Abstract
BACKGROUND AND PURPOSE
The designer drug 1-(4-methylphenyl)-2-methylaminopropan-1-one (4-methylmethcathinone, mephedrone) is reported to possess psychostimulant, entactogenic and hallucinogenic effects. The purpose of this study was to examine the effects of acute administration of mephedrone on extracellular levels of dopamine (DA) and 5-HT in the nucleus accumbens of awake rats and compare these effects with those induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and amphetamine.
EXPERIMENTAL APPROACH
Microdialysis sampling was performed while simultaneously recording locomotor activity in rats and the monoamines were determined by HPLC with electrochemical detection.
KEY RESULTS
Mephedrone (3 mg·kg−1 s.c.) and (+)-amphetamine (1 mg·kg−1 s.c.) caused rapid increases in extracellular DA levels of 496% and 412%, respectively, whereas MDMA (3 mg·kg−1 s.c.) showed only a moderate effect (235%). The corresponding 5-HT levels increased to 941% (mephedrone) and 911% (MDMA), but only to 165% following amphetamine. The calculated t1/2 values for elimination rate of mephedrone, MDMA and amphetamine-induced increases in extracellular DA levels were 25, 303 and 51 min, the corresponding t1/2 values for 5-HT were 26, 48 and 84 min, respectively. Locomotor activity was increased most by amphetamine, whereas both mephedrone and MDMA showed about three times lower and shorter-lasting effects.
CONCLUSIONS AND IMPLICATIONS
The neurochemical and functional properties of mephedrone resemble those of MDMA, but it also shows an amphetamine-like effect in that it evokes a rapid release and elimination of DA in the brain reward system, a feature that may contribute to its potent re-inforcing properties.
Keywords: 4-methylmethcathinone; cathinones; phenethylamines; 3,4-methylenedioxymethamphetamine; microdialysis; dopamine; 5-HT; serotonin; legal highs; psychostimulants
Introduction
Mephedrone (1-(4-methylphenyl)-2-methylaminopropan-1-one, 4-methylmethcathinone, 4-MMC) is a substituted phenethylamine, structurally a cathinone derivative that possesses powerful psychostimulant, entactogenic and hallucinogenic effects (see Europol–EMCDDA, 2010; Schifano et al., 2011). Recently, mephedrone has gained growing popularity as a ‘designer drug’ or a ‘research compound’ offered for sale under various names via Internet sites, head shops and recently, by street dealers (Schifano et al., 2011; Winstock et al., 2010a,b; 2011;). During the last 2 years, mephedrone has been banned in most of the EU countries. However, the unified international legislation and control of mephedrone as a substance of abuse is still missing. The users of mephedrone have described its psychomimetic effects being comparable to amphetamine, cocaine and 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) causing euphoria, elevated mood, stimulation, enhanced appreciation for music, decreased hostility, improved mental function and mild sexual stimulation (Europol–EMCDDA, 2010). A recent web-based survey from 1006 responders revealed that mephedrone users consider its effects best compared with those of MDMA (Carhart-Harris et al., 2011). However, mephedrone is associated with a high risk of triggering repetitive and uncontrolled drug intake subsequent to its initial administration. Excessive intake of mephedrone leads to acute intoxication, displaying the clinical features of acute sympathomimetic toxidrome (Wood et al., 2010a,b;); this has been linked to an increasing number of fatalities in European countries as reported recently (Gustavsson and Escher, 2009; Dickson et al., 2010; Winstock et al., 2010a; Lusthof et al., 2011). Based on the spectrum of psychostimulant effects described by the drug users and the chemical similarity of mephedrone to substituted methcathinone and methamphetamine it has been speculated that mephedrone may act via increased release and re-uptake inhibition of 5-HT (serotonin) and dopamine (DA) (Schifano et al., 2011). However, there are no data available to provide neurochemical evidence for the in vivo effects of mephedrone on DA and 5-HT transmission in the brain areas implicated in drug reinforcement.
The chemical structure of mephedrone, being a β-ketoamphetamine, indicates that it is closely related to cathinone, the active constituent of khat known for its psychostimulant properties (see Kalix, 1994). Indeed, cathinone was shown to exert similar effects to amphetamine, increasing locomotor activity (Kalix, 1992) and extracellular DA levels in the nucleus accumbens (NAcc) and caudate-putamen of rats (Pehek et al., 1990). These findings offer an initial hypothesis that mephedrone, besides its similarity to MDMA that affects mostly 5-HT release and 5-HT2 receptors (see Gudelsky and Yamamoto, 2008), may also act by increasing the release of DA and/or inhibiting its re-uptake in the mesolimbic structures including the NAcc. Both animal and human studies revealed that the acute re-inforcing effects of drugs, as well as their incentive and reward seeking behaviour, are anatomically linked to the NAcc and mesolimbic DA system (see Koob and Volkow, 2010). Further, the abuse potential of a drug is believed to be governed primarily by its t1/2, and the potency and the kinetics of the pharmacodynamic responses induced (see Nutt et al., 2007).
The rationale behind this study was to examine, by use of in vivo microdialysis and simultaneous recordings of locomotor activity, the effects of acute administration of mephedrone on extracellular levels of DA and 5-HT and their metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindole-3-acetic acid (5-HIAA) in the NAcc of awake rats and compare these effects with those induced by a single dose of (±)-3,4-methylenedioxymethamphetamine (MDMA) and (+)-amphetamine.
Methods
Chemicals and test substances
Dopamine.HCl (DA), 5-HT, the metabolites DOPAC, 5-HIAA and MDMA, as well as chemicals for mobile phase and perfusion medium preparations including sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate, sodium chloride, potassium chloride, calcium chloride, citric acid, sodium acetate and methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). EDTA-2Na was obtained from Dojindo (Kumamoto, Japan). (+)-Amphetamine sulphate salt was purchased from Apoteket AB (Stockholm, Sweden), mephedrone.HCl (illicit formulation, purity >95%) was kindly provided by Swedish National Laboratory of Forensic Science (Linköping, Sweden). De-ionized water was obtained from a MilliQ water purification system (Millipore, MA, USA). Standard solutions of monoamines, metabolites and drugs were prepared in water and kept frozen (−20°C) in amber-coloured test tubes. All drugs were dissolved in saline and administered s.c. in a volume of 1 mL·kg−1 in the scruff of the neck at the following doses (all in the respective salt form): mephedrone (1 or 3 mg·kg−1), MDMA (3 mg·kg−1) and (+)-amphetamine (1 mg·kg−1). The nomenclature of drugs and molecular targets conforms to ‘Guide to Receptors and Channels’ (Alexander et al., 2008).
Surgery and microdialysis experiments
Male Sprague-Dawley rats (weighing 300–350 g) were used in the study. The microdialysis experiments were carried out on awake rats following the protocol described elsewhere (Kehr, 1999; Kehr and Yoshitake, 2006). All animal care and experimental procedures were approved by the local ethical committee and complied with the guidelines of the ‘Principles of Laboratory Animal Care’ (NIH publication no. 8023) and the Council of the European Communities (86/809/EEC). The rats (three animals per cage) were maintained on a 12 h light-dark cycle (light at 7:00 h), room temperature 22 ± 2°C and humidity 50–55%. All efforts were made to minimize animal suffering and the number of animals used for the study.
Rats were anaesthetized with isoflurane (Forene®, Abbott Laboratories, Abbott Park, IL, USA) using a Univentor 400 anaesthesia unit (AgnThos, Lidingö, Sweden). The rat was placed in a stereotaxic frame (David Kopf Instruments, CA, USA) in a flat skull position with the incisor bar set to −3.2 mm. The isoflurane anaesthesia was maintained at 2% in the mixture of air, the air flow was 500 mL·min−1, the body temperature of the rat was controlled by a rectal thermometer and maintained at +37°C using a CMA/150 temperature controller (CMA/Microdialysis, Stockholm, Sweden). The middle scalp incision of 2–3 cm was made and the flaps were kept aside using the homeostatic forceps. After exposure of the skull, a hole for a probe and two holes for the fixing screws were drilled using a fine trephine drill. The guide cannula for a microdialysis probe (Eicom Corp., Kyoto, Japan) was implanted into the NAcc (AP +2.2 mm, L +1.1 mm, V −5.3 mm; from bregma and the dural surface, according to the stereotaxic atlas of Paxinos and Watson (2007). The guide cannula was fixed firmly to the skull surface using dental cement. Following 5–7 days of recovery, a microdialysis probe (Eicom CX-I; 0.22 mm outside diameter, 2 mm membrane length with 50 000 Da cut-off) was inserted into the guide cannula of the awake rat. The probe was perfused with Ringer's solution (NaCl, 147 mM; KCl, 4 mM; CaCl2, 2.3 mM) at a flow rate of 1 µL·min−1. After the initial stabilization period of 2–3 h, the microdialysis samples were collected in 20 min intervals. The first three samples were used for estimation of basal levels of DA, 5-HT, and DOPAC, homovanillic acid (HVA) and 5-HIAA. Thereafter, mephedrone (1 or 3 mg·kg−1), MDMA (3 mg·kg−1) (+)-amphetamine (1 mg·kg−1) or vehicle were injected s.c. to separate groups of rats and the fractions were collected for 180 min. At the end of the experiment, the animals were killed by an overdose of isoflurane and dislocation of the neck. The brains were removed and examined for correct placement of the probe (the probe track) in the rat brain.
Locomotor activity test
Locomotor activity was monitored by use of a single-beam activity frame (44 × 30 cm ACTIMO 10, Shintechno, Japan) placed around the lower part of the Macrolon III cage. This arrangement allowed for simultaneous recordings of locomotor activity and microdialysis sampling. The data were collected by counting and summarizing the overall activity (number of beam crossings) in 5 min intervals and further pooled into 20 min bins, thereby matching the frequency of microdialysis sampling.
HPLC determination of monoamines and acidic metabolites
Concentrations of DA and 5-HT in the brain microdialysis samples were determined by HPLC with electrochemical detection as described elsewhere (Kehr and Yoshitake, 2006). Briefly, the HPLC system consisted of a HTEC500 unit (Eicom, Kyoto, Japan), and a CMA/200 Refrigerated Microsampler (CMA Microdialysis, Stockholm, Sweden) equipped with a 20 µL loop and operating at +4°C. The potential of the glassy carbon working electrode was +450 mV versus the Ag/AgCl reference electrode. The separation was achieved on a 200 × 2.0 (inside diameter) mm Eicompak CAX column (Eicom) protected with a guard column CAX-GC2/20 (Eicom). The mobile phase was a mixture of methanol and 0.1 M phosphate buffer (pH 6.0) (30:70, v v−1) containing 40 mM potassium chloride and 0.13 mM EDTA-2Na. The chromatograms were recorded and integrated by use of a computerized data acquisition system Clarity (DataApex, Prague, Czech Republic).
The detection limit (signal-to-noise ratio = 3) for DA and 5-HT was 0.05 nM, that is, 0.75 fmol in 15 µL injected onto the column respectively. Concentrations of DOPAC, HVA and 5-HIAA in the brain microdialysis samples were determined by a separate HPLC system with electrochemical detection (HTEC500). The potential of the glassy carbon working electrode was +750 mV versus the Ag/AgCl reference electrode. The separation was achieved on a 150 × 3.0 (inside diameter) mm Eicompak SC-5ODS column (Eicom) protected with the guard column OPTI-GUARD C18 (Optimize Technologies, Oregon, OR, USA). The mobile phase was a mixture of methanol and 0.1 M citrate/0.1 M sodium acetate buffer solution (pH 3.5) (16:84, v v−1) and contained 210 mg·L−1 octanesulphonic acid sodium salt and 5 mg·L−1 EDTA-2Na. The detection limit (signal-to-noise ratio = 3) for DOPAC and 5-HIAA was 2 nM, that is, 10 fmol in 5 µL injected onto the column. The chromatograms were recorded and integrated by use of the computerized data acquisition system Clarity (DataApex).
Data presentation and statistical analysis
The basal concentrations of monoamines and metabolites were calculated from the mean values of three fractions collected from each individual animal during the pre-drug period (−60 to 0 min) and then expressed as a mean ± SEM, n = 4 rats for each treated group. The mean concentrations were taken as 100% and all values were recalculated as a percentage of these basal levels. The overall effects of drug treatments on DA and 5-HT levels, as well as on the levels of metabolites DOPAC and 5-HIAA were expressed for each analyte as the area under the curve (AUC(0–180 min)) calculated as the sum of relative changes in extracellular levels of DA, 5-HT, DOPAC and 5-HIAA over the 180 min post-treatment period (nine samples) and subtracted from the mean relative AUC(0–180 min) value of the vehicle-treated group. Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, CA, USA) statistical software. Mean basal levels were compared by use of one-way anova followed by Newman–Keuls multiple comparison test. Differences between the groups of treatment and interaction of treatment and time were analysed by repeated measures two-way repeated measures anova followed by Bonferroni's post-test. Differences between the AUC(0–180 min) values of DA, 5-HT, DOPAC, 5-HIAA and the ratio 5-HT : DA of the vehicle- and drug-treated groups were analysed by one-way anova followed by Newman–Keuls multiple comparison test. Elimination rates expressed as the t1/2 of the decays in drug-induced increases in extracellular DA and 5-HT levels and locomotor activity were calculated by use of a non-linear fit to the one phase exponential decay curve.
Results
Basal extracellular levels of DA, 5-HT, DOPAC and 5-HIAA in the rat NAcc
A typical placement of the guide cannula and the microdialysis probe in the NAcc is illustrated in Figure 1. As seen, the membrane of the microdialysis probe was positioned preferentially in the NAcc shell but protruded also to the core part of the nucleus. The basal concentrations (expressed in nM) of DA, 5-HT and metabolites DOPAC and 5-HIAA in the rat NAcc are summarized in Table 1. The basal levels of monoamines and metabolites did not significantly differ within the respective treated groups.
Figure 1.
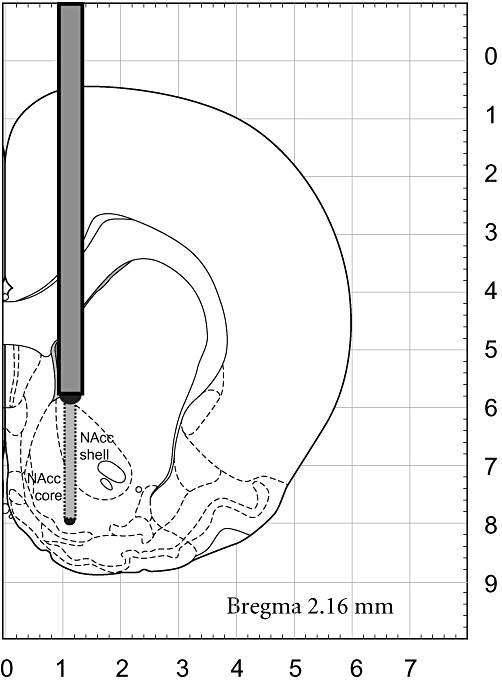
A representative placement of the microdialysis probe in the nucleus accumbens. The membrane of the microdialysis probe is targeting preferentially the shell but protruded also to the core part of the nucleus. Adapted from Paxinos and Watson (2007).
Table 1.
Basal levels of DA, 5-HT, DOPAC and 5-HIAA
| Vehicle | Mephedrone (1 mg·kg−1) | Mephedrone (3 mg·kg−1) | MDMA (3 mg·kg−1) | Amphetamine (1 mg·kg−1) | |
|---|---|---|---|---|---|
| DA | 1.73 ± 0.34 | 1.22 ± 0.16 | 1.48 ± 0.36 | 0.92 ± 0.39 | 1.91 ± 0.49 |
| 5-HT | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.02 | 0.09 ± 0.01 |
| DOPAC | 418.8 ± 144.4 | 366.3 ± 52.6 | 632.1 ± 58.4 | 399.3 ± 123.0 | 448.4 ± 34.9 |
| 5-HIAA | 81.1 ± 27.5 | 114.3 ± 22.7 | 74.8 ± 17.9 | 77.2 ± 13.5 | 64.5 ± 9.3 |
The results were calculated as nM and expressed as mean ± SEM, n = 4, in the microdialysates from rat nucleus accumbens The basal levels of monoamines and metabolites did not differ significantly within the respective treatment groups.
Effects of mephedrone, MDMA and amphetamine on DA and 5-HT levels
Administration of mephedrone at a dose of 3 mg·kg−1 s.c. caused a rapid increase in extracellular levels of DA reaching the peak levels of 496 ± 140% (P < 0.001) for DA at 40 min (Figure 2A) and 941 ± 102% (P < 0.001) for 5-HT levels already at 20 min (Figure 2B).
Figure 2.
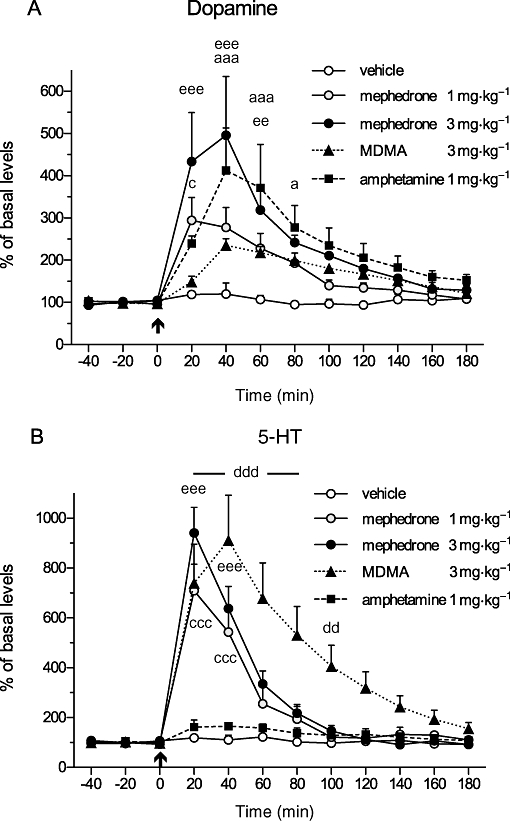
Effects of a single s.c. administration of mephedrone (1 and 3 mg·kg−1), MDMA (3 mg·kg−1) and amphetamine (1 mg·kg−1) on (A) extracellular levels of DA and (B) extracellular levels of 5-HT in the NAcc of awake rats. The arrow indicates the time of drug or vehicle administration. Repeated measures two-way anova followed by Bonferroni post-test; the drug-treated groups were compared with the vehicle group: mephedrone (1 mg·kg−1) cccP < 0.001, cP < 0.05; mephedrone (3 mg·kg−1) eeeP < 0.001, eeP < 0.01; MDMA dddP < 0.001, ddP < 0.01; amphetamine aaaP < 0.001, aP < 0.05; mean ± SEM, n = 4.
Mephedrone given at the lower (1 mg·kg−1) dose caused reduced but still significant increases in 5-HT levels to 709 ± 107% (P < 0.001) and DA levels to 295 ± 54% (P < 0.05). The DA levels between the groups were significantly different both for the treatment [F(4,15)= 5.26; P < 0.01] and the interaction of time and treatment [F(44,165)= 3.28; P < 0.001]. The corresponding values for the differences in 5-HT levels for the treatment were [F(4,15)= 9.45; P < 0.001] and the interaction of time and treatment [F(44,165)= 11.89; P < 0.001].
The increased concentrations of DA and 5-HT returned rapidly within the next 100–120 min to the basal levels. A single injection of MDMA (3 mg·kg−1 s.c.) caused only a moderate increase in DA levels to 235 ± 16%, which was not significant (two-way anova) from the vehicle group (Figure 2A). However, MDMA caused a massive increase in extracellular 5-HT levels with a peak value of 911 ± 180% (P < 0.001) at 40 min as shown in Figure 2B. Lastly, a single dose of amphetamine (1 mg·kg−1 s.c.) increased the DA levels to 412 ± 101% (P < 0.001) at 40 min, and the 5-HT levels to 165 ± 12% (not significant from the vehicle group) shown in Figure 2A and B, respectively.
Effects of mephedrone, MDMA and amphetamine on DOPAC and 5-HIAA levels
The same samples analysed for DA and 5-HT concentrations were also analysed for the content of metabolites DOPAC and 5-HIAA. The graph representing the overall effect on the metabolites DOPAC and 5-HIAA following acute treatments with mephedrone at 1 and 3 mg·kg−1, MDMA (3 mg·kg−1) and amphetamine (1 mg·kg−1), expressed as relative AUC(0–180 min) values, is shown in Figure 3. Following administration of mephedrone at the higher dose, the DOPAC levels were significantly reduced by 23.3 ± 4.2% (P < 0.01) compared with the AUC(0–180 min) value of the control group. A similar decrease by 27.6 ± 5.5% (P < 0.001) was observed for the MDMA effect, whereas amphetamine caused the most significant decrease of the DOPAC levels by 42.5 ± 3.6% (P < 0.001) of the corresponding value of the vehicle-treated group. Mephedrone (3 mg·kg−1) and MDMA decreased non-significantly the AUC(0–180 min) values of 5-HIAA by 14.9 ± 12.8% and 30.9 ± 3.0% respectively, whereas amphetamine caused a significant increase in 5-HIAA levels by 32.9 ± 14.3% (P < 0.05) of the vehicle-treated group.
Figure 3.
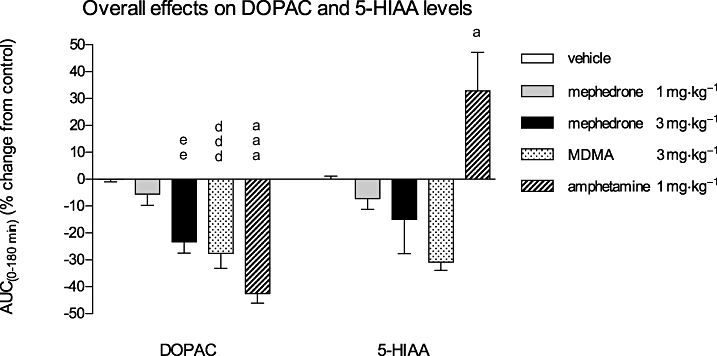
Overall effects of mephedrone (1 and 3 mg·kg−1), MDMA (3 mg·kg−1) and amphetamine (1 mg·kg−1) on DOPAC, and 5-HIAA levels in the NAcc of awake rats. The columns represent the AUC(0–180 min) values calculated as the differences in relative changes in DOPAC and 5-HIAA levels over a 3 h period between the drug- and vehicle-treated groups. One-way anova followed by Newman–Keuls multiple comparison test; drug-treated groups were compared with the vehicle group: mephedrone (3 mg·kg−1) eeP < 0.01; MDMA dddP < 0.001; amphetamine aaaP < 0.001; aP < 0.05; mean ± SEM, n = 4.
The overall effects of mephedrone, MDMA and amphetamine on DA and 5-HT levels
The overall effects of mephedrone, MDMA and amphetamine on the relative AUC(0–180 min) values of DA and 5-HT are depicted in Figure 4. Mephedrone (3 mg·kg−1) caused a marked and significant increase in the AUC(0–180 min) value of DA by 155.6 ± 35.5% (P < 0.01), which was similar to that induced by amphetamine: 148.5 ± 43.8% (P < 0.01). Further, mephedrone at 3 mg·kg−1 caused a marked increase in the AUC(0–180 min) of 5-HT by 197.8 ± 34.3% (P < 0.05), whereas the same dose of MDMA increased the 5-HT value up to 364 ± 89.4% (P < 0.001) of the vehicle group. The AUC(0–180 min) ratios of the 5-HT and DA values, calculated as mean ± SEM for each treatment group were: 1.01 ± 0.04 (vehicle), 1.42 ± 0.13 (mephedrone 1 mg·kg−1), 1.22 ± 0.2 (mephedrone 3 mg·kg−1), 2.68 ± 0.5 (MDMA 3 mg·kg−1) and 0.62 ± 0.14 for amphetamine (1 mg·kg−1). As seen, the 5-HT : DA values for mephedrone, and MDMA, indicate that these drugs are preferential releasers of 5-HT, whereas the 5-HT : DA ratio for amphetamine confirms its preferential effect on DA release.
Figure 4.
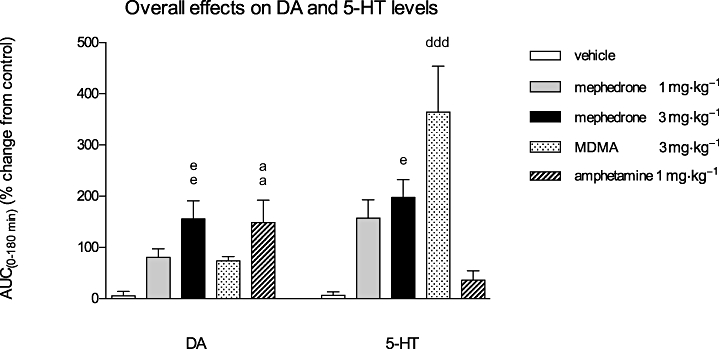
Overall effects of mephedrone (1 and 3 mg·kg−1), MDMA (3 mg·kg−1) and amphetamine (1 mg·kg−1) on DA, and 5-HT levels in the NAcc of awake rats. The columns represent the AUC(0–180 min) values calculated as the differences in relative changes in DA and 5-HT over a 3 h period between the drug- and vehicle-treated groups. One-way anova followed by Newman–Keuls multiple comparison test; drug-treated groups were compared with the vehicle group: mephedrone (3 mg·kg−1) eeP < 0.01, eP < 0.05; MDMA dddP < 0.001; amphetamine aaP < 0.01; mean ± SEM, n = 4.
Elimination rate of DA and 5-HT release induced by mephedrone, MDMA or amphetamine
The elimination rates of drug-induced increases in extracellular DA and 5-HT levels in the rat NAcc were calculated by use of a one-phase exponential decay curve fit to the experimental data and calculating the respective t1/2 values for DA and 5-HT. The calculated t1/2 values for the elimination rates of mephedrone-induced release of DA and 5-HT were 24.5 min and 25.5 min, respectively, for the dose of 3 mg·kg−1. The corresponding values for 1 mg·kg−1 mephedrone were 37.4 min and 24.8 min; for MDMA 302.5 min and 47.9 min, respectively, and for elimination of amphetamine-induced DA and 5-HT release, their respective t1/2 values were 51 min and 84.1 min. The calculated correlation factors (r2) for the curve fit of the DA and 5-HT decays for the mephedrone, MDMA and amphetamine-treated groups were for the DA curves: 0.595, 0.705 and 0.438, and for the 5-HT curves: 0.898, 0.664, 0.254, respectively.
Effects of mephedrone, MDMA and amphetamine on locomotor activity
Locomotor activity of vehicle- and drug-treated rats was monitored simultaneously during the microdialysis sampling period (Figure 5). The mephedrone-induced motor activation showed a peak level of 220.8 ± 34.7 counts in 20 min and returned to basal levels during the following 40 min. MDMA caused a similar, not significant motor activation (208.8 ± 29.3 beam crossings) in 40 min. Amphetamine induced a robust and long-lasting locomotor activation with a maximum of 554.5 ± 169.3 counts (P < 0.001) in the 40–60 min bin and the activation remained significantly elevated during the following 60 min, and, thereafter, slowly decreased until the end of the sampling period. Two-way repeated measures anova followed by Bonferroni multiple comparison test revealed significant differences for the treatment (F(4,15)= 6.04; P < 0.01) and the interaction of time and treatment (F(44,165)= 4.69; P < 0.001). The overall value of locomotor activation (AUC(0–180 min)) by amphetamine was 4.47 and 5.37 times larger than the corresponding AUC(0–180 min) values of MDMA and mephedrone, respectively. The rate of decline of drug-induced motor activation expressed as the t1/2 of each exponential curve calculated by the nonlinear regression analysis of the experimental data revealed a t1/2 of 7.3 min (r2 0.663) for mephedrone, 23.6 min (r2 0.702) for MDMA and 43.9 min (r2 0.252) for amphetamine.
Figure 5.
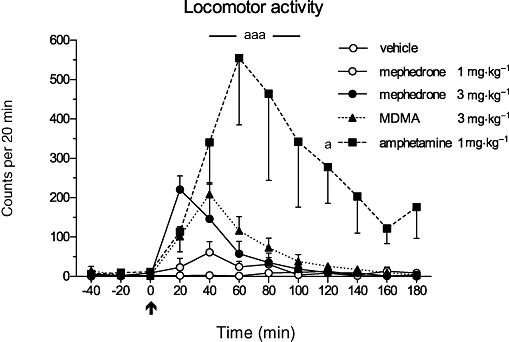
Effects of mephedrone (1 and 3 mg·kg−1), MDMA (3 mg·kg−1) and amphetamine (1 mg·kg−1) on locomotor activity recorded simultaneously with microdialysis sampling. The drug or vehicle was administered at time 0 min (arrow). Repeated measures two-way anova followed by Bonferroni post-test; the drug-treated groups were compared with the vehicle group: amphetamine: aaaP < 0.001, aP < 0.05; mean ± SEM, n = 4.
Discussion
Recently, numerous reports, comments and correspondence in medical literature have discussed and pointed out the health problems associated with growing use of novel synthetic stimulant drugs, so-called ‘legal highs’, which are relatively cheap and distributed under various names mostly via the Internet. Cathinone derivatives and, in particular, mephedrone has gained wide popularity as a research chemical and a party drug in several European countries (see Europol–EMCDDA, 2010) including Sweden (Gustavsson and Escher, 2009), England (Brandt et al., 2010a,b,c; Dargan et al., 2010; Schifano et al., 2011; Winstock et al., 2010a,b; 2011;), France (Debruyne et al., 2010), Ireland (McNamara et al., 2010; Nicholson et al., 2010), Scotland (Torrance and Cooper, 2010) and the Netherlands (Brunt et al., 2010). The users of mephedrone have compared its powerful psychostimulant, entactogenic and hallucinogenic properties to other abuse substances of this class including amphetamine, methamphetamine, cocaine and ecstasy (Europol–EMCDDA, 2010; Winstock et al., 2010a,b; 2011;). In a survey among dance drug users in the UK (Winstock et al., 2011), mephedrone was the sixth most frequently used drug after tobacco, alcohol, cannabis, cocaine and MDMA. However, the use of mephedrone is associated with a high risk of overdose, leading to uncontrolled and often fatal drug intoxication (Gustavsson and Escher, 2009; Dickson et al., 2010; James et al., 2010; Regan et al., 2010; Wood et al., 2010a,b; 2011; Lusthof et al., 2011).
Mephedrone as a substituted β-ketoamphetamine is expected to act primarily by increasing the release of monoamines and possibly via inhibition of their re-uptake (Schifano et al., 2011). The major finding of the present study is that mephedrone causes significant, rapid and dose-dependent increases in both 5-HT and DA levels in the NAcc. The overall effects of mephedrone injected at a higher dose on the 5-HT levels were comparable to the effects induced by the same dose of MDMA. Both mephedrone and MDMA preferentially increased 5-HT over the DA levels; however, the proportion of 5-HT increase (ratio 5-HT : DA) was at least two times higher for MDMA than for mephedrone. In addition, mephedrone but not MDMA, still potently increased the accumbal DA release to a level that was comparable to the effect induced by amphetamine. Amphetamine had only a minor effect on extracellular 5-HT concentrations.
The effects of MDMA and amphetamine on the release of DA and 5-HT in the rat NAcc observed in this study are in good agreement with earlier reports on the effects of these two drugs given at similar doses. Thus, a single i.p. injection of MDMA (2.5 or 3 mg·kg−1) increased accumbal DA to about 200 to 250% (Kankaanpääet al., 1998; O'Shea et al., 2005; Panos and Baker, 2010) and 5-HT levels only to about 350% (Kankaanpääet al., 1998) and 200% (O'Shea et al., 2005) of the baseline. However, in another paper, Kurling et al. (2008) reported that MDMA (5 mg·kg−1 free base, i.p.) increased the DA levels to about 700% and 5-HT levels to more than 1400% in NAcc of awake rats. Amphetamine sulphate increased accumbal DA to about 250% following a dose of 0.75 mg·kg−1 i.p. (Auclair et al., 2004) and to about 300% at a s.c. dose of 0.63 mg·kg−1 free base (Millan et al., 1999), to 650% following an i.p. injection of 1.6 mg·kg−1 sulphate salt (Pehek et al., 1990), to 350–550% in the Lewis and Fisher rats, respectively, following a s.c. dose of 1 mg·kg−1 sulphate salt (Cadoni and Di Chiara, 2007) and up to 700% at the same dose but calculated as a free base (Kankaanpääet al., 1998). The effects of amphetamine on accumbal 5-HT levels are negligible (Kankaanpääet al., 1998) or only moderate, increasing the 5-HT levels to about 130% of control values (Millan et al., 1999). The differences between the reported effects of systemic amphetamine on accumbal DA levels can be explained by the different routes of administration, doses given as salts or free bases, racemic or (+)-form of amphetamine and variations in the microdialysis protocols used by different investigators.
Microdialysis data provide valuable information on the in vivo pharmacodynamics of neurotransmitter release; however, the data do not allow a direct evaluation of a potential mechanism of action of drugs such as mephedrone. Mephedrone caused only minor and temporal decreases in the DOPAC levels, whereas the values of 5-HIAA were not significantly affected. These effects are similar to those induced by MDMA, whereas amphetamine significantly decreased the DOPAC levels, while the 5-HIAA levels significantly increased. Thus, the moderately reduced levels of DOPAC and 5-HIAA further indicate that the effect of mephedrone resembles that of MDMA, that is, it acts preferentially as a releaser of 5-HT, NA and DA, as reported for MDMA using in vitro methods (Rothman et al., 2001).
Addition of substituents to the ring of phenethylamine and its related derivatives at the ethylamine moiety including amphetamine causes a shift in the psychostimulant properties of these agents from acting as preferential releasers of NA and DA to release also 5-HT and/or exhibit binding affinity to 5-HT2 receptors, the features responsible for increased hallucinogenic effects of such compounds (Shulgin and Shulgin, 2007). Strategies based on increased 5-HT transmission, for example, by use of amphetamine analogues that release both DA and 5-HT (Rothman et al., 2001; Rothman and Baumann, 2006; Baumann et al., 2011), co-administration of 5-HT releasing drug fenfluramine (Wee and Woolverton, 2006) or co-administration of 5-HT2A antagonits or 5-HT2C receptors agonists (Bubar and Cunningham, 2008; Fletcher et al., 2011) were proposed to counteract self-administration of psychostimulant drugs in animal models. In a recent study, Baumann and colleagues (Baumann et al., 2011) compared four amphetamine analogues displaying similar in vitro potency as DA releasers but marked differences as 5-HT releasers. Although the microdialysis data on DA release in NAcc did not correlate with the in vitro predictions, the authors found good correlations between extracellular DA levels and locomotor activity. The increases in 5-HT release in NAcc were proportional to the decreased DA release and decreased locomotion, the most significant effect was observed for the p-methylamphetamine (PAL-313) analogue (Baumann et al., 2011). These data support the findings in our study, demonstrating that substituted phenethylamines mephedrone and MDMA markedly increase 5-HT release, but lower DA release and reduce locomotor activity when compared with the effects of amphetamine.
In this respect, it could be predicted that mephedrone and MDMA are weaker re-inforcers than amphetamine or cocaine. This conclusion is in line with a current report on mephedrone and cocaine users in the UK where about 56% of 947 responders (dance drug users) evaluated mephedrone as less addictive but giving a better high than cocaine, whereas 30% and 14% reported that it was equally or more addictive than cocaine (Winstock et al., 2011). On the other hand, it was reported that some users compulsively redose mephedrone, consuming their whole supply during a session (Europol–EMCDDA, 2010). One possible explanation for this behaviour could be a short duration (2–3 h) of the mephedrone effects (Europol–EMCDDA, 2010). This conclusion is in agreement with our data showing that mephedrone, given at a same dose as MDMA, is a more potent DA releaser than MDMA, whereas the elimination rate of mephedrone-induced DA release in the NAcc was almost 10 times faster than that of induced by MDMA and two times faster than that induced by amphetamine.
The calculated elimination rates of extracellular DA and 5-HT levels correlate well with the pharmacokinetic profiles of MDMA and amphetamine reported elsewhere. Thus, the estimated t1/2of MDMA was 47.4 min following a s.c. injection of 2 mg·kg−1 MDMA in the rat (Baumann et al., 2009). This value is in good agreement with the t1/2of 48 min reflecting the rate of elimination of MDMA-induced 5-HT efflux but does not agree with the t1/2of 303 min for decay of MDMA-induced DA release. This discrepancy can be due to a large standard error in estimated t1/2for elimination rate of MDMA-induced DA, alternatively, the slow elimination rate of DA may imply the effects of MDMA are evoked by its active metabolites such as 3,4-methylenedioxyamphetamine (MDA) (Baumann et al., 2009). The estimated t1/2of (+)-amphetamine following an i.v. bolus injection in the rat was 67 min (Hutchaleelaha et al., 1994). This can be compared with the pharmacodynamic effects of amphetamine expressed as t1/2of elimination rates of extracellular levels of DA (51 min) and 5-HT (84 min) reported in this study. There are no pharmacokinetic data available on mephedrone. Six mephedrone (phase I) metabolites have been identified in rat urine and seven in human urine (Meyer et al., 2010). The initial metabolic step for both species is N-demethylation of mephedrone to normephedrone. It is not known whether normephedrone possesses any psychomimetic properties. This possibility cannot be excluded, particularly when considering an analogous N-demethylation of methamphetamine to its active metabolite amphetamine (see Schep et al., 2010) and N-demethylation of MDMA to its active metabolite MDA (Baumann and Rothman, 2009). Interestingly, the calculated t1/2 for (+)-methamphetamine and its metabolite (+)-amphetamine in the male rat following i.v. administration of 1 mg·kg−1 (+)-methamphetamine were 73 min and 93 min, respectively (Milesi-Halléet al., 2005), and in another study (Rivière et al., 1999), 63 min and 98 min, respectively. The estimated t1/2of the cardiotoxic metabolite MDA formed from MDMA was 175 min, which is almost four times lower than the t1/2value for MDMA (Baumann et al., 2009). In conclusion, by referring to metabolism of methamphetamine (Rivière et al., 1999) and MDMA (Baumann et al., 2009), it can be hypothesized that a potential accumulation of mephedrone metabolites including normephedrone could contribute to the clinically-reported, toxicity of mephedrone binges.
The peak effect of mephedrone on DA release was similar to the effect of a 1 mg·kg−1 s.c. dose of amphetamine, which in turn, could be compared with the i.v. dose of 0.1 mg·kg−1 inducing self-administration in rats (Di Ciano et al., 1995). Here, the rats self-administered at about 0.7 mg amphetamine within the first 30 min of the session, which was accompanied by an increase in accumbal DA to the peak level of 550% in the 15–30 min fraction (Di Ciano et al., 1995), levels similar to those observed for the amphetamine and mephedrone doses in our study. However, further studies are necessary to demonstrate whether mephedrone can induce self-administration in rats.
From a comparison of the time courses of locomotor activation induced by mephedrone, MDMA and amphetamine it was concluded that the overall effect of mephedrone was equipotent to MDMA; however, the mephedrone-induced motor activation diminished about three, and six times faster than that induced by MDMA and amphetamine, respectively. Amphetamine caused a marked increase in locomotor activity that lasted for about 120 min; this finding is in good agreement with data reported elsewhere (Cadoni and Di Chiara, 2007; Kurling et al., 2008). Compared to its ability to release of DA, (+)-amphetamine is an even more potent at inducing the release of NA, while possessing similar efficacies for inhibition of DA and NA uptake (Rothman et al., 2001). This suggests that the differences in locomotor activation observed between (+)-amphetamine and mephedrone or MDMA could also account for the differences in activation of the noradrenergic system. Indeed, it was demonstrated that (+)-amphetamine-induced locomotor hyperactivity is markedly reduced in mice lacking α1B-adrenoceptors (Drouin et al., 2002). On the other hand, the amphetamine-induced locomotion could be almost completely abolished by the blockade of 5-HT2A receptors in the ventral tegmental area of the rat (Auclair et al., 2004). These data indicate a complex interplay between NA and 5-HT and their respective receptors in controlling the release of DA in the NAcc induced by various psychostimulant drugs.
In conclusion, the present data demonstrate for the first time that acute administration of mephedrone induces a rapid release of both 5-HT and DA in the NAcc of awake rats and this effect is accompanied by a short-lasting increase in locomotor activity. These results support the notion that mephedrone resembles the key neurochemical and functional properties of MDMA, confirming the similarities between mephedrone and MDMA effects reported by drug users. In addition, mephedrone-induced release and rapid elimination of DA in the NAcc were similar to the effect of amphetamine given at a dose relevant to its addictive properties. However, further studies are needed to elucidate the detailed mechanisms behind the reported risk of a compulsive binge intake of mephedrone and the risk for tolerance development.
Acknowledgments
The study was supported by the Swedish National Institute of Public Health and the Swedish Research Council (Grant 9459). The authors thank Anna Zackrisson from Swedish National Laboratory of Forensic Science, Linköping, Sweden for kind supply of mephedrone.
Glossary
Abbreviations
- 5-HIAA
5-hydroxyindolacetic acid
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- MDMA
(±)-3,4-methylenedioxymethamphetamine hydrochloride
- NA
noradrenaline
- NAcc
nucleus accumbens
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair A, Blanc G, Glowinski J, Tassin JP. Role of serotonin 2A receptors in the d-amphetamine-induced release of dopamine: comparison with previous data on alpha1b-adrenergic receptors. J Neurochem. 2004;91:318–326. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA) Int Rev Neurobiol. 2009;88:257–296. doi: 10.1016/S0074-7742(09)88010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Test Anal. 2010a doi: 10.1002/dta.204. accessed on 29 December 2010 [Epub ahead of print] PMID: 21191917. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test Anal. 2010b;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Second generation mephedrone. The confusing case of NRG-1. BMJ. 2010c;341:c3564. doi: 10.1136/bmj.c3564. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2010 doi: 10.1177/0269881110378370. accessed on 8 September 2010 [Epub ahead of print] PMID: 20826554. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.02.011. accessed on 21 March 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103:875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Courné MA, Le Boisselier R, Djezzar S, Gérardin M, Boucher A, et al. Mephedrone: a designer drug of recent use in France. Therapie. 2010;65:519–524. doi: 10.2515/therapie/2010077. (French) [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Coury A, Depoortere RY, Egilmez Y, Lane JD, Emmett-Oglesby MW, et al. Comparison of changes in extracellular dopamine concentrations in the nucleus accumbens during intravenous self-administration of cocaine or d-amphetamine. Behav Pharmacol. 1995;6:311–322. [PubMed] [Google Scholar]
- Dickson AJ, Vorce SP, Levine B, Past MR. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J Anal Toxicol. 2010;34:162–168. doi: 10.1093/jat/34.3.162. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europol–EMCDDA. Joint report on a new psychoactive substance: 4-methylmethcathinone (mephedrone) 2010. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Lisbon, Portugal. September.
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson D, Escher C. Mephedrone–Internet drug which seems to have come and stay. Fatal cases in Sweden have drawn attention to previously unknown substance. Lakartidningen. 2009;106:2769–2771. [PubMed] [Google Scholar]
- Hutchaleelaha A, Sukbuntherng J, Chow HH, Mayersohn M. Disposition kinetics of d- and l-amphetamine following intravenous administration of racemic amphetamine to rats. Drug Metab Dispos. 1994;22:406–411. [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J. 2010;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kalix P. Khat, an amphetamine-like stimulant. J Psychoactive Drugs. 1994;26:69–74. doi: 10.1080/02791072.1994.10472604. [DOI] [PubMed] [Google Scholar]
- Kankaanpää A, Meririnne E, Lillsunde P, Seppälä T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kehr J. Monitoring chemistry of brain microenvironment: biosensors, microdialysis and related techniques. Chapter 41. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Heidelberg: Springer-Verlag GmbH; 1999. pp. 1149–1198. [Google Scholar]
- Kehr J, Yoshitake T. Monitoring brain chemical signals by microdialysis. In: Grimes CA, Dickey EC, Pishko MV, editors. Encyclopedia of Sensors. Vol. 6. Valencia, CA: American Scientific Publishers; 2006. pp. 287–312. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurling S, Kankaanpää A, Seppälä T. Sub-chronic nandrolone treatment modifies neurochemical and behavioral effects of amphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in rats. Behav Brain Res. 2008;189:191–201. doi: 10.1016/j.bbr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in the Netherlands. Forensic Sci Int. 2011;206:e93–e95. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- McNamara S, Stokes S, Coleman N. Head shop compound abuse amongst attendees of the Drug Treatment Centre Board. Ir Med J. 2010;103:136–137. 134. [PubMed] [Google Scholar]
- Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397:1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, et al. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: acute mephedrone ‘meow’ myocarditis. Heart. 2010;96:2051–2052. doi: 10.1136/hrt.2010.209338. [DOI] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, et al. Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology. 2005;30:1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- Panos JJ, Baker LE. An in vivo microdialysis assessment of concurrent MDMA and cocaine administration in Sprague-Dawley rats. Psychopharmacology (Berl) 2010;209:95–102. doi: 10.1007/s00213-009-1774-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 6th edn. Amsterdam: Elsevier, Academic Press; 2007. [Google Scholar]
- Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29:1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J. 2010 doi: 10.1136/emj.2010.103093. accessed on 23 December 2010 [Epub ahead of print] PMID: 21183522. [DOI] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol (Phila) 2010;48:675–694. doi: 10.3109/15563650.2010.516752. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PiHKAL – Phenethylamines I Have Known and Loved: A Chemical Love Story. Berkeley, CA: Transform Press; 2007. [Google Scholar]
- Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202:e62–e63. doi: 10.1016/j.forsciint.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–343. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Marsden J, Mitcheson L. What should be done about mephedrone? BMJ. 2010a;340:c1605. doi: 10.1136/bmj.c1605. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Marsden J. Mephedrone: still available and twice the price. Lancet. 2010b;376:1537. doi: 10.1016/S0140-6736(10)62021-1. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010a;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010b;6:327–330. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]


