Abstract
BACKGROUND AND PURPOSE
Retinol-binding protein 4 (RBP4) is an adipocyte-secreted hormone proposed to link obesity with insulin resistance. However, the role of RBP4 in cardiovascular complications is yet to be fully understood. The present study is aimed to decipher the association between RBP4 with pro-inflammatory cytokines and low-density lipoprotein (LDL) cholesterol in diet-induced obese and hyperlipidaemic mice. To understand the correlation, rimonabant, an anti-obesity drug, has been used to relieve the atherosclerotic predisposition.
EXPERIMENTAL APPROACH
Adipose and/or aortic tissue expressions of RBP4, pro-inflammatory cytokine genes and circulating LDL levels were measured in high fat (HF)-fed female C57BL/6 and high cholesterol (HC)-fed apolipoprotein E3 (ApoE3) Leiden mice.
KEY RESULTS
Mice fed a HF diet had a significantly increased adipose expression of RBP4, TNF-α and monocyte chemoattractant protein 1 (MCP-1) and down-regulated adiponectin mRNA levels. A significant increase in aortic RBP4 and MCP-1 expression and circulating levels of LDL and C-reactive protein (CRP) was found in the ApoE3 mice fed a HC diet. Interestingly, rimonabant treatment lowered the elevated aortic RBP4, MCP-1 expressions and significantly reduced the serum levels of LDL, CRP, RBP4 and MCP-1.
CONCLUSION AND IMPLICATIONS
Our results indicate that RBP4 is positively associated with markers of inflammation in obese and pro-atherogenic conditions and could play a role in a predisposition to atherosclerosis. Furthermore, our results indicate that rimonabant may improve vascular function by modulating RBP4 along with pro-inflammatory cytokines.
Keywords: endothelial function, inflammation, LDL cholesterol, RBP4, rimonabant
Introduction
Patients with obesity have an increased risk of developing cardiovascular diseases and extensive atherosclerosis (Rimm et al., 1995; Eckel, 1997; Kopelman, 2000; McGill et al., 2002; Lavie et al., 2009). Over the past few years, accumulating evidence has indicated that increased cytokines secreted from the adipose tissue may be responsible for creating a pro-inflammatory state that in turn results in the development of both insulin resistance and endothelial dysfunction (ED; the initial stage in the development of atherosclerosis) (Berg and Scherer, 2005; Lau et al., 2005). In particular, cytokines such as IL-6 or TNF-α, soluble adhesion molecules and downstream acute phase reactants such as C-reactive protein (CRP) and serum amyloid A have been shown to predict the risk of cardiovascular events.
A recent protein of interest is retinol-binding protein 4 (RBP4), which is preferentially expressed in visceral compared with subcutaneous adipose tissues (Klöting et al., 2007) and appears to be up-regulated in obese rodents (Yang et al., 2005). Elevated serum RBP4 is associated with inflammation, insulin resistance, type 2 diabetes and metabolic abnormalities such as obesity, glucose intolerance, dyslipidaemia and hypertension (Basualdo et al., 1997; Yang et al., 2005; Cho et al., 2006; Erikstrup et al., 2006; Graham et al., 2006; Takashima et al., 2006; Solini et al., 2009; Usui et al., 2009) and is also closely involved in the development of cardiovascular disease (Ingelsson and Lind, 2009; Park et al., 2009).
Cannabinoid type 1 (CB1) receptors are expressed in adipocytes (Cota et al. 2003; Gary-Bobo et al. 2006) and appear to be up-regulated in the adipose tissue of animals with genetic modification- or diet-induced obesity (Yan et al. 2007; Bensaid et al., 2003). Deletion of CB1 receptors leads to leanness and resistance to diet-induced obesity (Cota et al. 2003). These experimental results suggest that the endocannabinoid system is crucial for the understanding of obesity and associated metabolic syndrome. As rimonabant, a CB1 receptor antagonist, has a beneficial effect on serum lipid parameters in humans as well as in animal models of obesity (Poirier et al., 2005), it is expected to prevent atherosclerosis. However, atherosclerosis is a multifactorial disease, and besides dyslipidaemia, inflammation is now recognized as one of the major factors influencing the course of the disease. Interestingly, rimonabant has been shown to reduce the levels of the pro-inflammatory cytokine TNF-α in models of obesity-associated hepatic steatosis (Gary-Bobo et al., 2007) and LPS-induced endotoxaemia (Croci et al., 2003). It also reduces platelet activation (Schäfer et al., 2008) and reduces the inflammatory component of atherosclerotic plaque progression (Dol-Gleizes et al., 2009).
Given the increased propensity of obese patients to develop macrovascular events, therapeutic strategies that limit inflammation in the vessel wall and reduce serum levels of inflammatory mediators as well as RBP4 might be a promising way of preventing vascular disease in this high-risk population. In the present study we assessed the potential anti-inflammatory effect of rimonabant, in addition to its metabolic effects, in particular on the vessel wall. We also investigated the adipose and aortic expression of RBP4 and its correlation with inflammatory and lipoprotein mediators associated with an increased risk of cardiovascular disease, and examined the effect of rimonabant on the expression of RBP4, in aortic tissues, and other pro-inflammatory markers in high cholesterol (HC)-fed apolipoprotein E3 (ApoE3) mice (a model of hypercholesterolaemia and atherosclerosis).
Methods
Animals
C57BL/6 and ApoE3 Leiden mice were housed individually in ventilated cages and given pelleted food (Standard Rodent diet, NIN, Hyderabad, India) and water ad libitum in a temperature- (25 ± 3°C) and humidity-controlled (50–70%) environment with a 12 h/12 h dark–light cycle. All animal care and experimental procedures complied with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals as stated by the National Institutes of Health and were approved by the Institutional Animal Ethics Committee, an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility.
The nomenclature of drug/molecular target conforms to the British Journal of Pharmacology's Guide to Receptors and Channels. (Alexander et al., 2008).
Experimental design
Female C57BL/6 mice were switched to the high-fat (HF) diet (Research Diet, New Brunswick, NJ, USA) for 8 weeks, 4 weeks or 0 days (control) before tissue collection. Blood samples were collected for biochemical estimations; white adipose tissues (WAT) were harvested and snap-frozen for mRNA expression of TNF-α, adiponectin, monocyte chemoattractant protein 1 (MCP-1) and RBP4, whereas aortic tissues were harvested for MCP-1 and RBP4 only using real-time PCR. In another set of experiments, 7- to 8-week-old female ApoE3 mice were kept on HC diet containing 1.5% (wt wt−1) cholesterol (SD Fine Chemicals, Mumbai, India) and 0.25% (wt wt−1) sodium cholate (SD Fine Chemicals) for 8 weeks. Blood was collected for estimation of biochemical parameters. For comparison of RBP4 and MCP-1 expression, animals were killed to isolate the aorta and snap-frozen for real-time PCR. In addition, 10 ApoE3 mice were fed a standard chow diet as control. All mice were 15–16 weeks of age at tissue collection to avoid possible age-related effects.
To investigate the effect of a single-dose rimonabant on RBP4 expression and lipid profiles, female C57BL/6 mice (7–8 weeks old), which were kept for 8 weeks on HF diet, were randomized and were deprived of food overnight to avoid any alterations in feeding behaviour. A single dose of rimonabant (10, 20 or 40 mg·kg−1) or vehicle (0.5% w v−1 Tween 80, SD Fine Chemicals, in water) was administered p.o., and 6 h later blood was collected for estimation of biochemical parameters. The animals were then killed, the abdomen opened and visceral WAT removed, isolated and flash-frozen for determination of mRNA expression of RBP4 using real-time PCR.
In a third set of experiments, female ApoE3 mice were fed with HC diet for 8 weeks. To investigate the chronic effect of rimonabant, 20 mg·kg−1 rimonabant or vehicle (0.5% w v−1 Tween 80 in water) was administered p.o. once daily for 28 days. Mice were weighed every week, and food intake was measured on the last day. At the end of the study, serum was collected and animals were killed; the aorta was removed and isolated and snap-frozen for use in gene expression studies. In addition, 10 ApoE3 mice were fed a standard chow diet containing 4% fat as control.
Cell culture and differentiation
3T3-L1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v v−1) FBS. Confluent cells were induced to differentiate to adipocytes by culturing for 72 h in DMEM containing 10% FBS, 1 mg·mL−1 insulin, 1 mM dexamethasone and 0.5 mM IBMX. The differentiated cells were then allowed to stabilize for 24 h in DMEM containing 10% FBS, after which the cells were treated with rimonabant for 6 h. All test reagents were diluted in culture medium. The cells were harvested for RNA extraction and the culture media were collected for the measurement of RBP4 protein.
RNA analysis and quantitative real-time polymerase chain reaction
WAT and aortic tissue samples were homogenized in TRIzol reagent (Invitrogen, Life Technolgies, Carlsbad, CA, USA) a Polytron hand-held homogenizer (Kinematica, Bohemia, NY, USA). Total RNA was extracted following the manufacturer's protocol. One microgram total RNA from each sample was taken for first-strand cDNA synthesis using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA; Part no. 4322171). An equal amount of cDNA from each sample was taken for quantitative real-time PCR using ABI-prism-7300 (Applied Biosystems, Foster City, CA, USA). FAM-labelled Taqman probes (Applied Biosystems) were used to determine the expression of adiponectin (Mm00456425_m1), TNF-α (Mm00443258_m1) and MCP-1 (Mm00441242_m1). Taqman Universal Mastermix (Cat. No: 430437) were procured from Applied Biosystems for expression profiling of the above-mentioned target genes. A VIC-labelled, housekeeping gene, mouse β-actin (Part No: 4352339E) probe was purchased from Applied Biosystems as 20× concentrate and was co-amplified in each sample with each target gene, for normalizing the results. Gene expression of RBP4 (F: 5′-ACTGGGGTGTAGCCTCCTTT −3′ and R: 5′-GGTGTCGTAGTCCGTGTCG −3′) was determined by use of SYBR Green quantitative real-time PCR using QIAGEN QuantiFast SYBR Green kit (Cat. no. 204052, Qiagen, Germantown, MD, USA). Mouse β-actin (F: 5′-TACAGCTTCACCACCACAGC-3′ and R: 5′-TCTCCAGGGAGG AAGAGGAT-3′) was used as an internal control for normalization of the results of SYBR Green PCR.
Measurement of RBP4 in aorta
Samples of aorta were homogenized in ice-cold Tris buffer containing 1 mM PMSF for RBP4 estimation. RBP4 levels were measured by elisa and were expressed as mg−1 tissue protein, measured using the biuret method (Pointe Scientific, Canton, MI, USA).
Western blotting
Adipose and aortic tissues were cut into small pieces and homogenized in PBS. The homogenate was centrifuged to sediment the tissue fragments. Next, a lysis buffer (25 mM HEPES, Triton-X 0.1%, 300 mM NaCl, 20 mM beta-glycerophosphate, 1.5 mM MgCl2, 0.2 mM EDTA, and 25 mM dithiothreitol) with protease inhibitors (4 mM sodium orthovanadate, 400 mM sodium fluoride, 20 mM benzamidine, 2 µg·mL−1 leupeptin, 4 µg·mL−1 aprotinin, and 500 µM PMSF) was added to the sample. The sample was then vortexed, incubated on ice for 30 min, centrifuged again, and the supernatant was stored at −70°C. For Western blotting, equal amounts of supernatant protein (30 µg) were denatured by boiling for 5 min in SDS sample buffer, separated by 8% SDS-PAGE and transferred to polyvinylidene difluoride membranes for immune blotting. The membranes were blocked with 5% skimmed milk in Tris-buffered saline with Tween 20 [10 mM Tris–HCl (pH 7.6), 150 mM NaCl and 0.5% Tween 20] and probed with RBP4 antibodies (1:2000) (Enzo Life Sciences, Inc., Plymouth Meeting, PA, USA). Bound antibodies were revealed with horseradish peroxidase-conjugated secondary antibodies (1:500) using an enhanced chemiluminescent solution (Pierce, Rockford, IL, USA).
Serum measurement
Serum triglyceride (Pointe Scientific), total cholesterol (Pointe Scientific), low-density lipoprotein (LDL; Randox Laboratories, Antrim, UK), high-density lipoprotein (HDL; Randox Laboratories, UK), free fatty acid (FFA) (Randox Laboratories) levels were determined by the colorimetric method using a biochemical kit. CRP (Randox Laboratories) levels were determined by the immunoturbidometric method using a biochemical kit. RBP4 (Phoenix Secretomics, Burlingame, CA, USA), MCP-1 (R&D System, Minneapolis, MN, USA) and adiponectin (B Bridge, Mountain View, CA, USA) levels in the serum were determined by elisa kits using the methods specified by the manufacturer. Interassay coefficient for RBP4 = 7.24–8.08, adiponectin = 4.6–7.2 and MCP-1 = 5.5–7.3 and intra-assay coefficients for RBP4 = 7.24–8.08, adiponectin = 2.5–5.8 and MCP-1 = 5.3–8.3.
Data and statistical analysis
Data are expressed as mean ± SEM. The statistical analysis was performed using one-way anova followed by Dunnett's test used for comparison of all the parameters between treatment and control groups. Differences in expression or biochemical parameters between two groups were compared using Student's t-test. Spearman's correlation coefficients (rS) were used to describe the linear association between variables. For each analysis, P-values less than 0.05 were considered to be statistically significant. All analyses were performed using GraphPad Prism software (GraphPad, La Jolla, CA, USA).
Results
HF diet alters the expression pattern of adipocytokines in WAT of C57BL/6 mice
Expressions of TNF-α, MCP-1 and adiponectin in WAT were measured at the start of the study and 4 and 8 weeks of HF exposure. As evident from Figure 1, both TNF-α and MCP-1 mRNAs were significantly increased (90 and 40%, respectively, P < 0.05) and adiponectin mRNA was significantly decreased (40%, P < 0.05) in HF-fed C57BL/6 mice after 8 weeks of exposure to HF diet. However, slight but not significant alterations in the expression of these cytokines were observed after 4 weeks of HF diet exposure (Figure 1). In the case of adipose tissue, the expression of RBP4 was increased early in the course of diet-induced obesity. RBP4 expression tended to be enhanced after 2 weeks (data not shown), was significantly increased (70%, P < 0.05) at 4 weeks and was 140% higher after 8 weeks in the group on an HF diet compared with the chow-fed control group (Figure 1).
Figure 1.

Analysis of the expression of TNF-α (A), MCP-1 (B), adiponectin (C) and RBP4 (D) mRNA in WAT of C57BL/6 mice after different weeks of HF diet exposure. Time is shown as weeks on diet. Fold changes in HF-fed C57BL/6 groups compared to the C57BL/6 control are presented. Each value represents mean ± SEM (n = 6 mice per group). *P < 0.05 compared with age- matched C57BL/6 wild type mice.
HF diet alters the lipid parameters in C57BL/6 mice
As shown in Table 1, after 8 weeks the HF-fed mice were 19% heavier than the chow-fed lean controls (body weight = 31.1 ± 1.6 vs. 25.4 ± 0.4 g; P < 0.05, n = 10 per group). There was a significant increase in LDL, triglyceride (TG), total cholesterol (TC) and FFAs, and decrease in HDL cholesterol in mice on a HF diet after 4 weeks and these remained consistent even after 8 weeks.
Table 1.
Effects of a high fat (HF) diet on the various serum biochemical parameters in C57BL/6 mice
| Variables | 0 week | 4 weeks | 8 weeks |
|---|---|---|---|
| LDL (mM) | 0.22 ± 0.01 | 0.27 ± 0.01* | 0.29 ± 0.01* |
| HDL (mM) | 1.40 ± 0.05 | 1.93 ± 0.07* | 1.83 ± 0.08* |
| NEFA (mM) | 1.15 ± 0.02 | 1.32 ± 0.06* | 1.66 ± 0.09* |
| Triglyceride (mM) | 0.79 ± 0.09 | 1.14 ± 0.11* | 1.21 ± 0.08* |
| Total cholesterol (mM) | 2.48 ± 0.09 | 3.4 ± 0.13* | 4.45 ± 6.7* |
| Body weight (g) | 25.4 ± 0.4 | 28.0 ± 1.4 | 31.1 ± 1.6* |
Values are shown as mean ± SEM.
P < 0.05 as compared to vehicle control.
Effect of single-dose rimonabant on adipose RBP4 and serum LDL levels in HF-fed C57BL/6 mice
Treatment with rimonabant reduced the circulating LDL in a dose-dependent manner (10 mg·kg−1: 5.4%, 20 mg·kg−1: 16.9%, 40 mg·kg−1: 29%; Figure 2A) without altering serum HDL cholesterol in HF-fed C57BL/6 mice. Parallel to its effect on LDL, rimonabant dose-dependently reduced the expression of RBP4 in WAT (10 mg·kg−1: 16.2%, 20 mg·kg−1: 24.8%, 40 mg·kg−1: 36.2%), Figure 3A. RBP4 proteins in adipose tissue also decreased in a dose-dependent fashion, which was confirmed by Western blot analysis (Figure 3B). A significant reduction in plasma LDL and RBP4 levels (0 h vs. 6 h) was observed in the 20 and 40 mg·kg−1 rimonabant treated groups (Figure 2B). There was a strong correlation observed between the expression of RBP4 and LDL (rS 1; P < 0.04) after rimonabant treatment.
Figure 2.
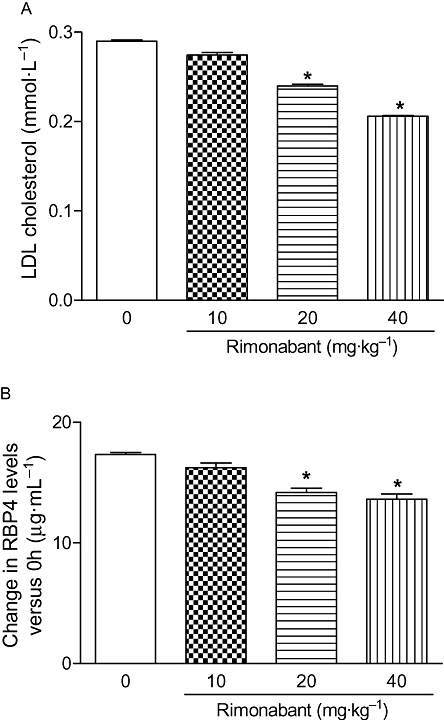
Single dose-response effect of rimonabant treatment on the LDL in HF-fed C57BL/6 mice. Changes in LDL in the HF-fed C57BL/6 mice with and without treatment are presented in (A). Baseline-corrected serum RBP4 levels of HF-fed C57BL/6 mice were analysed by elisa (B). The columns represent the change in the treatment groups compared with the vehicle control group. Each value represents mean ± SEM (n = 6). *P < 0.05 versus vehicle-treated group.
Figure 3.
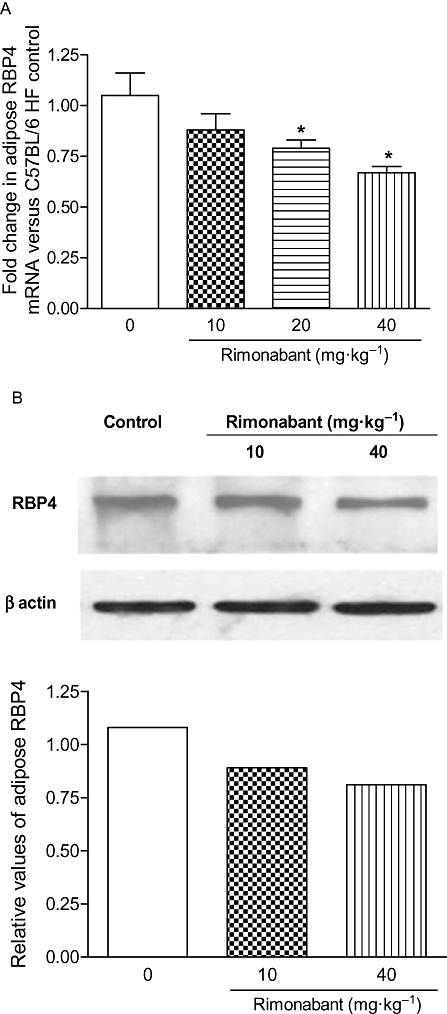
Single dose-response effect of rimonabant treatment on the expression of RBP4 mRNA in WAT of HF-fed C57BL/6 mice was determined by quantitative real-time PCR (A) and its protein was analysed by Western blotting (B). The columns represent the change in the treatment groups compared with the vehicle control group. Each value represents mean ± SEM (n = 6). *P < 0.05 versus vehicle treated group.
Treatment of 3T3-L1 adipocytes with rimonabant reduces the expression and secretion of RBP4
To investigate whether rimonabant has a direct effect on RBP4, fully differentiated 3T3-L1 cells were treated for 6 h with rimonabant 1 and 10 µM. Rimonabant reduced the cellular RBP4 mRNA expression and its secreted protein level in a dose-dependent manner as compared with untreated controls (P < 0.05) (Figure 4).
Figure 4.
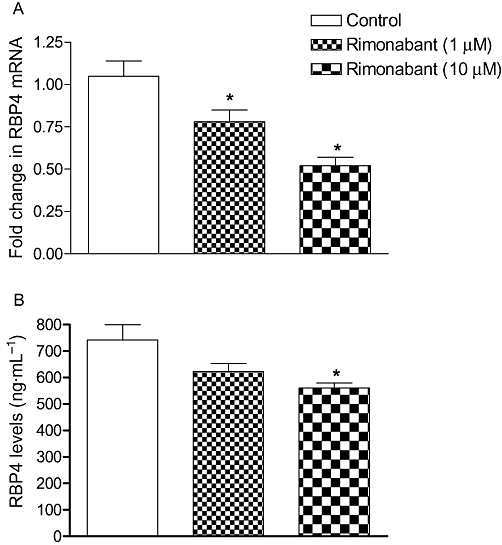
Effect of single doses of rimonabant on RBP4 mRNA (A) and its protein (B) levels in 3T3L1 cells. Supernatant RBP4 levels were analysed by elisa and RBP4 mRNA expressions (means± SEM) were measured by real-time PCR and normalized to the control samples (n = 3). *P < 0.05 versus untreated group.
Increased aortic RBP4, MCP-1 levels and serum inflammatory markers in HC- and HF-fed animals
Interestingly, RBP4 expression was found to be significantly higher (50%, P < 0.05, Figure 5A) in aortic tissues of HF-fed C57BL/6 mice than the chow-fed group. However, a marked increase in aortic RBP4 mRNA (170%, P < 0.05, Figure 5A) expression and its protein level (110%, P < 0.05, Figure 5B) were observed in HC-fed ApoE3 mice as compared with the chow-fed mice. Similar results were obtained in the blotting study where we detected the presence of RBP4 in the aorta, and higher protein levels were observed in HC-fed ApoE3 mice as compared with the chow-fed animals (Figure 5C). In response to HF both C57BL/6 nd ApoE3 mice had higher serum RBP4 levels than chow-fed animals (41 and 55%, respectively, P < 0.05) (Figure 5D). Overall, HC-fed ApoE3 mice had significantly higher RBP4 when compared with HF-fed C57BL/6 mice.
Figure 5.
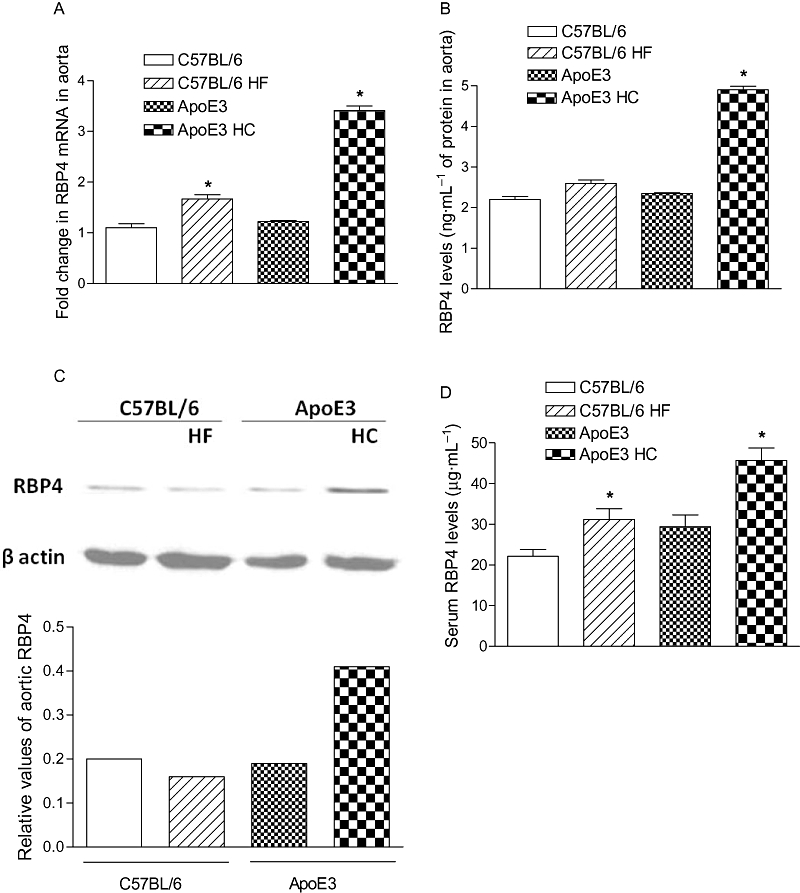
Expression of RBP4 mRNA in aortic tissue of HF-fed C57BL/6 and HC-fed ApoE3 mice, determined by quantitative real-time PCR (A). Aortic RBP4 (B) and serum levels (D) of HF-fed C57BL/6 and HC-fed ApoE3 mice were analysed by elisa. RBP4 protein in aorta was analysed by Western blotting (C). The columns represent the change in groups compared with the C57BL/6 control group, mean ± SEM (n = 6). *P < 0.05 compared with their respective control mice.
As shown in Figure 6, aortic MCP-1 expression was significantly increased (45%, P < 0.05) in HC-fed ApoE3 mice when compared with their chow-fed group. Furthermore, we investigated the CRP levels in the same ApoE3 mice, and as shown in Figure 7, HC-fed mice had significantly high circulatory CRP levels (600%) compared with their control animals indicating that the animals were at risk of cardiovascular complications.
Figure 6.
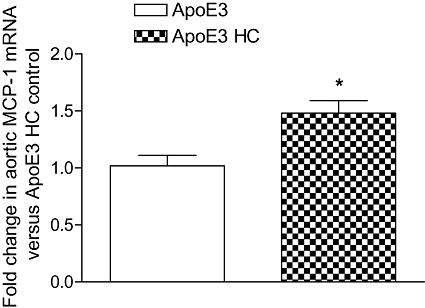
Expression of MCP-1 mRNA in aortic tissue of HC-fed ApoE3 mice, determined by quantitative real-time PCR. The columns represent the change in the HC-fed ApoE3 groups compared with their age matched ApoE3 control. Values are mean ± SEM (n = 6 mice per group). *P < 0.05, as compared with control ApoE3 mice.
Figure 7.
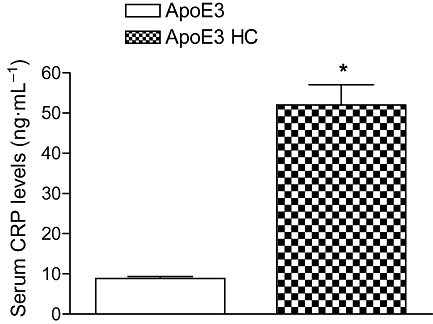
Analysis of the CRP in HC-fed ApoE3 mice using an immunoturbidometric method. The columns represent the change in the HC-fed ApoE3 groups compared with their age matched ApoE3 control. Values are mean ± SEM (n = 6 mice per group). *P < 0.05, as compared with ApoE3 mice.
Effect of chronic treatment with rimonabant on body weight, feed intake and lipid parameters in HC-fed ApoE3 mice
As shown in Table 2, 4 weeks' treatment with 20 mg·kg−1 rimonabant significantly decreased body weight gain compared with vehicle-treated animals. Food intake of the rimonabant-treated group was decreased in comparison with the control group. The average food intake of the rimonabant group was 3.1 g, whereas in control animals it was 16.4 g on the last day of the dosing. Rimonabant was also found to reduce the serum TG, TC, LDL and FFA levels significantly in HC-fed ApoE3 mice (Table 2).
Table 2.
Effects of rimonabant on serum biochemical parameters in HC-fed ApoE3 mice after 28 days treatment
| Metabolic parameters | Vehicle | Rimonabant (20 mg·kg−1) |
|---|---|---|
| Body weight (g) | 26.5 ± 0.81 | 23.9 ± 0.69* |
| LDL (mM) | 2.4 ± 0.21 | 1.61 ± 0.11* |
| HDL (mM) | 1.84 ± 0.06 | 1.95 ± 0.09 |
| Triglyceride (mM) | 2.62 ± 0.05 | 1.59 ± 0.07* |
| Total cholesterol (mM) | 14.93 ± 0.58 | 10.09 ± 0.41* |
| FFA (mM) | 1.89 ± 0.16 | 1.25 ± 0.10* |
| CRP (ng·mL−1) | 62.3 ± 7.10 | 48.3 ± 3.61* |
| MCP-1 (pg·mL−1) | 842.3 ± 42.1 | 645.8 ± 56.2* |
| Adiponectin (µg·mL−1) | 23.2 ± 2.2 | 27.6 ± 2.8 |
| RBP4 (µg·mL−1) | 57.3 ± 3.2 | 48.1 ± 2.1* |
Values are shown as mean ± SEM.
P < 0.05 as compared to vehicle control.
Effect of chronic treatment with rimonabant on RBP4, MCP-1 and CRP in HC-fed ApoE3 mice
As shown in Figure 8A and C, chronic rimonabant treatment decreased the expression of RBP4 in aortic tissues by 55% and MCP-1 by 29% versus control. Interestingly, RBP4 protein levels in the aorta of mice treated with 20 mg·kg−1 rimonabant were not significantly different from those in control animals, although they tended to decrease (Figure 8B). Four weeks' treatment with 20 mg·kg−1 rimonabant significantly decreased serum CRP levels in ApoE3 mice when compared with vehicle-treated animals (Table 2). In parallel with this reduction in CRP, serum MCP-1 and RBP4 levels were significantly reduced in the rimonabant-treated group in comparison with control animals (Table 2). However, we could not find any atherosclerotic lesions in these ApoE3 animals in spite of the higher levels of pro-inflammatory markers like CRP and MCP-1 associated with high concentrations of lipids in serum. A possible explanation is that the duration of exposure to the cholesterol diet was insufficient to produce atherosclerotic lesions.
Figure 8.

Effect of rimonabant (20 mg·kg−1) treatment on the expression of RBP4 determined by quantitative real-time PCR (A) and its protein (B) in aortic tissue of HC-fed ApoE3 mice measured using elisa. (C) Analysis of the expression of MCP-1 in aorta of HC-fed ApoE3 mice as determined by quantitative real-time PCR. The columns represent the fold change in the treatment groups compared with the vehicle control group, mean ± SEM (n = 6). *P < 0.05 versus vehicle treated group.
Discussion
In a recent study, we demonstrated that rimonabant reduces the expressions of RBP4 and the pro-inflammatory cytokines in adipose tissues of genetically altered obese (ob/ob) mice (Mohapatra et al., 2009). As an extension of this earlier study, we planned to explore the possible association between RBP4 and the inflammatory state of adipose tissue with varying degrees of obesity. Therefore, in the present study, we measured the expression of RBP4 and pro-inflammatory cytokines in WAT of diet-induced obese mice at different time intervals. Our data demonstrate that adipose RBP4 expression steadily increases with an increase in adiposity. The cytokine TNF-α is a well-established marker for inflammation, and MCP-1 is a chemoattractant molecule, which dictates the infiltration of macrophages into adipose tissue, contributing to the inflammatory state. In the present study, we observed a significantly higher expression of both TNF-α and MCP-1 mRNA in WAT of mice fed a HF diet for 8 weeks. Similar findings were obtained by Yao-Borengasser et al. (2007) and they found a significant positive relationship between adipose tissue MCP-1 and RBP4 expression in humans. We also observed that the expression of adiponectin, an anti-inflammatory adipokine, was significantly decreased in the HF-fed mice. Therefore, there was a positive association between the gene expression pattern of RBP4 with the expressions of MCP-1 and TNF-α and an inverse correlation with adiponectin in WAT of HF-fed mice, suggesting a possible role of RBP4 in adipose inflammation.
An important observation in the current study was that RBP4 expression was found to increase concomitantly with LDL, TC and FFA in HF-fed C57BL/6 mice. We examined the effects of the anti-obesity agent rimonabant, a selective CB1 antagonist, on LDL cholesterol and RBP4 expression in WAT of HF-fed mice. Surprisingly, a single dose of rimonabant similarly reduced LDL cholesterol and adipose RBP4 expression and their circulating levels in obese mice. This is the first study to show the acute effect of rimonabant on LDL and RBP4 expression in HF-fed mice. Similar to our observations, Erikstrup et al. (2006) reported a significant correlation of serum RBP4 with TG, LDL and HDL levels in patients with type 2 diabetes, indicating that a strategy that lowers LDL levels might also reduce RBP4 levels. It was, therefore, hypothesized that the RBP4 suppression could be either a consequence of LDL reduction or a direct effect of rimonabant on adipocytes. This was further clarified when rimonabant was added to cultured adipocytes and a decrease in RBP4 mRNA and its protein levels occurred, suggesting that this is a direct effect of the drug on the adipocyte. In similar lines, RBP4 homozygous knockout (RBP4–/–) mice demonstrate lower FFA levels, indicating the role of RBP4 in lipid metabolism (Quadro et al., 2003). However, more studies are required to examine the inter-relationship between RBP4 and LDL.
The observed positive correlation of adipose RBP4 with pro-inflammatory cytokines and LDL, which are strong predictors of cardiovascular disorders, led us to hypothesize that RBP4 may be involved in cardiovascular complications. To explore this hypothesis, we investigated the aortic expression of RBP4 and its protein levels and its correlation with established cardiovascular markers in response to HF/HC diet in C57BL/6 and ApoE3 mice respectively.
The effect of diet, HF in the case of C57BL/6 mice and HC for ApoE3 mice, was pronounced, and there was a dramatic increase in the RBP4 expression, suggesting a pro-atherogenic role of RBP4. We confirmed that the RBP4 protein was indeed present in the aorta at very low levels, as determined by Western blot, and increased after HF/HC diet in C57BL6 and ApoE3 mice. Similarly, we found elevated serum levels of RBP4 in both HF-/HC-fed C57BL/6 and ApoE3 mice compared with normal controls. Support for a pro-atherogenic role of RBP4 was obtained by finding that HC-fed ApoE3 mice also had a significant elevation of aortic MCP-1 transcript along with serum CRP levels. CRP levels have been reported to be increased in vascular inflammation (Dutta et al., 2009), ED (Stokes et al., 2009) and atherosclerosis (Tuomainen et al., 2008; Zhang et al., 2008) in mice, emphasizing the role of CRP in cardiovascular diseases. MCP-1, like CRP, is known to be a marker of inflammation, and its levels reportedly increase in cardiovascular diseases (Niu and Kolattukudy, 2009). Balagopal et al. (2007) described a study in obese children, in which a correlation was found between RBP4 levels and plasma levels of inflammatory marker as CRP and IL-6. As CRP and MCP-1 are established inflammatory markers corroborating with RBP4 and correlate with cardiovascular disease risk, our new findings add further support for the concept that RBP4 may be linked with cardiovascular disease.
Furthermore, we investigated the effect of rimonabant on cardiovascular complications and features of associated metabolic syndrome (inflammation, dyslipidaemia) in HC-fed ApoE3 mice. Our results demonstrate that rimonabant ameliorates dyslipidaemia, a major biochemical disorder associated with cardiovascular diseases. Rimonabant treatment of ApoE3 mice reduced serum levels of cholesterol, FFAs and TG, and, importantly, decreased the LDL. On a molecular level, rimonabant increases adiponectin (Bensaid et al., 2003) that is known to have an anti-inflammatory as well as an anti-atherosclerotic effect (Han et al., 2009). In the current study, serum adiponectin was not significantly different from that in the control, although there was a tendency for it to be increased after rimonabant treatment. Our results also showed that rimonabant treatment reduced the elevated levels of CRP and MCP-1 (tissue and circulatory levels), suggesting its anti-inflammatory role. These findings are in agreement with in vivo data from other laboratories (Nissen et al., 2008; Schäfer et al., 2008). Recently, Dol-Gleizes et al. (2009) reported that there was reduction in pro-inflammatory markers such as IL-12, VCAM-1 and MCP-1 after rimonabant treatment; these parameters remained unaltered in the pair-fed group. Hence, it is speculated that in the present study, thereduction in CRP and MCP-1 was due to rimonabant not because of the reduction in body weight mediated by food restriction. Therefore, elevated levels of aortic RBP4 in ApoE3 mice may be associated with cardiovascular disease and its normalization after rimonabant treatment may represent one possible explanation by which rimonabant ameliorates the cardiovascular complications in HC-fed ApoE3 mice.
To summarize, the present study showing increased expression of RBP4 in association with inflammatory markers and LDL levels in obese and atherogenic conditions further indicates that RBP4 is involved in the cardiometabolic complications associated with these conditions. However, further studies are needed to validate these associations and to evaluate the clinical implications of our findings.
Acknowledgments
This work was supported by the Zydus Research Centre, Ahmedabad, India.
Glossary
Abbreviations
- ApoE3
apolipoprotein E3
- CB1
cannabinoid type 1
- CRP
C-reactive protein
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- HC
high cholesterol
- HDL
high-density lipoprotein
- HF
high fat
- LDL
low-density lipoprotein
- MCP-1
monocyte chemoattractant protein 1
- RBP4
retinol-binding protein 4
- WAT
white adipose tissue
Conflict of interest
There are no conflicts of interest involved in the study.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V. Reduction of elevated serum retinol binding protein (rbp4) in obese children by lifestyle intervention: association with sub-clinical inflammation. J Clin Endocrin Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16:39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dol-Gleizes F, Paumelle R, Visentin V, Marés A, Desitter P, Hennuyer N, et al. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor–deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. doi: 10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- Dutta K, Nandi D, Bishayi B. Repeated systemic Escherichia coli infection enhances anti-oxidant response in hypercholesterolemic mice inducing cardiovascular inflammation. Inflammation. 2009;32:89–98. doi: 10.1007/s10753-009-9107-5. [DOI] [PubMed] [Google Scholar]
- Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96:3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- Erikstrup C, Mortensen OH, Pedersen BK. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1393–1394. [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Scatton B, Le Fur G, Oury-Donat F, Bensaid M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol. 2006;69:471–478. doi: 10.1124/mol.105.015040. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog Cardiovasc Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lau DCW, Bikramjit Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Millani RV, Ventura HO. Obesity and cardiovascular disease. risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- Mohapatra J, Sharma M, Singh S, Pandya G, Chatterjee A, Balaraman R, et al. Involvement of adipokines in rimonabant-mediated insulin sensitivity in ob/ob mice. J Pharm Pharmacol. 2009;61:1493–1498. doi: 10.1211/jpp/61.11.0008. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodés-Cabau J, Cannon CP, Deanfield JE, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanism and clinical implications. Clin Sci (Lond) 2009;117:95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- Park SE, Kim DH, Lee JH, Park JS, Kang ES, Ahn CW, et al. Retinol-binding protein-4 is associated with endothelial dysfunction in adults with newly diagnosed type 2 diabetes mellitus. Atherosclerosis. 2009;204:23–25. doi: 10.1016/j.atherosclerosis.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24:421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Pfrang J, Neumüller J, Fiedler S, Ertl G, Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol. 2008;154:1047–1054. doi: 10.1038/bjp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solini A, Santini E, Madec S, Rossi C, Muscelli E. Retinol-binding protein-4 in women with untreated essential hypertension. Am J Hypertens. 2009;22:948–949. doi: 10.1038/ajh.2009.116. [DOI] [PubMed] [Google Scholar]
- Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- Takashima N, Tomoike H, Iwai N. Retinol-Binding Protein 4 and Insulin Resistance. N Engl J Med. 2006;355:1392. doi: 10.1056/NEJMc061863. [DOI] [PubMed] [Google Scholar]
- Tuomainen AM, Jauhiainen M, Kovanen PT, Metso J, Paju S, Pussinen PJ. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microb Pathog. 2008;44:111–117. doi: 10.1016/j.micpath.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Usui S, Ichimura M, Ikeda S, Okamoto M. Association between serum retinol-binding protein 4 and small dense low-density lipoprotein cholesterol levels in young adult women. Clin Chim Acta. 2009;399:45–48. doi: 10.1016/j.cca.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Yan ZC, Liu DY, Zhang LL, Shen CY, Ma QL, Cao TB, et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferatoractivated receptor-d. Biochem Biophys Res Commun. 2007;354:427–433. doi: 10.1016/j.bbrc.2006.12.213. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee M, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chang Z, Yang J, Wang Q. Antiatherogenic property of triterpenoids-enriched extract from the aerial parts of Salvia miltiorrhiza. Phytother Res. 2008;22:1040–1045. doi: 10.1002/ptr.2426. [DOI] [PubMed] [Google Scholar]


