Abstract
The external surfaces of the body, such as the skin and the gastrointestinal mucosal membrane, are an important line of defence preventing the invasion of microorganisms and their products. Mucosal immune cells, especially intraepithelial lymphocytes, are involved in maintaining the integrity of these epithelial barriers. They contribute towards the tolerance to commensal organisms, which occupy these same sites, and to the immune responses against harmful organisms and their products. The composition of the microbiota is influenced by immune cells as well as external environmental factors, especially the use of antibiotics and diet. There is an increasing appreciation that the microbiota affects systemic immune responses in addition to local immunity. Failure to control the occupancy by microorganisms may result in the disruption of the delicate homeostasis between beneficial and harmful microorganisms and contribute to inflammatory pathologies. This review will discuss some of our current understanding of the impact of immune cells and diet on the microbiota.
Keywords: diet, intraepithelial lymphocytes, microbiota, mucosal immunity, oral immunology, signalling
Introduction
The main interfaces between the host and its external environment are the skin, and the gastrointestinal and respiratory tracts. These barriers are under constant threat of disease from invading microorganisms and their products. Not surprisingly, these sites are under intense scrutiny of the immune system and the upkeep of this barrier is paramount. Epithelial surfaces are more than simple physical obstructions; they constitute complex chemical and biological obstacles. The acidity of the stomach and the mucus lining, as well as microbicidal enzymes in the gastrointestinal tract are often sufficient to prevent microbial invasion. But, epithelial surfaces are not only under threat of potential harmful pathogens, they also harbour many beneficial microorganisms. Many of the microbes that collectively make up the microbiota are highly beneficial for our health and well-being. Colonization commences immediately upon birth by the largest population of symbiotic bacteria. This community is comprised of approximately 1 × 1014 bacteria (archeal, fungal and viral species are also present), with a least 2000 individual species.1,2 Nevertheless, predominantly four bacterial phyla have adapted to the intestinal niche: the Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria.3–5
The host and its symbionts seem to have co-evolved towards a mutually beneficial state of co-existence.6 Their cross-talk facilitates the digestive and anabolic functions of the host and protects the epithelial barriers from colonization by pathogens.7 The microbes compete for nutrients and attachment sites to epithelial cells (ECs). Some can even engage in chemical warfare and produce (bacteriocins) or induce antimicrobial substances, directly killing competitors. Alterations in the composition of the microbiota, dysbiosis, may contribute to the development of various inflammatory disorders. Tight management of the immune response is therefore critical for host function and survival, maintaining an optimal balance between tolerance to harmless compounds as well as the commensal flora while efficiently clearing pathogens and their toxins.8
Structural organization of mucosal surfaces
The mucosal immune system can be divided into inductive and effector sites. Inductive sites are constituted by organized mucosa-associated lymphoid tissue as well as mucosa-draining lymph nodes whereas effector sites are represented by the lamina propria, the stroma of exocrine glands and surface epithelia.9,10 Limiting the contact between the luminal microbial community and the intestinal surface can be considered the first line of defence (Fig. 1). Glycoproteins, forming a mucosal layer, gradients of immunoglobulins (IgA) and antimicrobial peptides prevent the penetration of most bacteria. Although the microbicidal action of antimicrobial peptides is often considered mild, concentrations in the crypts can reach levels sufficient for strong bacterial lysis.11
Figure 1.
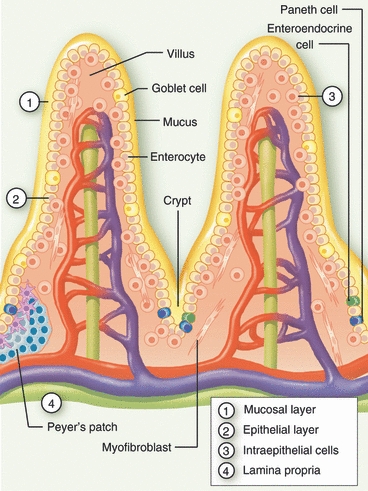
Structural organization of intestinal mucosa. Schematic representation of the small intestinal mucosal barrier, consisting of a mucosal layer (1) and gradients of IgA and antimicrobial factors, the epithelial cells (2) made up of enterocytes, Paneth cells, goblet cells and enteroendocrine cells, intraepithelial lymphocytes (3), and the lamina propria (4). Additional secondary lymphoid structures, such as cryptopatches and Peyers’ patches are present in the lamina propria.
The second line of defence is formed by the monolayer of the ECs themselves (Fig. 1), interconnected through tight junctions by which barrier permeability can be regulated. This monolayer is composed of four lineages that arise from a single epithelial stem cell; absorptive enterocytes, mucus-producing goblet cells, hormone-producing enteroendocrine cells and the microbicidal factors-producing Paneth cells. It was originally thought that these epithelial cells only function to keep luminal microbes from invading the sterile tissues. However, there exists a much more intricate and mutual beneficial relationship between ECs and the microbiota. The intestinal mucosa encourages microbial colonization by providing an ideal environment, and more directly by shifting its energy source to favour some microbes over others. For example, fucosylation of glycans allows some bacterial species, which use this as their energy source, to outcompete others.12 Once these species have gained a competitive advantage and established themselves, they take control of their environment. This involves the recruitment of ‘accessory’ species that offer benefits to the initial colonists,13 and the induction of morphological changes to the hosts’ intestinal barrier, e.g. the induction of bactericidal factors that keep competitors at bay.14 The microbiota in return enhances host nutrient metabolism, especially via the breakdown of complex carbohydrates into monosaccharides and short-chain fatty acids, which regulate growth and differentiation of the hosts’ ECs.
The lamina propria could be considered the fourth and final barrier before systemic immunity is required.15 It lies beneath the intestinal epithelium and is comprised of a supportive layer of conjunctive tissue (Fig. 1). This contains distinct lymphoid structures that can detect and restrain microbes through the presence of dendritic cells, macrophages and scattered lymphoid cells further supported by stromal cells. Most of the lymphoid tissue is organized in several different structures, such as isolated cryptopatches, aggregates termed isolated lymphoid follicles, or in larger clusters more resembling lymph nodes, the Peyer's patches. CD4+ lymphoid tissue inducer (LTi) cells and stromal organizer cells are crucial for the development of lymphoid structures.16,17 The LTi cells are a subtype of innate lymphoid cells. They derive from haematopoietic progenitor cells in the fetal liver, seed the developing lymphoid tissues during fetal development and initiate the formation of lymphoid organs. Later in life, a population of cells similar to embryonic LTi cells, LTi-like cells, supports the formation of organized lymphoid structures in the intestine.16 Whereas differentiation of Peyer's patches is induced before birth by LTi cells,17 isolated lymphoid follicles develop after birth in response to the microbiota or inflammation.18–20 Notably, the development of cryptopatches and intestinal lymph nodes is independent of the stimulation by microorganisms.21–23
The intraepithelial lymphoid cells
The epithelial layers of the mammalian surface lining contain a specialized lymphoid population, the intraepithelial lymphocytes (IELs), which are directly involved in host defence as well as barrier maintenance; constituting the third line of defence (Fig. 1). The IELs populate all the body's surfaces. They represent one of the largest lymphocyte populations found in mammals, yet are the least well understood, and are a heterogeneous population mainly composed of unconventional T cells. The IELs may share many properties with conventional T cells, which express a T-cell receptor (TCR) consisting of the TCR-α and TCR-β chains, but there are crucial differences. In contrast to conventional T cells, IELs express antigen receptors with a limited diversity,24 and are kept in a heightened state of activation, avoiding the need for a priming-step before full activation and unnecessary delay.25 In addition, they can respond without the need for clonal expansion of a rare precursor cell that expresses a particular TCR that recognizes an antigen of interest. Upon activation, IELs immediately release cytokines that contribute to the activation and the recruitment of innate immune cells, and which may contribute to the orchestration of a subsequent adaptive immune response.
Lymphocytes expressing an alternative TCR, comprised of the TCR-γ and TCR-δ chains, are a relatively minor population in vertebrates, but are highly enriched at all epithelial sites. In the epidermis TCR-γδ T cells are the sole population of lymphocytes whereas in the respiratory and intestinal tracts they cohabit with TCR-αβ T cells. The small intestinal IELs, in addition to TCR-γδ T cells, comprise TCR-αβ T cells of an unconventional nature, expressing a CD8αα homodimeric receptor or neither CD4 nor CD8.26 Intriguingly, the tissue distribution of TCR-γδ T cells in the mouse is according to the expression of their TCR γ-chain. In the gut, the clonal repertoire of murine TCR-γδ T cells is restricted to the TCR-Vγ5 coupled with Vδ4 or Vδ6.27 The epidermal TCR-γδ population is solely restricted to TCR-Vγ3 coupled with TCR-Vδ1 and shows very limited, if any, variability. The equivalent to TCR-Vγ3 IELs is not present in humans because of the absence of the thymic stromal immunoglobulin-like determinant skint1.28 TCR-Vγ4 have a seemingly wider distribution, occupying the epithelial layers of the lungs, the female reproductive organs and the tongue.
Discriminating good from bad?
The interaction between the ECs and the microbes generally does not result in the initiation of inflammatory responses, instead such interactions, of an as yet largely unidentified nature, are highly beneficial. Microbial colonization is paramount for the development of the intestinal microvasculature and induction of the expression of a myriad of genes involved in metabolism, immune response, and intestinal barrier integrity.29,30 The rejuvination of the total EC lining, achieved within a week, depends on the proliferation of intestinal stem cells,31 and is half the normal rate in the absence of microbes.32 The result of decreased proliferation is increased susceptibility to epithelial cell damage. However, our limited understanding of how the microbiota influences our epithelial barriers is highlighted by the observations that susceptibility to damage is negatively influenced by both the absence as well as the presence of microbial components.15,33,34 This relates to the requirement of microorganisms for (structural) development and priming of the immune system, the absence of which may result in an inability to mount a response, whereas the particular presence of other microorganisms will negatively contribute to immunopathology 35 (Fig. 2).
Figure 2.
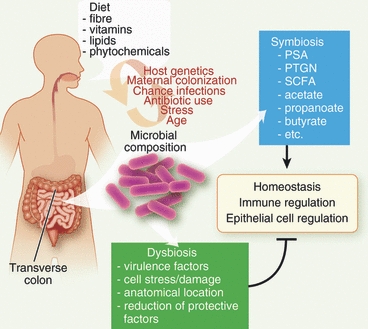
Influences affecting the intestinal microbial composition and barrier homeostasis. Besides host genetics, chance encounters, stress and aging effects, the diet has a major influence on the intestinal microbial composition. Especially the fibre content, and their metabolic products such as short chain fatty acids (SCFA), acetate, propanoate and butyrate have a major impact on the mutually beneficial relationship (symbiosis) between the host and the microbiota. In addition, microbial factors such as polysaccharide A (PSA) and peptidoglycans (PTGN) make a direct contribution this relationship. Alterations in the microbial make up, dysbiosis, may result in epithelial barrier damage and stress as the result of a reduction in protective microbial factors and an increase in pathogenic microorganisms.
The immune system is equipped with pattern recognition receptors (PRRs) that are able to recognize macromolecular ligands from non-mammalian origin. PRR ligands, microorganism-associated molecular patterns, encompass commensal- and symbiont-derived molecules in addition to those derived from pathogens. Recent years have increasingly focused on the role of PPRs as mediators in the inter-kingdom communication between microorganisms and the host. Most PRRs are present in the intestine, although the cellular origin is not well defined, and expression levels are modified by the luminal microbial content and by immune-mediated signals.36 Triggering of PRRs would normally result in the initiation of an inflammatory response, which upon encountering commensals would be highly disadvantageous for the host. Distinguishing luminal antigens from those that have invaded the sterile tissues may in part be accomplished by the polarized structure of the barrier sites or by alternative wiring of the signalling machinery and tuning by negative regulators in some cell types. The epithelium not only tolerates microorganisms, it actively requires their presence for optimal functioning and wound repair.37–41In vitro experiments and observations made in injury models suggest that PRRs may also be important in the induction of members of the family of epidermal growth factors such as epidermal growth factor, transforming growth factor-α, epiregulin and amphiregulin.32,42,43
It seems unlikely that ‘friendly’ bacteria posses special attributes uniquely responsible for immune suppression or induction of tolerance. Lateral transfer of genes between bacteria is common and although many bacterial species are beneficial to their host, they remain a risk and could at any time unilaterally abandon a mutual beneficial relationship. However, some of their products, such as polysaccharide A, do seem to enhance immune protection.44 Other microbial products, such as meso-diaminopimelic acid containing peptidoglycans, may actively, and selfishly, contribute to immune activation against competing pathogens45 (Fig. 2). Location and context may be the most important mechanism discriminating between pathobionts, which can breach the epithelial barrier, and symbionts and commensals, which generally do not cross this barrier. Tissue damage and stress responses may fine tune the initial innate immune response and determine if a more robust response against a harmful antigen, which is causing cell death, or a more tolerogenic response against benign microorganisms having taken an accidental wrong turn, is most appropriate. Indeed it was recently highlighted that inflammasome activation is involved in intestinal homeostasis, balancing the protection of the epithelial layer via induction of EC proliferation and thereby preventing bacterial translocation with immune activation and inflammation.46–49
Maintaining the barrier
In contrast to the skin, which forms a tight but not impregnable seal, the ECs of the intestine have a prominent role in the exchange of nutrients and fluids and form more leaky barriers. The bacterial load and metabolic processes inherently pose a risk for a single cell barrier, and the ECs are rapidly replaced.31 This process takes place at the bottom of the small intestine and colon crypts where intestinal stem cells proliferate.50 It has become clear that the microbiota can influence growth, survival, inflammatory control and permeability of the epithelial layer thereby shaping the local ecosystem.51,52 For example, some Bifidobacteria species harbour specific carbohydrate transporters allowing them to catabolize fructose instead of glucose, which is low in the distal colon, and to produce acetate as a consequence, which protects ECs.53,54 How acetate protects the ECs is not clear, but also ECs would suffer in the distal colon from reduced glucose levels. Interestingly, they once more turn to the microbiota for an alternative source of energy, using bacterially produced butyrate.55 As a result of the lack of microbial cross-talk, the EC proliferation rate is approximately halved in germ-free animals, and in contrast to conventionally raised mice, villus capillaries are poorly developed.32
In contrast to conventional T lymphocytes, IELs populate the epithelial barrier sites before birth.24 It is during and shortly after birth that mammals are exposed to microorganisms and acquire their microbiota. The luminal microorganisms thereafter influence the development and function of the IELs. Germ-free reared mice harbour reduced numbers of IELs, and TCR-γδ-bearing IELs show diminished cytolytic capacity in the absence of microbes.56,57 Studies from several laboratories indicate that IELs play a unique role in maintaining EC homeostasis and responses to tissue repair and malignancy. The intertwined relationship between IELs, the epithelial barrier and the microbiota is further illustrated by the ability of IELs, in addition to the microbiota, to support EC growth and turnover.58,59 This suggests that IELs, via the production of cytokines, chemokines and growth factors, are important in maintaining epithelial barriers and may indirectly influence the intestinal microbial communities (Fig. 3).
Figure 3.

Maintaining the ‘fence’. Balancing epithelial barrier health via cross-talk between epithelial cells and the luminal microorganisms, and the cells of the immune system, especially intraepithelial lymphocytes (IELs), themselves maintained via dietary derived aryl hydrocarbon receptor (AhR) ligands such as indole-3-carbinol (I3C). The IELs maintain the epithelial barrier via release of growth factors and support in the activation of antimicrobial peptides. Upon barrier breakthrough, IELs are directly involved in the cytolytic immune response, removing infected cells, and orchestrating subsequent adaptive immunity as well as the barrier repair response.
An important gap in our knowledge are the signals that govern IEL biology. One aspect is the nature of the molecules able to activate their TCR. Although TCR-γδ can interact with non-classical MHC molecules, this does not seem to depend on the presence of peptide,60 and may not be a prerequisite for TCR-γδ cell activation. IELs express gene products located in the ‘NK locus’. This includes activating receptors expressed by natural killer (NK) cells, such as NKG2D, which contains a C-type lectin-like domain capable of recognizing protein ligands.61 Human intestinal IELs can recognize the non-classical MHC molecules and NKG2D ligands MICA and MICB, and the distantly related human MHC class I molecules, the ULBPs. These former are expressed on ECs, endothelium and fibroblasts in conditions of damage or disease62 but the latter are ubiquitously expressed on endothelial and epithelial cells independently of activation status.63 In the mouse, NKG2D interacts with molecules similar to the ULBPs and distantly related to MHC class I, which are four members of the retinoic acid early inducible (Rea)-1 family,64 and the minor histocompatibility antigen, H-60.65 Similar to MICA and MICB, Rea-1 and H-60 are expressed in cells under duress.66 Engagement of NKG2D on IELs with ligands expressed on ECs results in cytolytic activity and killing of the target cell.62 The response to EC stress signals will enable IELs, despite limited TCR diversity, to respond to a wide range of inflammatory conditions rapidly supporting EC growth and differentiation to maintain the epithelial barrier (Fig. 3). However, the nature of the stress molecule(s) remains elusive.
Dietary polysaccharides
In addition to gender and age, the diet has a major influence on the composition of the gut microbiota.67–69 In return, alterations in the microbial composition and their metabolites can have sometimes unexpected effects on local and systemic immunity of the host, something which has only recently been recognized.45,70–73 As such, the diet may constitute a major contributor to the dramatic increase in inflammatory diseases such as asthma, multiple sclerosis, type 1 diabetes and Crohn's disease as recorded over the past 50 years.74,75 Such a connection was already made with swaying epidemiological data on the association between obesity and diabetes,76 obesity and asthma,77 diet and asthma,78 and diets low in fruit and vegetable content and inflammatory bowel disease (IBD).79–81 If diet is such a major contributor to intestinal barrier integrity and to the rapid rise in allergies and autoimmune disorders in the developed world, this predicts that the microbiota composition is substantially different in populations in Europe and rural Africa. Indeed, African children consume a diet with much higher fibre content, and their microbiota is enriched in specific symbiotic Bacteriodetes species and reduced in Firmicutes compared with European children.67
The intestinal microbiota is in large part derived from the mother during the birthing process but modified thereafter by diet and environmental chance encounters such as infections and use of antibiotics. Initial intake is exclusively milk, consisting of a diversity of polysaccharides modified in the mammary gland to contain lactose branches. Of particular importance are the combinations of lactose with sialic acid. As mentioned, fucosylation of intestinal epithelial glycans encourages bacterial colonization with particular species.12 Milk oligosaccharides, which cannot be absorbed or digested by the host and are similar in structure to EC glycans, act as neutralizing ligands preventing the attachment of some bacteria over the desired symbiotic Bifidobacteria species. Indeed, mice reared on milk missing sialylated oligosaccharides show an altered composition of their microbiota, which impacts on their capacity to maintain their epithelial barrier.82
A substantial microbial change occurs when solids are introduced, and polysaccharides, in the form of plant fibres, and their metabolites, short-chain fatty acids, acetate, butyrate and propanoate, positively influence the microbial make-up of the intestine (Fig. 2). Receptors for short-chain fatty acidss are G protein-coupled receptors, such as GPR41 and GPR43, the latter of which is expressed on cells of the innate immune system. Surprisingly, the absence of GPR43 results in an exacerbated and poorly resolving immune response,72 similar to those observed in germ-free mice, which have few short-chain fatty acids.35,72,83 Of note is the observation that levels of short-chain fatty acids are generally lower in IBD patients.81
Dietary vitamins and phytochemicals
The microbiota and diet constitute essential substrates to the biosynthesis and metabolism of important vitamins, especially cobalamin (vitamin B12), structurally complicated and currently still only produced through bacterial fermentation synthesis, and vitamin K, synthesized in leafy green vegetables as phylloquinone it requires intestinal bacteria for the conversion to several forms of vitamin K and their absorption. Two additional vitamins have recently been directly implicated in maintaining intestinal barrier integrity, vitamins D and A. The first, and its receptor (VDR), seem important for the development of one of the intestinal IEL populations, the TCR-αβ+ CD8αα+, without affecting the TCR-γδ IELs.84 The absence of VDR or vitamin D results in increased inflammation of the gastrointestinal tract, which may be a result of multifaceted requirements for this vitamin. The decrease in TCR-αβ CD8αα IELs may reduce barrier homeostasis and cytolytic activity, but TCR-γδ IELs would be able to compensate. However, vitamin D is also required for the production of the microbicidal cathelicidins,85 the absence of which could additionally contribute to an altered intestinal microbial load or composition.
Metabolically active derivatives of vitamin A include retinoic acid, which bound to its nuclear hormone receptors can induce transcription of genes. Initial reports suggested that retinoic acid is involved in maintaining a balanced intestinal TCR-αβ+ CD4+ T helper (Th) cell compartment, increasing anti-inflammatory regulatory T cells while inhibiting pro-inflammatory Th17 cells.86–90 Although, this suggested that vitamin A consumption is anti-inflammatory, two recent studies have challenged this.91,92 Retinoic acid seems important for general Th-mediated responses, in its absence mice fail to mount a robust Th1 and Th17 response.92 Location and context may also be key to the effect of vitamin A. Concentrations of vitamin A may be highest at their point of absorption, the intestinal mucosae, which may have different biological effects.93 More important, the presence of microorganism-associated molecular patterns may give context to a vitamin A signal.94 Interestingly, the presence of IL-15, an essential survival factor for IELs, and retinoic acid seem to create particularly favourable conditions for Th1 and Th17 development, potentially providing a mechanistic insight into the cause of coeliac disease.91
Foodstuffs that affect intestinal homeostasis have strong links with the dietary vegetable component, providing both vitamins and fibre. Our recent data highlights yet another vegetable-derived phytochemical that has a surprising and dramatic influence on barrier immunity. Indole-3-carbinol is produced by the breakdown of the glucosinolate glucobrassicin, generated during photosynthesis from tryptophan. It is present at high levels in basilica plants and forms part of the diet via the cruciferous vegetables such as cabbages and broccoli. Stomach acids condense indole-3-carbinol to diindolylmethane and indolocarbazole, two high-affinity ligands for the aryl hydrocarbon receptor (AhR). Interestingly, all IELs express relatively high levels of AhR and have a cell autonomous requirement for its activation, without which they fail to survive in their target tissues.71 Although Th17 and systemic TCR-γδ do express AhR,95,96 its absence only results in the selective disappearance of the intestinal TCR-γδ and TCR-αβ CD8αα IELs and TCR-Vγ3 epidermal IELs, and mice fed a diet low in vegetable content lose the majority of their intestinal IELs.71
Intraepithelial lymphocytes are reported to release anti-microbial peptides upon epithelial injury, interact with the cell types of the epithelial layer, and may thereby directly limit the translocation and dissemination of intestinal microorganisms upon barrier damage.97 Interestingly, AhR-deficient mice, or those on a diet low in vegetable material, show increased CD4-mediated interferon-γ production, EC hyperplasia, reduced epithelial turnover, apical cytoplasmic mucin distention, reduced expression of anti-microbial peptides, and an increased bacterial load with a enhanced contribution of species in the phylum Bacteroidetes.71 These observations are all hallmarks of IBD,98,99 and are in line with the premise that IBD develops in genetically susceptible hosts but that environmental factors precipitate the onset or reactivation and severity of the disease.
Conclusions and perspectives
The host controls EC responsiveness towards occupying bacteria via cross-talk with these same bacteria, thereby limiting inflammation at epithelial barrier sites. This is largely responsible for a state of immune tolerance enhancing and cultivating the mutually beneficial interactions between the host and its microbial occupants, unless barriers are broken and damage is inflicted. Deregulation of this cross-talk is associated with loss of EC homeostasis and disruption of the intricate network of interdependent microbial species occupying these surfaces. The outcome is activation of pro-inflammatory pathways, with the potential to propagate the imbalance, and if not resolved, ultimately resulting in chronic inflammation and immunopathology.
Epidemiological studies provided initial insights into deregulation at epithelial barriers and the increasing incidence of inflammatory diseases in the developed world with diet. Recent findings have confirmed the importance of the microbiota on tuning systemic immunity, and have added important new insights on a molecular level. Polysaccharides play a pivotal role, creating the initial niche for the first colonizers, preventing the attachment of undesired others, and subsequently cultivating a healthy and balanced microbiota which in turn maintains the epithelial barrier.12,82 At the basolateral side of the barrier other compounds, via the activation of the AhR, maintain the IEL populations.71 While patrolling the epithelial barriers, IELs respond to EC stress signals, orchestrate immunity as well as tolerance, thereby influencing the intestinal bacterial load and composition. Importantly, IELs are also directly involved in EC growth, homeostasis and wound repair. Carefully designed and controlled human trials are needed to substantiate results from mouse studies and observations in humans. However, rather than developing additional anti-inflammatory drugs, changing diets which are currently often stored and transported for a significant amount of time, highly processed and low in vegetable content, may be a more cost effective way towards health and well-being.
Acknowledgments
The authors wish to acknowledge funding from the BBSRC (M.V.) and the Wiener-Anspach Fondation (E.M.).
Disclosures
The authors have no conflicting financial interests.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature. 2007;449:811–8. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–51. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–90. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 8.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–7. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–7. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 11.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 12.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–62. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slack E, Hapfelmeier S, Stecher B, et al. Innate and adaptive immunity cooperate flexibly to maintain host–microbiota mutualism. Science. 2009;325:617–20. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 18.Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2:478–85. doi: 10.1038/mi.2009.114. [DOI] [PubMed] [Google Scholar]
- 19.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pabst O, Herbrand H, Worbs T, et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 21.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 22.Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing RORγt+ innate lymphoid cells. Immunology. 2011;132:453–65. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji M, Suzuki K, Kitamura H, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 25.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 26.Pennington DJ, Silva-Santos B, Shires J, et al. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat Immunol. 2003;4:991–8. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 27.Takagaki Y, DeCloux A, Bonneville M, Tonegawa S. Diversity of γδ T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989;339:712–4. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- 28.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–62. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–34. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–5. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–12. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 32.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki A, Kanai T, Ishikura T, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 34.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 36.Lundin A, Bok CM, Aronsson L, et al. Gut flora, Toll-like receptors and nuclear receptors: a tripartite communication that tunes innate immunity in large intestine. Cell Microbiol. 2008;10:1093–103. doi: 10.1111/j.1462-5822.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 37.Fukata M, Michelsen KS, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 38.Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–36. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 40.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–7. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–21. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraud AS. X. Trefoil peptide and EGF receptor/ligand transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2000;278:G501–6. doi: 10.1152/ajpgi.2000.278.4.G501. [DOI] [PubMed] [Google Scholar]
- 44.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupaul-Chicoine J, Yeretssian G, Doiron K, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–78. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–91. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–13. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host–microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 52.Wick MJ, Madara JL, Fields BN, Normark SJ. Molecular cross talk between epithelial cells and pathogenic microorganisms. Cell. 1991;67:651–9. doi: 10.1016/0092-8674(91)90061-3. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 54.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–52. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-γδ+ intraepithelial lymphocytes. Science. 1989;243:1716–8. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 57.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of αβ T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–7. [PMC free article] [PubMed] [Google Scholar]
- 58.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 59.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 1995;92:6147–51. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chien YH, Hampl J. Antigen-recognition properties of murine γδ T cells. Springer Semin Immunopathol. 2000;22:239–50. doi: 10.1007/pl00006752. [DOI] [PubMed] [Google Scholar]
- 61.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 62.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 63.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 64.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 65.Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998;161:3501–9. [PubMed] [Google Scholar]
- 66.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 67.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva E, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011 doi: 10.1016/j.cell.2011.09.025. DOI: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 72.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 75.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 76.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 77.Sin DD, Sutherland ER. Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63:1018–23. doi: 10.1136/thx.2007.086819. [DOI] [PubMed] [Google Scholar]
- 78.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 79.Amre DK, D'Souza S, Morgan K, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am J Gastroenterol. 2007;102:2016–25. doi: 10.1111/j.1572-0241.2007.01411.x. [DOI] [PubMed] [Google Scholar]
- 80.D'Souza S, Levy E, Mack D, et al. Dietary patterns and risk for Crohn's disease in children. Inflamm Bowel Dis. 2008;14:367–73. doi: 10.1002/ibd.20333. [DOI] [PubMed] [Google Scholar]
- 81.Galvez J, Rodriguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601–8. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 82.Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–54. doi: 10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr. 1986;116:1772–6. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 84.Bruce D, Cantorna MT. Intrinsic requirement for the vitamin D receptor in the development of CD8αα-expressing T cells. J Immunol. 2011;186:2819–25. doi: 10.4049/jimmunol.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 86.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 89.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 90.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DePaolo RW, Abadie V, Tang F, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–4. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall JA, Cannons JL, Grainger JR, et al. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor α. Immunity. 2011;34:435–47. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184:5519–26. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 95.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 96.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 97.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and γδ intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–54. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 99.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]


