Abstract
BACKGROUND AND PURPOSE
Expression of inducible NOS (iNOS) is important in certain inflammatory diseases. We determined if the hormone aldosterone, a mineralocorticoid receptor (MR) agonist, affects LPS activation of iNOS expression in rat aortic smooth muscle cells (RASMC).
EXPERIMENTAL APPROACH
Cultured RASMC were treated with LPS, with or without agonists/antagonists of steroid receptors. iNOS expression was determined by nitrite assays on culture medium removed from treated cells and by immunoblotting of cell protein extracts.
KEY RESULTS
LPS (1 µg·mL−1) increased nitrite and iNOS protein above that in control (untreated) cells. These effects of LPS were reduced by aldosterone (0.1–10 µM). The MR antagonists, eplerenone (10 µM) and spironolactone (10 or 50 µM), did not inhibit these actions of 1 µM aldosterone, but the latter were prevented by 10 µM mifepristone, a glucocorticoid (GR) and progestogen receptor (PR) antagonist. Mifepristone also prevented the reduction of LPS-induced nitrite increase produced by 1 µM dexamethasone (GR agonist) and 10 µM progesterone (PR agonist). Spironolactone (10–50 µM) by itself decreased LPS-induced increases in nitrite and iNOS protein. Mifepristone (10 µM) partially reversed these effects of 10 µM spironolactone, but not those of 50 µM; the effects of 50 µM spironolactone were also unchanged when mifepristone was increased to 50 µM.
CONCLUSIONS AND IMPLICATIONS
This pharmacological profile suggests that aldosterone, and possibly 10 µM spironolactone, use mechanisms that are dependent on PR and/or GR, but not MR, to inhibit iNOS induction in RASMC. With 50 µM spironolactone, other inhibitory mechanisms requiring further investigation may become predominant.
Keywords: rat aorta, smooth muscle cells, nitric oxide, nitric oxide synthase, lipopolysaccharide, steroid receptors, aldosterone, spironolactone, mifepristone, eplerenone
Introduction
NO, an important vasodilator generated in blood vessels, is synthesized by three main isoforms of NOS enzyme, two of which are eNOS found mainly in endothelial cells (Dudzinski et al., 2006) and neuronal NOS in ‘nitrergic’ nerves (Toda and Okamura, 2003). The expression of a third form (‘inducible’ NOS or iNOS) is activated by inflammatory agents, including bacterial cell components (e.g. LPS) and cytokines produced by immune system cells. The cell signalling and nuclear transcription factors controlling mRNA production and iNOS protein expression are of much interest (Aktan, 2004), with the complexity of these pathways sometimes varying with cell type (Kleinert et al., 2004). iNOS can be induced in macrophages, endothelial and smooth muscle cells in blood vessels (Shah, 2000) producing NO at much greater rates than other NOS enzymes and potentially causing excessive vasodilatation. NO also reacts with superoxide anion generating peroxynitrite as a cell damaging reactive oxidant species (Pacher et al., 2007). Septic shock is an acute pathological condition whereby NO from iNOS expression in vascular cells contributes to causing life-threatening hypotension and poor blood perfusion of vital organs in patients with this condition (Titheradge, 1999). iNOS expression has also been observed in atherosclerotic vascular tissue although its role in the chronic development of this disease is less clear (Ginnan et al., 2008).
The ‘renin-angiotensin-aldosterone’ system regulates BP, fluid volume and salt haemostasis, with aldosterone (ALDO) acting upon mineralocorticoid receptors (MR) to promote availability of Na+ channels and transporters (Na+/K+-ATPases) in kidney tubular epithelial cells, allowing Na+ recapture from urinary fluid into the blood with concurrent urinary K+ excretion. Abnormally high plasma ALDO concentrations (in congestive heart failure) can adversely affect the vasculature by producing endothelial dysfunction (partly by impairing eNOS formation of NO) and by inducing pro-inflammatory factors contributing to vasculopathy (Cachofeiro et al., 2008). Some of these actions of ALDO can be inhibited by MR antagonists (Schiffrin, 2006).
Few studies of ALDO effects upon iNOS in vascular cells exist. Ikeda et al. (1995) showed that ALDO inhibited the induction of iNOS by IL-1β in rat cultured aortic smooth muscle cells (RASMC), without identifying the involvement of MR. Here, we show that ALDO also inhibits iNOS expression induced by LPS in RASMC and have used antagonists to investigate which steroid receptor(s) are involved in this effect.
Methods
Cell culture
Rat aortic smooth muscle cells were grown in 6-well culture trays from explants of endothelium-denuded thoracic aortas of adult male Sprague-Dawley rats, killed by cervical dislocation according to UK Government (Home Office) regulations. Explants were covered with ‘complete medium’ (CM) containing the following as (v/v) percentages and from initial stock concentrations (in parentheses): 82% Medium 199 (M199), 10% heat-inactivated fetal bovine serum (FBS), 4% L-glutamine (200 mM), 2% combined penicillin and streptomycin (10 000 U·mL−1, and 10 mg·mL−1, respectively), 2% amphotericin B (250 µg·mL−1). Trays were incubated at 37°C in a humidified atmosphere of 5% CO2/95% air. Cell outgrowth beginning after about 4 days was maintained with changes of CM every 2–3 days to allow cells to proliferate into overlapping layers of the ‘hill-and-valley’ morphology of cultured RASMC, mostly in their elongated ‘contractile’ phenotype. Cell lines from each rat were maintained in separate trays, subcultured by passaging using trypsin/EDTA treatment, then centrifuged and resuspended in CM. Cells were added to two wells of a 6-well tray to maintain growing cell lines, and also seeded in identical volumes into 12-well trays to grow confluent cultures (used from passage 3 to 15) for studying iNOS expression.
Nitrite assays
Rat aortic smooth muscle cells were treated at 37°C for 24 h with 1.5 mL CM per well containing LPS and/or steroids, using duplicate wells per treatment on each tray. Control cells were incubated with CM alone. LPS 1 µg·mL−1 was prepared in CM. Ethanol was used to make stock solutions of ALDO (200 µM), progesterone (PROG) (10 mM), dexamethasone (DEXA) (10 mM), spironolactone (SPIRO) (10 mM) and mifepristone (MIF) (10 mM), whereas dimethylsulphoxide (DMSO) was the solvent for eplerenone (EPLER) (10 mM). Subdilutions were then made in CM to give final concentrations added to cells. Ethanol/DMSO concentrations were equalized in drug solutions for each particular study, by adjusting with additional solvent where appropriate. Maximum solvent concentrations were 1.5% (v/v) for ethanol alone or 0.5% ethanol + 0.1% DMSO (if both were required).
Nitrite (the oxidation product of cumulative NO production by cells) was determined in samples of medium taken from wells, using the spectrophotometric ‘Griess reaction’ assay as described before (Robertson et al., 2004). Blank assays used CM incubated without exposure to cells. Absorbance values were converted to nitrite concentrations by using standard curves made with known concentrations of potassium nitrite prepared in CM. Preliminary tests showed that standard curves were not significantly altered by preparing potassium nitrite in CM containing the highest concentration of each individual drug used in the cell treatments.
Cell viability (MTT assay)
After the nitrite samples had been obtained, residual medium was removed from wells and replaced with MTT (3-[4,5-dimethylthiazol-2-yl]−2,5-diphenyltetrazolium bromide) solution in CM (lacking phenol red indicator). After 2 h incubation, Mosmann's (1983) assay for cell viability was carried out as previously described (Robertson et al., 2004).
Cell protein extracts and Western blotting
Cell suspensions were seeded equally into 6-well trays and grown to confluence in a separate tray for each rat cell line. Drug solutions (LPS, steroids, etc.) made as above were added (2.5 mL per well) in duplicate wells per treatment. After incubation (24 h, 37°C), duplicate samples of medium were taken from each well for immediate use in nitrite assays, while the trays (with residual medium on cells) were returned to the incubator. After completion of nitrite assays, protein extracts were made from pooled cells from each treatment on the tray, essentially as before (Robertson et al., 2004) but with minor differences. Cell layers were harvested into PBS and centrifuged. Cell pellets were mixed with 120 µL lysis buffer containing protease inhibitor cocktail. After vortexing and centrifugation, supernatant protein concentrations were determined by Bradford protein assay. Protein samples were stored at −20°C before use.
Protein extracts were run by SDS-PAGE on 12-lane mini-gels [resolving and stacking gels of 8% and 4% (v/v) polyacrylamide, respectively]. Lanes were loaded with 25 µL comprising 12.5 µL Laemmli buffer (0.125 M tris, pH 6.8; 20% glycerol; 10% mercaptoethanol; 0.004% bromphenol blue) and 12.5 µL of protein sample. Protein was equalized for each lane on a given gel, by diluting original samples with lysis buffer where necessary, to lie in a range from 20–40 µg. Sometimes, one lane ran protein lysate (5 µL) from LPS/interferon-γ activated mouse macrophages containing iNOS as a positive control. Proteins were transferred from gels onto PVDF membranes by semi-dry blotting. Membranes were incubated in ‘blocking buffer’ (5% w/v dried skimmed milk in Tween/Tris buffered saline) as described before (Robertson et al., 2004). Enhanced chemiluminescence was used to detect iNOS after incubation of membranes with blocking buffer containing mouse antibodies (used at 1:833 and 1:5000 v/v dilution, respectively) against iNOS and actin (the latter to monitor for evenness of protein loading), followed by horseradish peroxidase-labelled anti-mouse IgG (1:667 dilution). Where more than one gel is shown for an experiment, they were not necessarily run at the same time. Densitometry of gel band images was done with the free online ‘ImageJ’ software (NIH, US Government).
Statistical analysis
Results for each experiment are summarized as mean (±SEM) for n different RASMC lines (each from an individual rat). Data were used so that each tray's cell line provided a single nitrite value per treatment, being the average of measurements from its duplicate wells per treatment. Similarly, MTT results on each tray were averaged from corresponding duplicate treatment wells. Drug and control treatments were applied in parallel on each tray, within the same cell line. Statistical analysis upon treatment groups in each study used ‘repeated measures’anova, incorporating Mauchly's Test of Sphericity upon the data to correct degrees of freedom for the anova when sphericity was found to be violated (SPSS statistics package version 17.0). Where anova significance of P < 0.05 was obtained, comparisons between particular pairs of groups were carried out as post hoc paired t-tests, with significance levels adjusted according to Bonferroni in relation to the number of pairwise tests done within the experiment. For gel analysis, ratios of each iNOS band relative to its corresponding actin band intensity were computed before statistical comparison between group means.
Materials
Male Sprague-Dawley rats came from an on-site breeding colony. M199 (Earle's Salts), amphotericin B, penicillin/streptomycin solution, L-glutamine, were from PAA laboratories (Yeovil, UK), bovine fetal serum (heat-inactivated) from Sera Laboratories (Haywards Heath, UK) and Phenol Red-free M199 from Gibco Invitrogen (Paisley, UK). Protease inhibitor cocktail tablets were from Roche Diagnostics (Mannheim, Germany) and dye reagent for protein assay from Bio-Rad (Hemel Hempsted, UK). LPS (Escherichia coli serotype 055:B5), ALDO, DEXA, MIF, PROG and SPIRO were from Sigma-Aldrich (Poole, UK); EPLER from Tocris (Avonmouth, UK). Murine-derived (IgG1) primary antibodies were anti-iNOS (Cat. No: 610431) and anti-actin Ab-5 (Cat. No: 612656) from Becton Dickinson (Cowley, UK), as also was iNOS ‘positive control’ mouse macrophage lysate (Cat. No: 611473). Secondary antibody was horseradish peroxidase-conjugated rabbit anti-mouse IgG (Cat. No: 31450) from Pierce Biotech. via Thermo Fisher (CramLington, UK). All drug and molecular target nomenclature conforms to The British Journal of Pharmacology's‘Guide to Receptors and Channels’ (Alexander et al., 2009).
Results
Concentration-dependent inhibitory effects of ALDO on LPS-induced nitrite and iNOS production
In this and later experiments, LPS (1 µg·mL−1) treatment consistently and significantly increased nitrite above the minor amounts from control cells. ALDO (0.1 and 1 µM) acted in a concentration-dependent way to inhibit this action of LPS (Figure 1A). ALDO alone (without LPS) did not alter nitrite from control amounts. In MTT assays (Figure 1B), there were no significant differences between groups, showing that drug treatments were not toxic. This was also the case for studies described in later Figures, so no other MTT results are given in this way. In further work (not shown), where cultures were treated with lower and higher ALDO concentrations, 10 nM gave a very small but non-significant inhibition of LPS-induced nitrite. Direct comparison between 1 and 10 µM ALDO showed 10 µM having a slightly but not significantly greater inhibitory effect than 1 µM, so the latter was chosen for all routine use here.
Figure 1.
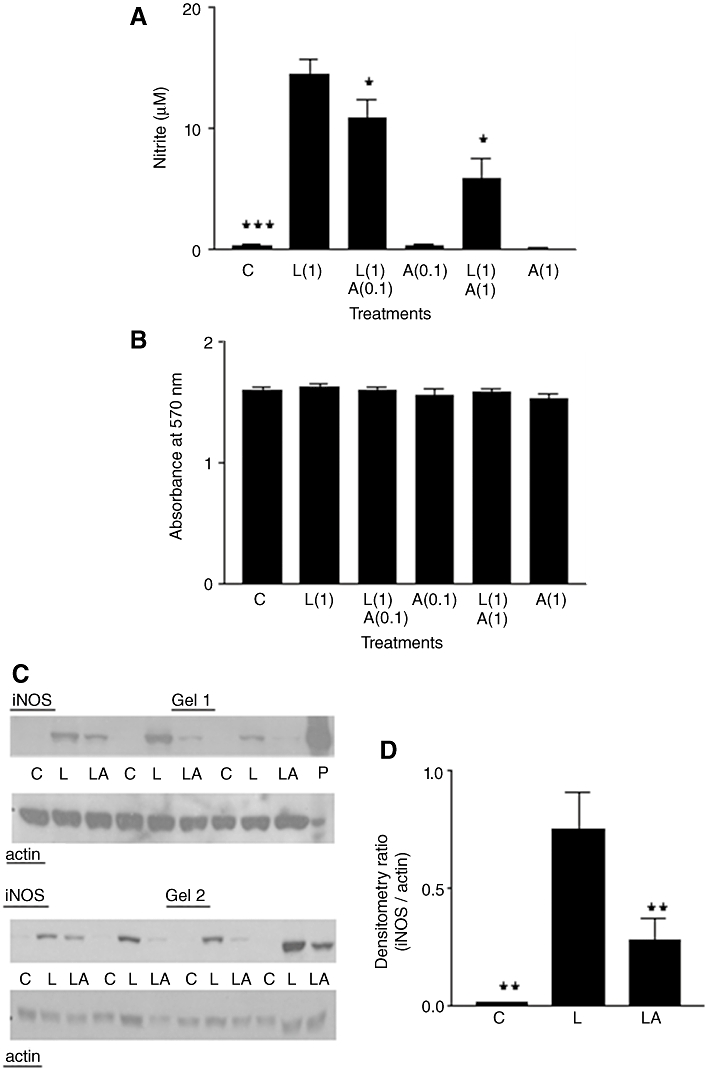
(A) Nitrite production in rat aortic smooth muscle cells. Results are means ± SEM of individual cultures from six rats. Treatment groups: control (C), LPS (L), aldosterone (A). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) or µM (aldosterone). (B) MTT results from the same cultures. (C) Immunoblotting of seven individual cultures run over two gels. From left to right, groups of three adjacent lanes show corresponding treatments for each culture (C = control; L = 1 µg·mL−1 LPS; LA = L with 1 µM aldosterone). ‘P’ represents sample of mouse macrophage protein extract containing iNOS used as positive control. (D) Treatment group means (±SEM) of iNOS/actin band intensity ratios from densitometry. *P < 0.05, **P < 0.01, ***P < 0.001, comparing LPS alone with controls or with LPS/steroid mixtures.
Western blotting (Figure 1C) showed either a very weak iNOS band, or no band, with control cells. In contrast, iNOS expression clearly occurred in LPS-treated cells and this was inhibited (sometimes almost completely) in cells treated with LPS and 1 µM ALDO. Mean values (±SEM) from nitrite assays before making protein extracts from these cultures were (in µM): control, 0.17 ± 0.07; LPS, 4.89 ± 1.58; LPS/ALDO, 2.1 ± 0.80. Densitometry (Figure 1D) showed significantly higher iNOS expression in LPS-treated cells compared with controls or with those treated with LPS plus ALDO.
In most of our work, RASMC were grown in CM containing FBS, and drugs were also made up in this CM/FBS for treating cells. FBS could naturally contain hormones including steroids, so other studies (not shown) determined if the actions of ALDO upon LPS-induced nitrite production were altered when drugs were applied in CM containing FBS previously treated with dextran-coated charcoal to remove such hormones. Comparisons were made with the same cultures treated concurrently with drugs in CM and normal FBS. Although LPS-induced nitrite was roughly halved in cells exposed to ‘charcoal treated’ compared with ‘normal’ FBS, 1 µM ALDO still decreased LPS-induced nitrite significantly to a similar proportional extent in both types of FBS. MTT assays following these treatments did not indicate any toxicity or differences in cell numbers when comparing with corresponding control cells under the two FBS conditions.
Concentration-dependent effects of SPIRO, in the presence and absence of ALDO, upon LPS-induced nitrite and iNOS production
Aldosterone is a MR agonist, so we determined if SPIRO, a MR antagonist, could prevent the effects of ALDO. Figure 2A/B shows 1 µM ALDO inhibiting LPS-induced nitrite production. However, when LPS-induced nitrite was determined in the presence of 10 µM SPIRO, the latter by itself surprisingly inhibited to a similar extent as ALDO (Figure 2A), whereas 50 µM SPIRO almost totally prevented LPS-induced nitrite production (Figure 2B). LPS-induced nitrite was inhibited when combining ALDO with 10 µM SPIRO, to a similar extent as when using either steroid alone (Figure 2A). Inhibition by 50 µM SPIRO combined with ALDO did not differ significantly from that obtained with their use as individual steroids (Figure 2B). Neither of the SPIRO concentrations by themselves affected background nitrite levels.
Figure 2.
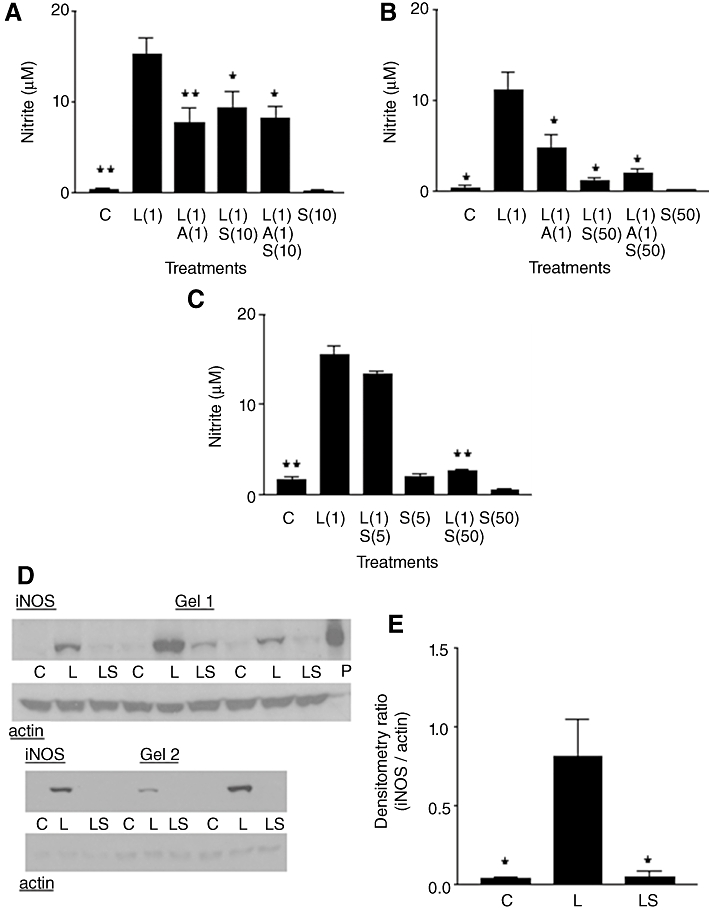
(A, B and C) Nitrite production in rat aortic smooth muscle cells. Results are means ±SEM of individual cultures from six rats. Treatment groups: control (C), LPS (L), aldosterone (A), spironolactone (S). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (aldosterone or spironolactone). (D) Immunoblotting of six individual cultures run over two gels. From left to right, groups of three adjacent lanes show corresponding treatments for each culture (C = control; L = 1 µg·mL−1 LPS; LS = L with 50 µM spironolactone). ‘P’ represents sample of mouse macrophage protein extract containing iNOS. (D) Treatment group means (±SEM) of iNOS/actin band intensity ratios from densitometry. *P < 0.05, **P < 0.01, comparing LPS alone with controls or with LPS/steroid mixtures.
To study concentration-dependent effects of SPIRO further, 5 and 50 µM were compared concurrently on the same culture trays (Figure 2C). SPIRO 5 µM gave a small but non-significant reduction in LPS-induced nitrite, while inhibition by 50 µM was considerably greater. In other studies (not shown), 100 µM SPIRO almost completely blocked LPS-induced nitrite but now showed cell toxicity (approximately 50% reduction in MTT values when compared with corresponding control cells).
Western blotting was initially used to investigate the effects of 50 µM SPIRO on LPS-induced iNOS expression (Figure 2D). Control cells had very weak or no iNOS bands, whereas iNOS expression clearly occurred in all LPS-treated cultures. In cells treated with LPS plus SPIRO, bands were either much weaker than with LPS alone or were not detectable. Mean nitrite values (±SEM) in these cultures were (in µM): control, 0.10 ± 0.03; LPS, 7.27 ± 3.54; LPS/SPIRO, 0.27 ± 0.06. There was significantly higher iNOS expression in LPS-treated cells compared either with controls or with cells treated with LPS plus SPIRO (Figure 2E).
Effects of MIF on the ability of ALDO to inhibit LPS-induced nitrite and iNOS expression
Mifepristone is a steroid antagonist of the glucocorticoid receptor (GR) and progestogen receptor (PR) but without activity upon the MR (Cadepond et al., 1997). The usual suppressive effect of ALDO (1 µM) on LPS-induced nitrite was observed (Figure 3A). However, when ALDO was combined with MIF (10 µM), the inhibitory action of ALDO was prevented. MIF by itself had no effect on LPS-induced nitrite, nor did MIF alter background nitrite from that seen in control cells.
Figure 3.
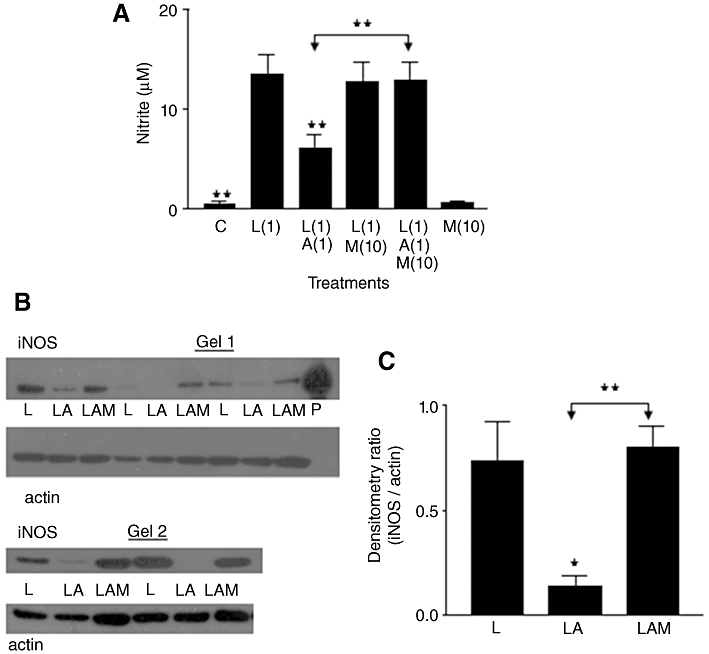
(A) Nitrite production in rat aortic smooth muscle cells. Results are means ±SEM of individual cultures from six rats. Treatment groups: control (C), LPS (L), aldosterone (A), mifepristone (M). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (aldosterone or mifepristone). (B) Immunoblotting of five individual cultures run over two gels. From left to right, groups of three adjacent lanes show corresponding treatments for each culture (L = 1 µg·mL−1 LPS; LA = mixture of L with 1 µM aldosterone; LAM = mixture of LA with 10 µM mifepristone). ‘P’ represents sample of mouse macrophage protein extract containing iNOS. (C) Treatment group means (±SEM) of iNOS/actin band intensity ratios from densitometry. *P < 0.05, **P < 0.01, comparing LPS alone with controls or with LPS/steroid mixtures, except where other group comparisons are indicated by bridging arrows.
For Western blot analysis (Figure 3B), three treatments (LPS; LPS with 1 µM ALDO; LPS with 1 µM ALDO and 10 µM MIF) were applied to cells. We omitted ‘no drug’ control treatments here to free up additional lanes on each gel to focus upon the specific relationship between iNOS bands following LPS treatment alone, in comparison with LPS plus steroids. Mean values (±SEM) for nitrite in the cultures used were (in µM): LPS, 8.95 ± 2.29; LPS/ALDO, 4.59 ± 1.56; LPS/ALDO/MIF, 8.33 ± 2.01. iNOS bands were obtained with LPS treatment in all cultures, although quite weakly in one case. Corresponding bands for ‘LPS/ALDO’ treatment were either weaker or absent, compared with those for ‘LPS alone’, whereas band intensity in cells treated with LPS/ALDO/MIF was much greater than with LPS/ALDO. Densitometry of bands showed that ALDO significantly decreased band intensity from that in LPS-treated cells, whereas additional inclusion of MIF prevented the action of ALDO (Figure 3C).
Effects of MIF on the ability of SPIRO to inhibit LPS-induced nitrite and iNOS expression
Initial studies (Figure 4A) determined effects of 10 µM SPIRO and 10 µM MIF on LPS-induced nitrite production. LPS treatment increased nitrite above controls and this effect of LPS was moderately and significantly inhibited by 10 µM SPIRO. When SPIRO was combined with MIF in LPS-treated cells, MIF at least partially counteracted the inhibitory effect of SPIRO. However, this apparent increase in nitrite caused by MIF was not a complete reversal of the action of SPIRO and resulted in levels of nitrite intermediate between those for LPS/SPIRO and those for LPS alone (and for LPS/MIF). MIF by itself did not alter LPS-induced nitrite production, nor did it affect background nitrite.
Figure 4.
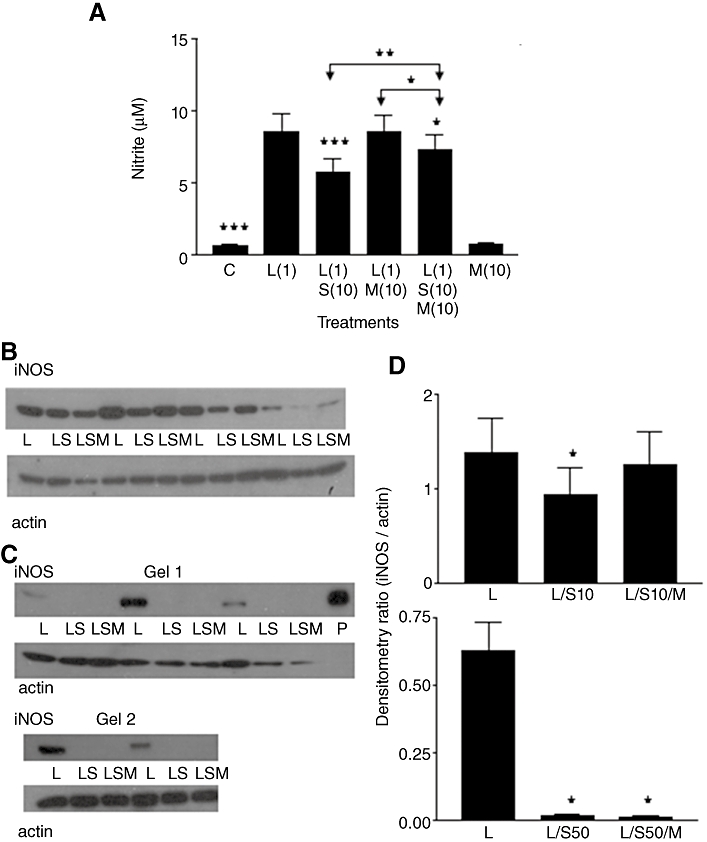
(A) Nitrite production in rat aortic smooth muscle cells. Results are means ± SEM of individual cultures from 14 rats. Treatment groups: control (C), LPS (L), spironolactone (S), mifepristone (M). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (spironolactone or mifepristone). (B) Immunoblotting of four individual cultures run on one gel. From left to right, groups of three adjacent lanes show corresponding treatments for each culture (L = 1 µg·mL−1 LPS; LS = mixture of L with 10 µM spironolactone; LSM = mixture of LS with 10 µM mifepristone. (C) Immunoblotting of five cultures run over two gels. Same abbreviations as used in (B) but treatments included 50 µM spironolactone. ‘P’ represents sample of mouse macrophage protein extract containing iNOS. (D) Treatment group means (±SEM) of iNOS/actin band intensity ratios from densitometry. Abbreviations the same as in (B/C), but S10 and S50 denote 10 µM spironolactone and 50 µM spironolactone respectively. *P < 0.05, **P < 0.01, ***P < 0.001, comparing LPS alone with controls or with LPS/steroid mixtures, except where other group comparisons are indicated by bridging arrows.
Figure 4B shows the Western blot obtained with 10 µM SPIRO along with LPS and 10 µM MIF. Mean values (±SEM) for nitrite in these cultures were (in µM): LPS, 9.45 ± 2.67; LPS/SPIRO, 7.48 ± 2.17; LPS/SPIRO/MIF, 8.33 ± 2.07, giving a generally similar pattern of drug effects upon nitrite to that in Figure 4A where many more (14) different cultures were used. Densitometry of gel bands (Figure 4D, upper graph) indicated significantly less iNOS in cells treated with LPS/SPIRO compared with those treated with LPS alone. However, like the nitrite study of Figure 4A, densitometry suggested that the mean values for iNOS expression in cells treated with LPS/SPIRO/MIF were intermediate but in this case did not differ significantly from the LPS or the LPS/SPIRO group (Figure 4D, upper graph).
In contrast, experiments using 50 µM SPIRO were more conclusive (Figure 4C), showing virtually complete inhibition of LPS-induced iNOS expression (similar to that depicted earlier in Figure 2D), and there was no sign of 10 µM MIF reversing this inhibitory effect of 50 µM SPIRO. Mean values (±SEM) for nitrite in these cultures were (in µM): LPS, 7.19 ± 1.90; LPS/SPIRO, 0.19 ± 0.09; LPS/SPIRO/MIF, 0.22 ± 0.09. Densitometry showed significantly greater iNOS expression in LPS-treated cells compared with those treated with LPS/SPIRO or with LPS/SPIRO/MIF (Figure 4D, lower graph).
To investigate this interaction between SPIRO and MIF further, we used both SPIRO and MIF at 50 µM to restore equally high concentrations of the steroids (Figure 5). LPS-induced nitrite was almost completely blocked by 50 µM SPIRO and this did not change if MIF was combined with SPIRO. MIF alone did not alter background nitrite from that of control cells. When combined with LPS (but without SPIRO), MIF did produce a moderate, although not statistically significant, inhibition of LPS-induced nitrite. None of the drug treatments significantly changed cell viability in MTT assays (not shown).
Figure 5.
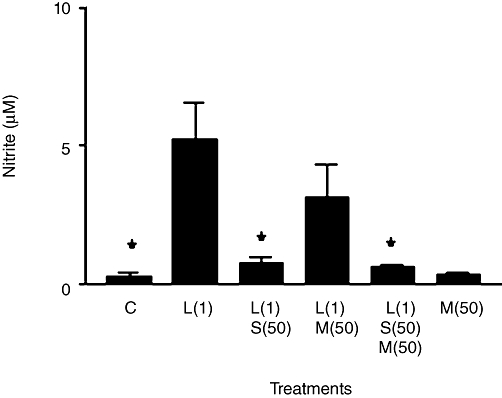
Nitrite production in rat aortic smooth muscle cells. Results are means ± SEM of individual cultures from seven different rats. Treatment groups: control (C), LPS (L), spironolactone (S) and mifepristone (M). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (spironolactone or mifepristone). *P < 0.05, comparing LPS alone with controls or with LPS/steroid mixtures.
Effects of MIF on the abilities of DEXA and PROG to inhibit LPS-induced nitrite production
As MIF has GR and PR antagonist properties, we determined if relatively selective agonists (DEXA and PROG, respectively) at these receptors can also suppress LPS-induced nitrite in a manner that is antagonized by 10 µM MIF. Preliminary studies (not shown) with various steroid concentrations showed that significant inhibitory effects on LPS-induced nitrite (without toxicity upon cells) were obtained with DEXA (0.1–10 µM) and PROG (10–50 µM), such that 1 µM DEXA and 10 µM PROG had actions similar in magnitude to those obtained with 1 µM ALDO. In the absence of LPS, neither DEXA nor PROG altered background nitrite from that seen with control cells. Figure 6A/B shows effects of MIF used with 1 µM DEXA and 10 µM PROG, respectively, in which LPS-induced nitrite was significantly decreased by DEXA or PROG. This effect of DEXA was largely reversed by MIF with no significant difference being found between LPS alone in comparison with LPS/DEXA/MIF. However, a comparison of the means for the LPS/DEXA group and the LPS/DEXA/MIF group gave a probability (P = 0.065) just above the normal level for statistical significance. MIF also appeared partially to reverse the inhibitory effect of PROG upon LPS-induced nitrite, although again this effect was not significant (P = 0.085; LPS/PROG compared with LPS/PROG/MIF). Furthermore, nitrite in the LPS/PROG/MIF group was moderately but significantly lower than in the group for LPS alone.
Figure 6.
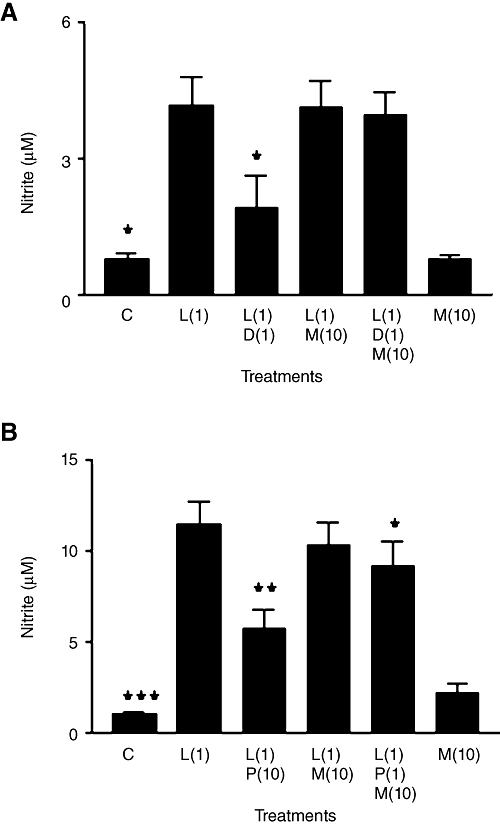
(A and B) Nitrite production in rat aortic smooth muscle cells. Results are means ± SEM of individual cultures from six different rats. In (A), treatment groups were control (C), LPS (L), dexamethasone (D), mifepristone (M), whereas progesterone (P) replaced dexamethasone in (B). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (dexamethasone, progesterone or mifepristone). *P < 0.05, **P < 0.01, ***P < 0.001, comparing LPS alone with controls or with LPS/steroid mixtures.
Effects of EPLER on the ability of ALDO to inhibit LPS-induced nitrite production
Additional studies on ALDO also investigated its interactions with EPLER, which is a more selective MR antagonist than SPIRO (Struthers et al., 2008). The usual inhibitory effects of 1 µM ALDO against LPS-induced nitrite production were not significantly changed by 10 µM EPLER (Figure 7). Also, in the absence of ALDO, EPLER had no effect on LPS-induced nitrite, nor did it alter background nitrite from that in control cells.
Figure 7.
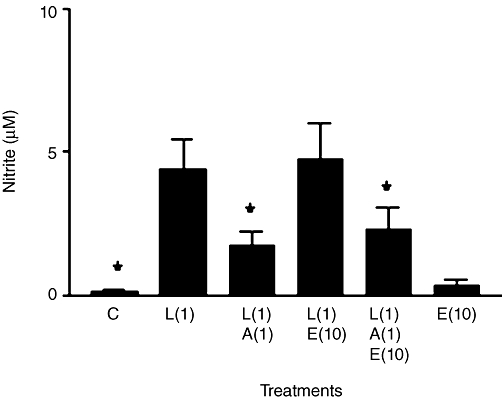
Nitrite production in rat aortic smooth muscle cells. Results are means ± SEM of individual cultures from eight different rats. Treatment groups: control (C), LPS (L), aldosterone (A) and eplerenone (E). Numbers beside drug abbreviations are concentrations in µg·mL−1 (LPS) and µM (aldosterone or eplerenone). *P < 0.05, comparing LPS alone with controls or with LPS/steroid mixtures.
Discussion
There are few publications describing the effects of ALDO on iNOS expression. Harizi et al. (2008) reported that ALDO (up to 100 nM) did not affect LPS (1 µg·mL−1)-induced iNOS expression in mouse cultured macrophages. However, Chun et al. (2003) found that 10 and 100 nM ALDO significantly inhibited iNOS-derived nitrite induced by 2 ng·mL−1 IL-1β in rat cardiomyocytes and concluded that a small inhibitory effect of 1 nM ALDO involved a MR agonist action because it was blocked by SPIRO (1–100 nM).
Following Ikeda et al. (1995), who showed that ALDO inhibited nitrite production and iNOS expression in RASMC treated with interleukin-1β (10 ng·mL−1), we have now demonstrated that ALDO exhibits such actions when LPS is the iNOS activating agent. ALDO (100 nM–10 µM) acted concentration-dependently, with 1 µM ALDO substantially inhibiting nitrite production, and with Western blotting showing that this was due to prevention of iNOS protein expression.
We concluded that the classical MR was unlikely to be involved in this action. Firstly, 10 nM ALDO produced very little inhibition of LPS-induced nitrite, whereas concentrations of 0.1 µM ALDO and greater were needed for a statistically significant effect, representing a range of concentrations higher than would be expected to allow saturation binding to a MR population, where ALDO is a very high affinity MR agonist with a KD of 0.5–1 nM (Farman and Bocchi, 2000). However, ALDO is also an agonist with lower affinity (KD about 10 nM) at the GR (Farman and Bocchi, 2000), so binding to the latter could provide an alternative explanation for the ALDO activity seen here. The lowest ALDO concentrations used in our study were certainly supraphysiological, but are somewhat nearer to pathological levels (around 10–15 nM) reported for plasma ALDO in patients with congestive heart failure, or within cardiac tissue samples from such patients (Weber, 2001; Chai et al., 2005). Secondly, the use of classical MR antagonists did not suggest ALDO acts as a MR agonist either. SPIRO is a well-established MR antagonist, but neither 10 nor 50 µM SPIRO opposed the inhibitory effects of ALDO on LPS-induced nitrite and iNOS expression. Unexpectedly, we found that SPIRO (10–50 µM) by itself significantly inhibited nitrite and iNOS expression, as discussed in more detail below. The threshold concentration for this effect to occur appeared to be around 5 to 10 µM SPIRO. EPLER (a highly selective MR antagonist) used at 10 µM did not prevent the inhibitory effect of ALDO on LPS-induced nitrite, but in major contrast to SPIRO, LPS-induced nitrite was not inhibited by EPLER.
In general, the ‘classical’ MR mediates many actions of ALDO through genomic regulation of cellular function, with ALDO binding to its cytoplasmic MR and causing translocation of the steroid receptor complex to the cell nucleus to bind ‘steroid responsive’ units on DNA, either to promote or repress gene transcription. Alternatively, the complex may interact with other transcription factors to promote or hinder their DNA binding (Connell and Davies, 2005). Similar mechanisms are exhibited by other agonist steroids on their cognate receptors. Interest has focussed recently on ‘non-genomic’ receptors for ALDO (and other steroids). These receptors may be in the cell plasma membrane to modulate cell functions without activating gene transcription, thus creating very rapid effects. The pharmacology of the non-genomic MR for ALDO is not fully established, with SPIRO and/or EPLER showing antagonism of some effects, but more usually being inactive against others (Connell and Davies, 2005). For example, ALDO (0.1–10 nM) acts non-genomically to increase intracellular cytosolic Ca2+ concentrations in RASMC and this is unaffected by 10 µM SPIRO (Wehling et al., 1995). Induction of iNOS by LPS and cytokines is a relatively slow genomic mechanism, requiring several hours for iNOS mRNA and protein to appear in RASMC (Beasley and Eldridge, 1994), making it potentially difficult to distinguish between a role for non-genomic versus genomic effects of ALDO on iNOS induction. Although a non-genomic MR cannot be ruled out here, other results suggest that another class of steroid receptor is involved.
The use of MIF, an antagonist with high affinity for both the GR and PR, but weak affinity if any for the MR (Rupprecht et al., 1993; Cadepond et al., 1997), suggested that the ‘classical’ GR and/or PR could be involved. As discussed above, ALDO activates the GR with lower affinity binding than at the MR, which may explain why inhibition by ALDO of LPS-induced nitrite and iNOS expression was prevented by 10 µM MIF. Similarly, ALDO can show agonist activity at the PR (Leo et al., 2004), which could also account for the inhibitory action of MIF. We have not directly investigated other steroid receptor types here, but from an extensive literature survey we have not seen reports of ALDO having significant agonist activity at oestrogen or androgen receptors.
Both 10 µM PROG and 1 µM DEXA inhibited LPS-induced nitrite production, agreeing with other reports that LPS and/or cytokine induction of iNOS in RASMC is inhibited by these steroids (Katsuyama et al., 1999; Zancan et al. 1999; Matsumara et al., 2001). The latter also reported that DEXA prevented NF-κB activation, a transcription factor required to promote iNOS induction in RASMC (Zhou et al., 1999), and this action of DEXA was antagonized by MIF. Although Western blotting was not used in this phase of our study, MIF was able at least in part, to reduce the inhibitory effects of DEXA and PROG on LPS-induced nitrite. DEXA is a strong agonist at the GR, but it also has some agonist activity at the MR and PR (Rupprecht et al., 1993; Leo et al., 2004). A high concentration of PROG (10 µM) was needed here to show significant inhibition of LPS-induced nitrite production, compared with the low nanomolar range at which this steroid would bind physiologically to its cognate PR. However, PROG is also a weak agonist at the GR and a very high affinity antagonist at the MR (Rupprecht et al., 1993; Krattenmacher, 2000). Thus, inhibition by MIF of DEXA and PROG actions on LPS-induced nitrite production again would point to a possible role of the GR and/or PR, but not the MR, in these effects. In this regard, MR, GR and PR have been reported to be present in RASMC, including these cells in culture (Nichols et al., 1985; Lee et al., 1997; Molnar et al., 2008).
In contrast to our finding that ALDO has an ‘anti-inflammatory’ effect on iNOS induction in RASMC via a non-MR mechanism, a ‘pro-inflammatory’ action to induce COX-2 was found by others treating rat cultured neonatal cardiomyocytes with ALDO in concentrations up to and including 1 µM. At the latter concentration, the effect of ALDO (shown to be mediated by MR) was prevented by SPIRO (10 µM) but not by (MIF 1 µM), whereas ALDO did not induce COX-2 in rat cardiac fibroblasts, suggesting some complexity in ALDO actions on inflammatory mediators within different cardiovascular cell types (Rebsamen et al., 2004).
The ability of SPIRO (10–50 µM) to inhibit LPS-induced nitrite and iNOS expression is difficult to explain fully by its known interactions with steroid receptor types. SPIRO is a much weaker antagonist at the GR than the MR, with no evidence for it being an agonist at either of them (Rupprecht et al., 1993). Also, SPIRO is an antagonist at androgen receptors, with some agonist activity at the PR (Garthwaite and McMahon, 2004). This suggests that SPIRO activation of a PR might contribute to its inhibition of iNOS expression, so we investigated if MIF could prevent the actions of SPIRO. MIF 10 µM appeared to produce a partial reversal of the modest inhibitory effect of 10 µM SPIRO on LPS-induced nitrite and iNOS expression, although this only reached statistical significance in the corresponding nitrite studies (when individual cell preparations from a large number of different rats were incorporated). In contrast, the use of 50 µM SPIRO almost totally suppressed LPS-induced nitrite and iNOS expression, such that 10 µM MIF was unable to reverse these effects of SPIRO at all. The latter might result if 10 µM MIF had an antagonist action that became relatively weaker when competing for receptor binding (putatively at the PR) with a higher (agonist) concentration of SPIRO (50 µM), so this was investigated by using both MIF and SPIRO at equally high (50 µM) concentrations. If competitive bindings were involved, 50 µM MIF might be expected to regain an ability to counter SPIRO inhibition of LPS-induced nitrite. However, 50 µM MIF did not change the inhibitory extent of 50 µM SPIRO, suggesting more complex interactions or properties of the steroids, including the possibility that 50 µM MIF by itself may inhibit LPS-induced nitrite production (but less strongly than 50 µM SPIRO).
Inhibition by SPIRO of iNOS expression is interesting because other reports have shown similar SPIRO concentrations to have pharmacological properties (in some cases unrelated to steroid receptor activation) that might be relevant. For example, Sorrentino et al. (2000) showed SPIRO (30–100 µM) inhibiting contractions of rat isolated aortic rings, produced either by the α1-adrenoceptor agonist, phenylephrine, or by KCl-induced depolarization of the vascular smooth muscle, concluding that SPIRO inhibited voltage-gated Ca2+ channels in the muscle cells. Some drugs that block such channels also inhibit LPS- and cytokine-induced iNOS expression in cultured RASMC (Szabóet al., 1993; Chou et al., 2002). Immunomodulatory effects of SPIRO (0.4−100 µM) are also known that are independent of MR involvement, including inhibition of NF-κB activation, suppression of cytokine production and modulation of many immune and inflammatory response genes in cultured cells (Bendtzen et al., 2003; Sønder et al., 2006a,b;), although effects specifically on iNOS gene expression have not previously been described. Whatever the underlying mechanisms for the effects of SPIRO shown here, we have also found that 50 µM SPIRO almost completely inhibits nitrite production in RASMC treated with IL-1β at 5 ng·mL−1 (GA Lyles and KL Sim, unpublished results).
In conclusion, our studies showing regulation of iNOS expression in vascular smooth muscle by ALDO and related steroids may be relevant to the proposed involvement of iNOS in certain vascular disorders. The demonstration that SPIRO, a MR antagonist used clinically to treat hyperaldosteronism and heart failure, can also inhibit iNOS expression is of potential interest because although the mechanisms underlying this action require more investigation, our findings complement other studies that indicate an ‘anti-inflammatory’ component of this drug's pharmacological properties.
Acknowledgments
This work was supported in part by Anonymous Trust funds donated to Dundee University Medical School.
Glossary
Abbreviations
- ALDO
aldosterone
- CM
complete medium
- DEXA
dexamethasone
- EPLER
eplerenone
- FBS
fetal bovine serum
- GR
glucocorticoid receptor(s)
- iNOS
inducible form of NOS
- M199
medium 199
- MIF
mifepristone
- MR
mineralocorticoid receptor(s)
- MTT
3-[4,5-dimethylthiazol-2-yl]−2,5-diphenyltetrazolium bromide
- PR
progestogen receptor(s)
- PROG
progesterone
- RASMC
rat aortic smooth muscle cells
- SPIRO
spironolactone
Conflicts of interest
None.
References
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D, Eldridge M. Interleukin-1β and tumor necrosis factor-α synergistically induce NO synthase in rat vascular smooth muscle cells. Am J Physiol. 1994;266:R1197–R1203. doi: 10.1152/ajpregu.1994.266.4.R1197. [DOI] [PubMed] [Google Scholar]
- Bendtzen K, Hansen PR, Rieneck K. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-alpha and interferon-gamma, and has potential in the treatment of arthritis. Clin Exp Immunol. 2003;134:151–158. doi: 10.1046/j.1365-2249.2003.02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachofeiro V, Miana M, de las Heras N, Martín-Fernández B, Ballesteros S, Fernández-Tresguerres J, et al. Aldosterone and the vascular system. J Steroid Biochem Mol Biol. 2008;109:331–335. doi: 10.1016/j.jsbmb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Cadepond F, Ulman A, Baulieu E-E. RU 486 (MIFEPRISTONE): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Chai W, Garrelds IM, de Vries R, Batenburg WW, van Kats JP, Danser AHJ. Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension. 2005;46:701–706. doi: 10.1161/01.HYP.0000182661.98259.4f. [DOI] [PubMed] [Google Scholar]
- Chou TC, Yang SP, Pei D. Amlodipine inhibits pro-inflammatory cytokines and free radical production and nitric oxide synthase expression in lipopolysaccharide/interferon-gamma-stimulated cultured vascular smooth muscle cells. Jpn J Pharmacol. 2002;89:157–163. doi: 10.1254/jjp.89.157. [DOI] [PubMed] [Google Scholar]
- Chun T-Y, Bloem LJ, Pratt JH. Aldosterone inhibits inducible nitric oxide synthase in neonatal rat cardiomyocytes. Endocrinology. 2003;144:1712–1717. doi: 10.1210/en.2002-220956. [DOI] [PubMed] [Google Scholar]
- Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthases. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- Farman N, Bocchi B. Mineralocorticoid selectivity: molecular and cellular aspects. Kidney Int. 2000;57:1364–1369. doi: 10.1046/j.1523-1755.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite SM, McMahon SG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27–31. doi: 10.1016/j.mce.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ginnan R, Guikema BJ, Halligan KE, Singer HA, Jourd'heuil D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative tissues. Free Radic Biol Med. 2008;44:1232–1245. doi: 10.1016/j.freeradbiomed.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, Mormède P, Corcuff J-B. Inter-strain differences in glucocorticoid and mineralocorticoid effects on macrophage and lymphocyte functions in mice. J Neuroimmunol. 2008;204:38–42. doi: 10.1016/j.jneuroim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Kanbe T, Nakayama I, Kawahara Y, Yokoyama M, Shimada K. Aldosterone inhibits nitric oxide synthesis in rat vascular smooth muscle cells induced by interleukin-1β. Eur J Pharmacol. 1995;290:69–73. doi: 10.1016/0922-4106(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Katsuyama K, Shichiri M, Kato H, Imai T, Marumo F, Hirata Y. Differential inhibitory actions by glucocorticoid and aspirin on cytokine-induced nitric oxide production in vascular smooth muscle cells. Endocrinology. 1999;140:2183–2190. doi: 10.1210/endo.140.5.6718. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Krattenmacher R. Drosperinone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62:29–38. doi: 10.1016/s0010-7824(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Lee WS, Harden JA, Yoshizami M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- Leo JCL, Guo C, Woon CT, Aw SE, Lin VCL. Glucorticoid and mineralocorticoid cross-talk with progesterone receptor to induce focal adhesion and growth inhibition in breast cancer cells. Endocrinology. 2004;145:1314–1321. doi: 10.1210/en.2003-0732. [DOI] [PubMed] [Google Scholar]
- Matsumara M, Kakashita H, Suzuki M, Banba N, Hattori Y. Dexamethasone suppresses iNOS gene expression by inhibiting NF-κB in vascular smooth muscle cells. Life Sci. 2001;69:1067–1077. doi: 10.1016/s0024-3205(01)01196-1. [DOI] [PubMed] [Google Scholar]
- Molnar GA, Lindschau C, Dubrovska G, Mertens PR, Kirsch T, Quinkler M, et al. Glucocorticoid-related signaling effects in vascular smooth muscle cells. Hypertension. 2008;51:1372–1378. doi: 10.1161/HYPERTENSIONAHA.107.105718. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Nguyen HH, Meyer WJ. Physical separation of aortic corticoid receptors with type I and type II specificities. J Steroid Biochem. 1985;22:577–582. doi: 10.1016/0022-4731(85)90208-0. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen MC, Perrier E, Gerber-Wicht C, Benitah J-P, Lang U. Direct and indirect effects of aldosterone on cyclooxygenase-2 and interleukin-6 expression in rat cardiac cells in culture and after myocardial infarction. Endocrinology. 2004;145:3135–3142. doi: 10.1210/en.2003-1544. [DOI] [PubMed] [Google Scholar]
- Robertson DAF, Hughes GA, Lyles GA. Expression of inducible nitric oxide synthase in cultured smooth muscle cells from rat mesenteric lymphatic vessels. Microcirculation. 2004;11:503–515. doi: 10.1080/10739680490476321. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, et al. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol. 1993;247:145–154. doi: 10.1016/0922-4106(93)90072-h. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- Shah AM. Inducible nitric oxide synthase and cardiovascular disease. Cardiovasc Res. 2000;45:148–155. doi: 10.1016/s0008-6363(99)00316-8. [DOI] [PubMed] [Google Scholar]
- Sønder SUS, Mikkelsen M, Rieneck K, Hedegaard CJ, Bendtzen K. Effects of spironolactone on human blood mononuclear cells: mineralocorticoid receptor independent effects on gene expression and late apoptosis induction. Br J Pharmacol. 2006a;148:46–53. doi: 10.1038/sj.bjp.0706700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønder SU, Woetmann A, Odum N, Bendtzen K. Spironolactone induces apoptosis and inhibits NF-kappaB independent of the mineralocorticoid receptor. Apoptosis. 2006b;11:2159–2165. doi: 10.1007/s10495-006-0286-3. [DOI] [PubMed] [Google Scholar]
- Sorrentino L, Autore G, Cirino G, d'Emanuele de Villa Bianca R, Calignano A, Vanasia M, et al. Effect of spironolactone and its metabolites on contractile property of isolated rat aorta rings. J Cardiovasc Pharmacol. 2000;36:230–235. doi: 10.1097/00005344-200008000-00013. [DOI] [PubMed] [Google Scholar]
- Struthers A, Krum K, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–158. doi: 10.1002/clc.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Mitchell JA, Gross SS, Thiemermann C, Vane JR. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1993;265:674–680. [PubMed] [Google Scholar]
- Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- Toda M, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev. 2003;55:271–324. doi: 10.1124/pr.55.2.3. [DOI] [PubMed] [Google Scholar]
- Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- Wehling M, Neylon CB, Fullerton M, Bobik A, Funder JW. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- Zancan V, Santagati S, Bolego C, Vegeto E, Maggi A, Puglisi L. 17Beta- estradiol decreases nitric oxide synthase II synthesis in vascular smooth muscle cells. Endocrinology. 1999;140:2004–2009. doi: 10.1210/endo.140.5.6694. [DOI] [PubMed] [Google Scholar]
- Zhou J, Struthers AD, Lyles GA. Differential effects of some cell signalling agents upon nitric oxide synthase expression and nuclear factor-κB activation induced by lipopolysaccharide in rat aortic smooth muscle cells. Pharmacol Res. 1999;39:363–373. doi: 10.1006/phrs.1998.0450. [DOI] [PubMed] [Google Scholar]


