel electrophoresis can be used with phospho-specific stains, controlled against total protein staining, to identify differential phosphorylation events, which can be identified by mass spectrometry. Protein and peptide ‘chips’ can be incubated with extracts from infected and uninfected cells and the ability of the active kinases to incorporate radiolabeled ATP onto specific proteins can be measured. Additionally, liquid chromatography tandem-mass spectrometry can also be used, following enrichment for phosphoproteins using techniques such as immunoprecipitation or immobilized metal ion affinity chromatography. Moreover, microarray approaches may also be useful in identifying differentially phosphorylated proteins. From a set of gene expression data, the responsible transcription factors and upstream signaling events can be inferred, particularly using pathway analysis approaches. Data regarding the role of phosphorylation sites and kinases on proteins in these pathways can then be searched for targets of existing compounds. Modem proteomic, functional genomic and bioinformatics approaches are likely to play key roles in the identification of novel targets for antiviral therapeutics.
Although more research needs to be performed on kinase inhibitors, such as genistein, these drugs show therapeutic potential by inhibiting phosphorylation signals that are necessary for arenavirus entry into host cells and the arenavirus replication cycle (Figure 2) and ameliorate symptoms induced by arenavirus infection, such as vascular leakage. In conclusion, arenavirus infection of host cells induces phosphorylation events that are triggered by specific kinases and appear to be essential for productive infection. These phosphorylation events can potentially be targeted by kinase inhibitors to inhibit arenaviral infection and ameliorate an individual who is suffering from arenavirus-induced hemorrhagic fever.
Executive summary.
Kinases are involved in arenavirus trafficking within the host cell
Arenaviruses are rodent borne, bipartite negative-stranded RNA viruses that are characterized into Old World (Lymphocytic choriomeningitis complex) or New World (Tacaribe complex) viruses.
The arenaviruses Machupo virus, Guanarito virus, Junin virus, Sabia virus and Lassa virus are capable of causing hemorrhagic fever and mortality in humans, and thus are a potential bioterrorism threat.
Arenavirus entry and productive infection in host cells triggers, and appears to be dependent on, a myriad of phosphorylated cellular proteins, such as Eps15 and Dyn2.
Kinase inhibitors as potential antivirals
Genistein, a tyrosine kinase inhibitor, has been used to inhibit arenaviral infection.
Inhibited levels of CREB and ATF2 phosphorylation occur in Pichindé virus-infected cells pretreated with genistein when compared with mock-treated infected cells.
Future perspective
Several clinical trials have been performed with general and specific kinase inhibitors in various fields of medicine.
Kinase inhibitors can realistically be employed as antivirals to ameliorate an arenavirus-infected individual.
Utilization of high-throughput methods can be exploited to identify potential kinase targets necessary for arenavirus infection.
Acknowledgments
This work was made possible through the NIAID Training in Emerging and Reemerging Infectious Diseases T32 AI07536 and NIH U01-AI054827 grants.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Eric M Vela, Battelle Biomedical Research Center (BBRC), 505 King Avenue, JM8–1-096, Columbus, OH 43201-2693, USA Tel.: +1 614 424 7998; Fax: +1 614 458 7998; velae@battelle.org.
Gavin C Bowick, Department of Pathology, Center for Biodefense & Emerging Infectious Diseases, Institute for Human Infections & Immunity, The University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0609, USA Tel.: +1 409 772 4769; Fax: +1 409 747 2437; gabowick@utmb.edu.
Norbert K Herzog, Department of Pathology, Center for Biodefense & Emerging Infectious Diseases, Institute for Human Infections & Immunity, The University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0609, USA Tel.: +1 409 772 3938; Fax: +1 409 747 2437; nherzog@utmb.edu.
Judith F Aronson, Department of Pathology, Center for Biodefense & Emerging Infectious Diseases, Institute for Human Infections & Immunity, The University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0609, USA Tel.: +1 409 772 6547; Fax: +1 409 772 9350; jaronson@utmb.edu.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Charrel R, Feldmann H, Fulhorst C, et al. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem. Biophys. Res. Commun. 2002;296:1118–1124. doi: 10.1016/s0006-291x(02)02053-3. [DOI] [PubMed] [Google Scholar]
- 2.Bolken T, Laquerre S, Zhang Y, et al. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antiviral Res. 2006;69:86–97. doi: 10.1016/j.antiviral.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrion R, Patterson J, Johnson C, et al. A ML29 reassortant virus protects guinea pigs against a distantly related Nigerian strain of Lassa virus and can provide sterilizing immunity. Vaccine. 2007;25:4093–4102. doi: 10.1016/j.vaccine.2007.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castilla V, Larzabal M, Sgalippa N, Wachsman M, Coto C. Antiviral mode of action of a synthetic brassinosteroid against Junin virus replication. Antiviral Res. 2005;68:88–95. doi: 10.1016/j.antiviral.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Enria D, Briggiler A, Sanchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78(1):132–139. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gowen B, Barnard D, Smee D, et al. Interferon Alfacon-1 protects hamsters from lethal Pichinde virus infection. Antimicrob. Agents Chemother. 2005;49:2378–2386. doi: 10.1128/AAC.49.6.2378-2386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowen B, Wong M, Jung K, et al. In vitro and vivo activities of T-705 against arenavirus and Bunyavirus infections. Antimicrob. Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vela E, Bowick C, Herzog N, Aronson J. Genistein treatment of cells inhibits arenavirus infection. Antiviral Res. 2008;77:153–156. doi: 10.1016/j.antiviral.2007.09.005. •• Demonstrates that arenavirus infection is inhibited in cells treated with kinase inhibitors.
- 9. Bowick G, Fennewald S, Scott E, et al. Identification of differentially activated cell-signalling networks associated with Pichinde virus pathogenesis by using systems kinomics. J. Virology. 2007;81:1923–1933. doi: 10.1128/JVI.02199-06. •• Identifies the involvement and activation of cellular kinases in response to Pichindé virus Infection.
- 10.Kuijl C, Savage NDL, Marsman M, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–730. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- 11.Ceo W, Henry M, Borrow P, et al. Identification of alpha-dystoglygan as a receptor for Lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 12.Borrow P, Oldstone M. Mechanism of Lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 13.Glushakova S, Lukashevich I. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch. Virol. 1989;104:157–161. doi: 10.1007/BF01313817. [DOI] [PubMed] [Google Scholar]
- 14.Radoshitzky S, Abraham J, Spiropoulou C, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446:92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez M, Cordo S, Candurra N. Characterization of Junin arenavirus cell entry. J. Gen. Virol. 2007;88:1776–1784. doi: 10.1099/vir.0.82808-0. • Characterized the endosomal entry pathway utilized by the arenavirus Junín virus.
- 16. Vela F, Zhang L, Colpitts T, Davey R, Aronson J. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology. 2007;369:1–11. doi: 10.1016/j.virol.2007.07.014. • Suggests that arenaviruses enter cells through a clathrin-medlated endocytic mechanism and has led to other studies that utilize kinase inhibitors to inhibit clathrin-mediated entry.
- 17.Castilla V, Merisch S. Low pH-induced fusion of Vero cells infected with Junin virus. Arch. Virol. 1996;141:1307–1317. doi: 10.1007/BF01718832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Simone C, Zandonatti M, Buchmeier M. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology. 1994;198:455–465. doi: 10.1006/viro.1994.1057. [DOI] [PubMed] [Google Scholar]
- 19.Di Simone C, Buchmeier M. Kinetics and pH dependence of acid-induced structural changes in the Lymphoytic choriomeningitis virus glycoprotein complex. Virology. 1995;209:3–9. doi: 10.1006/viro.1995.1225. [DOI] [PubMed] [Google Scholar]
- 20.Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- 21.Conner S, Schmid S. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 22.Torrisi M, Lotti L, Beileudi F, et al. Eps 15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell. 1997;10:417–434. doi: 10.1091/mbc.10.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNiven M, Ceo H, Pitts K, Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 24.Bowick G, Fennewald S, Elsom B, et al. Differential signaling networks induced by mild and lethal hemorrhagic fever virus infections. J Virology. 2006;80:10248–10252. doi: 10.1128/JVI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fennewald S, Scott E, Zhang L, et al. Thioaptamer decoy targeting of AP-1 proteins influences cytokine expression and the outcome of arenavirus infections. J Gen. Virol. 2007;88:981–990. doi: 10.1099/vir.0.82499-0. [DOI] [PubMed] [Google Scholar]
- 26. Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. • Characterized genistein as a specific tyrosine kinase Inhibitor.
- 27.Damm E, Pelkmsns L, Kartenbeck J, et al. Clathrin- and caveolin-l -independent endocytosis: entry of simain virus 40 into cells devoid of caveolae. J. Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV4O-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 29. Duan W, Kuo I, Selvarajan S, et al. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am. J. Respir. Crit. Care Med. 2003;167:185–192. doi: 10.1164/rccm.200205-420OC. • Utilized the kinase inhibitor genistein to inhibit kinase activity in the guinea pig animal model.
- 30.Tseng C, Lo H, Teng H, Lo W, Ker C. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Quinn T, Peters K, De Vries C, Ferrara N, Williams L. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Fit-1 (VEGFR-l) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 33.Deininger M, Druker B. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev. 2003;55:401–423. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- 34.Das J, Chen P, Norris D, et al. 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N- (2-chloro-6-methylphenyl)-2- [[6-] 4-(hydroxyethyl) -1 -piperazinyl)]-2-methyl-4-pyrimidinyl] amino)]-1,3-thiazole-5-carboxamide (dasatnib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med. Chem. 2006;49:6819–6832. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Khng J, Jasinghe V, et al. In vivo activity of ABT-869, a multi-target kinase inhibitor, against acute myeloid leukemia with wild-type FLT3 receptor. Leukemia Res. 2007 doi: 10.1016/j.leukres.2007.11.025. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 36.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Soler R, Chachoua A, Hammond L, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. Am. J. Clin. Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 38.Lee M, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr. Med. Chem. 2005;12:2979–2994. doi: 10.2174/092986705774462914. [DOI] [PubMed] [Google Scholar]
- 39.Castoldi R, Pennella G, Saturno C, et al. Assessing and managing toxicities induced by kinase inhibitors. Curr. Opin. Drug Discov. Devel. 2007;10:53–57. [PubMed] [Google Scholar]
- 40.Schang L, St.Vincent M, Lacasse J. Five years of progress on cyclin-dependent kinases and other cellular proteins as potential targets for antiviral drugs. Antivir. Chem. Chemother. 2006;17:293–320. doi: 10.1177/095632020601700601. [DOI] [PubMed] [Google Scholar]
- 41.Schleiss M, Eickhoff J, Auerochs S, et al. Protein kinase inhibitors of the quinazoline class exert anti-cytomegaloviral activity in vitroand in vivo. Antiviral Res. 2008 doi: 10.1016/j.antiviral.2008.01.154. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Huong S, Chlu M, Raab-Traub N, Huang E. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]