Abstract
BACKGROUND AND PURPOSE
Infusion of corticotropin-releasing factor (CRF)/urocortin (Ucn) family peptides suppresses feeding in mice. We examined whether rats show peripheral CRF/Ucn-induced anorexia and determined its behavioural and pharmacological bases.
EXPERIMENTAL APPROACH
Male Wistar rats (n = 5–12 per group) were administered (i.p.) CRF receptor agonists with different subtype affinities. Food intake, formation of conditioned taste aversion and corticosterone levels were assessed. In addition, Ucn 1- and Ucn 2-induced anorexia was studied in fasted CRF2 knockout (n = 11) and wild-type (n = 13) mice.
KEY RESULTS
Ucn 1, non-selective CRF receptor agonist, reduced food intake most potently (∼0.32 nmol·kg−1) and efficaciously (up to 70% reduction) in fasted and fed rats. The peptides' rank-order of anorexic potency was Ucn 1 ≥ Ucn 2 > >stressin1-A > Ucn 3, and efficacy, Ucn 1 > stressin1-A > Ucn 2 = Ucn 3. Ucn 1 reduced meal frequency and size, facilitated feeding bout termination and slowed eating rate. Stressin1-A (CRF1 agonist) reduced meal size; Ucn 2 (CRF2 agonist) reduced meal frequency. Stressin1-A and Ucn 1, but not Ucn 2, produced a conditioned taste aversion, reduced feeding efficiency and weight regain and elicited diarrhoea. Ucn 1, but not Ucn 2, also increased corticosterone levels. Ucn 1 and Ucn 2 reduced feeding in wild-type, but not CRF2 knockout, mice.
CONCLUSIONS AND IMPLICATIONS
CRF1 agonists, Ucn 1 and stressin1-A, reduced feeding and induced interoceptive stress, whereas Ucn 2 potently suppressed feeding via a CRF2-dependent mechanism without eliciting malaise. Consistent with their pharmacological differences, peripheral urocortins have diverse effects on appetite.
Keywords: corticotropin-releasing factor, corticotropin-releasing hormone, CRF or CRH receptor, urocortin, stressin1-A, meal pattern or microstructure, food intake, appetite, anorexia, conditioned taste aversion, corticosterone, body weight, obesity
Introduction
Since the isolation of corticotropin-releasing factor (CRF) in 1981 (Vale et al., 1981), several other mammalian CRF-like peptides with potential roles in energy homeostasis have been identified, including urocortins 1, 2 and 3 (Ucn 1, Ucn 2 and Ucn 3). Two mammalian genes encode class B G-protein-coupled CRF receptors (CRF1 and CRF2) (Hsu and Hsueh, 2001; Lewis et al., 2001; Reyes et al., 2001) that are targets of CRF and related peptides. Ucn 1 (Vaughan et al., 1995) can potently activate both CRF receptor subtypes. In contrast, Ucn 2 and Ucn 3 are 100- to 1000-fold selective as agonists of CRF2 receptors, respectively (Lewis et al., 2001; Reyes et al., 2001).
The anorexic effects of central administration of CRF receptor agonists are recognized and widely studied (Spina et al. 1996, Cullen et al. 2001, Inoue et al. 2003, Ohata and Shibasaki 2004, Pelleymounter et al. 2004, Zorrilla et al. 2004, de Groote et al. 2005, Cottone et al. 2007, Fekete et al. 2007; reviewed in Fekete and Zorrilla, 2007). For example, i.c.v. administration of Ucn 1 (Spina et al., 1996), Ucn 2 (Inoue et al., 2003) and Ucn 3 (Fekete et al., 2007) reduced food intake in fasted rats without producing malaise. However, much less is known about the effects of peripheral administration of urocortins on ingestion. In addition to brain sites (Fekete and Zorrilla, 2007), urocortins are present in several peripheral tissues relevant to energy homeostasis, including the gastrointestinal tract, where they are expressed in the stomach (Kozicz and Arimura, 2002; Chen et al., 2004), intestine (Harada et al., 1999; Lewis et al., 2001; Saruta et al., 2004; 2005; Yamauchi et al., 2005), muscularis mucosa layer (Hsu and Hsueh, 2001; Saruta et al., 2005) and enteric nervous system (Bittencourt et al., 1999; Harada et al., 1999). Urocortins are also present in endocrine tissue [such as the adrenals (Fukuda et al., 2005; Yamauchi et al., 2005), anterior pituitary (Iino et al., 1997; Yamauchi et al., 2005) and pancreatic β-cells (Li et al., 2003)], adipose tissue (Seres et al., 2004) and skeletal muscle (Chen et al., 2004). CRF receptors are correspondingly expressed in these tissues (De Souza, 1995; Van Pett et al., 2000).
Systemic infusion of CRF family peptides, including the urocortins, suppresses feeding in mice (Hsu and Hsueh, 2001; Wang et al., 2001) but has not been widely studied in other species. The behavioural mechanism of peripheral CRF/Ucn-induced anorexia and the receptor subtypes underlying these effects are also unknown. The present study tested the hypothesis that systemic (i.p.) administration of CRF receptor agonists suppress food intake in rats. The behavioural mechanism of action was explored via meal microstructure analysis and by determining whether anorexia was accompanied by malaise- or stress-like responses. The receptor subtype involved in the various actions was explored by comparing the anorexic potency (i.e. the minimal dose needed to observe an anorexic effect) and efficacy (i.e. the maximal intake reduction achieved) of agonists with selective CRF1 affinity (stressin1-A, a synthetic peptide analogue of CRF) (Rivier et al., 2007), selective CRF2 affinity (Ucn 2 and Ucn 3) and joint CRF1/CRF2 affinity (Ucn 1). To test the putative CRF2 subtype of pharmacological action further, the effects of peripheral administration of Ucn 1 and Ucn 2 on food intake were compared in fasted CRF2 knockout and wild-type mice. Ucn 1 and stressin1-A, peptides with high CRF1 affinity, reduced food intake and feeding efficiency in both fasted and fed rats and also induced a conditioned taste aversion (CTA), increased corticosterone (CORT) levels and diarrhoea. In contrast, peripheral Ucn 2 potently suppressed feeding, but not feeding efficiency, via a CRF2-dependent mechanism by reducing meal frequency without eliciting signs of malaise. The present results support the hypothesis that peripheral CRF2 agonists have specific appetite-suppressing properties, whereas peripheral CRF1 agonists produce interoceptive stress potentially relevant to the pathophysiology of functional gastrointestinal disorders.
Methods
Animals
The animals used were: 337 adult (200–225 g on arrival) pair-housed, male Wistar rats (Charles River, Hollister, CA), 10 adult male and 1 adult female CRF2 receptor knockout mice (25–29 g at study onset; Crhr2tm1Klee/Crhr2tm1Klee; Bale et al., 2000), and 10 adult male and 3 adult female wild-type littermate mice (25–27 g at study onset). Mice were studied on a mixed C57BL/6J × 129/Sv background as described previously (Bale et al., 2000). Results were pooled across sex because sex ratios were similar across genotype, and sex differences on treatment effects were not observed. Animals were housed under a 12 h/12 h dark/light cycle (dark onset at 8 h 00 min) in a humidity- (60%) and temperature-controlled (22°C) vivarium. Standard rodent chow [LM-485 Diet 7012, 65% (kcal) carbohydrate, 13% fat, 21% protein, metabolizable energy 3.41 kcal·g−1; Harlan Teklad, Madison, WI] and tap water were available ad libitum unless otherwise indicated. Animals were acclimatized to the vivarium for at least 1 week prior to the start of the experiments. All animal care and experimental procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85–23, revised 1996) and ‘Principles of Laboratory Animal Care’ and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Stressin1-A, rat/murine Ucn 1, murine Ucn 2 and murine Ucn 3 were synthesized manually using solid phase methodology, purified using HPLC and fully characterized using capillary zone electrophoresis, HPLC and MS (Reyes et al., 2001; Rivier et al., 2002; 2007;).
Feeding studies
Food deprivation-induced free-feeding
For studies of deprivation-induced feeding, rats (n = 158) or mice were habituated to individual test cages, which were constructed identically to their home cages, in a sound-attenuated test room for 6 h (dark cycle, 9 h 00 min to 15 h 00 min), after which they were returned to the vivarium. Food and tap water were available ad libitum during habituation, so that the test room would not be associated with food deprivation stress. Chow was removed at the end of habituation period, and the animals were returned to the vivarium to begin a 20 h pretreatment fast. On the next day, the animals were re-acclimatized to their respective test cages, which contained bedding from the previous day, for 2 h. Test injections were then administered at 11 h 00 min (3 h into the dark cycle), after which rats were immediately returned to the test cages with pre-weighed chow and tap water. Rats received one of the following i.p. doses in a between-subjects design: stressin1-A [n = 72; 0.0 (vehicle), 0.64, 1.28, 5.12, 10.24, 20.48 or 40.96 nmol·kg−1; (equivalent to 0.0, 3.14, 6.28, 25.12, 50.28, 100.56 or 201.12 µg·kg−1, respectively)], Ucn 1 [n = 24; 0, 0.32, 0.64 or 1.28 nmol·kg−1 (equivalent to 0.0, 1.5, 3 or 6 µg·kg−1, respectively)], Ucn 2 [n = 24; 0, 0.64, 1.28 or 5.12 nmol·kg−1 (equivalent to 0.0, 2.64, 5.28 or 21.13 µg·kg−1, respectively)] and Ucn 3 [n = 38; 0, 0.64, 1.28, 5.12, 40.96 or 81.92 nmol·kg−1 (equivalent to 0.0, 2.66, 5.32, 21.28, 170.24 or 340.48 µg·kg−1, respectively)]. Nominal molar concentrations were calculated based on the gross peptide weight. Actual molar concentrations based on net peptide weight are probably lower, but the net peptide proportions should not differ across the studied peptides. Mice (wild-type male, n = 5, female, n = 3; CRF2 knockout male, n = 4, female, n = 1) received doses of Ucn 1 (0.0, 0.32, 0.64 nmol·kg−1); separate mice (wild-type male, n = 5; CRF2 knockout male, n = 6) received doses of Ucn 2 (0.0, 1.28, 5.12 nmol·kg−1) in a within-subjects design with six intervening drug-free days between each treatment and food deprivation session. Food and water were post-weighed after 2 h. Peptides were initially solubilized in a small volume (µg equivalent µL) of nanopure water and then diluted to the appropriate final doses in sterile saline to attain injection volumes of 1 mL·kg−1 and 3 mL·kg−1 for rats and mice, respectively (Rivier et al., 2007). Peptides were dissolved immediately before testing and kept on ice.
Microstructure of ingestion
To identify the effects of stressin1-A (CRF1 agonist), Ucn 2, Ucn 3 (CRF2 agonists) and Ucn 1 (CRF1/CRF2 agonist) on the microstructure of ingestion, rats (n = 53) were housed in ventilated, sound-attenuating Plexiglas chambers equipped with a wire-mesh floor under a 12 h/12 h reverse light–dark cycle with lights off at 10:00 AM. Rats obtained individual corn syrup-sweetened, palatable chow pellets (45 mg, Formula A/I; Research Diets, New Brunswick, NJ) from a trough replenished by an automated pellet dispenser. Water aliquots (100 µL) were obtained by making nose pokes into a hole adjacent to a delivery reservoir. Responses for food or water were recorded by a computer with 10 ms resolution, which allowed detailed temporal resolution of discrete units of food and water intake, as described previously (Zorrilla et al., 2005a,b; Fekete et al., 2007). Testing began once responding stabilized (±10% food responding for 3 days). For testing, 23 h food-deprived rats received i.p. doses of stressin1-A [0.0 (vehicle), 0.64, 1.28 or 5.12 nmol·kg−1, (equivalent to 0.0, 3.14, 6.28 or 25.12 µg·kg−1, respectively); n = 12], Ucn 1 [0.0, 0.32, 0.64 or 1.28 nmol·kg−1 (equivalent to 0.0, 1.5, 3 or 6 µg·kg−1, respectively); n = 6], Ucn 2 [0.0, 0.64, 1.28 or 5.12 nmol·kg−1 (equivalent to 0.0, 2.64, 5.28 or 21.13 µg·kg−1, respectively); n = 6] or Ucn 3 [0.0, 40.96 nmol·kg−1 (equivalent to 0.0, 170.24 µg·kg−1 respectively); n = 6] in a within-subjects design. Treatments were given at the onset of the dark cycle with six intervening drug-free days between each treatment and food deprivation. Food was removed at the end of the daily chamber maintenance, and rats remained in their familiar holding cage during fasting. To assess the potential effect of feeding state on stressin1-A or Ucn-evoked anorexia, separate groups of ad libitum-fed rats received i.p. doses of stressin1-A, Ucn 1, Ucn 2 (same doses as applied on fasted rats; n = 6, n = 6, n = 5, respectively) and Ucn 3 [0.0, 10.24, 20.48 or 40.96 nmol·kg−1 (equivalent to 0.0, 42.56, 85.12 or 170.24 µg·kg−1 respectively); n = 6]. Treatments in rats fed ad libitum were given per a within-subjects design at the onset of the dark cycle with two intervening drug-free days between each treatment. Incremental 2 h and 4 h food and water intake were calculated, and food intake was normalized for metabolic demands of different body weights per Kleiber's 0.75 mass exponent (Kleiber and Rogers, 1961).
Based on the time course of changes in intake, analyses of meal and bout microstructure were performed for the first 4 h of observation of deprivation-induced feeding for peptides showing anorexic actions across this period (i.e. stressin1-A, Ucn 1 and Ucn 2). For meal pattern analysis, an empirically validated, drinking-inclusive meal definition was used (Zorrilla et al., 2005a,b; Fekete et al., 2007), whereby meals were defined as consecutive responses for food or water that contained at least five food-directed responses (0.225 g) and in which ingestive responses occurred within 5 min of the previous response. Defined in this manner (Zorrilla et al., 2005a), meals are hypothesized to be a unit of ingestion relevant to satiation (defined as the mechanisms through which feeding episodes come to end) and satiety (defined as the mechanisms through which completed feeding episodes are not subsequently resumed). The duration of eating and drinking within meals was defined separately as the duration of consecutive responses for food and water. Meal sizes for eating and drinking were calculated separately as the average number of food- or water-directed responses during meals. The rates of eating and drinking within meals were calculated by dividing each meal size by its respective duration.
To analyse changes in bout microstructure, a bout (i.e. the individual bursts of sustained feeding that collectively comprise a meal; Zorrilla et al., 2005a; Fekete et al., 2007) was defined as a sequence of at least three feeding responses in which no inter-feeding interval exceeded 15 s. This bout criterion was derived from inspection of the frequency histogram of inter-feeding intervals. Analogous analyses of drinking bouts applied a 10 s inter-drinking interval criterion. Thus, ‘bouts’ reflected within-meal episodes of sustained feeding that were not interrupted by prandial pauses (e.g. to drink). Bouts reflect a lower-level organization of ingestive behaviour than meals and reveal other aspects of ingestive behaviour, including whether feeding is sustained when present (e.g. by positive drive processes such as hunger or perceived food palatability) (Kissileff, 2000). Each rat's central tendency and, where appropriate, variability (within-subject means and SD, respectively) were calculated for the number of feeding and drinking bouts, the size and duration of bouts, the rate of ingestive events within bouts and the duration of inter-bout intervals. Finally, mean bout waveforms were calculated by aligning bout onsets and averaging event density function peaks for bouts emitted within the first four post-treatment hours. Mean bout waveforms appear as rounded peaks with a steep initial rise and gradual decay of ingestion rate. The time and number of ingestive events before and after the peak of the average waveform were then calculated.
CTA
To determine whether anorexic doses of stressin1-A, Ucn 1 or Ucn 2 produced a malaise-like state, individually housed rats (n = 99) were tested in a sensitive, 12 day, multiple-pairing, two-bottle choice CTA test (Inoue et al., 2003; Fekete et al., 2007). Rats were acclimated for 1 week to acquire their daily fluid intake during brief, defined periods by providing them with limited (25 min) home cage access to two sipper tube bottles, each containing tap water, beginning at 9:30 AM. On days 8 and 10 (preconditioning and post-conditioning 1), rats had limited access to one bottle with a 7.31 mM sodium saccharin solution (0.15% w/v; Sigma, St. Louis, MO) and one bottle with tap water. Immediately after the conclusion of saccharin access, subjects were injected i.p. with stressin1-A [0.0 (vehicle), 1.28, 5.12, 10.24 or 20.48 nmol·kg−1 (equivalent to 0.0, 6.28, 12.56 or 25.12 µg·kg−1, respectively); n = 35], Ucn 1 [0.0, 0.32, 0.64 or 1.28 nmol·kg−1 (equivalent to 0.0, 1.5, 3 or 6 µg·kg−1, respectively); n = 39] or Ucn 2 [0.0, 1.28 or 2.56 nmol·kg−1 (equivalent to 0.0, 2.64, 5.28 or 10.56 µg·kg−1, respectively); n = 25] in a between-subjects design, receiving the same dose on each day. Tap water was available in both bottles on days 9 and 11. On day 12 (post-conditioning 2), each subject again chose between the saccharin solution and tap water in a treatment-free state. The initial position (side) of the saccharin bottle was counterbalanced across subjects and alternated daily thereafter. This paradigm is sensitive to gastrotoxic doses of LiCl (Fekete et al., 2007), wherein a relative decrease in saccharin preference ratio is interpreted as the formation of CTA.
Plasma corticosterone measurement
To determine whether equally anorexic doses of Ucn 1 and Ucn 2 produced a stress-like adrenocortical response, rats (n = 27) were either handled (no injection) or injected (i.p.) with vehicle, 0.64 nmol·kg−1 Ucn 1 or 1.28 nmol·kg−1 Ucn 2 in a between-subjects design. The urocortin doses were selected to elicit similar degrees of anorexia. Forty-five minutes later, tails were nicked, and ∼200 µL of tail blood was collected in pre-chilled polypropylene tubes containing 15 µL 0.5 M EDTA (pH 8.0). After centrifugation, rat plasma, diluted 1:200 in assay buffer, was assayed for CORT immunoreactivity using a double antibody-125I radioimmunoassay kit (#07120103; MP Biomedicals, Irvine, CA) as per the manufacturer's instructions. The minimum detectable dose of CORT was 7.7 ng·mL−1.
Statistical analysis
In free-feeding experiments in rats, the effects of stressin1-A, Ucn 1, Ucn 2 or Ucn 3 treatment on food and water intake were analysed using mixed-design anova with time as the within-subjects factor and dose as the between-subjects factor. In mouse studies of intake, a mixed-design anova was used, with genotype as the between-subjects factor and dose as the within-subjects factor. Quantitative and microstructure measures of food and water intake in the nose poke test cages were analysed using repeated-measures anovas, with dose and, where relevant, time as the within-subject factors. Linear contrast anovas were used to identify the monophasic dose-response effects of the peptides on feeding or drinking parameters, defined by a log-linear dose–response function (Rosner, 1995). Saccharin preference on post-conditioning day 2 was analysed by one-way anova, with dose as the between-subjects factor. Corticosterone concentration was analysed by one-way anova, with drug as the between-subjects factor. Following significant omnibus tests, pairwise comparisons for all anovas were conducted using Fisher's protected least significant difference (LSD) test (Ott, 1988; Levin et al., 1994). Post-treatment weight gain and feeding efficiency in the nose poke studies were analysed by the Wilcoxon's signed-rank test due to non-Gaussian distributions and inhomogeneity of variance. The software packages used were SPSS 12.0 (SPSS, Chicago, IL) and InStat (GraphPad, San Diego, CA, USA). Bout microstructure analyses were performed with the assistance of Orbital Spike 4.0 (http://www.blki.hu/~szucs/OS3.html). All results are expressed as mean ± SEM.
Results
Effects of i.p. stressin1-A or Ucn administration on nocturnal free feeding in fasted rats
As shown in Figure 1, the peptides reduced food intake with a rank-order potency of Ucn 1 > Ucn 2 > > stressin1-A ≥ Ucn 3. Ucn 1 significantly and in a log-linear dose-dependent manner reduced cumulative 2 h and 4 h nocturnal food intake (linear contrast anova; 2 h, F1,23= 21.63, P < 0.001; 4 h, F1,23= 6.71, P < 0.05). Pairwise comparisons showed reduced intake compared with vehicle treatment at the 0.64 and 1.28 nmol·kg−1 doses (Figure 1B). Stressin1-A decreased cumulative food intake in a log-linear dose-dependent manner (2 h, F1,71= 14.94, P < 0.001; 4 h, F1,71= 27.17, P < 0.001), but much less potently than Ucn 1 (16- to 64-fold higher doses of stressin1-A than Ucn 1 were required to reduce food intake; Figure 1A). The selective CRF2 agonists Ucn 2 (Figure 1C; 2 h, F1,23= 6.61, P < 0.02; 4 h, F1,23= 4.12, P = 0.056) and Ucn 3 (Figure 1D; 2 h, F1,32= 16.63, P < 0.0005; 4 h, F1,32= 4.70, P < 0.05) also reduced cumulative deprivation-induced feeding in a log-linear dose-dependent manner. Ucn 2 was only slightly less potent than Ucn 1 in reducing food intake and 8- to 64-fold more potent than Ucn 3. Ucn 2 reduced 2 h re-feeding at the 5.12 nmol·kg−1 dose and 4 h food intake at the 1.28 nmol·kg−1 dose (Figure 1C), whereas Ucn 3 required doses of >40 nmol·kg−1 (Figure 1D).
Figure 1.
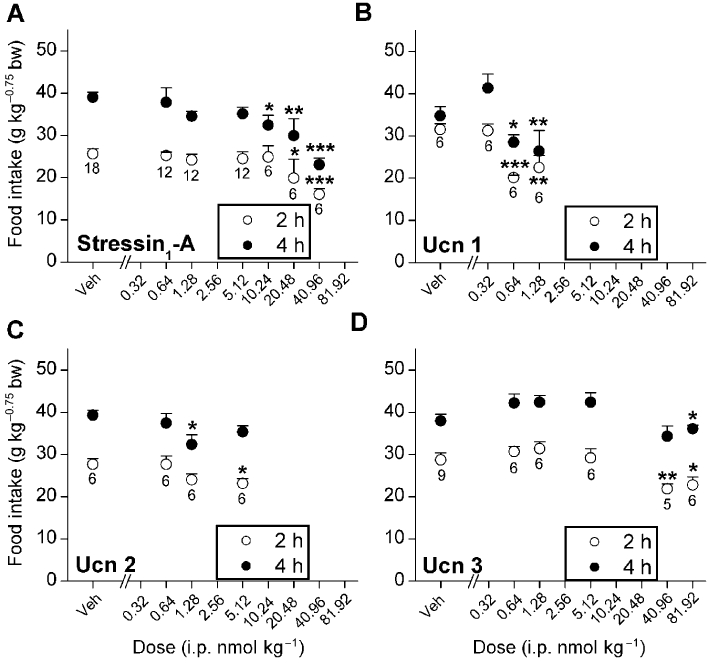
Effects of i.p. treatment with stressin1-A, Ucn 1, Ucn 2 and Ucn 3 on mean (±SEM) cumulative 2 h and 4 h food intake in 20 h food-deprived male Wistar rats. Food intake was normalized for body weight per Kleiber's mass exponent (0.75). *P < 0.05, **P < 0.01, ***P < 0.001, lower than respective vehicle treatment (Fisher's protected LSD test). Sample size (n) is indicated beneath each dose's symbol.
Effects of stressin1-A, Ucn 1 and Ucn 2 on the microstructure of nocturnal ingestion in fasted rats
The free-feeding experiments showed that i.p. injection of stressin1-A, Ucn 1 and Ucn 2 reduced 4 h nocturnal re-feeding with greater potency than Ucn 3. Studies were then performed on rats housed in test cages that detect intake as nose poke events to study the microstructure mode of action by which stressin1-A, Ucn 1, Ucn 2 but not Ucn 3 reduce nocturnal ingestion and to examine the peptides' effects on weight regain and feeding efficiency following food deprivation.
Total quantity of intake
Figure 2 shows that i.p. administration of stressin1-A, Ucn 1 and Ucn 2 but not Ucn 3 reduced cumulative nocturnal deprivation-induced food intake in the microstructure test cages. Ucn 1 potently reduced re-feeding through 4 h (Figure 2C; dose, F1,5= 81.68, P < 0.001; time × dose interaction, F1,5= 11.55, P < 0.05) in a log-linear dose-dependent manner (2 h, F1,5= 64.49, P < 0.0001; 4 h, F1,5= 60.79, P < 0.001). Significant reductions in food intake were evident at all doses (0.32, 0.64 and 1.28 nmol·kg−1). The high dose of Ucn 1 continued to reduce incremental food intake through the second 2 h period (vehicle, 9.0 ± 1.2, 1.28 nmol·kg−1 2.0 ± 1.7 g·kg−0.75 bw; F3,15= 5.77, P < 0.01, LSD P < 0.05). Stressin1-A was at least one order less potent than Ucn 1 (Figure 2A), only reducing 2 h food intake at the 5.12 nmol·kg−1 dose (F1,11= 5.53, P < 0.05). In contrast, Ucn 2 also potently reduced re-feeding (Figure 2C; dose, F1,5= 11.77, P < 0.05; dose × time interaction, F1,5= 10.62, P < 0.05) in a log-linear dose-dependent manner (F1,5= 15.72, P < 0.05). Pairwise comparisons indicated that the 0.64, 1.28 and 5.12 nmol·kg−1 doses of Ucn 2 significantly reduced 2 h intake. Cumulative anorexia persisted at the 5.12 nmol·kg−1 dose through 4 h. Ucn 3 was the least potent anorexic peptide as a 40.96 nmol·kg−1 dose did not reduce 2 h or 4 h food intake in fasted rats (Figure 2G). Closer inspection of the data showed that rats treated with high doses of Ucn 3 decreased their food intake within the first 10 min of re-feeding (vehicle, 5.9 ± 0.6 vs. 40.96 nmol·kg−1, 5.1 ± 0.6 g·kg−0.75 bw; Student's paired t-test P < 0.001).
Figure 2.
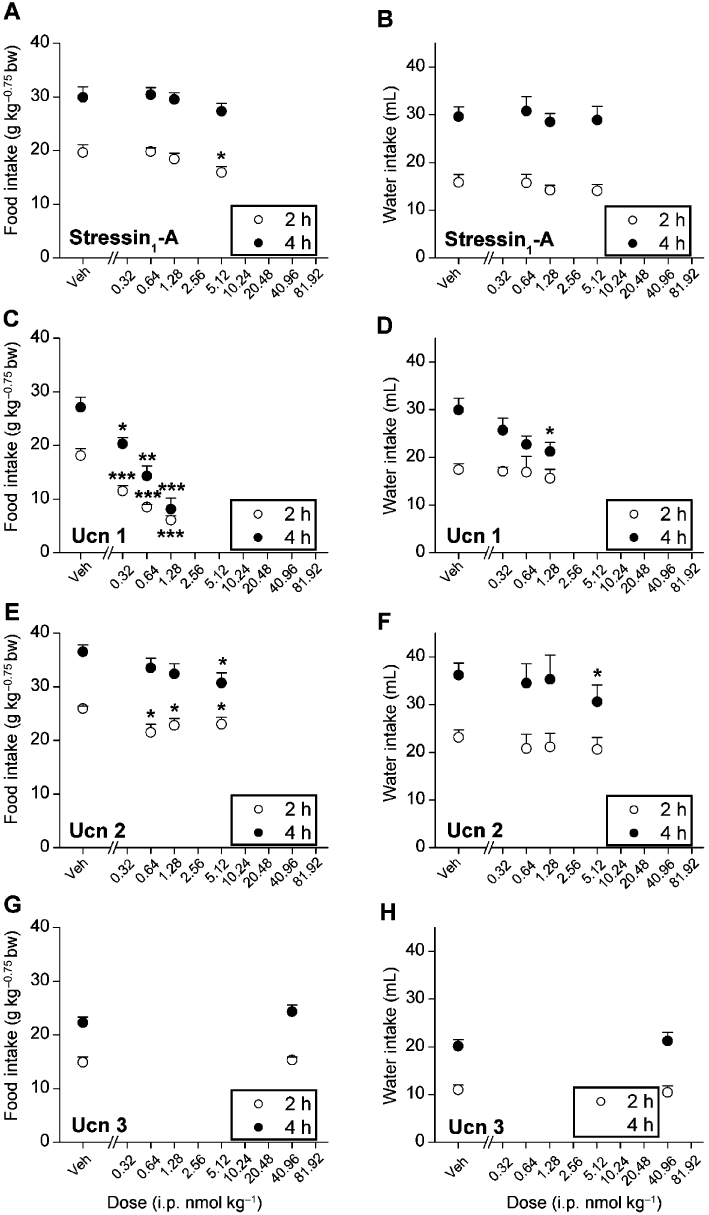
Effects of i.p. treatment with stressin1-A, Ucn 1 and Ucn 2 on mean (±SEM) cumulative food or water intake (2 and 4 h) in 24 h food-deprived male Wistar rats (stressin1-A, n = 12; Ucn 1 and Ucn 2, n = 6 per group) housed in automated test cages that detect intake as nose poke events. Food intake was normalized for body weight per Kleiber's mass exponent (0.75). *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle treatment (within-subjects Fisher's protected LSD test).
Significant reductions in responding for water accompanied the anorexic actions of Ucn 1 (Figure 2D; time × dose interaction, F1,5= 17.62, P < 0.01) and Ucn 2 (Figure 2F; F1,5= 6.78, P < 0.05) but were not seen following stressin1-A or Ucn 3 administration (Figure 2B and H). Pairwise comparisons revealed reductions in 4 h water intake at the 1.28 nmol·kg−1 dose of Ucn 1 and the 5.12 nmol·kg−1 dose of Ucn 2.
Weight regain and feeding efficiency
As shown in Figure 3A and C, treatment with stressin1-A (0.64 and 5.12 nmol·kg−1; Wilcoxon's sign-rank test, P < 0.01, n = 12) and Ucn 1 (1.28 nmol·kg−1; Wilcoxon's sign-rank test, P < 0.05, n = 6) decreased the body weight regain of previously fasted rats on the test day. This action of Ucn 1 persisted throughout the post-test day (vehicle, 8.3 ± 0.5 vs. 1.28 nmol·kg−1 dose 6.0 ± 0.3% weight gain; Wilcoxon's sign-rank test, P < 0.05, n = 6). Additionally, as shown in Figure 3B, the feeding efficiency [body weight gain (g)/food intake (g)] of rats was significantly reduced on the test day following stressin1-A injection (0.64 and 5.12 nmol·kg−1; Wilcoxon's sign-rank test, P = 0.051 and P < 0.01, respectively). Feed efficiency was reduced on the second post-test day following Ucn 1 administration (vehicle, 0.53 ± 0.03, 0.64 nmol·kg−1, 0.47 ± 0.03; Wilcoxon's sign-rank test, P < 0.05, n = 6). Ucn 2 and Ucn 3 treatment (Figure 3E–H) did not share these effects on weight regain or feeding efficiency, suggesting a CRF1 mechanism of action. Prior to drug administration, the fasting-induced weight loss did not differ significantly between treatment groups, as desired (Supplementary Figure S1A–D).
Figure 3.
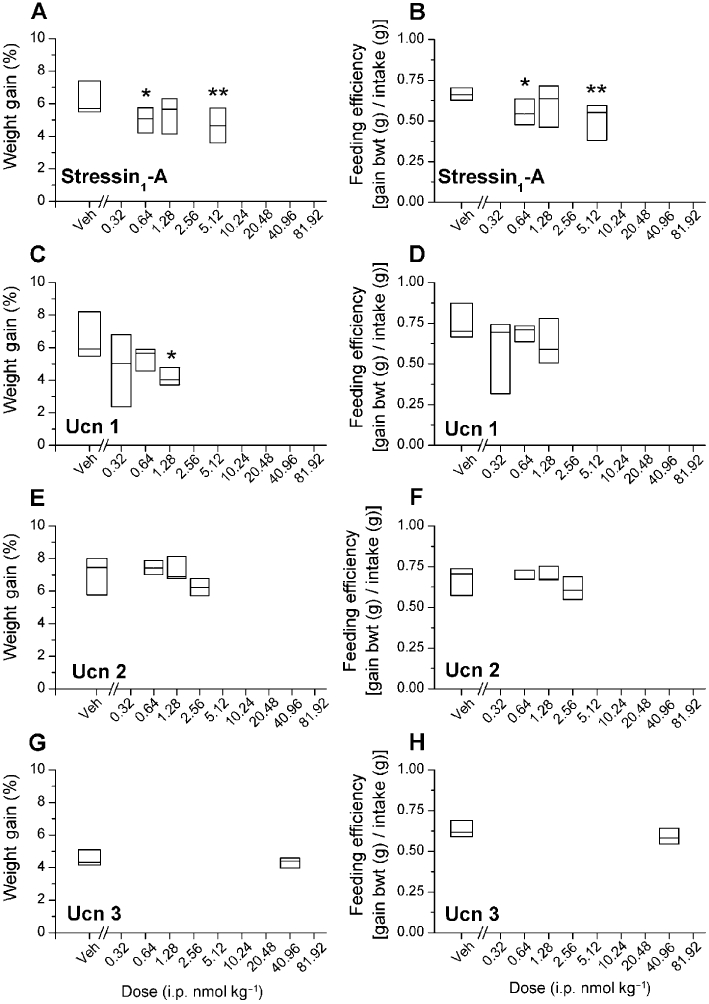
Effects of i.p. stressin1-A (n = 12), Ucn 1, Ucn 2 or Ucn 3 (n = 6 per group) on mean (±SEM) cumulative weight gain and feeding efficiency during re-feeding in 24 h fasted male Wistar rats housed in automated test cages that detect intake as nose poke events. Data expressed in median and 25 and 75 percentile. Stressin1-A and Ucn 1, but not Ucn 2 and Ucn 3, decreased weight gain and feeding efficiency during re-feeding after a fast. *P < 0.05, **P < 0.01, compared with vehicle (Wilcoxon's signed-rank test). Since the food intake did not change following Ucn 3 treatment, the BWT of the rats have not been recorded on the post-test day.
Meal microstructure
Microstructure analysis was performed for peptides that showed anorexic effects for at least 2–4 h. As shown in Table 1, the highest dose of Ucn 1 made rats eat fewer meals (F1,5= 17.74, P < 0.01) with longer inter-meal intervals (F3,15= 29.13, P < 0.005) during the 4 h observation period. Ucn 1 also reduced the size of the first meal (F1,5= 8.49, P < 0.05), with pairwise comparisons showing a significant reduction from vehicle treatment at the 1.28 nmol·kg−1 dose (vehicle, 6.0 ± 0.9 g; 0.32 nmol·kg−1, 5.3 ± 0.5 g; 0.64 nmol·kg−1, 4.2 ± 0.3 g; 1.28 nmol·kg−1, 3.3 ± 0.4 g). Meal size also tended to be reduced across the entire 4 h period after Ucn 1 treatment (F3,15= 3.03, P = 0.06), with a significant reduction seen at the intermediate 0.64 nmol·kg−1 dose (Table 1). Unlike Ucn 1, stressin1-A did not alter meal frequency. Rather, stressin1-A tended to reduce meal size (F1,11= 3.89, P = 0.07); pairwise comparisons showed that the highest dose (5.12 nmol·kg−1) of stressin1-A tended to reduce the size of the first meal (6.6 ± 0.6 g vs. 5.2 ± 0.4 g, P = 0.08) and significantly reduced the average meal size for food within the 4 h observation window (Table 1). In contrast, Ucn 2 did not reduce meal size, but rather tended to reduce meal frequency within the first 2 h (F3,15= 2.53, P = 0.09), especially at the 0.64 nmol·kg−1 dose (P = 0.09). Rats treated with Ucn 2 ate ∼1 fewer meals within the first four post-treatment hours than did vehicle controls (Table 1).
Table 1.
Effect of i.p. injection of stressin1-A, Ucn 1 and Ucn 2 on 4 h nocturnal meal microstructure in fasted male rats
| Microstructure parameters | ||||
|---|---|---|---|---|
| Meal frequency (number of meals) | Average inter-meal interval (min) | Average meal size Food (g) | Water (mL) | |
| Stressin1-A | ||||
| Vehicle | 4.8 ± 0.4 | 44.9 ± 3.4 | 3.3 ± 0.3 | 5.4 ± 0.6 |
| 0.64 nmol·kg−1 | 5.5 ± 0.6 | 38.6 ± 3.8 | 2.9 ± 0.2 | 4.6 ± 0.5 |
| 1.28 nmol·kg−1 | 5.4 ± 0.8 | 52.4 ± 8.9 | 3.3 ± 0.3 | 5.2 ± 0.7 |
| 5.12 nmol·kg−1 | 5.2 ± 0.3 | 42.0 ± 3.8 | 2.7 ± 0.2* | 4.4 ± 0.4 |
| Ucn 1 | ||||
| Vehicle | 4.8 ± 0.7 | 57.5 ± 17.4 | 3.6 ± 0.5 | 6.3 ± 1.7 |
| 0.32 nmol·kg−1 | 4.1 ± 0.6 | 65.3 ± 14.5 | 2.9 ± 0.3 | 5.0 ± 0.8 |
| 0.64 nmol·kg−1 | 4.3 ± 0.6 | 66.5 ± 11.9 | 2.0 ± 0.1* | 2.8 ± 0.5 |
| 1.28 nmol·kg−1 | 1.6 ± 0.3** | 182.5 ± 42.1** | 3.1 ± 0.4 | 4.4 ± 0.6 |
| Ucn 2 | ||||
| Vehicle | 4.8 ± 0.7 | 45.9 ± 10.5 | 3.8 ± 0.6 | 6.9 ± 0.8 |
| 0.64 nmol·kg−1 | 4.0 ± 0.4 | 50.6 ± 8.0 | 3.9 ± 0.3 | 7.9 ± 1.0 |
| 1.28 nmol·kg−1 | 3.6 ± 0.4 | 58.5 ± 6.9 | 4.3 ± 0.6 | 8.4 ± 0.6 |
| 5.12 nmol·kg−1 | 3.6 ± 0.2# | 50.9 ± 6.0 | 4.0 ± 0.2 | 7.8 ± 1.0 |
P < 0.05
P < 0.01, #P < 0.09, different from vehicle condition.
Bout microstructure
Consistent with the reduction in meal frequency, i.p. administration of Ucn 1 reduced the number of feeding bouts (Figure 4A; F1,5= 31.07, P < 0.001) and drinking bouts (Figure 4B; F1,5= 6.29, P = 0.05) during the 4 h period in a log-linear dose-dependent manner. Pairwise reductions compared with vehicle treatment were seen at the 0.64 and 1.28 nmol·kg−1 doses for feeding bouts and 1.28 nmol·kg−1 dose for drinking bouts. Ucn 1 treatment prolonged the intervals between feeding bouts (Figure 4C; 0.64 and 1.28 nmol·kg−1 doses; F1,5= 13.51, P < 0.05) and drinking bouts (Figure 4D; 1.28 nmol·kg−1 dose; F1,5= 11.96, P < 0.05). Stressin1-A did not significantly alter the mean number of ingestive bouts or the duration of intervals between them, whereas Ucn 2 (5.12 nmol·kg−1) reduced the number of drinking bouts (data not shown). As shown in Figure 5, each dose of Ucn 1 significantly reduced the time spent feeding (Figure 5A and C; F1,5= 18.94, P < 0.0001) and the amount eaten after the peak of the feeding bout (Figure 5E; F1,5= 13.68, P < 0.05), suggesting a facilitated termination of feeding bouts. In contrast, the average time (Figure 5B) and amount eaten before (Figure 5D) the bout peak were unaffected by Ucn 1 treatment. Ucn 1 administration did not alter the form of drinking bouts (data not shown). Ucn 1 administration decreased the serial regularity of feeding, but not drinking, within bouts (see Supplemental Results and Figure S2).
Figure 4.
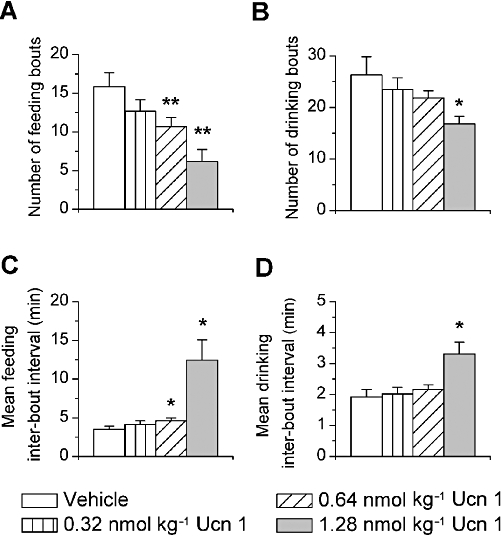
Effects of i.p. Ucn 1 administration on the number of (A) feeding bouts and (B) drinking bouts and the mean duration of intervals between bouts of (C) feeding and (D) drinking. Ucn 1 decreased the number of feeding and drinking bouts and increased the duration of intervals between them. *P < 0.05, **P < 0.01, compared with vehicle treatment.
Figure 5.
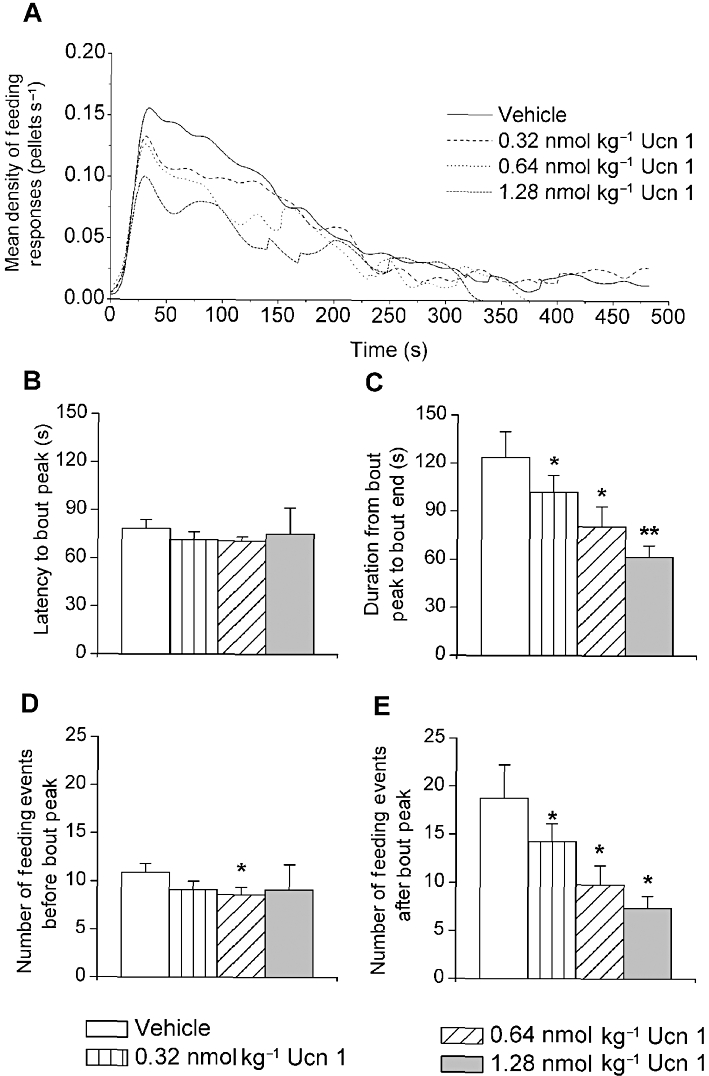
Effects of i.p. Ucn 1 administration on (A) the density of feeding events (‘eating rate’) in a 4 h bout of feeding (B) the latency until and (C) the time after the peak in an average feeding bout and the number of feeding events (D) before and (E) after the peak of the average bout in 24 h food-deprived male Wistar rats during re-feeding. Ucn 1 treatment dose-dependently reduced the sustained eating rate within bouts and the amount of time and feeding events that followed the peak of the feeding bout. The number of feeding events before the bout peak also decreased following 0.64 nmol·kg−1 Ucn 1 treatment. *P < 0.05, **P < 0.01, lower than respective vehicle treatment (Fisher's protected LSD test, n = 6).
Effects of stressin1-A, Ucn 1, Ucn 2 and Ucn 3 on food intake in non-fasted rats
Ucn 1 induced anorexia (Figure 6C; dose, F3,15= 5.54, P < 0.01; dose × time interaction, F3,15= 16.99, P < 0.001) in rats fed ad libitum in a log-linear dose-dependent manner (dose, F1,5= 20.25, P < 0.01, dose × time interaction, F1,5= 32.45, P < 0.01). Pairwise comparisons indicated that the 0.64, 1.28 and 5.12 nmol·kg−1 doses of Ucn 1 significantly reduced 4 h cumulative intake. The reduction in food intake following Ucn 1 treatment did not attain significance within the first 2 h (dose, F1,5= 4.53, P = 0.087, NS), unlike what was seen in food-deprived rats. However, the anorexic effect was significant in the subsequent 2 h at the 1.28 and 5.12 nmol·kg−1 doses (vehicle: 10.0 ± 1.0, 1.28 nmol·kg−1: 5.0 ± 0.9, 5.12 nmol·kg−1: 2.4 ± 1.0 g·kg−0.75 bw, dose F3,15= 11.96, P < 0.001, LSD P < 0.05). Ucn 2 also elicited cumulative 4 h anorexia (Figure 3E; dose, F3,12= 5.11, P < 0.05, linear contrast dose 4 h, F1,4= 8.82, P < 0.05) at a fourold higher dose (5.12 nmol) than was required for Ucn 1. Stressin1-A did not significantly reduce intake at the tested doses in rats fed ad libitum. Although anova analyses indicated that Ucn 3 administration did not reduce food intake (F1,15= 2.15, P = 0.16, NS), pairwise comparisons indicated that the highest dose tended to decrease intake as compared with the vehicle (40.96 nmol·kg−1, P = 0.07; Figure 6A and G). Thus, the acute anorexigenic effects of stressin1-A, Ucn 1 and Ucn 2 appeared to be blunted in magnitude or potency and slightly delayed in onset in fed rats as compared with fasted rats. No treatment significantly altered water intake in the rats fed ad libitum (Figure 6B, D, F and H).
Figure 6.
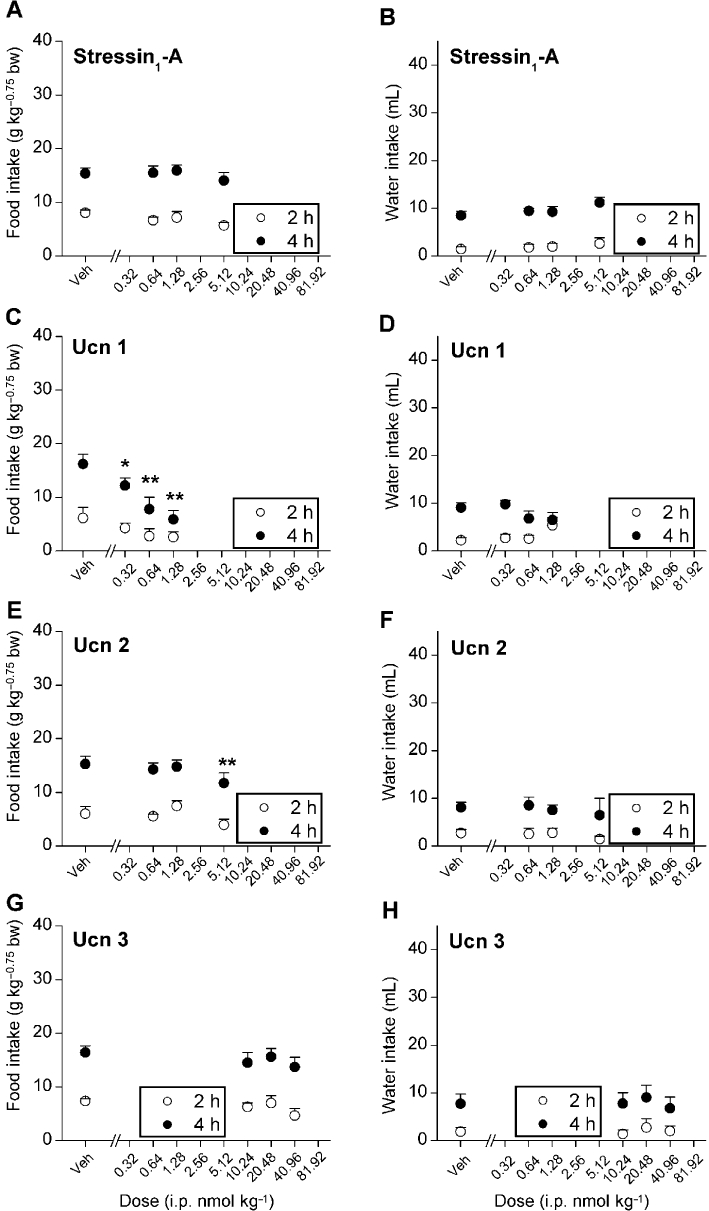
Effects of i.p. treatment with stressin1-A, Ucn 1,Ucn 2 and Ucn 3 on mean (±SEM) cumulative food or water intake (2 and 4 h) in male Wistar rats fed ad libitum (stressin1-A, Ucn 1 and Ucn 3, n = 6 per group and Ucn 2, n = 5 per group) housed in automated test cages that detect intake as nose poke events. Food intake was normalized to body weight per Kleiber's mass exponent (0.75). *P < 0.05, **P < 0.01, compared with vehicle treatment (within-subjects Fisher's protected LSD test).
CTA
Anorexic doses of both stressin1-A (F4,34= 3.06, P < 0.05) and Ucn 1 (F3,38= 38.617, P < 0.001) produced a CTA (Figure 7A, B). Conditioning with the 10.24 and 20.48 nmol·kg−1 doses of stressin1-A significantly decreased saccharin preference compared with vehicle treatment, with a similar trend evident for the 5.12 nmol·kg−1 dose (P = 0.07). Each dose of Ucn 1 (0.32, 0.64 and 1.28 nmol·kg−1) yielded a significant decrease in saccharin preference. In contrast, anorexic doses of Ucn 2 did not produce a significant taste aversion (Figure 7C). Subjects treated with stressin1-A and Ucn 1, but not Ucn 2, also exhibited watery diarrhoea, similar to a previous report (Tachéet al., 2002).
Figure 7.
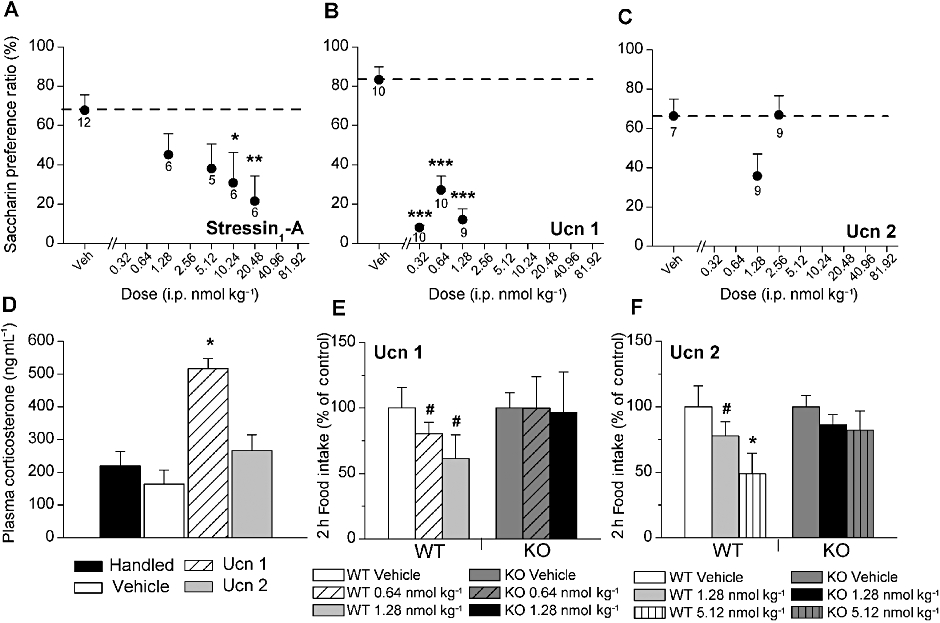
Effects of i.p. administration of (A) stressin1-A (B) Ucn 1 and (C) Ucn 2 on the formation of a CTA. A relative decrease in the saccharin preference ratio is interpreted as the formation of a CTA. Data are expressed as the mean (±SEM) preference ratio for a 7.31 mM saccharin solution over water as a function of having received two prior pairings of i.p. stressin1-A, Ucn 1 or Ucn 2 injections immediately after access to a previously novel saccharin solution. *P < 0.05, **P < 0.01, preference ratios significantly lower after stressin1-A (10.24 and 20.48 nmol, respectively). ***P < 0.001, Ucn 1 (0.32, 0.64 and 1.28 nmol, respectively) compared with respective vehicle treatment (between-subjects LSD test). Sample size is indicated beneath each symbol. (D) Effect of handling (no injection; n = 7), vehicle (n = 7), 0.64 nmol Ucn 1 (n = 7) and 1.28 nmol Ucn 2 (n = 6) on plasma corticosterone level. *P < 0.05, corticosterone level significantly higher after Ucn 1, but not Ucn 2, treatment compared with vehicle treatment (between-subjects LSD test). (E–F) Effects of Ucn 1 (E) or mouse Ucn 2 (F) on the mean (±SEM) 2 h food intake in wild-type (WT, Ucn1, n = 8, Ucn 2, n = 5) and CRF2 receptor knockout (KO, Ucn 1, n = 5, Ucn 2, n = 6) mice. Statistics were performed on the raw data but are presented as % of vehicle intake (g·kg−0.75 bw) changes to facilitate comparison. Two-hour intake for vehicle-treated subjects were 51.8 or 24.6 g·kg−0.75 bw for Ucn 1 or Ucn 2 studies, respectively. *P < 0.05, # P = 0.06, Ucn 1 and Ucn 2 significantly decreased food intake in WT mice but not in CRF2 KO mice.
Plasma corticosterone measurement: comparison of equally anorexic doses of Ucn 1 and Ucn 2
As shown in Figure 7D, an anorexic dose of Ucn 1 (0.64 nmol·kg−1, i.p.) significantly increased plasma CORT (F3,26= 14.63, P < 0.001) to levels greater than those of handling controls, vehicle controls and subjects treated with an equally anorexic dose of Ucn 2. In contrast, CORT levels of Ucn 2-treated subjects did not differ from those of controls.
Effects of Ucn 1 and Ucn 2 on feeding in fasted CRF2 knockout and wild-type mice
As shown in Figure 7E, Ucn 1 significantly altered 2 h re-feeding in fasted WT mice (F2,14= 4.34, P < 0.05). Pairwise comparisons showed that both the 0.64 and 1.28 nmol·kg−1 doses tended to reduce intake in WT mice (P = 0.06) but not CRF2 knockout mice. As shown in Figure 7F, Ucn 2 reduced 2 h deprivation-induced food intake in WT mice (dose, F1,4= 7.81, P < 0.05; LSD post hoc test, P < 0.05 for 5.12 nmol·kg−1 vs. vehicle and P = 0.06 for 1.28 nmol·kg−1 vs. vehicle), but not in CRF2 knockout mice (F1,5= 2.06, P > 0.05), leading to a significant difference in the 2 h intake in WT vs. CRF2 knockout mice after the 5.12 nmol·kg−1 dose (P < 0.05). Ucn 1 also significantly decreased water intake in WT mice (F1,6= 9.50, P < 0.05, pairwise LSD post hoc test 1.28 nmol·kg−1 vs. vehicle P < 0.05), but not in CRF2 knockout mice. Ucn 2 treatment did not significantly alter water intake in either genotype (data not shown).
Discussion
The present study indicates that systemic Ucn 2 administration can potently suppress food intake in rodents at doses that do not evoke signs of malaise or stress. Systemic Ucn 2 reduced feeding in wild-type, but not CRF2 knockout mice, indicating a CRF2 mechanism of action. In contrast, systemic treatment with Ucn 1 and stressin1-A, ligands with CRF1 affinity, not only reduced feeding but also decreased feeding efficiency and produced signs of interoceptive stress.
Ucn 1, a non-selective CRF receptor agonist, was the most potent at reducing food intake (at doses as low as 0.32 nmol·kg−1) and most efficacious (up to 70% reduction) in fasted and fed rats. The anorexic potency of Ucn 1 via the i.p. route was of the same order of magnitude as that of cholecystokinin-8 (Lo et al., 2007; Cooper et al., 2008) and amylin (Lutz et al., 1995), recognized anorexic hormones, for reducing acute (∼1–2 h) re-feeding in fasted rats and was even more potent than others, such as oxyntomodulin (∼10 nmol·kg−1; Dakin et al., 2004), apolipoprotein A-IV (∼2 nmol·kg−1; Lo et al., 2007), glucagon-like peptide-1 (>10 nmol·kg−1; Dakin et al., 2004) and leptin (>1.5 nmol·kg−1; Patel and Ebenezer, 2008). Stressin1-A, a selective CRF1 agonist, produced the second largest suppression of re-feeding (up to 40%) in fasted rats but required substantially higher doses (10.24–20.48 nmol·kg−1) than Ucn 1 and did not reduce feeding in fed rats. In contrast, Ucn 2, a selective CRF2 agonist, was similarly potent to Ucn 1 (0.64–5.12 nmol·kg−1) and the other anorexic hormones but produced much smaller reductions in 2 h intake (∼15%). Ucn 3, another selective CRF2 agonist, also reduced food intake by only 15% but at 32–64-fold higher doses than were needed for Ucn 2. Thus, the rank-order potency of the peptides to reduce deprivation-induced re-feeding was Ucn 1 ≥ Ucn 2 > > stressin1-A > Ucn 3, whereas the rank-order for anorexic efficacy was Ucn 1 > stressin1-A > Ucn 2 = Ucn 3. The rank-ordered anorexic activity of Ucn 1 > Ucn 2 > Ucn 3 observed here in rats is also consistent with previous studies in mice (Tanaka et al., 2009). In the fed state, unlike the Ucns, stressin1-A was ineffective. However, the anorexigenic activity of Ucn 1 and Ucn 2 was delayed in onset by ∼2 h to a time when baseline food intake was otherwise greater.
Several pharmacological differences might explain Ucn 1's greater anorexic activity than the other peptides. Firstly, Ucn 1 is the only peptide tested that activates both CRF receptor subtypes with subnanomolar affinity, providing two potential mechanisms for anorexia (Fekete and Zorrilla, 2007). Secondly, Ucn 1 also binds the CRF binding protein with high affinity and a slow rate of dissociation. This is relevant because CRF binding protein/ligand complexes might have direct or indirect (via agonist liberation) signalling properties (Chan et al., 2000; Ungless et al., 2003; Fekete and Zorrilla, 2007), and because association with the CRF binding protein might protect the peptide from degradation, prolonging its duration of action.
Several pharmacological properties might also explain the 30- to 60-fold lower potency of Ucn 3 to reduce food intake compared with Ucn 2. Firstly, Ucn 3, although more selective than Ucn 2 at the CRF2 receptor, is at least one order of magnitude less potent at binding the CRF2 receptor and the rodent CRF binding protein than Ucn 2 (Fekete and Zorrilla, 2007). Secondly, Ucn 3 does not show CRF1-like agonism, whereas Ucn 2 is a full agonist, albeit of very low affinity, of the CRF1 receptor (Fekete and Zorrilla, 2007). Thirdly, Ucn 3 may less effectively engage certain CRF2-mediated signal transduction pathways than Ucn 2. For example, in previous in vitro studies, Ucn 3 less effectively activated MAPK pathways than did Ucn 1 and Ucn 2 (Brar et al., 2004; Chen et al., 2005). Accordingly, signal transduction mechanism specificity for different ligands has recently been suggested for CRF receptors (Berger et al., 2006; Beyermann et al., 2007). Similar to the present findings, others have also found a decreased ability of peripheral Ucn 3 (vs.Ucn 2) to produce putative CRF2-dependent effects in vivo, including slowing of gastric emptying, a result potentially relevant to the anorexia observed in the present study (Martinez et al., 2002; 2004;). The briefer duration of anorexic action of Ucn 3 in rats (10 min) seen here as compared with the other CRF-family peptides is consistent with the short duration of action reported for Ucn 3 previously in mouse (20 min) (Tanaka et al., 2009), results potentially consistent with greater degradation/inactivation of Ucn 3. Finally, Ucn 3 does not appreciably cross the intact blood–brain barrier, in contrast to reports of moderate spontaneous passage by Ucn 2 (Kastin and Akerstrom, 2002), and a latent transport system for Ucn 1 that is induced by anorexics such as leptin, TNF-α and glucose pretreatment (Pan and Kastin, 2008).
Ucn 1 reduced food intake with a rapid onset in fasted rats and also tended to reduce intake within 2 h in rats fed ad libitum, culminating in a 65% reduction in intake by 4 h. Ucn 1 reduced intake in fasted rats by decreasing both meal frequency and meal size. Bout analyses showed that Ucn 1-treated rats had fewer bouts of both eating and drinking with longer inter-bout intervals. Unlike Ucn 1, stressin1-A reduced meal size but not meal frequency, whereas Ucn 2 tended to reduce meal frequency but not meal size. Ucn 1-treated rats also showed decreased and less regular eating rates. Finally, Ucn 1 also decreased the quantity and duration of eating following the peak of feeding bouts, a profile consistent with facilitated bout termination. The relationship between the peptides' pharmacological differences (e.g. receptor specificities, CRF binding protein affinity, blood–brain barrier penetration) and their different effects on feeding microstructure remains unclear.
The present results obtained after peripheral administration of Ucn peptides to fasted rats contrast with those from studies in which Ucn 2 was administered centrally to rats fed ad libitum, where it was found that Ucn 2 induced a delayed anorexia and did so by selectively reducing meal size (Inoue et al., 2003; Cottone et al., 2007). The route of administration (systemic vs. central) or feeding state (fasted vs. fed) might account for these different effects of Ucn 2 treatment. Somewhat larger reductions in chow intake following peripheral Ucn 2 administration than those seen here have been reported previously in mice (Wang et al., 2001; Gourcerol et al., 2007); differences in the species (mouse or rat) or experimental diet (chow vs. palatable, sweetened chow) may account for these different outcomes.
In accord with our findings, Ucn 2 was shown to suppress food intake in fasted mice (Wang et al., 2001; Gourcerol et al., 2007), but the doses required to reduce food intake in the present study were higher. Systemic Ucn 1- and Ucn 2-induced anorexia were also shown to occur in rats and to be absent in CRF2 knockout mice, demonstrating a CRF2 mode of action. The latter finding is consistent with previous results showing a role for CRF2 receptors in the synergistic anorexic effect of combined Ucn 2-cholescystokinin administration (Gourcerol et al., 2007). A possible biological mechanism of peripheral CRF2-related anorexia is slowed gastric emptying (Wang et al., 2001; Martinez et al., 2004), which results from CRF2-dependent reductions in gastric and ileal motility (Porcher et al., 2005). The presence of urocortins in the gastrointestinal tract (Harada et al., 1999; Kozicz and Arimura, 2002; Yamauchi et al., 2005), pancreatic β-cells (Li et al., 2003) and adipose tissue (Seres et al., 2004) is consistent with a possible endogenous role for peripheral urocortins in the control of food intake. Potential targets through which peripherally administered urocortins might control food intake include CRF receptors in the stomach, duodenum, ileum, colon and liver, as well as CRF2 receptors in the nodose ganglion, which subserves visceral afferent functions and contains cell bodies for vagal fibres (Lawrence et al., 2002; Chatzaki et al., 2004a,b; 2006; Porcher et al., 2005; 2006; Simopoulos et al., 2009). Endocrine signalling to the CNS is also a conceivable mode of urocortin action, because Ucn 2 can spontaneously reach brain parenchyma at a moderate rate (Kastin and Akerstrom, 2002), and Ucn 1 transport across the blood–brain barrier can be induced by leptin, TNF-α and glucose (Pan and Kastin, 2008). As a substituted fragment of CRF (Rivier et al., 2007), stressin1-A might similarly share the ability of CRF to access brain parenchyma from the periphery via saturable transport, unlike Ucn 3 (Kastin and Akerstrom, 2002). The brain penetration of Ucn 1 involves CRF1 and CRF2 receptors localized on cerebral microvessels; the latter play a key role in Ucn 1 passage following induction by leptin (Pan et al., 2004; Tu et al., 2007). CRF2 receptors are expressed in close proximity to cerebral microvessels in appetite-regulatory structures, including the area postrema and medial nucleus tractus solitarius/dorsal vagal complex (Bittencourt and Sawchenko, 2000; Van Pett et al., 2000) and activation of brain CRF2 receptors inhibits food intake (Wang and Kotz, 2002; Fekete et al., 2007). In support of an endogenous anorexic role for CRF2 systems in food intake control, CRF2 null mice were found to eat more sweet chow (Tabarin et al., 2007) and a higher-fat diet (Bale et al., 2003) than WT mice (Kozicz and Arimura, 2002).
Both Ucn 1 and stressin1-A, but not Ucn 2, reduced weight regain and feeding efficiency for 1–2 days following systemic administration. One possible contributing mechanism is CRF1-mediated increases in excretion because the tested doses of Ucn 1 and stressin1-A, but not Ucn 2, acutely elicited diarrhoea. Changes in energy expenditure or fuel substrate utilization may also be involved, but such data have been controversial. One study reported that i.p. doses of Ucn 1 similar to those studied here reduced oxygen consumption in lean mice (Asakawa et al., 2001). In contrast, another study found that central administration of Ucn 1 increased energy expenditure (Spina et al., 1996; De Fanti and Martinez, 2002). Additionally, intra-PVN Ucn 1 administration induced brown adipose tissue uncoupling protein-1 mRNA synthesis and increased relative utilization of fat as an energy substrate (Kotz et al., 2002).
Peripheral Ucn 1 and Ucn 2 administration also reduced water intake at doses that reduced food intake in fasted rats. Ucn 1 made rats drink more irregularly in the meal and take fewer drinking bouts that were separated by longer pauses. Similar to the present findings, i.c.v. administration of Ucn 2 and Ucn 3 decreased water intake (Zorrilla et al., 2004; Fekete et al., 2007), and intra-hypothalamic administration of Ucn 3 reduced prandial water intake, partly by reducing drinking bout frequency (Fekete et al., 2007). Whether the present hypodipsic actions of peripheral Ucn 1 and Ucn 2 are primary or secondary to reduced food intake remains unclear.
Both stressin1-A and Ucn 1 elicited signs of an aversive state at anorexic doses. Each peptide produced a conditioned taste aversion and high levels of defecation/diarrhoea. When administered i.p. Ucn 1 also increased circulating corticosterone levels. These effects were not shared by similarly anorexic doses of the selective CRF2 agonist Ucn 2, suggesting a role for CRF1 receptors in the negative interoceptive state. The present results are consistent with findings by Taché and colleagues showing that i.p. administration of CRF or stressin1-A, similar to stressors, stimulated defecation and diarrhoea by increasing colonic transit (Zorrilla et al., 2003; Martinez and Tache, 2006; Yuan et al., 2007) and increased visceral nociception (Martinez et al., 2004; Nijsen et al., 2005). CRF and stressin1-A stimulate colonic function via a direct action on colonic cholinergic and nitrergic myenteric neurons that express CRF1 receptors (Yuan et al., 2007). The results support the hypothesis that peripheral CRF1 receptors are involved in the aversive pathophysiology of functional gastrointestinal disorders, such as irritable bowel syndrome (Taché and Brunnhuber, 2008).
Consistent with our finding that i.p. administration of Ucn 1 increased CORT levels, previous studies found that i.v. Ucn 1 infusion activated the hypothalamic-pituitary-adrenal axis via a CRF1-dependent mechanism (Vetter et al., 2002) and that systemic stressin1-A administration increased adrenocorticotropic hormone (ACTH) release in rats (Rivier et al., 2007). In contrast, peripheral administration of a similarly anorexic dose of Ucn 2 did not increase CORT levels in the present study, consistent with the dearth of CRF2 receptors on ACTH-secreting pituitary corticotrophs (Lovenberg et al., 1995) and reports showing that peripheral Ucn 3 administration does not increase CORT secretion (Venihaki et al., 2004). The results support a facilitatory role for peripheral CRF1, but not CRF2, receptors in CORT secretion.
In summary, systemic Ucn 2 administration potently suppressed food intake via a CRF2-dependent mechanism without eliciting signs of malaise. Systemic Ucn 1 and stressin1-A injection likewise reduced feeding but also decreased feeding efficiency and produced interoceptive stress signs, including malaise-like behaviour, diarrhoea and hypothalamic–pituitary–adrenal axis activation. Peripheral Ucn 3 was relatively ineffective at reducing both re-feeding following fasting and feeding in rats fed ad libitum. Consistent with their pharmacological differences, our results show that the effects of peripheral urocortins on appetite are also diverse.
Acknowledgments
The authors thank Robert Lintz for technical support and Michael Arends for editorial assistance. This is publication number 20694 from The Scripps Research Institute. This work was supported by National Institutes of Health grants DK64871, DK026741, DK070118 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. ÉMF was supported by a Hungarian State Eötvös Scholarship.
Glossary
Abbreviations
- CORT
corticosterone
- CRF
corticotropin-releasing factor
- CRF1
CRF type 1 receptor
- CRF2
CRF type 2 receptor
- CTA
conditioned taste aversion
- LSD
Fisher's protected least significant difference
- Ucn
urocortin
Conflicts of interest
Wylie Vale, PhD
The following have been licensed by The Salk Institute for Biological Studies and/or The Clayton Foundation: CRF to Ferring Pharmaceuticals, CRFR1 and Ucn 2 to Neurocrine Biosciences and Ucn 3 to Johnson & Johnson. WWV is a co-founder, member of the Board of Directors and a shareholder of Neurocrine Biosciences, a company that is developing small molecule antagonists of corticotropin releasing factor and has licensed Ucn 2 as a potential treatment for acute congestive heart failure. The work described in this manuscript is supported by the National Institutes of Health and private sources and is independent of Neurocrine Biosciences.
Jean Rivier, PhD
JR is founder and president of Sentia Medical Sciences Inc., a company that has licensed from the Salk Institute, CRF peptide antagonists for their effects on the gastrointestinal, cardiovascular, skin and central nervous systems. The work described in this manuscript is supported by the National Institutes of Health and private sources and is independent of Sentia Medical Sciences Inc.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Prior to administration of stressin1-A (n = 12), Ucn 1, Ucn 2 or Ucn 3 (n = 6 per group), fasting-induced body weight loss did not differ in relation to peptide treatment or dose, as desired. Data are expressed as medians (line) with the surrounding interval indicating the 25th and 75th percentiles.
Figure S2 Illustration of (A–D) the serial regularity within feeding bouts after i.p. administration of Ucn 1 and (E, F) serial entropy. Density plots of the ‘within-bout’ inter-feeding interval (IFI) return maps (joint IFI probability distribution) are constructed from ‘within-bout’ IFIs from one representative rat (# 2) during the first four post-injection hours (A–D). With vehicle treatment, rats showed serial regularity from pellet to pellet, reflected in a ‘clustered’ return map. Ucn 1, but not stressin1-A or Ucn 2 (data not shown), increased the serial irregularity of feeding within bouts of feeding, calculated as increased entropy of the probability joint return map for feeding compared with the respective vehicle treatment and illustrated as an increased spread on the return map. Warmer colours (towards red) indicate higher local probabilities. *P < 0.05, **P < 0.01, compared with vehicle treatment.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Asakawa A, Inui A, Ueno N, Makino S, Fujimiya M, Fujino MA, et al. Urocortin reduces oxygen consumption in lean and ob/ob mice. Int J Mol Med. 2001;7:539–541. doi: 10.3892/ijmm.7.5.539. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- Berger H, Heinrich N, Wietfeld D, Bienert M, Beyermann M. Evidence that corticotropin-releasing factor receptor type 1 couples to Gs- and Gi-proteins through different conformations of its J-domain. Br J Pharmacol. 2006;149:942–947. doi: 10.1038/sj.bjp.0706926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, et al. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br J Pharmacol. 2007;151:851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004a;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, et al. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J Neurochem. 2004b;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Lambropoulou M, Constantinidis TC, Papadopoulos N, Tache Y, Minopoulos G, et al. Corticotropin-releasing factor (CRF) receptor type 2 in the human stomach: protective biological role by inhibition of apoptosis. J Cell Physiol. 2006;209:905–911. doi: 10.1002/jcp.20792. [DOI] [PubMed] [Google Scholar]
- Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–2457. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, et al. Mouse corticotropin-releasing factor receptor type 2 gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Reeve JR, Jr, Abdalla MO, Moyer L, Raboin SJ, Green GM, et al. Cholecystokinin-33 is more effective than cholecystokinin-8 in inhibiting food intake and in stimulating the myenteric plexus and dorsal vagal complex. Brain Res. 2008;1205:27–35. doi: 10.1016/j.brainres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol. 2007;583:487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology. 2001;142:992–999. doi: 10.1210/endo.142.3.7989. [DOI] [PubMed] [Google Scholar]
- Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- De Fanti BA, Martinez JA. Central urocortin activation of sympathetic-regulated energy metabolism in Wistar rats. Brain Res. 2002;930:37–41. doi: 10.1016/s0006-8993(01)03401-1. [DOI] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, et al. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Takahashi K, Suzuki T, Saruta M, Watanabe M, Nakata T, et al. Urocortin 1, urocortin 3/stresscopin and corticotropin-releasing factor receptors in human adrenal and its disorders. J Clin Endocrinol Metab. 2005;90:4671–4678. doi: 10.1210/jc.2005-0090. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Wang L, Wang YH, Million M, Tache Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology. 2007;148:6115–6123. doi: 10.1210/en.2007-0678. [DOI] [PubMed] [Google Scholar]
- de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, Demura H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci Lett. 1999;267:125–128. doi: 10.1016/s0304-3940(99)00349-3. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Iino K, Sasano H, Oki Y, Andoh N, Shin RW, Kitamoto T, et al. Urocortin expression in human pituitary gland and pituitary adenoma. J Clin Endocrinol Metab. 1997;82:3842–3850. doi: 10.1210/jcem.82.11.4371. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, et al. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- Kissileff HR. Ingestive behavior microstructure, basic mechanisms and clinical applications. Neurosci Biobehav Rev. 2000;24:171–172. doi: 10.1016/s0149-7634(99)00071-8. [DOI] [PubMed] [Google Scholar]
- Kleiber M, Rogers TA. Energy metabolism. Annu Rev Physiol. 1961;23:5–36. doi: 10.1146/annurev.ph.23.030161.000311. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Wang C, Levine AS, Billington CJ. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R546–R551. doi: 10.1152/ajpregu.00436.2001. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Arimura A. Distribution of urocortin in the rat's gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides. 2002;23:515–521. doi: 10.1016/s0196-9781(01)00639-8. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol. 2002;136:896–904. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J, Serlin R, Seaman M. A controlled, poweful multiple-comparison strategy for several situations. Psychol Bull. 1994;115:153–159. [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, et al. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1490–R1494. doi: 10.1152/ajpregu.00329.2007. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Million M, Rivier J, Tache Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1744. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Nijsen M, Ongenae N, Meulemans A, Coulie B. Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil. 2005;17:423–432. doi: 10.1111/j.1365-2982.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Ott L. An Introduction to Statistical Methods and Data Analysis. Boston, MA: PWS-Kent; 1988. [Google Scholar]
- Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ. Modulation of feeding-related peptide/protein signals by the blood-brain barrier. J Neurochem. 2004;90:455–461. doi: 10.1111/j.1471-4159.2004.02502.x. [DOI] [PubMed] [Google Scholar]
- Patel JD, Ebenezer IS. The effect of intraperitoneal administration of leptin on short-term food intake in rats. Eur J Pharmacol. 2008;580:143–152. doi: 10.1016/j.ejphar.2007.10.046. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, Canini F, et al. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. 2006;27:1464–1475. doi: 10.1016/j.peptides.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, Perrin MH, et al. Stressin(1)-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. Boston, MA: Duxbury Press; 1995. [Google Scholar]
- Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- Saruta M, Takahashi K, Suzuki T, Fukuda T, Torii A, Sasano H. Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides. 2005;26:1196–1206. doi: 10.1016/j.peptides.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, et al. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- Simopoulos C, Christodoulou E, Lambropoulou M, Tsaroucha AK, Kakolyris S, Polychronidis A, et al. Neuropeptide urocortin 1 and its receptors are expressed in the human liver. Neuroendocrinology. 2009;89:315–326. doi: 10.1159/000187136. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, et al. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, et al. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Brunnhuber S. From Hans Selye's discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: implication in stress-related functional bowel diseases. Ann N Y Acad Sci. 2008;1148:29–41. doi: 10.1196/annals.1410.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Million M, Maillot C. Role of corticotropin-releasing factor receptor subtype 1 in stress-related functional colonic alterations: implications in irritable bowel syndrome. Eur J Surg Suppl. 2002;587:168–170. [PubMed] [Google Scholar]
- Tanaka C, Asakawa A, Ushikai M, Sakoguchi T, Amitani H, Terashi M, et al. Comparison of the anorexigenic activity of CRF family peptides. Biochem Biophys Res Commun. 2009;390:887–891. doi: 10.1016/j.bbrc.2009.10.069. [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Pan W. Corticotropin-releasing hormone receptor (CRHR)1 and CRHR2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. 2007;21:700–711. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, et al. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2002;283:R358–R367. doi: 10.1152/ajpregu.00558.2001. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Rivier JE, Tache Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- Yamauchi N, Otagiri A, Nemoto T, Sekino A, Oono H, Kato I, et al. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol. 2005;17:656–663. doi: 10.1111/j.1365-2826.2005.01354.x. [DOI] [PubMed] [Google Scholar]
- Yuan PQ, Million M, Wu SV, Rivier J, Tache Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, et al. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005a;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology (Berl) 2005b;177:324–335. doi: 10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


