Abstract
Norovirus (NoV) -derived virus-like particles (VLPs) resemble empty shells of the virus and NoV P-particles contain only protruding domains of the NoV capsid. Both NoV-derived subviral particles show similar functionality and antigenicity in vitro and are considered to be potential vaccine candidates against NoV gastroenteritis. BALB/c mice were immunized with baculovirus-produced GII-4 VLPs or the corresponding Escherichia coli-produced P-particles by the intramuscular or intradermal route and the NoV-specific antibody and T-cell immune responses were compared. Elevated antibody levels were induced with a single VLP immunization, whereas P-particle immunization required a boost. High avidity antibodies were raised only by VLP immunization. VLP immunization resulted in a balanced T helper type 1/type 2 immune response whereas P-particles induced a T helper type 2-biased response. Only VLP immunization primed T cells for interferon-γ production. Most importantly, cross-reactive B and T cells were induced solely by VLP immunization. In addition, VLP antiserum blocked the binding of heterotypic VLPs to human histo-blood group antigen receptor and saliva. The findings in this study are relevant for the development of NoV vaccines.
Keywords: histo-blood group antigen blocking, immune response, norovirus, P-particles, virus-like particles
Introduction
Noroviruses (NoVs) are the leading cause of non-bacterial acute gastroenteritis worldwide. Most of the NoVs that infect humans belong to the genogroups GI and GII1 and genetic variation among NoV genotypes is wide.2 At present most NoV outbreaks and sporadic cases are caused by genogroup GII viruses, especially by genotype GII-4.3 The NoVs are non-enveloped viruses and have an outer capsid consisting of a single structural protein, the capsid protein (VP1) organized into 90 dimers, which exhibit T = 3 icosahedral symmetry.4 The capsid proteins have two major domains, a shell (S) domain and protrusion domains (P), which are linked together by a short hinge (H) region. The S-domain is responsible for the formation of the interior shell from which arch-like P-dimers extend outwards.5 The P-domain consists of P1 and P2 subdomains located on the surface of the capsid. The majority of the genetic variation is identified in the P2 domain.4,5 Mutagenesis6 and X-ray crystallography7 studies have shown that P2-domain binds to histo-blood group antigen (HBGA), which is the putative NoV docking site and receptor for entry into the host cell.8
Norovirus VP1 proteins have the ability to self-assemble to form virus-like particles (VLPs) deprived of viral genetic material, which morphologically and antigenically resemble the native virus.9 Different expression systems have been developed to produce the capsid in the form of VLPs. Most commonly, recombinant baculoviruses are used to express NoV capsid proteins in insect cells.9 P-domains alone have also been expressed in vitro, typically in a bacterial Escherichia coli-based cloning and expression system.10–12 The P-domain monomers form dimers in vitro which can further self-assemble into larger complexes, P-particles, consisting of 12 P-dimers having the total molecular weight of 830 000.13 The relevance of the E. coli system is that large quantities of a recombinant protein can be produced at low cost.11 Furthermore, linking the P-domain genetically with an affinity tag makes the purification process reasonably straightforward.
Morphological and biological characterization of NoVs has been challenging because of the lack of a cell culture system.14 Use of the two subviral particles, VLPs and P-particles, has added greatly to the understanding of the NoV structure and biology. Several studies, including our own, showed similar functionality and antigenic properties of recombinant NoV VLPs produced by the baculovirus expression system and recombinant P-particles produced in E. coli.10,11,13,15 In particular, HBGA binding studies revealed that P-particles are able to bind to the HBGA receptor with intensity similar to that of the corresponding VLPs.15 In addition, a hyperimmune animal serum raised against the P-domain is able to recognize NoV VLPs and can be used, for example, in various enzyme immunoassays.10,15 The P-particles have also been proposed as an alternative to VLP-based vaccine development against NoV gastroenteritis because they have been shown to induce antibody responses in mice able to block binding of NoV VLPs to the HBGA receptor.15,16 In general, VLP are very immunogenic and various VLP-based vaccine candidates to other viruses (i.e. hepatitis B virus, human papillomavirus, influenza virus, parvovirus, HIV etc.) have been found to be safe, immunogenic and effective in pre-clinical and clinical trials and some of them have already been commercialized.17
The present study compares the in vivo immunogenicity of the two potential NoV subunit vaccine candidates, GII-4 VLPs and GII-4 P-particles in BALB/c mice. Despite earlier findings of similar antigenic and receptor-binding properties described above, our results demonstrate the superiority of the VLPs in the induction of a T helper type 1 (Th1) and Th2 balanced cross-reactive immune response compared with the P-particles.
Materials and methods
Production and purification of baculovirus-expressed NoV VLPs and E. coli-expressed P-particles
The NoV GII-4 (1999, GenBank ID: AF080551), GII-4 New Orleans (GII-4 NO, 2010, GenBank ID: GU445325), GII-12 (1998, GenBank ID: AJ277618) and GI-3 (2002, GenBank ID: AF414403) VLPs used in immunizations and as antigens in ELISAs were expressed in a BV–insect cell system and purified by sucrose gradients as described earlier.10,18 Polyhistidine-tagged P-proteins were produced in E. coli and the protein was isolated by Ni-NTA affinity chromatography as described in detail elsewhere.10 The purity of the VLPs and P-proteins was verified by SDS–PAGE.10,18 The morphology and the integrity of the VLPs and the P-protein formation in P-particles were verified by electron microscopy (Fig. 1). The double-stranded DNA (dsDNA) content of the VLP preparation was determined by the Quant-it dsDNA Broad-Range Assay kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and found to be 10 ng/dose. The functional and antigenic properties of both products were tested in an HBGA binding assay, Western blot and ELISA methods as published earlier.10,18,19
Figure 1.
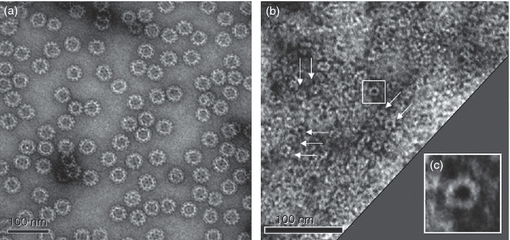
Electron microscopy images of purified norovirus (NoV) capsid GII-4 virus-like particles (VLPs) (a) and P-particles (b). Typical ring-shaped structures of P-particles are indicated with arrows. An enlarged image of a single P-particle (squared in panel b) is shown (c). VLPs and P-particles were negatively stained with 3% uranyl acetate (pH 4.5) and the preparations were examined using FEI Tecnai F12 electron microscope operating at 120 kV.
Study animals, immunization and sample collection
Female BALB/c OlaHsd mice were obtained from Harlan Laboratories (Horst, the Netherlands). The mice were 7 weeks old at the time of the first immunization. All procedures were authorized and performed according to the guidelines by the Finnish National Animal Experiment Board. The mice were anaesthetized before immunization with a formulation of Hypnorm (VetaPharma Limited, Leeds, UK) and Dormicum (Roche Pharma AG, Grenzach-Wyhlen, Germany). The mice were immunized (four to five mice/experimental group) twice, at week 0 and week 3 with 10 μg GII-4 VLPs or P-particles by a needle injection administered intramuscularly (IM) or intradermally (ID). Blood samples were collected from the tail vein at study weeks 0 (pre-immunization bleed), 2, 3 and 4. Sera from mice receiving no antigen (naive mice) were used as a negative control. Mice were killed at study week 5 and whole blood and lymphoid tissue were collected.
Blood and lymphoid tissue preparation
Tail blood and whole blood samples were centrifuged (Himac CT15RE; Hitachi, Twinsburg, OH) at 3500 g 20 min and the serum was separated and stored at −20°. Spleens from killed mice were collected in Hanks’ balanced salt solution (HBSS) (Sigma-Aldrich, St Louis, MO). The structure of the spleen was disrupted with a scalpel and a single-cell suspension was prepared using a 70-μm cell strainer (Becton, Dickinson and Company, Franklin Lakes, NJ). Cell suspensions were centrifuged 300 × g for 10 min (Multifuge 3SR Plus; Heraeus, Wehrheim, Germany) and the cells were resuspensed in HBSS. The HBSS diluted 1 : 10 and pH balanced with 7·5% sodium bicarbonate (Sigma-Aldrich) was added to lyse the red blood cells and the molarity of the suspension was recovered with 2 × HBSS. The cells were washed in HBSS, counted in Bürker's chamber and stored in liquid nitrogen in sterile freezing medium (RPMI-1640 supplemented with 40% fetal bovine serum (FBS) and 10% DMSO, all from Sigma-Aldrich) for further use.
Synthetic peptides
Peptides that were 15 amino acids long covering a region of the P1 capsid domain of NoV GII-4 (CLLPQEWVQHFYQEA), GII-4 NO (CLLPQEWVQYFYQEA) and GII-12 (CLLPQEWIQHLYQES) corresponding to a T-cell epitope (amino acids 461–475) published earlier by LoBue et al.20 and a rotavirus 14-mer VP6 peptide (amino acids 289–302, RLSFQLMRPPNMTP21) were synthesized by Proimmune Ltd. (Oxford, UK). The lyophilized peptides were dissolved in DMSO (Sigma-Aldrich) and diluted in working stock aliquots (0·1 mg/ml) in sterile PBS (Sigma-Aldrich) and stored at −80° for further use.
Serum IgG and IgG subtype ELISA
Sera from immunized and naive mice were tested for total IgG, IgG1 and IgG2a by ELISA. NoV GII-4, GII-4 NO, GII-12 and GI-3 VLPs in PBS were used to coat 96-well Nunc Immuno Maxisorp plates (Thermo Fisher Scientific Inc., Waltham, MA) (0·4–1 μg/ml). After blocking with 5% milk (Sigma-Aldrich) in PBS the serum samples diluted 1 : 200 or serial twofold dilutions were added to the plates and incubated for 1 hr at 37°. All the serum and secondary antibody dilutions were prepared in 1% milk + 0·05% Tween-20 in PBS and added to the wells at a volume of 100 μl. Between each step the plates were washed six times with 0·05% Tween-20 in PBS. For the detection of antigen specific IgGs, horseradish peroxidase (HRP) -conjugated anti-mouse IgG (Sigma-Aldrich) was used at a dilution of 1 : 4000. Anti-GII-4 IgG subtype responses were determined using goat anti-mouse IgG1 or IgG2a HRP conjugate (Invitrogen) at a dilution of 1 : 6000. The secondary antibody was incubated for 1 hr at 37° and O-phenylenediamine dihydrochloride (SIGMAFAST OPD, Sigma-Aldrich) at a concentration of 0·4 mg/ml was used as a substrate for HRP. After 30 min the colour reaction was stopped with 2 m sulphuric acid. Absorbance (optical density, OD) at a wavelength of 490 nm was measured in a microplate reader (Victor2 1420; Perkin Elmer, Waltham, MA). Each plate, in addition to test sera, contained blank wells, a NoV GII-4/GII-4 NO/GII-12/GI-3-positive mouse serum and naive mouse serum in duplicate. Background signal (mean OD value from blank wells) was subtracted from all readings on the plate. Cut-off value was calculated from the OD values of the naive mice sera as follows: mean OD + 3 × SD. A sample was considered positive if the net OD value was above the set cut-off and at least 0·100 OD. End-point antibody titres were defined as the highest dilution of serum giving an OD above the set cut-off value. A Th2/Th1 response ratio was calculated by dividing the end-point titre of IgG1 response with the corresponding IgG2a titre.
Antibody avidity
An avidity assay to detect high avidity NoV antibodies was adopted from Rockx et al.22 Briefly, the IgG ELISA was conducted as described in the previous section except that after the incubation of serum samples (1 : 200 dilution) on NoV GII-4 VLP-coated microtitre plates the samples were aspirated and 8 m urea (Sigma-Aldrich) was added (250 μl/well). Two 5-min incubation steps with urea were used to remove the low avidity antibodies. The avidity index was calculated as (OD with urea/OD without urea) × 100% and an index value of ≥ 50% was considered to be high avidity.
Denaturing and native Western blot analysis
Norovirus capsids (VLPs) were separated by 12% denaturing SDS–PAGE or non-denaturing (native) PAGE and transferred under similar conditions onto the nitrocellulose paper. Native PAGE was run similarly to the SDS–PAGE with the exception that no β-mercaptoethanol and SDS were added and the protein samples were not boiled. In non-denaturing (native) Western blot the proteins were transferred on nitrocellulose paper at 4° to avoid protein denaturing. The staining of proteins on the nitrocellulose paper was performed as described elsewhere.10 Briefly, transferred proteins were detected using sera from mice immunized with NoV GII-4 VLPs or P-particles (dilution 1 : 500), bound IgGs were detected with HRP-conjugated goat anti-mouse IgG (Sigma Aldrich).
Antibody blocking assays
The blocking assays using synthetic carbohydrate HBGAs and human saliva were conducted as described by others8,15 with some modifications. Binding of NoV GII-4 and GI-3 VLPs to synthetic carbohydrate HBGA receptors H-type-3 and Leb (Lectinity Holdings, Inc., Moscow, Russia) was tested before conducting blocking assays. Both VLPs bound to H-type-3 but not to the control carbohydrate Leb (ref. 18 and data not shown). In the blocking assay all the dilution buffers, incubation conditions and washing steps were identical to those described for ELISA unless otherwise specified. Microtitre plates were coated overnight at 4° with either GII-4 (200 ng/well) or GI-3 (50 ng/well) VLPs. The sera were serially diluted starting from 1 : 200 for the detection of homotypic blocking or heterotypic cross-blocking. After the incubation of sera, the biotinylated H-type-3 was added at a concentration of 40 μg/ml (100 μl/well) and incubated at 37° for 4 hr. The bound carbohydrate was detected using 1 : 2000 diluted streptavidin-conjugated HRP (Thermo Fisher Scientific Inc.) and OPD substrate. In the saliva blocking assays human type A saliva was diluted 1 : 3000 in PBS and coated on a microtitre plate. For the type-specific blocking assays GII-4 VLPs (final concentration 0·2 μg/ml) were pre-incubated with twofold titrated serum samples (1 : 100 to 1 : 12 800 dilutions) at 37° for 1 hr before adding to the plates. For the cross-blocking, GII-4 NO VLPs (0·1 μg/ml) were pre-incubated with the sera diluted 1 : 10 to 1 : 640. The plates were incubated for 1 hr and developed and measured as described above. An OD reading from the wells lacking serum was considered as a maximum signal for the binding of VLPs to the synthetic HBGAs or saliva. Blocking index was calculated as the percentage of the maximum binding blocked by a certain serum dilution and was calculated as 100% – ([OD wells with serum/OD wells without serum] × 100%).
ELISPOT interferon-γ assay
Enzyme-linked immunosorbent spot (ELISPOT) assay was used to measure NoV-specific T-cell response by quantification of interferon-γ (IFN-γ) production from the splenocytes. Multiscreen HTS-IP filter plates (Millipore, Billerica, MA) were coated with 100 μl anti-mouse IFN-γ monoclonal antibody (Nacka Strand; Mabtech AB, Sweden) at 2·5 μg/ml. After washing with cell culture grade PBS (Bio-Whittaker; Lonza, Basel, Switzerland) the plates were blocked with cell culture medium (CM) (RPMI-1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μm 2-mercaptoethanol and 2 mm l-glutamine, all purchased from Sigma-Aldrich) containing 10% FBS (Sigma-Aldrich) for 3 hr at room temperature. The NoV-capsid-derived peptides (GII-4, GII-4 NO and GII-12 specific) and a negative control peptide (rotavirus VP6 specific), were added to the plates at a final concentration of 5 μg/ml. Liquid nitrogen frozen splenocytes were thawed, washed, suspended in CM with 10% FBS and added to the wells (0·1 × 106 cells/well). Cells without the peptides (CM alone) and cells stimulated with concanavalin A (Sigma-Aldrich) at 10 μg/ml were used as controls. After 24 hr of incubation at 37° and 5% CO2 the cells were discarded and the plates were washed first with PBS and then with PBS containing 0·05% Tween and 1% FBS. Biotinylated Anti-Mouse IFN-γ monoclonal antibody (Mabtech) was added in PBS containing 10% FBS at 2 μg/ml and incubated for 16–18 hr at 4°. After incubation the plates were washed and streptavidin-HRP in PBS/10% FBS diluted 1 : 500 was added (50 μl/well). After 1 hr of incubation the plates were washed and the spots were developed with a 3-amino-9-ethylcarbazole substrate staining kit (Sigma-Aldrich). The reaction was stopped after 8 min with tap water. The spots were counted under a dissection microscope by two independent counters and the results are expressed as mean spot-forming cells (SFC) per 106 splenocytes of duplicate wells. The result was considered positive if the mean SFC with the specific peptide was above the mean SFC of the negative control peptide + 3 × SD.
Statistical analyses
Mann–Whitney U-test was used to assess the intergroup differences in antibody magnitude and blocking ability. All hypothesis testing was two-tailed. Statistical significance was defined as P<0·05.
Results
High levels of NoV-specific IgG antibodies were induced by a single VLP immunization
Genogroup II (GII)-4 VLP immunization induced a strong GII-4-specific IgG response in all the study animals by both immunization routes (Fig. 2a). Although GII-4 P-particle immunization also resulted in a relatively high level of IgG, the antibody responses of the groups immunized with the P-particles by either of the routes were significantly lower than in the groups immunized with the VLPs (all P<0·05) (Fig. 2a). No GII-4-specific antibodies were detected in the naive mice. To determine the kinetics of the GII-4-specific IgG antibody response development tail blood samples were taken at several time-points during the study and the level of antibodies was determined by ELISA (Fig. 2b). A sharp increase of IgGs was detected 2 weeks after the first immunization with the GII-4 VLPs and the levels rose slightly after the second immunization. The first immunization with the GII-4 P-particles induced low levels of GII-4-specific IgGs at study week 3. The antibody levels were on average sixfold lower (P=0·01) than what was observed with the VLP immunization at the corresponding time-point. At the time of terminations and after receiving the second dose of the P-particles, the IgG levels had risen to levels similar to those induced by the VLPs after the first immunization, indicating that a booster immunization was needed with the P-particles.
Figure 2.
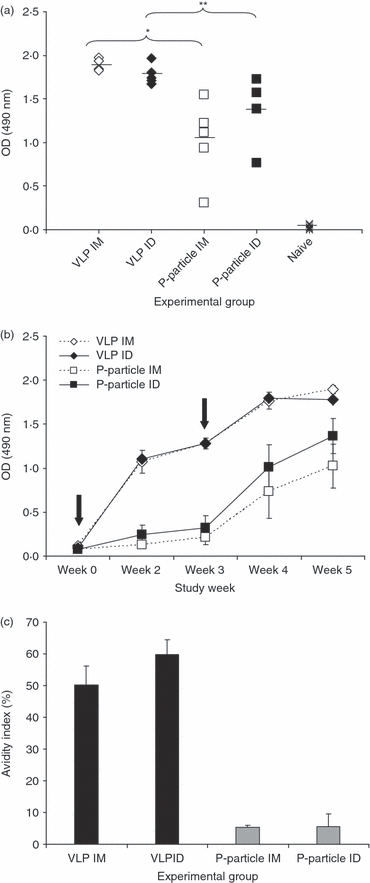
Anti-GII-4 IgG response. BALB/c mice were immunized intradermally (ID) or intramuscularly (IM) twice with 10 μg of norovirus (NoV) GII-4 virus-like particles (VLPs), GII-4 P-particles, or no immunogen (naive). (a) Termination sera were tested in ELISA at 1 : 200 dilutions. The values shown are the optical density (OD) values of each individual serum and the mean OD of the group (horizontal bar). Groups were compared by Mann–Whitney U-test; *P=0.016, **P=0.009. (b) Kinetics of norovirus GII-4-specific serum IgG response. Serum samples collected at study weeks 0, 2, 3, 4 and 5 were tested for GII-4-specific IgGs in ELISA at 1 : 200 dilutions. Mean OD values of the groups with standard errors are shown. Immunizations at week 0 and week 3 are indicated with arrows. (c) Mean avidity indices (%) of norovirus GII-4-specific serum IgG antibodies. Termination sera are tested at the dilution of 1 : 200. Bars show the mean avidity index of an experimental group.
High avidity IgGs were induced by the VLPs
The avidity of GII-4-specific antibodies in the termination serum was assayed from all immunized mice and the mean avidity indices of each group are presented in Fig. 2(c). The VLP immunization by both IM and ID routes resulted in IgG antibodies with high avidity (mean avidity indices 50·2 ± 5·9% and 59·7 ± 4·7%, respectively). Instead, extremely low avidity of IgGs for the specific antigen was obtained in groups immunized with the P-particles (mean avidity indices 5·3 ± 0·7% and 5·6 ± 4·0%). It is noteworthy that, in both groups immunized with the P-particles four of the five mouse serum IgGs were stripped down completely by the urea treatment (OD value 0·003–0·036).
Th1/Th2 balanced immune response induced by the VLPs
The systemic NoV GII-4-specific IgG response was further characterized into Th1 and Th2 responses by measuring IgG antibody subtypes IgG2a and IgG1,23 respectively. Figure 3 shows the GII-4-specific IgG1 and IgG2a antibody levels of immunized mice measured in an end-point serum titration ELISA. The VLPs induced high levels of both IgG subtypes resulting in identical end-point titres (1 : 204 800) of IgG1 and IgG2a and therefore in a Th2/Th1 ratio of 1 (Fig. 3a) as described in the Materials and methods section. In contrast, P-particle immunization elicited relatively high levels of IgG1 (end-point titre 1 : 51 200) but low levels of IgG2a (end-point titre 1 : 1600) resulting in a Th2/Th1 ratio of 32 (Fig. 3b). Mice immunized with the VLPs produced on average 128-fold higher levels of IgG2a than mice immunized with the P-particles as judged from the end-point titres.
Figure 3.
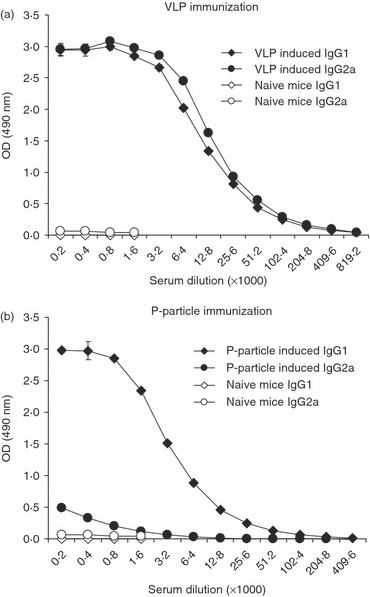
End-point serum titrations of GII-4 specific IgG1 and IgG2a subtype antibody responses of groups of mice immunized intramuscularly with GII-4 virus-like particles (VLPs) (a) or P-particles (b). Mean (± SEM) titres of the sera from each group are shown.
The difference in the induction of Th1 responses by VLPs and P-particles was further studied by measuring IFN-γ production from mice splenocytes in an ELISPOT assay. Cells of mice immunized by the VLPs produced IFN-γ when stimulated in vitro with synthetic 15-mer peptides representing a T-cell epitope20 of the P1 capsid domain of GII-4 (mean 551 ± 73 SFC/106 cells), and a corresponding sequence in GII-4 NO capsid (mean 318 ± 41 SFC/106 cells) and GII-12 capsid (mean 372 ± 47 SFC/106 cells) (Fig. 4). In contrast, P-particle immunization did not induce IFN-γ production by T cells when stimulated with any of the NoV peptides (Fig. 4). Cell viability was similar in all groups controlled by concanavalin A stimulation. No response to the negative control peptide (rotavirus VP6 derived) was detected in any of the groups.
Figure 4.
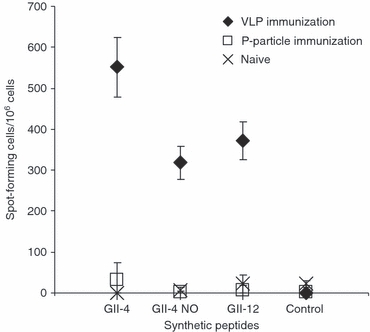
Norovirus (NoV) -specific interferon-γ (IFN-γ) response after immunization with GII-4 virus-like particles (VLPs) or P-particles. Splenocytes from the immunized or naive mice were stimulated with the synthetic NoV-specific peptides from the three different NoV genotypes (GII-4, GII-4 New Orleans and GII-12) and a negative control peptide (rotavirus VP6-specific) and analysed for IFN-γ production with the ELISPOT. The mean spot-forming cells (SFC)/106 cells with the error bars are shown.
P-particle antisera did not detect the unfolded structure of the GII-4 capsid proteins in Western blot analysis
The immunological properties of the serum antibodies were further characterized by Western blot analysis. Sera from the mice immunized with the GII-4 VLPs or the P-particles were used to detect the denatured or non-denatured NoV capsid proteins. As shown in Fig. 5(a) under denaturing conditions, VLP antisera detected the unfolded capsid proteins with a great intensity but no binding was detected with the P-particle immunized mouse serum. However, when the proteins retained their native conformation (Fig. 5b) sera from both immunized groups were able to detect NoV capsids with equal intensity. No binding of naive serum to capsid proteins was detected (data not shown).
Figure 5.
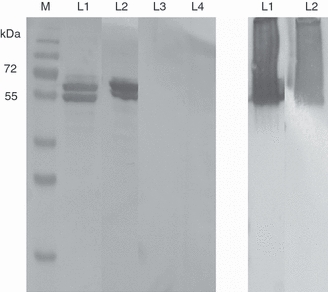
Detection of norovirus GII-4 capsid protein in a Western blot. (a) Sera from mice immunized with GII-4 virus-like particles (VLPs) intramuscularly (IM; L1) or intradermally (ID; L2) or immunized with P-particles IM (L3) or ID (L4) were used to detect denatured GII-4 capsid proteins. M; protein weight marker. (b) Sera from mice immunized ID with GII-4 VLPs (L1) or with P-particles (L2) were used to detect GII-4 capsid proteins in their native (non-denatured) conformation. Native PAGE in which the protein samples were not denaturized was run in a similar manner to the denaturing SDS–PAGE with the exception that no β-mercaptoethanol and SDS were added, the protein samples were not boiled and the transfer of the proteins on nitrocellulose paper was conducted at 4° to avoid protein denaturation.
Heterotypic immune response was generated by the VLP but not the P-protein immunization
To examine the cross-reactivity of the antibodies induced by the GII-4 VLPs or P-particles the termination sera were assayed against heterologous GII-4 NO, GII-12 and GI-3 VLPs in an ELISA (Fig. 6a). VLP immunization induced cross-reactive antibodies to all of the heterologous VLPs. In contrast, P-particle immunization did not stimulate a cross-reactive antibody response to any of the heterologous antigens and the level of cross-reactive antibodies in the sera was comparable to that in the sera of naive mice (all P>0·05). Slightly elevated NoV GII-4 NO-specific IgG levels were detected in both P-particle-immunized groups (Fig. 6a) as the mean ODs of the groups exceeded the cut-off value and therefore the magnitude of the NO-specific response was further analysed by an end-point titration assay (Fig. 6b). The mean end-point titres of GII-4 NO-specific antibodies were 1 : 400 (IM immunization) and 1 : 1600 (ID immunization). The corresponding mean end-point titres of the NO-specific sera for VLP immunized mice were 1 : 12 800 and 1 : 102 400. Interestingly, the cross-reactivity of the IgG antibodies observed with the VLP immunization was directed at NoV capsid antigens within the genogroup and between the genogroups. Moreover, only the VLP immunized mice showed T-cell responses to GII-4 NO and GII-12-derived synthetic peptides (Fig. 4) as described in detail in the section on the Th1/Th2 balanced immune response induced by the VLPs.
Figure 6.
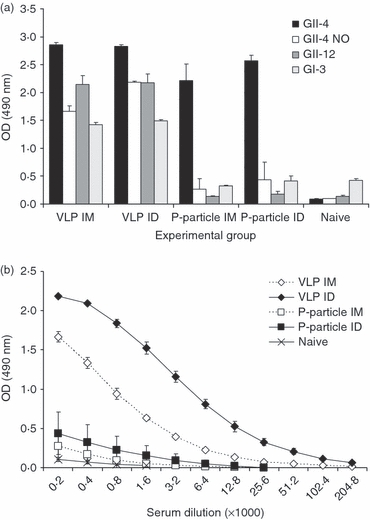
Levels of cross-reactive serum IgG antibodies to norovirus (NoV) GII-4 NO, GII-12 and GI-3-derived virus-like particles (VLPs). Mice were immunized intramuscularly (IM) or intradermally (ID) with GII-4 VLPs or P-particles and termination sera were tested in ELISA with homologous and heterologous VLPs. Naive mice were used as negative controls. (a) The mean IgG levels (± SEM) for different VLPs used are shown for each experimental group. (b) GII-4 NO-specific IgG responses were analysed in serum end-point titration ELISA. The mean optical density (OD) values of each serum dilution and the SEMs for each experimental group are shown.
VLP antiserum showed greater blocking activity than P-particle antiserum
Histo-blood group antigen blocking assays were conducted to test GII-4 VLP and P-particle antiserum blocking of GII-4 VLPs binding to the synthetic H-type-3 receptor (Fig. 7a,b) and to human type A saliva (Fig. 7c,d). In the synthetic H-type-3 blocking assay high blocking ability (maximum blocking, 83%) at serum dilutions 1 : 1600 (IM route) and 1 : 3200 (ID route) was detected for the VLP antiserum. To obtain maximum blocking with the P-particle antiserum eightfold and fourfold (Fig. 7a,b, respectively) more concentrated serum dilutions were needed compared with the VLP antiserum. Maximum blocking of the naive mouse serum was 1·2% at a 1 : 200 dilution (data not shown). A similar difference in the blocking activities of GII-4 VLP antisera and P-particle antisera was observed in saliva blocking assays for IM-immunized groups (Fig. 7c) and ID-immunized groups (Fig. 7d). In addition to the type-specific blocking described above, the blocking activity of the cross-reactive antibodies to GII-4 NO was tested in a saliva blocking assay. As shown in Fig. 7(e,f) only VLP immunization resulted in cross-blocking antibodies against GII-4 NO. Furthermore, the blocking activity of GI-3-specific antibodies detected after VLP immunization was tested in an assay in which GII-4 VLP antiserum was used to block the GI-3 VLPs binding to the synthetic H-type-3 receptor. A blocking index of 53% was obtained with a serum dilution of 1 : 400 and a serum dilution of 1 : 800 still blocked 27% of the specific binding (data not shown). The results from these assays indicate that NoV VLP but not the P-particle immunization induces cross-blocking antibodies.
Figure 7.
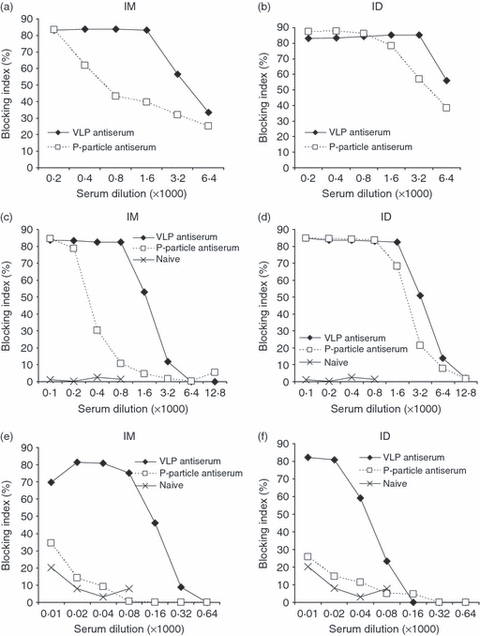
Blockage of norovirus (NoV) GII-4 virus-like particles (VLPs) binding to histo-blood group antigen (HBGA) H-type-3 receptor (a and b) or to human type A saliva (c and d) by sera of mice immunized with the GII-4 VLPs or P-particles. Blockage of NoV GII-4 New Orleans (NO) VLPs binding to human type A saliva (e and f) by sera of mice immunized with the GII-4 VLPs or P-particles. The panels on the left show the results of intramuscular (IM) immunizations and those on the right show the results of intradermal (ID) immunizations. The blocking indices (%) from group-wise pooled and twofold titrated sera are shown.
Discussion
The functional and antigenic properties of NoV VLPs and P-particles have previously been characterized.10,15,16,18 Although P-particles contain only the P-domains of the NoV capsid and lack the conserved shell domain, several studies have demonstrated the similar antigenic and receptor-binding properties of the two NoV subunit particulate structures.13,15 NoV VLPs induce systemic and mucosal immune responses in small animal models24,25 and in humans.26–29 P-particle immunogenicity studies have been conducted in mice.15,16 Both NoV VLPs and P-particles possess unique immunogenicity-enhancing features including particulate nature and repetitive, high density display of antigens and both are attractive candidates for NoV vaccine development.30–32 However, no studies have been conducted to compare the antibody-mediated and cellular immune responses induced by the VLPs compared with P-particles. Our data demonstrate the superiority of the NoV GII-4 VLPs in the induction of a high quality heterotypic immune response compared with the GII-4 P-particles.
Consistent with the results of others15,16,24,25 high levels of type-specific IgG antibodies were obtained by both NoV GII-4 VLP and P-particle immunizations of BALB/c mice, but the quality of the immune response was different. A strong GII-4-specific IgG response was achieved only after two doses of the P-particles, whereas a similar response was obtained after one dose of the VLPs. Furthermore, IgG subtypes obtained with the P-particles were predominantly of IgG1 isotype, indicative of a Th2 immune response, whereas VLPs were strong inducers of both subtypes, IgG1 and IgG2a, indicative of a mixed and balanced Th1/Th2 response. As Th1 cytokine IFN-γ production was induced only in the cells of mice immunized with the VLP the result confirmed that only VLPs were able to activate T cells and stimulate cell-mediated immunity even in the heterologous strains. LoBue et al.20 also showed that IFN-γ secretion was achieved when the cells were stimulated with the peptides corresponding to homologous and heterologous T-cell epitopes. Here we showed that IFN-γ production from the T cells can be induced when stimulated by a genetically related genotype (GII-4 NO) and a distant genotype (GII-12) derived peptides having up to three amino acids different in a single T-cell epitope (GII-12), which in part supports the hypothesis of GII NoV T-cell epitope conservation.20 In a NoV challenge study33 conducted in humans the authors discussed that activation of Th1 responses may have been associated with protection against NoV infection in some volunteers. Moreover, a murine norovirus challenge study revealed that the clearance of the virus from the tissues was achieved only when CD4+ and CD8+ T cells were activated along with the B cells.34 The results described above20,33,34 strongly suggest that T-cell responses are an important factor in protective NoV immunity.
The dramatic difference observed between the Th1 responses induced by the VLPs and P-particles indicates that there may be different immunological mechanisms involved in antigen processing. Possibly the macromolecular structure of VLPs is preferred in the endocytic process of the antigen-presenting cells, especially dendritic cells and macrophages, thereby activating particulate T-cell responses. Possibly another set of antigen-presenting cells, other than dendritic cells, or another dendritic cell subset is responsible for the antigen presentation of P-particle, which leads to a strongly Th2-biased response.35,36 However, it is not excluded that the trace amount of DNA found in the VLP preparations (10 ng/dose, respectively) could have influenced the induction of IgG2a as earlier described by Jegerlehner et al.37
The immunological properties of serum antibodies were also characterized by Western blot analysis. All antibodies induced by the P-particle immunization were presumably formed against conformational epitopes of NoV P-domain as they did not react in Western blot with denatured NoV capsid proteins. On the contrary, antibodies induced by VLPs reacted against both conformational and linear epitopes of the NoV capsid. The reason for this observation may be that immunodominant linear epitopes are formed by the shell domain of the capsid, which is lacking in the P-particles. The role of these antibodies in NoV infection is not known but linear epitopes of feline calicivirus capsid protein have been shown to induce neutralizing antibodies.38 This leads to the conclusion that conserved as well as variable domains of the capsid protein should be included in NoV subunit vaccines.
Avidity or affinity maturation of antibodies is the indicator for how morphologically precise the antigen-binding epitope is and usually the avidity increases in time and as a function of antigen exposures.39 High avidity antibodies appear to correlate with protection of the disease in the case of several viral infections40–43 and lower titres of antibodies are needed to neutralize the virus when the avidity of antibodies is high.44,45 The induction of high avidity IgGs by NoV GII-4 VLPs shown here and poor avidity IgGs by P-particles towards NoV indicates that there probably are some critical elements in the repetitive structure of the VLPs affecting the antibody binding site formation. Possibly the antibodies formed toward linear epitopes of an antigen possess more avidity or high avidity antibody formation may be NoV capsid shell domain dependent.
Different genotypes of NoVs fluctuate in a year-by-year manner and an effective NoV vaccine should provide protection against a number of existing and future genotypes, within and between genogroups.30,31 We studied the cross-reactivity of antibodies induced by the monovalent GII-4 VLPs and P-particle immunizations using GII-4 NO as a representative genotype in the GII-4 genocluster, GII-12 VLPs as a representative genotype in the GII genogroup distinct from the GII-41 and GI-3 VLPs as a representative genotype in the GI genogroup as antigens in ELISA. The VLP immunization induced a heterotypic response with cross-reactive antibodies towards all the heterotypic VLPs used in this study. In contrast, no statistically significant cross-reactive antibody response was induced by the P-particles, indicating a type-specific immune response only. Furthermore, only VLPs but not P-particles could stimulate the cell-mediated immune response towards heterotypic NoV genotypes (GII-4 NO and GII-12). We suggest that a Th1/Th2 balanced immune response has a role in the observed cross-reactivity. Stimulation of cell-mediated immunity reflected by the production of IFN-γ as well as the induction of high IgG2a antibody levels by VLP immunization may be important for the induction of heterotypic immunity in a fashion similar to that of the proteins of influenza virus and rotavirus.46,47
As NoV has been shown to attach to HBGA carbohydrates displayed on the surface of mucosal cells or as free antigens in biological fluids48 these are considered to serve as a receptor for NoV entry in host cells.8,49,50 The neutralizing antibodies raised against NoV after a natural infection or in an animal model have been shown to block the binding of NoV VLP to the synthetic HBGA receptor8,15,51 and to human saliva16 containing a number of different HBGAs. Moreover, recent studies have suggested that HBGA blocking ability is an important factor in achieving protective immunity against NoV19,52 and the present vaccine approaches are focused on stimulating heterotypic HBGA blocking antibodies.28,30,31 Our results demonstrated that both the VLP and P-particle antisera were able to block the GII-4 VLPs binding to HBGA receptor and A-type human saliva. However, in contrast to studies by others,15 our data showed that depending on the immunization route and blocking assay used, a twofold to eightfold greater titre of P-particle antiserum was needed to achieve maximum type-specific blocking compared with the VLP antiserum. Furthermore, the important finding was the ability of GII-4 VLP antiserum to cross-block GII-4 NO and GI-3 VLPs binding to human saliva or to the synthetic H-type-3 receptor, a desirable feature in NoV vaccines. The difference of the blocking activity between VLP and P-particle antisera was somewhat smaller for ID immunized animals compared with IM immunized animals. This difference might be partly explained by the abundance of professional antigen-presenting cells, dendritic cells, present in the epidermis compared with the muscle and therefore more efficient antigen presentation.
The results of the present study show that the quality of immune response should be comprehensively studied when searching for vaccine candidates against NoV disease. We believe that an efficient NoV vaccine should induce cross-reactive antibody responses with neutralizing activity as well as T-cell immunity. We showed that these features are conferred by the VLPs but not the P-particles.
Acknowledgments
We thank Eeva Jokela for technical assistance during the study. We are also grateful to Eija Jokitalo and Helena Vihinen for the guidance and technical help in transmission electron microscopy.
Disclosures
None of the authors have conflict of interest.
References
- 1.Zheng D, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–23. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Hansman GS, Natori K, Shirato-Horikoshi H, et al. Genetic and antigenic diversity among noroviruses. J Gen Virol. 2006;87:909–19. doi: 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 3.Siebenga JJ, Vennema H, Zheng DP, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009;200:802–12. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 4.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–90. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 5.Bertolotti-Ciarlet A, White LJ, Chen R, Prasad BV, Estes MK. Structural requirements for the assembly of Norwalk virus-like particles. J Virol. 2002;76:4044–55. doi: 10.1128/JVI.76.8.4044-4055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan M, Huang P, Meller J, Zhong W, Farkas T, Jiang X. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J Virol. 2003;77:12562–71. doi: 10.1128/JVI.77.23.12562-12571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JM, Hutson AM, Estes MK, Prasad BV. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc Natl Acad Sci U S A. 2008;105:9175–80. doi: 10.1073/pnas.0803275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol. 2002;76:12335–43. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–32. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koho T, Huhti L, Blazevic V, et al. Production and characterization of virus-like particles and the P domain protein of GII.4 norovirus. J Virol Methods. 2011 doi: 10.1016/j.jviromet.2011.05.009. doi: 10.1016/j.viromet.2011.05.009 in press. [DOI] [PubMed] [Google Scholar]
- 11.Tan M, Zhong W, Song D, Thornton S, Jiang X. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J Med Virol. 2004;74:641–9. doi: 10.1002/jmv.20228. [DOI] [PubMed] [Google Scholar]
- 12.Yoda T, Terano Y, Shimada A, et al. Expression of recombinant Norwalk-like virus capsid proteins using a bacterial system and the development of its immunologic detection. J Med Virol. 2000;60:475–81. doi: 10.1002/(sici)1096-9071(200004)60:4<475::aid-jmv17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Tan M, Jiang X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–30. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 15.Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, Jiang W, Jiang X. Noroviral P particle: structure, function and applications in virus–host interaction. Virology. 2008;382:115–23. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Huang P, Xia M, et al. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85:753–64. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–76. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 18.Huhti L, Blazevic V, Nurminen K, Koho T, Hytonen VP, Vesikari T. A comparison of methods for purification and concentration of norovirus GII-4 capsid virus-like particles. Arch Virol. 2010;155:1855–8. doi: 10.1007/s00705-010-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurminen K, Blazevic V, Huhti L, Rasanen S, Koho T, Hytonen VP, Vesikari T. Prevalence of norovirus GII-4 antibodies in Finnish children. J Med Virol. 2011;83:525–31. doi: 10.1002/jmv.21990. [DOI] [PubMed] [Google Scholar]
- 20.LoBue AD, Lindesmith LC, Baric RS. Identification of cross-reactive norovirus CD4+ T cell epitopes. J Virol. 2010;84:8530–8. doi: 10.1128/JVI.00727-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banos DM, Lopez S, Arias CF, Esquivel FR. Identification of a T-helper cell epitope on the rotavirus VP6 protein. J Virol. 1997;71:419–26. doi: 10.1128/jvi.71.1.419-426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rockx B, Baric RS, de Grijs I, Duizer E, Koopmans MP. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J Med Virol. 2005;77:439–46. doi: 10.1002/jmv.20473. [DOI] [PubMed] [Google Scholar]
- 23.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 24.Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345–53. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75:9713–22. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–8. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 27.El-Kamary SS, Pasetti MF, Mendelman PM, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, Moe CL, Baric RS. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24:5220–34. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 29.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–7. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 30.Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010;9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinje J. A norovirus vaccine on the horizon? J Infect Dis. 2010;202:1623–5. doi: 10.1086/657088. [DOI] [PubMed] [Google Scholar]
- 32.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and Rotavirus VP6 as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29:8126–33. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following snow mountain virus challenge. J Virol. 2005;79:2900–9. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008;4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon S, Agrawal A, Van Dyke T, et al. A toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-fos in dendritic cells. J Immunol. 2004;172:4733–43. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 37.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–20. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 38.Almanza H, Cubillos C, Angulo I, Mena I, Borrego B, Barcena J. Santa Cruz, Chile: 2010. Fine mapping of linear epitopes on the capsid protein of feline calicivirus (FCV) recognized by neutralizing and non-neutralizing monoclonal antibodies in: Fourth International Conference on Caliciviruses. Poster presentation. [Google Scholar]
- 39.Murphy K, Travers P, Walport M. Immunological memory. In: Masson S, Lawrence E, Lucas G, Goatly B, editors. Janeway's immunobiology. 7th edition. New York, Abington: Garland Science; 2008. pp. 442–55. [Google Scholar]
- 40.Bachmann MF, Kalinke U, Althage A, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–7. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 41.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;9:1209–13. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 43.Salmi AA. Antibody affinity and protection in virus infections. Curr Opin Immunol. 1991;3:503–6. doi: 10.1016/0952-7915(91)90011-o. [DOI] [PubMed] [Google Scholar]
- 44.Leonova GN, Pavlenko EV. Characterization of neutralizing antibodies to far eastern of tick-borne encephalitis virus subtype and the antibody avidity for four tick-borne encephalitis vaccines in human. Vaccine. 2009;27:2899–904. doi: 10.1016/j.vaccine.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 45.Ray R, Meyer K, Banerjee A, et al. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J Infect Dis. 2010;202:862–6. doi: 10.1086/655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor PM, Askonas BA. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–20. [PMC free article] [PubMed] [Google Scholar]
- 47.VanCott JL, Prada AE, McNeal MM, et al. Mice develop effective but delayed protective immune responses when immunized as neonates either intranasally with nonliving VP6/LT(R192G) or orally with live rhesus rotavirus vaccine candidates. J Virol. 2006;80:4949–61. doi: 10.1128/JVI.80.10.4949-4961.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, et al. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–77. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P, Farkas T, Marionneau S, et al. Noroviruses bind to human ABO, lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 50.Hutson AM, Atmar RL, Marcus DM, Estes MK. Norwalk virus-like particle hemagglutination by binding to histo-blood group antigens. J Virol. 2003;77:405–15. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, Vinje J. Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol. 2009;83:5363–74. doi: 10.1128/JVI.02518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]


