Abstract
Cells of the female reproductive tract (FRT) can present antigen to naive and memory T cells. However, the effects of oestrogen, known to modulate immune responses, on antigen presentation in the FRT remain undefined. In the present study, DO11.10 T-cell antigen receptor transgenic mice specific for the class II MHC-restricted ovalbumin (OVA) 323–339 peptide were used to study the effects of oestradiol and pathogen-associated molecular patterns on antigen presentation in the FRT. We report here that oestradiol inhibited antigen presentation of OVA by uterine epithelial cells, uterine stromal cells and vaginal cells to OVA-specific memory T cells. When ovariectomized animals were treated with oestradiol for 1 or 3 days, antigen presentation was decreased by 20–80%. In contrast, incubation with PAMP increased antigen presentation by epithelial cells (Pam3Cys), stromal cells (peptidoglycan, Pam3Cys) and vaginal cells (Pam3Cys). In contrast, CpG inhibited both stromal and vaginal cell antigen presentation. Analysis of mRNA expression by reverse transcription PCR indicated that oestradiol inhibited CD40, CD80 and class II in the uterus and CD40, CD86 and class II in the vagina. Expression in isolated uterine and vaginal cells paralleled that seen in whole tissues. In contrast, oestradiol increased polymeric immunoglobulin receptor mRNA expression in the uterus and decreased it in the vagina. These results indicate that antigen-presenting cells in the uterus and vagina are responsive to oestradiol, which inhibits antigen presentation and co-stimulatory molecule expression. Further, these findings suggest that antigen-presenting cells in the uterus and vagina respond to selected Toll-like receptor agonists with altered antigen presentation.
Keywords: antigen presentation, epithelial cells, oestradiol, pathogen-associated molecular patterns, uterus
Introduction
It is well documented that epithelial cells lining the female reproductive tract (FRT) play an important role in the innate immune responses by providing a physical barrier, and producing antimicrobial peptides, cytokines and chemokines that recruit immune cells.1–3 Less well recognized are the findings that epithelial cells process and present antigen to naive and memory T cells.4–9 We and others have found that enterocytes in the gastrointestinal tract and epithelial cells in the human and rat uterus express HLA-DR (MHC class II) and co-stimulatory molecules that function in antigen-specific T-cell activation by epithelial cells.10–15 More recently, we found that mouse uterine epithelial cells as well as antigen-presenting cells (APC) in the uterine stroma and vagina are able to present ovalbumin (OVA) and OVA323–339 peptide to naive T and memory T cells from DO11.10 T-cell antigen receptor (TCR) transgenic mice.9
Unique to the mucosa of the FRT is the dynamic regulation of innate and adaptive immune processes by sex steroid hormones.16 With regards to antigen presentation, we have previously shown in the rat model that oestradiol exerts differential effects, i.e. enhancing antigen presentation in the uterus while on the other hand reducing antigen presentation in the vagina.17–19 In further studies, we showed that the actions of oestradiol on antigen presentation were in part the result of biologically active transforming growth factor-β produced by epithelial cells.20,21 These findings demonstrate the competence of epithelial cells of the uterus and vagina in antigen presentation, and further point to possible indirect effects of sex hormones on this process through soluble immune factors produced by epithelial cells. More recently, we have defined the spectrum of soluble factors including cytokines, chemokines and growth factors produced by epithelial cells of the FRT.22–29 How these specific factors either independently or in concert regulate antigen presentation by epithelial cells of the uterus and vagina remains to be determined.
Critical steps in the process of antigen presentation include; (i) primary signal delivered through the triad interaction between antigen-specific receptor and MHC class II–antigen complex on APC, and (ii) second signal delivered by the binding of co-stimulatory molecules expressed by APC with the cognate antigen-specific receptors (see review in ref. 30). A number of co-stimulatory molecules have been described, with the members of the CD28/B7 family being the best characterized.31 Additional co-stimulatory signal is through the interaction of CD40, a member of the tumour necrosis factor super family expressed on APC, and its ligand (CD40L or CD154) on T cells.32,33 The pattern of expression of co-stimulatory molecules by APC is, therefore, an essential determinant of antigen presentation and hence the generation of adaptive immune responses. For example, immature dendritic cells with lower surface expression of CD80 and CD86 induce anergic T-cell responses and peripheral immune tolerance.34–36 In the context of antigen presentation in the FRT, we previously reported that in the rat model, specific blockade of MHC II, CD80 and CD86 abrogates the stimulatory effect of oestradiol on antigen presentation by uterine epithelial cells.18,19,37 Similarly, in the murine model, we reported a 30–40% decrease in antigen presentation by uterine epithelial and stromal cells and by vaginal cells upon specific antibody blockade of CD80 and CD86.9 These initial findings suggested the involvement of MHC II, CD80 and CD86 molecules in the process of antigen presentation by uterine epithelial cells. We undertook the current study to fully understand the mechanisms by which oestradiol regulates antigen presentation in the uterus and vagina.
Further complexity to understanding the biology of antigen presentation within the FRT mucosa stems from the fact that the underlying molecular regulatory mechanisms may be fundamentally different depending on whether the tissue is in a normal physiological state or is undergoing inflammation because of infection with mucosal pathogens.38 Cells of the FRT, including epithelial cells, detect pathogen invasion using innate immune receptors that recognize pathogen-associated molecular patterns (PAMP).2 Among such innate immune receptors are the Toll-like receptors (TLRs), which are type I transmembrane receptor proteins that are highly evolutionarily conserved.39 Cross-linking of TLR with either PAMP or synthetic agonists results in a cellular signalling cascade that leads to production of pro-inflammatory cytokines paralleled by cellular up-regulation of activation markers and co-stimulatory molecules (reviewed in ref. 40). The TLR-mediated activation has been shown to enhance antigen presentation by dendritic cells.41–43 Studies both in humans and in rodent models have described the expression of functional TLRs by uterine and vaginal epithelial cells.22,26,44–46 It is, therefore, possible that TLR-mediated activation may represent a mechanism for regulating antigen presentation by APC of the FRT.
In the present study, we sought to determine the role of oestradiol in regulating antigen presentation by uterine epithelial and stromal cells, and by vagina cells. Our objectives were to: (i) determine whether oestradiol regulates the ability of freshly prepared mouse uterine epithelial and stromal cells as well as vaginal cells to process and present recall antigen to OVA-specific memory T cells, (ii) evaluate whether oestradiol mediates the expression of MHC class II and co-stimulatory molecules, and (iii) establish whether PAMP influence antigen presentation by mouse uterine and vaginal APC.
Materials and methods
General procedures
Sexually mature, BALB/cAnNCr, female mice weighing between 15 and 20 g were purchased from the National Cancer Institute colony at Charles River Laboratories (Kingston, NY). Transgenic BALB/c-TgN(DO11.10)10Loh mice, which express the mouse α-chain and β-chain TCR that pairs with the CD4 co-receptor and is specific for chicken OVA323–339 peptide in the context of I-Ad (class II-restricted) were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained in a constant-temperature room with fixed light/dark intervals (12 hr) and allowed food and water ad libitum. Animals underwent ovariectomy 5–7 days before each experiment. For each experiment, animals were killed using CO2, and uteri and/or vaginae were pooled from 8 to 16 animals. All procedures involving animals were conducted after approval of the Dartmouth College Institutional Animal Care and Use Committee.
Preparation of CD4+ memory T cells
The CD4+ T cells from TCR transgene homozygous mice were prepared from spleens and peripheral lymph nodes of 6- to 10-week-old mice as previously described.9 Briefly, splenic cells were dispersed by gentle grinding between two sterilized glass slides in Hanks’ balanced salt solution (HBSS; Gibco/Invitrogen, Grand Island, NY). Following centrifugation for 8 min at 500 g, erythrocytes were lysed by NH4Cl treatment. The CD4+ subpopulation of T cells was purified by negative selection using a Mouse T-Cell CD4 Subset Column kit (R&D Systems, Inc., Minneapolis, MN). To prepare memory cells, T cells were stimulated with 0·3 μm OVA peptide and incubated with irradiated BALB/c splenocytes, added at 20 : 1 as APC in Iscove's modified Dulbecco's medium (Sigma, St Louis, MO) containing 10% heat-inactivated (56° for 30 min) fetal bovine serum (defined FBS; Hyclone, Logan, UT) supplemented with 100 μm non-essential amino acids, 1 mm sodium pyruvate (both from Gibco/Invitrogen), 50 μm 2-mercaptoethanol (Sigma), 2 mm l-glutamine, (Mediatech, Inc., Herndon, VA) and 100 mg/ml Primocin (InvivoGen, San Diego, CA). Cultures of 2 ml (5 × 106 cells per well) were expanded at 72 hr by threefold dilution into fresh medium. Between days 7 and 10, when T cells were considered to be resting as determined by minimal [3H]thymidine uptake, they were harvested, washed and frozen at −80° in Iscove's modified Dulbecco's medium containing 50% FBS and 10% DMSO (Sigma) until used.
Preparation of whole tissues, uterine epithelial and stromal cells, and vaginal cells
Whole tissues
For whole tissue experiments uteri and vaginae were collected from adult animals. Tissues were isolated, cut into small fragments and pooled (50–100 mg) before being snap frozen on dry ice and stored in liquid nitrogen until further analysis.
Uterine epithelial cells
Uterine epithelial and stromal cell suspensions were prepared by enzymatic digestion and screen disruption as described previously.9 To prepare isolated epithelial cells, uteri were removed, cut open lengthwise, and incubated with 46 500 units trypsin (Sigma) per ml 2·5% pancreatin (Gibco/Invitrogen), at 20 ml/g tissue, for 60 min at 4° and 60 min at 22°. Following transfer to ice-cold (3°) HBSS, uteri were vortexed to release sheets of epithelial cells. Uterine tissues were rinsed and vortexed twice more and resulting cell suspensions were combined. Pooled epithelial sheets were recovered by passing the cell suspension through a 20-μm mesh nylon screen (Small Parts, Inc., Miami Lakes, FL), collected, and centrifuged (500 g) for 5 min. Epithelial sheets were resuspended in culture medium consisting of Dulbecco's modified Eagle's medium (DMEM)/Ham F-12 nutrient mixed 1 : 1 (without phenol red; Gibco/Invitrogen) containing 10% charcoal/dextran-stripped FBS (Hyclone) and supplemented with 20 mm HEPES (Gibco/Invitrogen), 2 mm l-glutamine and 100 μg/ml Primocin. Sheets were aspirated through 20-gauge needles to prepare isolated cells for experimentation. The purity of epithelial cells was evaluated by three criteria9: (i) epithelial cells used in these studies were examined by microscopy and found to comprise epithelial sheets of 50–500 cells that were free of isolated cell contaminants, (ii) when grown on cell inserts, mouse uterine epithelial cells achieve high transepithelial resistance (> 2000 ohms/well; background resistance 150 ohms/well), which would not be possible if there was stromal cell contamination, and (iii) staining of cell inserts with anti-CD45 antibody indicated that epithelial cells accounted for more than 99·5% cells present on each insert.
Uterine stromal cells
To isolate stromal cells, pooled uteri, following the removal of epithelial cells, were incubated for 30 min at 37° in 0·05% trypsin and 530 μm EDTA in HBSS (Mediatech) with 400 units/ml deoxyribonuclease I (Worthington, Lakewood, NJ), using 2 ml per tissue as previously described.9 Tissues in culture medium were dispersed by gentle rubbing on a 40-μm mesh nylon screen (Small Parts) and the resulting cell suspension was centrifuged (500 g) for 10 min. Stromal cells were resuspended in DMEM/F-12 with 10% stripped FBS.
Vaginal cells
Vaginal cells from BALB/c mice were prepared by incubation of tissues, which were hemi-sectioned lengthwise, with 46 500 units of trypsin/ml, 2·5% pancreatin, using 2 ml/vagina, for 60 min at 4° and 60 min at 22° with constant rotation (100 r.p.m.) before separation by gentle rubbing in DMEM/F-12 culture medium on a 40-mm mesh nylon screen. Cells were aspirated through 20-gauge needles to prepare isolated cells for experimentation.
Antigen presentation assays
To measure antigen presentation by freshly isolated cells, OVA peptide-specific T cells (1 × 105 cells/100 μl) in DMEM/F-12 culture medium were seeded in triplicate wells in 96-well flat-bottom microtitre plates (Nalge Nunc International, Rochester, NY) with irradiated uterine epithelial, uterine stromal or vaginal APC (1 × 105 cells/100 μl) in the presence of OVA (APC + T + OVA). The APC were irradiated before the start of antigen presentation with 2000 rads to prevent their proliferation. Controls included in all experiments were APC incubated with T cells in the absence of antigen (APC + T), APC incubated with antigen (APC + OVA) and T cells incubated with antigen (T + OVA). Following 48 hr of incubation at 37°, T-lymphocyte proliferation was measured by [3H]thymidine uptake as previously described.9,19,21 Each well received 1 μCi (50 μl medium) 20–24 hr before the termination of each experiment. Cells in wells were transferred individually onto glass fibre filter-mats with a cell harvester (Skatron/Molecular Devices, Sunnyvale, CA). Radioactivity incorporated into cells was measured in a liquid scintillation counter (Packard, Meriden, CT).
Treatment of reproductive tract cells with PAMP
To determine whether PAMP affected antigen presentation by uterine and vaginal cells, PAMP were added to individual wells in antigen presentation assays at the start of each experiment. To measure the effect of PAMP on co-stimulatory molecule expression, PAMP were added to epithelial cells, stromal cells and vaginal cells (5 × 105 cells/100 ml) at the start of each 24 hr incubation period as previously described.28,44 The TLR agonists used in these studies included TLR1/TLR2: Pam3Cys-Ser-(Lys)4 (P3C, EMC Microcollections, Tübingen, Germany) at a final concentration of 10 μg/ml; TLR2/TLR6: Peptidoglycan from Staphylococcus aureus (InvivoGen), 10 μg/ml; TLR3: Poly(I:C) (InvivoGen), 25 μg/ml; TLR4: ultra-pure lipopolysaccharide from Salmonella minnesota (LPS; List Biological Laboratories, Inc., Campell, CA), 1 μg/ml; TLR7: Loxoribine (InvivoGen), 100 μm; and TLR9: CpG oligonucleotide (InvivoGen), 1 μm. The indicated concentrations of TLR agonists used in this study have previously been shown by our group to induce production of cytokines and chemokines by mouse uterine epithelial cells.28,44 Wells containing media alone were included in all experiments as controls. The TLR agonists were tested for LPS contamination using the Limulus assay (BioWhittaker, Inc., Walkersville, MD) according to manufacturer's instructions before being used in the cell culture assays and were found to be below the level of detection.44
Reverse transcription PCR analysis and TaqMan real-time reverse transcription PCR
For isolation of RNA from whole uteri and vaginae, tissues were homogenized in TRIzol Reagent (1 ml/sample) according to the manufacturer's instructions (Invitrogen) using the PowerGen 125 tissue homogenizer (Fisher Scientific Inc., Pittsburgh, PA). RNA was purified using an RNeasy Mini kit (Qiagen Sciences, Valencia, CA) and treated with DNase (Qiagen). After testing the RNA for integrity and possible contaminants, 600 ng total RNA was reverse transcribed using the iScript cDNA synthesis kit according to the manufacturer's recommendations (BioRad, Hercules, CA) in 20 ml and stored at −20° until analysed. Total RNA from uterine and vaginal cell preparations was recovered by adding 250 ml/well TRIzol Reagent before pooling extracts from two to three wells into a 1-ml final volume. RNA was isolated using the RNeasy Micro kit (Qiagen) and reverse transcribed to cDNA.
For real-time reverse transcription PCR (RT-PCR) analysis, murine co-stimulatory molecules, MHC class II and polymeric immunoglobulin receptor (pIgR) cDNA was amplified using the Platinum PCR Supermix (Invitrogen) with specific primers (Table 1) designed to be specific for each molecule of interest and to ensure that PCR products would have a size of approximately 600 bp. Thirty-five cycles of PCR amplification were performed on the PTC-100 Thermal Cycler (MJ Research, Waltham, MA) as follows: denaturing at 94° for 30 s, annealing at 55° for 30 s, extension at 72° for 1 min. The absence of DNA contamination in RNA preparations was verified by PCR amplification without reverse transcriptase. The RT-PCR products were visualized on a 1·5% agarose gel.
Table 1.
PCR primer pairs used for amplification of co-stimulatory molecules, MHC class II and polymeric immunoglobulin receptor (pIgR) mRNA
| Product | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|
| CD40 | GTGCCAGCCAGGAAGCCGACT GAC | CCTCCTGTGTTGACAGGCTGACACC | 676 |
| CD80 | CATCCTGGTCCTGGTCCTTTCAGACC | GCTTCTTCAGGCCCGAAGGTAAGGCTG | 578 |
| CD86 | GACGCGTAAGAGTGGCTCCTG TAGGCAC | CGGGTGACCTTGCTTAGACATGCAGGTC | 580 |
| MHC II | GATCTGCTGGCCTGCTGGGATCCAGA | GCCCAGGCCCAGGGTGGCTGC AGACAC | 542 |
| pIgR | TGGCAAACGAGGCCAAATATCTGTGCCGGA | TCATTCTTCCGATGTCGGACTCTGGGCACC | 1239 |
Relative expression levels of co-stimulatory molecules, MHC class II and pIgR were measured using the 5′ fluorogenic nuclease assay in quantitative real-time RT-PCR and TaqMan chemistry on the ABI 7700 Prism real-time PCR instrument (Applied Biosystems, Foster City, CA). Primer/MGB probe sets were obtained from ABI assays-on-demand (CD40, ID no. Mn00441891 m1, CD80, Mn00711660 m1, CD86, Mn00444543 m1, MHC class II, Mn00783707 s1, pIgR, Mn00465049 m1 and β-actin, Mn00607939 s1, respectively). The PCR was conducted using the following cycle parameters: 95° 12 min for one cycle, (95° for 20 s, 60° for 1 min) for 40 cycles. Analysis was carried out using the sequence detection software supplied with the ABI 7700. The threshold cycle (Ct) for each set of duplicate reactions was used to quantify the amount of starting template. A difference in Ct values (ΔCt) was calculated for each gene of interest by subtracting the mean Ct for endogenous β-actin control of each cDNA sample. Assuming that each reaction functions at 100% PCR efficiency, a difference of one Ct represents a twofold difference. Relative expression levels were expressed as a fold-change in mRNA expression and calculated using the formula 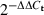 .
.
Hormone treatment
17β-Oestradiol-from Calbiochem (La Jolla, CA) was initially dissolved in 100% ethanol, evaporated to dryness, and then resuspended in 0·9% saline as previously described.21 Oestradiol injection was performed with 0·1 μg hormone/50 μl/mouse via the subcutaneous route. We previously found this particular dose of oestradiol to exert an optimal effect on antigen presentation in the rat model.19 Control animals received 50 μl saline subcutaneously. To correct for the alcohol present in the oestradiol preparation, an equivalent amount of ethanol was evaporated in flasks used to prepare the saline control.
Statistics
Statistical analysis was performed using unpaired t-test. One-way analysis of variance followed by a Tukey multiple comparison (GraphPad Prism version 5.0; GraphPad Software Inc., San Diego, CA) was used to determine differences between treatment conditions. A value of P < 0·05 was considered significant.
Results
Effect of oestradiol on antigen presentation of OVA by uterine and vaginal APC from ovariectomized mice
Previous studies from our laboratory have shown that mouse uterine epithelial cells as well as APC in the uterine stroma and vagina are able present OVA and OVA323–339 peptide to naive T cells and memory T cells from DO11.10 TCR transgenic mice.9 To more fully characterize antigen presentation, studies were undertaken to determine whether oestradiol regulates antigen presentation in the FRT of the mouse. Mice underwent ovariectomy and were treated with either saline or oestradiol (0·1 μg/day) for 3 days before being killed 24 hr after the last injection. Uterine epithelial cells, uterine stromal cells and vaginal cells were prepared from non-transgenic BALB/c mice as previously described.47,48 To study antigen presentation, isolated cells were incubated with memory T cells from DO11.10 transgenic mice.49 As seen in Fig. 1, oestradiol treatment inhibited uterine epithelial and stromal cell antigen presentation by 50–70% relative to that seen in control animals that received vehicle. Similarly, the administration of oestradiol significantly decreased antigen presentation by APC in the vagina. As uterine and vaginal antigen presentation was measured in the same groups of animals, these studies indicate that oestradiol has a similar effect on antigen presentation throughout the FRT.
Figure 1.
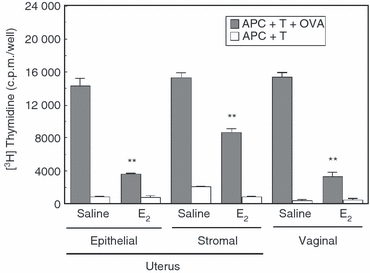
Effect of oestradiol (E2) on antigen presentation of ovalbumin (OVA) to CD4+ DO11.10 memory T cells by antigen-presenting cells (APC) from the uterus and vagina. Ovariectomized animals (6–10 BALB/c mice/group) were treated with either saline (50 μl) or oestradiol (0·1 μg/50 μl) daily for 3 days before killing. Uterine and vaginal tissues were pooled and cells were prepared by enzymatic digestion. Memory T cells were prepared by incubating spleen cells from DO11.10 mice with OVA323–339 peptide for 6–8 days in culture as described in Materials and methods. Epithelial, stromal, or vaginal cells (APC; 1 × 105 cells/100 μl) were incubated with memory T cells (T; 1 × 105 cells/100 μl) and OVA (300 μg/ml) for 3 days. [3H]Thymidine was added for the last 24 hr of incubation. Values shown are [3H]thymidine incorporation for APC + T + OVA as well as control groups APC + T incubations expressed as mean ± SE (three or four wells per group). **Significantly (P < 0·01) lower than saline control. Representative of two experiments.
To more fully characterize the onset of the inhibitory response of oestradiol in vivo in the uterus and vagina, ovariectomized animals were injected with a single dose of oestradiol (0·1 mg) and killed 24 hr later. As seen in Fig. 2, inhibition of antigen presentation was measurable at 24 hr following hormone treatment, relative to saline controls, but was less pronounced than that seen after 3 days of hormone treatment (Fig. 1). We found that antigen presentation in response to oestradiol was inhibited by 15–25% of that seen with saline controls within 24 hr of hormone treatment in the uterus and by 50% in the vagina. These studies indicate that the effects of oestradiol on antigen presentation in the uterus and vagina are relatively rapid and that this animal model can be used to examine the mechanisms through which oestradiol regulates antigen presentation in the FRT.
Figure 2.
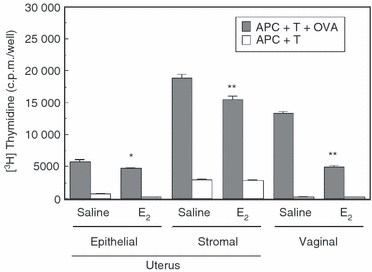
Rapid effect of oestradiol (E2) on antigen presentation by uterine epithelial and stromal cells and vaginal cells. Following a single injection of saline (50 μl) or oestradiol (0·1 μg), ovariectomized mice were killed 24 hr later. Uteri and vaginae (9–10 animals/group) were pooled and reproductive tract antigen-presenting cells (APC) were prepared as described in Materials and methods. Uterine and vaginal APC (1 × 105 cells/100 μl) were incubated with CD4+ memory T cells (1 × 105 cells/100 μl) in the presence or absence of ovalbumin (OVA) for 3 days. Values shown are the mean ± SE (three or four wells per group). [3H]Thymidine was added for the last 24 hr of incubation. *Significantly (P < 0·05) lower than saline control (APC + T + OVA). **Significantly (P < 0·01) lower than saline control (APC + T + OVA).
Expression of co-stimulatory molecules, MHC II and pIgR in tissues of the mouse FRT
We have previously shown in functional studies that antigen presentation by uterine and vaginal cells is dependent on MHC II and co-stimulatory molecules.18,19,37 In the current study, we have now extended these investigations to specifically examine the expression of these molecules in mouse uterine and vaginal tissue using RT-PCR. After isolating RNA from whole tissues and reverse transcription, cDNA was amplified using the specific PCR primer pairs described in Table 1. Visualization on a 1·5% agarose gel showed that co-stimulatory molecules CD40, CD80 and CD86 as well as MHC II and the pIgR, used as a positive control, responsible for transporting IgA from tissue to lumen are expressed in both the uterus (Fig. 3) and the vagina (not shown). Having demonstrated that the uterus and vagina express mRNA for these molecules, we used Taqman real-time RT-PCR to examine the relative expression of co-stimulatory molecules in each tissue. As seen in Fig. 4(a), CD40 was expressed at the highest levels in the uterus followed by CD86 and CD80. Analysis of co-stimulatory molecule expression in the vagina indicated that expression of CD40 was similar to CD80 and CD86 (Fig. 4b). In contrast, pIgR expression in the uterus and vagina was significantly higher than co-stimulatory molecule and class II expression. When co-stimulatory molecule, class II and pIgR mRNA expression in the uterus and the vagina were compared (Fig. 4c), CD40 expression in the uterus was higher relative to that seen in the vagina. In contrast, the expression of CD80, CD86, MHC class II and pIgR in the uterus were comparable to that seen in the vagina (within two- to fourfold).
Figure 3.
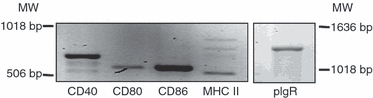
Constitutive expression of co-stimulatory molecules in the uterus of the mouse. Expression of CD40, CD80, CD86 and MHC II was determined by reverse transcription-PCR amplification of total RNA isolated from pooled tissues of 10–12 mice. The primer sequences and expected amplicon sizes are summarized in Table 1. MW indicates molecular weight marker. Real-time reverse transcription-PCR (lower panel) was used to determine the relative levels of expression of CD40, CD80, CD86 and MHC II mRNA in the uterine and vaginal tissues, normalized against an endogenous β-actin control. Representative of two experiments.
Figure 4.
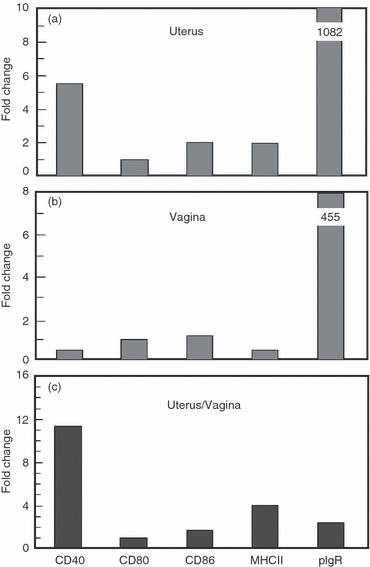
Expression of co-stimulatory molecules, MHC II and polymeric immunoglobulin receptor (pIgR) in tissues of the mouse female reproductive tract. Uteri and vaginae from 10–12 mice were pooled and homogenized before RNA isolation and reverse transcription as described in Materials and methods. CD40, CD80, CD86, MHC II and pIgR specific cDNA was quantified using real time reverse transcription-PCR. The profile of expression in the uterus and vagina is relative to their respective CD80 levels, which are set to one. The lower panel shows a comparison of CD40, CD80, CD86, MHC II and pIgR expression levels between uterus and vagina. Graphs shown are representative of two separate experiments.
Effect of oestradiol on co-stimulatory molecule expression in the uterus and vagina
Having determined that intact uterine and vaginal tissues express co-stimulatory molecules, we used real-time RT-PCR to examine the effect of oestradiol on expression in uterine and vaginal tissues from ovariectomized animals treated with either saline or oestradiol (0·1 μg/day) for 3 days before being killed 24 hr after the last injection. As seen in Fig. 5, oestradiol treatment lowered the expression of CD40 and CD80 and class II in the uterus and CD40, CD86 and MHC class II in the vagina. Analysis of pIgR expression indicated that whereas oestradiol inhibited expression in the vagina, it had a slight stimulatory effect on pIgR expression in the uterus.
Figure 5.
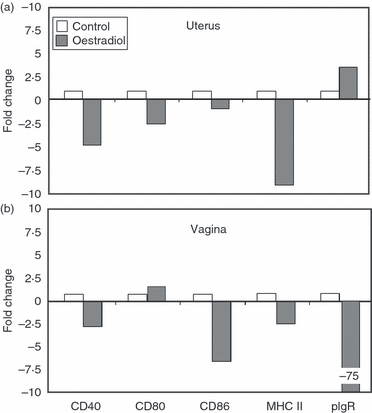
Effect of oestradiol on expression of co-stimulatory molecules in the uterus and vagina of ovariectomized mice. Animals (6–10/group) were treated with either saline (50 μl) or oestradiol (0·1 μg/50 μl) daily for 1–3 days before being killed. Uterine (a) and vaginal (b) tissues were pooled before RNA isolation and reverse transcription as described in the Materials and methods. CD40, CD80, CD86, MHC II and polymeric immunoglobulin receptor (pIgR) specific cDNA was quantified using real-time reverse transcription-PCR. The profile of expression is shown as fold change, with saline control tissues set to one. Graphs shown are representative of three separate experiments.
The inhibition of co-stimulatory molecule expression in uterine and vaginal tissues following oestradiol treatment prompted us to examine whether oestradiol exerts its inhibitory effects on isolated APC. Following treatment of ovariectomized animals with saline or oestradiol daily for 3 days, uteri and vaginae were pooled before the preparation of isolated uterine epithelial and stromal cells as well as vaginal cells by enzymatic digestion, isolation of RNA and reverse transcription. As seen in Fig. 6, when co-stimulatory molecules, MHC II and pIgR mRNA was quantified using real-time RT-PCR and compared with isolated cells from control animals (saline), oestradiol lowered the expression of CD40, CD80 (3- to 40-fold) CD86 and MHC class II in all cell populations tested. We also found that whereas oestradiol stimulated pIgR expression in uterine epithelial cells, it had a marked inhibitory effect on pIgR expression in vaginal cells. In other studies (not shown), we found that oestradiol inhibition of co-stimulatory molecule expression could be measured in isolated cells following a single injection of oestradiol. These findings suggest that oestradiol regulation of co-stimulatory expression most probably accounts for the inhibitory effects seen when reproductive tract APC are incubated with OVA-specific T cells in the presence of OVA (Fig. 1).
Figure 6.
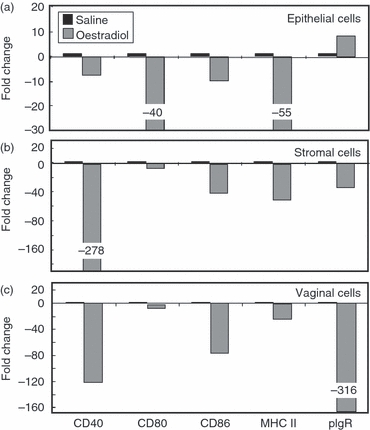
Influence of oestradiol on co-stimulatory molecule expression by isolated uterine epithelial and stromal cells and vaginal cells. Ovariectomized animals (6–10 mice/group) received either saline (50 μl) or oestradiol (0·1 μg/50 μl) daily for 3 days before being killed. Uterine and vaginal tissues were pooled before preparation of isolated cells by enzymatic digestion. RNA isolation and reverse transcription were performed as described in the Materials and methods. Co-stimulatory molecules, MHC II and polymeric immunoglobulin receptor (pIgR) specific cDNA was quantified using real-time reverse transcription-PCR. The profile of expression is shown as fold change with cells from control animals (saline) set to one. Graphs shown are representative of two experiments.
Effect of PAMP on antigen presentation and co-stimulatory molecule expression by uterine and vaginal cells
Previous studies from our laboratory have shown that mouse uterine and vaginal tissues express TLR1–9 mRNA, with TLR8 and TLR4 being the most highly expressed TLRs in the uterus and TLR4 and TLR5 being the most highly expressed TLRs in the vagina.44 As part of these studies, we found that exposure of primary uterine epithelial cells to several TLR agonists resulted in secretion of monocyte chemotactic protein-1 and enhanced α-defensin (AD2), β defensins (BD1, -2 and -4) and secretory leucocyte protease inhibitor mRNA expression. To determine whether TLR agonists influence antigen presentation by isolated APC from the uterus and vaginal, uterine epithelial and stromal cells as well as vaginal cells from intact animals were prepared and incubated with OVA-specific memory T cells and OVA, along with PAMP for 3 days. Figure 7 shows the effect of PAMP on the antigen presentation by uterine epithelial cells and APC from the uterus and vagina. Treatment with Pam3Cys (TLR1/TLR2 agonist) increased antigen presentation by uterine epithelial cells, whereas other TLR agonists had no effect. In contrast, when uterine stromal cells were analysed, peptidoglycan (PGN; TLR2/TLR6) and Pam3Cys increased antigen presentation while CpG decreased stromal antigen presentation. With vaginal cells, Pam3Cys increased antigen presentation and CpG decreased vaginal antigen presentation. These results suggest that PAMP responsiveness throughout the FRT varies with the site analysed and the APC present.
Figure 7.
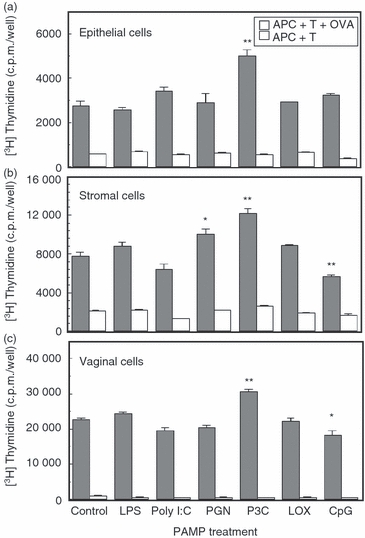
Effect of pathogen-associated molecular patterns (PAMP) on antigen presentation of ovalbumin (OVA) to T cells by isolated uterine and vaginal cells. Uterine epithelial and stromal (a, b) cells as well as vaginal cells (c) from 15 intact animals were prepared as described in Materials and methods. Reproductive tract antigen-presenting cells (APC) were incubated with OVA-specific memory T cells and OVA, along with PAMP for 3 days. PAMP used were Toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS) at 1 μg/ml, TLR3 agonist Poly(I:C) at 25 μg/ml, TLR2 (1/6) agonist Pam3Cys-Ser-(Lys)4 (P3C) at 10 μg/ml, TLR2 (1/6) agonist peptidoglycan (PGN) at 10 μg/ml, TLR7 agonist LOX at 100 μm and TLR9 agonist CpG at 1 μm. [3H]Thymidine was added to each well for the last 24 hr. Values are mean ± SE of three or four wells per group. *Significantly (P < 0·05) different than control group. **Significantly (P < 0·01) different than control. Representative of two experiments.
As part of these studies, we evaluated the effect of PAMP on co-stimulatory molecule expression. Uterine epithelial cells and stromal cells, and vaginal APC were incubated for 24 hr with TLR agonists. The relative expression of CD40, CD80, CD86, MHC II and pIgR was then measured by real-time RT-PCR (Fig. 8). We found that the expression of CD40 by uterine epithelial cells and vaginal cells was increased following stimulation with LPS, Poly(I:C), PGN and Pam3Cys (Fig. 8a,c). In contrast, CD80 expression by uterine stromal and vaginal cells was unaffected by the TLR agonists used in this study except for LPS stimulation, which modestly induced its expression by uterine epithelial cells (Fig. 8a). The expression of CD86 was induced (twofold or higher) in uterine stromal cells and vaginal cells by Poly(I:C) and LPS, respectively (Fig. 8a,c). On the other hand, MHC class II expression was induced in uterine epithelial cells and stromal cells by Pam3Cys (Fig. 8a,b). In addition, MHC expression by uterine stromal cells was increased by PGN stimulation (Fig. 8b). Further analysis showed that in vaginal cells, MHC class II expression was increased by Poly(I:C) and PGN stimulation (Fig. 8c). Finally, the expression of pIgR was induced with LPS, PGN and Pam3Cys in uterine epithelial and stromal cells and vaginal APC (Fig. 8). Our finding that some genes were not affected by PAMP following 24 hr of exposure does not exclude the possibility that some may have been transiently altered and then return to baseline within hours of exposure. What is clear from these studies is that many genes involved in antigen presentation by uterine epithelial, uterine stromal and vaginal cells from the FRT are altered with PAMP exposure. In general, PAMP stimulation appears to exert subtle changes in the expression of co-stimulatory molecules, MHC II and pIgR by uterine epithelial cells, uterine stromal cells and vaginal cells.
Figure 8.
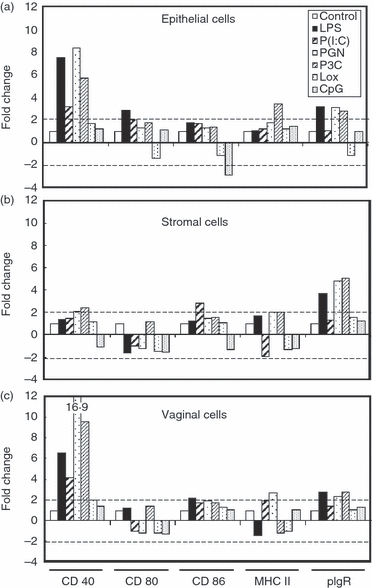
Effect of pathogen-associated molecular patterns (PAMP) on co-stimulatory molecule expression by isolated uterine and vaginal cells in culture. Uterine epithelial cells (a), uterine stromal cells (b) and vaginal cells (c) (5 × 105 cells/100 μl) from 15 intact animals were incubated in 96-well plates along with PAMP (100 μl) for 24 hr before RNA isolation and reverse transcription as described in the Materials and methods. Toll-like receptor (TLR) agonists and concentrations are the same as those indicated in Fig. 7. CD40, CD80, CD86, MHC II and polymeric immunoglobulin receptor (pIgR) specific cDNA were quantified using real-time reverse transcription-PCR and profiles are expressed as fold change relative to expression of control cells, the values of which are set to one. Hatched lines in each panel indicate the minimal level of detection (twofold). Graphs shown are representative of two experiments.
Discussion
The goal of this study was to investigate the mechanisms by which oestradiol regulates antigen presentation by APC of the FRT. Our studies, conducted in the murine model, demonstrate that oestradiol decreases antigen presentation by the uterine epithelial cells and stromal cells and by vaginal APC. To more fully define the effects of oestradiol on antigen presentation, we examined the pattern of expression of MHC II, co-stimulatory molecules (CD40, CD80 and CD86) and pIgR by uterine and vaginal cells. The RT-PCR analyses showed constitutive expression of these molecules in the uterus and vagina, with subtle tissue site-specific quantitative and qualitative differences in the patterns of expression. Additional analyses indicated that the expression of MHC II, CD80, CD86 by isolated uterine and vagina cells was decreased with oestradiol treatment. With regards to pIgR expression, although oestradiol treatment stimulated its expression in the uterus, the hormone had an inhibitory effect on pIgR expression in the vagina. Lastly, studies conducted to determine the effects of PAMP stimulation showed that specific TLR agonists selectively increased antigen presentation in the uterus and vagina, possibly as a result of increased expression of co-stimulatory molecules.
The capacity of various cells of the FRT to present antigen has previously been demonstrated.1,17–21 We have previously shown in the mouse model that uterine epithelial and stromal cells as well as vaginal cells process and present both OVA and peptides.9 Further, we found that antigen presentation by primary polarized uterine epithelial cells was surface-dependent, predominantly occurring at the basolateral surface of polarized cells.9 These initial studies clearly demonstrated that uterine epithelial cells posses the necessary cellular machinery for processing and presentation of specific antigens. Less clear are the mechanisms that regulate antigen presentation in the FRT. We now provide evidence that oestradiol inhibits antigen presentation by uterine epithelial and stromal cells, and by vaginal cells. Further, the kinetics of oestradiol responses indicate relatively rapid inhibitory activity on antigen presentation. Our previous work in the rat showed that oestradiol exerted a differential effect on antigen presentation by uterine and vaginal cells.17–20 On the one hand, oestradiol stimulated antigen presentation by uterine epithelial cells but on the other inhibited antigen presentation by vagina cells. The results of the current study, obtained in the murine model, have corroborated our previous observation of oestradiol-mediated inhibition of antigen presentation by vagina cells. However, the discordant effect of oestradiol on antigen presentation in the uterus in the rat and murine model may highlight unique species-specific effects of the hormone.
In other studies we proposed a working hypothesis to explain how HIV evades FRT mucosal immune protection. We were drawn to the conclusion that during the menstrual cycle, oestradiol and possibly progesterone suppress aspects of the innate, humoral and cell-mediated immune systems to optimize conditions for conception and thereby create a window of vulnerability (7–10 days following ovulation) during which the potential for viral infectivity in the FRT is enhanced.50 On the one hand, our findings in the present study extend this working hypothesis by showing that oestradiol, which peaks at ovulation in the mouse and human, suppresses APC, which are the essential key regulators of adaptive immunity. The practical implication of these findings is that the likelihood of immune responses to spermatozoa, which are allogeneic to the female, are suppressed to optimize chances for successful fertilization in future reproductive cycles. On the other hand, our findings that oestradiol suppresses antigen presentation provides insight into the complexity of inducing local immunity to FRT vaccines. Our study suggests that, in generating adaptive immune responses in the genital mucosa, attention needs to be paid to the stage of the menstrual cycle when vaccines are applied.
We hypothesized in this study that oestradiol inhibition of antigen presentation by cells of the FRT involves modulation of the expression of MHC II and co-stimulatory molecules, which are integral components of the process of antigen presentation. Our previous studies in the rat model,17–19 and more recently in the murine model,1 showed that antigen presentation by uterine epithelial cells and stromal cells and vaginal cells is mediated by MHC II and is dependent on co-stimulatory molecules CD80 (B7.1) and CD86 (B7.2). We therefore sought to (i) characterize the expression of MHC II, CD40, CD80, CD86 and pIgR, and (ii) determine the effects of oestradiol treatment on the expression of these molecules by uterine and vaginal cells. Whereas the expression of MHC II by FRT tissues has been documented, the expression of co-stimulatory molecules is less certain.11,13,14,51,52 This may be partly because of the low sensitivity of procedures that rely on antibody staining, especially for detecting less stable molecules that are weakly expressed by some APC.53,54 Using a highly sensitive RT-PCR analysis, we were able to demonstrate constitutive expression of MHC II, CD40, CD80, CD86 and pIgR in uterine and vaginal tissues. When compared with the uterus, vagina expressed significant higher CD40 mRNA. The biological significance of this observation remains to be determined. Interestingly, CD40-dependent antigen presentation in the vagina has been linked to the induction of immune tolerance.55 Whether this finding is related to the fact that the vaginal mucosa is colonized by commensal bacteria,56 which may bias immune responses towards tolerance within this mucosal tissue, remains to be determined. More importantly, our findings indicate that uterine and vaginal tissues do express co-stimulatory molecules in addition to MHC II, and therefore are poised to serve as immune induction sites.
As part of these studies, we found that oestradiol decreased mRNA for MHC II, CD40, CD80 and CD86 in uterine and vaginal tissue. Similar findings were obtained with isolated uterine epithelial cells, uterine stromal cells and vaginal APC. To the best of our knowledge, this is the first description of oestradiol-mediated inhibition of expression of MHC II and co-stimulatory molecules by cells in the FRT. The molecular mechanisms by which oestradiol exerts these effects remain to be determined. With regards to MHC II expression, recent studies indicate that oestradiol-mediated inhibition of this molecule involves activation of the c-Jun N-terminal kinase pathway or by modification of the histone acetylation status of the class II promoter.57,58 Whether similar mechanisms mediate oestradiol effects on MHC II expression by FRT cells remains to be investigated. These findings collectively suggest that oestradiol regulation of antigen presentation is probably mediated by its inhibitory effects on MHC II and co-stimulatory molecules. Finally, analysis of pIgR expression demonstrated that oestradiol stimulates its expression in the uterus and inhibits expression in the vagina. These results are consistent with our earlier data in the rat model showing a differential effect of oestradiol on pIgR expression in the uterus and vagina.59–61 The physiological significance of the differential effect of oestradiol on pIgR expression in the uterus and vagina remains unknown. However, our previous studies have shown that in immunized (sheep red blood cells) ovariectomized rats, oestradiol treatment results in increased accumulation of IgA and IgG in the uterus with concomitant decreased levels of these antibodies in vaginal secretions.62,63 These previous observations may now be interpreted in light of the present findings of the differential effects of oestradiol on pIgR expression by uterine and vaginal tissues. However, the probable impact of oestradiol treatment on antibody-mediated protection in the uterus and vagina remains unclear. These findings do, however, provide insight into the mechanisms responsible for mid-cycle suppression of IgA that occurs in cervical–vaginal secretions of women during the menstrual cycle.64
The mechanisms that underlie antigen presentation in the FRT may be fundamentally distinct depending on whether the FRT is in a normal physiological state or whether it is responding to pathogens (e.g. infection of the tract).38 To address this question, we investigated the effects of various PAMP on antigen presentation by uterine and vaginal cells. In general, we found that PAMP exerted selective stimulatory effects on antigen presentation by uterine epithelial and stromal cells, and by vaginal APC. The observed effects of PAMP on antigen presentation may be directly related to the pattern of expression of TLR by uterine and vaginal APC.28,44,65 An unexpected observation in this study was the finding that LPS had no effect on antigen presentation by uterine epithelial cells, uterine stromal cells or vaginal cells. This is despite the fact that uterine epithelial cells and stromal cells respond to LPS stimulation with enhanced secretion of cytokines and chemokines.44,66 Hence, it appears that LPS has no direct effect on antigen presentation in the FRT. Overall, our studies indicate that reproductive tract immune cells are responsive to LPS in terms of cytokine/chemokine secretion while not responding to signals that might otherwise result in over-reaction to selective organisms. Whether this is a unique characteristic of mucosal surfaces that normally have LPS in the lumen remains to be determined.
To further explore the mechanisms for TLR-induced increase in antigen presentation, we determined the pattern of expression of MHC II, CD40, CD80, CD86 and pIgR by isolated uterine and vaginal cells following stimulation with PAMP. The expression of MHC II and pIgR were increased by treatment with several PAMP. Furthermore, the expression of co-stimulatory molecules was selectively affected by specific PAMP. None of the PAMP analysed had any significant effect on CD80 expression by uterine stromal and vaginal APC. However, PGN and Pam3Cys increased expression of CD40 by uterine epithelial and stromal cells as well as vaginal cells. The enhanced expression of CD40 coupled with increased MHC II expression following stimulation with PGN and Pam3Cys may account for the increased antigen presentation by uterine and vaginal cells. Additional analyses showed that expression of CD40 and CD80 by uterine epithelial cells as well as the expression of CD40 by vaginal APC was similarly enhanced by LPS and Poly(I:C). The finding that LPS increased uterine epithelial expression of CD40 and CD80 without concomitant increase in antigen presentation, suggests that TLR-induced increase in expression of co-stimulatory molecules may not be an adequate signal for increasing antigen presentation. Further analysis showed that CpG stimulation had no effect on the expression of co-stimulatory molecules, although it decreased antigen presentation by uterine stromal cells and vaginal APC. These findings suggest that TLR-induced increases in antigen presentation may occur through mechanisms that are independent of changes in expression of co-stimulatory molecules. Indeed recent studies with dendritic cells have shown that TLR stimulation has additional proximate effects on antigen presentation, including cytoskeletal rearrangements, antigen uptake and processing, antigen–MHC complex translocation to the plasma membrane and modulating the strength of immunological synapses.41–43,67–69 Hence, TLR are being considered as potential vaccine adjuvants (reviewed in ref. 70). Our study shows that within the mucosa of the FRT, TLR activation potentiates antigen presentation by a mechanism that is partly the result of modulation of expression of MHC II and co-stimulatory molecules.
In conclusion, our study provides insight into the mechanisms of antigen presentation by APC of the FRT. The finding that oestradiol rapidly inhibits antigen presentation suggests that critical levels of oestradiol during different phases of the menstrual cycle serve to facilitate reproductive functions (e.g. tolerance of allogeneic spermatozoa and fetal allograft) of the FRT when decreased immunity is a desirable outcome. In addition, our finding that TLR agonists increase antigen presentation has particular significance for designing adjuvants for mucosal vaccines against sexually transmitted infections.
Acknowledgments
The authors gratefully thank Drs Gisela Soboll and Mimi Ghosh, Department of Physiology and Neurobiology for critical feedback during the preparation of the manuscript. We also thank Jennifer Fields, Dartmouth-Hitchcock Medical Center for her assistance in carrying out some of the experiments in this study. This work was supported by research grants AI-13541 and AI-51877 from National Institutes of Health and in part by the Norris Cotton Cancer Center Support Grant CA-23108.
Glossary
Abbreviations:
- APC
antigen-presenting cells
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- FRT
female reproductive tract
- HBSS
Hanks’ balanced salt solution
- OVA
ovalbumin
- PAMP
pathogen-associated molecular patterns
- PGN
peptidoglycan
- pIgR
polymeric immunoglobulin receptor
- TCR
T-cell receptor
- TLR
Toll-like receptor
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wira CR, Grant-Tschudy KS, Crane-Godreau M. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:1–12. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 2.Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Women's Health Rev. 2008;4:102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiserlian D, Vidal K, Revillard JP. Murine enterocytes can present soluble antigen to specific class II-restricted CD4+ T cells. Eur J Immunol. 1989;19:1513–1516. doi: 10.1002/eji.1830190827. [DOI] [PubMed] [Google Scholar]
- 5.Mayer L, Panja A, Li Y. Antigen recognition in the gastrointestinal tract: death to the dogma. Immunol Res. 1991;10:356–359. doi: 10.1007/BF02919721. [DOI] [PubMed] [Google Scholar]
- 6.Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, Nepom GT. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debbabi H, Ghosh S, Kamath AB, Alt J, Demello DE, Dunsmore S, Behar SM. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274–L279. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 9.Wira CR, Rossoll RM, Young RC. Polarized uterine epithelial cells preferentially present antigen at the basolateral surface: role of stromal cells in regulating class II-mediated epithelial cell antigen presentation. J Immunol. 2005;175:1795–1804. doi: 10.4049/jimmunol.175.3.1795. [DOI] [PubMed] [Google Scholar]
- 10.Mayer L, Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166:1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabibzadeh SS, Mortillo S, Gerber MA. Immunoultrastructural localization of Ia antigens in human endometrium. Arch Pathol Lab Med. 1987;111:32–37. [PubMed] [Google Scholar]
- 12.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100:3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 13.Bjercke S, Brandtzaeg P. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA-DR in the human endometrium throughout the menstrual cycle. Hum Reprod. 1993;8:1420–1425. doi: 10.1093/oxfordjournals.humrep.a138271. [DOI] [PubMed] [Google Scholar]
- 14.Wallace PK, Yeaman GR, Johnson K, Collins JE, Guyre PM, Wira CR. MHC class II expression and antigen presentation by human endometrial cells. J Steroid Biochem Mol Biol. 2001;76:203–211. doi: 10.1016/s0960-0760(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 15.Fahey JV, Wallace PK, Johnson K, Guyre PM, Wira CR. Antigen presentation by human uterine epithelial cells to autologous T cells. Am J Reprod Immunol. 2006;55:1–11. doi: 10.1111/j.1600-0897.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 16.Wira C, Fahey J, Wallace P, Yeaman G. Effect of the menstrual cycle on immunological parameters in the human female reproductive tract. J Acquir Immune Defic Syndr. 2005;38(Suppl. 1):S34–S36. doi: 10.1097/01.qai.0000167040.58181.d5. [DOI] [PubMed] [Google Scholar]
- 17.Prabhala RH, Wira CR. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive tract mucosal tissues. J Immunol. 1995;155:5566–5573. [PubMed] [Google Scholar]
- 18.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- 19.Wira CR, Rossoll RM, Kaushic C. Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells. Endocrinology. 2000;141:2877–2885. doi: 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- 20.Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells: role of TGF-β as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143:2872–2879. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- 21.Wira CR, Rossoll RM. Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-β production by epithelial cells in mediating antigen-presenting cell function. Immunology. 2003;109:398–406. doi: 10.1046/j.1365-2567.2003.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crane-Godreau MA, Wira CR. CCL20/macrophage inflammatory protein 3α and tumor necrosis factor alpha production by primary uterine epithelial cells in response to treatment with lipopolysaccharide or Pam3Cys. Infect Immun. 2005;73:476–484. doi: 10.1128/IAI.73.1.476-484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant-Tschudy KS, Wira CR. Effect of oestradiol on mouse uterine epithelial cell tumour necrosis factor-α release is mediated through uterine stromal cells. Immunology. 2005;115:99–107. doi: 10.1111/j.1365-2567.2005.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer TM, Fahey JV, Wright JA, Wira CR. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C) Am J Reprod Immunol. 2005;54:193–202. doi: 10.1111/j.1600-0897.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Soboll G, Crane-Godreau MA, Lyimo MA, Wira CR. Effect of oestradiol on PAMP-mediated CCL20/MIP-3α production by mouse uterine epithelial cells in culture. Immunology. 2006;118:185–194. doi: 10.1111/j.1365-2567.2006.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 32.van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 33.Elgueta R, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 37.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526–4534. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]
- 38.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 39.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 41.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 42.Watts C, Zaru R, Prescott AR, Wallin RP, West MA. Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol. 2007;19:73–78. doi: 10.1016/j.coi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 43.West MA, Prescott AR, Chan KM, Zhou Z, Rose-John S, Scheller J, Watts C. TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J Cell Biol. 2008;182:993–1005. doi: 10.1083/jcb.200801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75:131–139. doi: 10.1095/biolreprod.106.050690. [DOI] [PubMed] [Google Scholar]
- 45.Young SL, Lyddon TD, Jorgenson RL, Misfeldt ML. Expression of Toll-like receptors in human endometrial epithelial cells and cell lines. Am J Reprod Immunol. 2004;52:67–73. doi: 10.1111/j.1600-0897.2004.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 47.Grant KS, Wira CR. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNFα and TGFβ release in culture. Biol Reprod. 2003;69:1091–1098. doi: 10.1095/biolreprod.103.015495. [DOI] [PubMed] [Google Scholar]
- 48.Grant-Tschudy KS, Wira CR. Effect of estradiol on mouse uterine epithelial cell transepithelial resistance (TER) Am J Reprod Immunol. 2004;52:252–262. doi: 10.1111/j.1600-0897.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 49.Gorham JD, et al. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci U S A. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ljunggren G, Anderson DJ. Cytokine induced modulation of MHC class I and class II molecules on human cervical epithelial cells. J Reprod Immunol. 1998;38:123–138. doi: 10.1016/s0165-0378(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 52.Sallinen K, Verajankorva E, Pollanen P. Expression of antigens involved in the presentation of lipid antigens and induction of clonal anergy in the female reproductive tract. J Reprod Immunol. 2000;46:91–101. doi: 10.1016/s0165-0378(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 53.Damoiseaux JG, Yagita H, Okumura K, van Breda Vriesman PJ. Costimulatory molecules CD80 and CD86 in the rat; tissue distribution and expression by antigen-presenting cells. J Leukoc Biol. 1998;64:803–809. doi: 10.1002/jlb.64.6.803. [DOI] [PubMed] [Google Scholar]
- 54.Schrijver IA, Melief MJ, van Meurs M, Companjen AR, Laman JD. Pararosaniline fixation for detection of co-stimulatory molecules, cytokines, and specific antibody. J Histochem Cytochem. 2000;48:95–103. doi: 10.1177/002215540004800110. [DOI] [PubMed] [Google Scholar]
- 55.Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, Edwards RP. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 56.Larsen B, Monif GR. Understanding the bacterial flora of the female genital tract. Clin Infect Dis. 2001;32:e69–e77. doi: 10.1086/318710. [DOI] [PubMed] [Google Scholar]
- 57.Adamski J, Ma Z, Nozell S, Benveniste EN. 17β-Estradiol inhibits class II major histocompatibility complex (MHC) expression: influence on histone modifications and cbp recruitment to the class II MHC promoter. Mol Endocrinol. 2004;18:1963–1974. doi: 10.1210/me.2004-0098. [DOI] [PubMed] [Google Scholar]
- 58.Adamski J, Benveniste EN. 17β-estradiol activation of the c-Jun N-terminal kinase pathway leads to down-regulation of class II major histocompatibility complex expression. Mol Endocrinol. 2005;19:113–124. doi: 10.1210/me.2004-0270. [DOI] [PubMed] [Google Scholar]
- 59.Kaushic C, Richardson JM, Wira CR. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology. 1995;136:2836–2844. doi: 10.1210/endo.136.7.7789308. [DOI] [PubMed] [Google Scholar]
- 60.Richardson JM, Kaushic C, Wira CR. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488–498. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- 61.Kaushic C, Frauendorf E, Wira CR. Polymeric immunoglobulin A receptor in the rodent female reproductive tract: expression in vagina and tissue specific mRNA regulation by sex hormones. Biol Reprod. 1997;57:958–966. doi: 10.1095/biolreprod57.5.958. [DOI] [PubMed] [Google Scholar]
- 62.Wira CR, Sandoe CP. Specific IgA and IgG antibodies in the secretions of the female reproductive tract: effects of immunization and estradiol on expression of this response in vivo. J Immunol. 1987;138:4159–4164. [PubMed] [Google Scholar]
- 63.Wira CR, Sandoe CP. Effect of uterine immunization and oestradiol on specific IgA and IgG antibodies in uterine, vaginal and salivary secretions. Immunology. 1989;68:24–30. [PMC free article] [PubMed] [Google Scholar]
- 64.Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 65.Yao XD, Fernandez S, Kelly MM, Kaushic C, Rosenthal KL. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. J Reprod Immunol. 2007;75:106–119. doi: 10.1016/j.jri.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Sheldon IM, Roberts MH. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS ONE. 2010;5:e12906. doi: 10.1371/journal.pone.0012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 68.Blander JM. Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol. 2007;28:19–25. doi: 10.1016/j.it.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Weck MM, Grünebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 70.Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine. 2008;26:6777–6783. doi: 10.1016/j.vaccine.2008.09.045. [DOI] [PubMed] [Google Scholar]


