Abstract
Objective
Previous studies have shown that exposure to parental verbal abuse (VA) in childhood was associated with higher rates of adult psychopathology and alterations in brain structure. Here we examine the potential consequences of exposure to peer VA during childhood.
Method
A total of 848 young adults (ages 18 to 25 years) with no history of exposure to domestic violence, sexual abuse, or parental physical abuse rated their childhood exposure to parental and peer VA and completed a self-report packet that included the Kellner Symptom Questionnaire, the Limbic Symptom Checklist-33, and the Dissociative Experiences Scale. Diffusion tensor images were collected on a subset of 63 young adults with no history of abuse or exposure to parental VA selected for varying degrees of exposure to peer VA. Images were analyzed using tract based spatial statistics
Results
Analysis of covariance revealed ‘dose-dependent’ effects of peer VA on anxiety, depression, anger-hostility, dissociation, ‘limbic irritability’, and drug use. Peer and parental VA were essentially equivalent in effect size on these ratings. Path analysis indicated that peer VA during middle school years had the most significant effect on symptom scores. Degree of exposure to peer VA correlated with increased mean and radial diffusivity and decreased fractional anisotropy in corpus callosum and corona radiata.
Conclusions
These findings parallel previous reports of psychopathology associated with childhood exposure to parental VA, and support the hypothesis that exposure to peer VA is an aversive stimulus associated with increased symptom ratings and meaningful alterations in brain structure.
INTRODUCTION
Exposure to trauma in childhood is associated with increased vulnerability to psychiatric disorders (1-3). This has been shown to be true for early exposure to childhood sexual abuse (CSA), physical abuse (PA), witnessing domestic violence (WDV) and composite scores reflecting exposure to multiple forms of trauma (4). Previously, we reported that parental verbal abuse (VA) is an important, but often overlooked, form of childhood adversity that was more strongly associated with symptom ratings such as depression and anger-hostility than parental PA (5, 6).
Exposure to physical and verbal aggression from peers is also a highly prevalent form of childhood stress perpetrated by other children who are not siblings and are not necessarily agemates (7). Their attacks may be actual physical blows or psychological attacks in the form of verbal taunts or social ostracism.
Victims of peer aggression show the scars — increased rates of depression, suicidal ideation, loneliness and even psychosis (7-9). Their grades are lower and absentee rates higher (10). They are more likely to carry weapons to school, and to engage in fights (11). They are likely to suffer more injuries, abuse over-the-counter medications, intentionally hurt animals and other people, and use weapons that could seriously harm others (10).
In the present study, we sought to ascertain what the effects are in adulthood. More specifically, we questioned whether childhood exposure to peer VA in the absence of physical bullying was associated with elevations in psychiatric symptoms, similar to what we observed with the effects of childhood exposure to parental VA. Lastly, we examined diffusion tensor imaging (DTI) scans from a group of healthy subjects to ascertain if the integrity of white matter tracts might be affected by exposure to peer VA, as we had recently observed in individuals exposed to parental VA (12).
METHODS
Subjects
Detailed ratings of symptoms and exposure to emotional abuse and trauma were collected and analyzed from our multi-study community database of 1662 young adults (636M/1026F) 18-25 years of age who responded to advertisement entitled “Memories of Childhood”. All subjects gave informed consent prior to participation. We focused on a group 848 subjects (363M/485F mean age 21.8±2.1years) who had no exposure to DV, CSA, parental PA or peer physical bullying and a subset of 707 subjects (298M/409F; mean age 21.9+2.1years) who in addition had no exposure to either maternal or paternal VA (defined by a maternal or paternal score on the verbal abuse questionnaire (5) greater than or equal to 40).
DTI was collected on a separate sample of 63 subjects (23M/40F, 21.9 ± 1.9 years) with no history of exposure to CSA, PA, WDV, peer physical bullying, harsh corporal punishment or significant parental VA, and no history of Axis I psychiatric disorders, who were recruited as healthy normal controls for other studies. These subjects did however differ in their degree of exposure to peer VA.
Assessments
Abuse and Trauma Ratings
History of exposure to trauma was obtained using previously described methods (5) for the evaluation of PA and CSA. History of WDV was assessed using the questions: “Have you ever witnessed serious domestic violence?” “...heard domestic violence in you family?”, “...watched your mother (father) threatened or assaulted”, “...heard your mother (father) threatened or assaulted”. Ratings of exposure to parental or peer VA were assessed using the Verbal Abuse Questionnaire (VAQ) which consists of 15 items that cover the key components of verbal abuse – scolding, yelling, swearing, blaming, insulting, threatening, demeaning, ridiculing, criticizing, screaming, belittling, etc (see Appendix). In a preliminary sample of 48 college students, the VAQ showed high internal consistency as applied to reports of both maternal behaviors (Cronbach's alpha = 0.98) and paternal behaviors (Cronbach's alpha = 0.94). In the present sample the VAQ also showed high internal consistency for female (Cronbach's alpha = 0.95) and male peer VA (Cronbach's alpha = 0.96).
Symptom Ratings
As in our previous studies of subjects exposed to VA (5, 6, 12), self-report ratings were obtained using Kellner's Symptom Questionnaire (SQ; (13)), the Dissociative Experience Scale (DES; (14)) and the Limbic System Checklist-33 (LSCL-33; (15)). The SQ provides four symptom scales (depression, anxiety, anger-hostility, somatic complaints). Depression and anxiety scores ≥ 12 are considered clinically significant (5, 13). DES scores > 30 are considered clinically significant and warrant further investigation (16). The LSCL-33 evaluates the frequency with which subjects experience symptoms often encountered as ictal temporal lobe epilepsy (TLE) phenomena (17). In our clinical experience scores ≥ 40 are often associated with electroencephalographic abnormalities (spikes, sharp waves, paroxysmal slowing).
Demographics
Race, ethnicity, subject's education, paternal education, family income, and perceived financial sufficiency (PFS) during childhood (1-much less than enough money to meet our needs ... 5-much more than enough money to meet our needs) were collected. We included PFS as an alternative to family income, as subjects were often uncertain of their parent's income, and family income could mean very different things depending on locale, family size, and parental spending habits. In all cases, PFS explained a greater share of the variance in symptom ratings than combined family income.
Image Acquisition
MRI examinations were conducted at the Brain Imaging Center. Heads were stabilized with cushions and tape to help minimize movement. Multiple diffusion-weighted images (DWIs), with 12 encoding directions and an additional T2-weighted scan, were acquired using a 3T Siemens Trio scanner with standard single shot, spin echo, echo planar acquisition sequence with eddy current balanced diffusion weighting gradient pulses to reduce distortion (18). Scan parameters were: b=1000 sec/mm2, TE/TR=81msec/5sec; matrix=128×128 on 220mm×220mm FOV; slices 5mm without gap resulting in voxels of 1.71875×1.71875×5mm. Four magnitude averages provided sufficient signal-to-noise ratios. Volumetric T1-weighted anatomic reference images were acquired using an MP-RAGE sequence (TE/TR/TI=2.74ms/2.1s/1/1s; 256×256×128 matrix for 1×1×1.3 mm voxels).
Data Analysis
Effects of exposure to peer VA were assessed in the subjects with no exposure to CSA, WDV, parental PA or parental VA (n = 707). Maximal degree of exposure to male or female peer VA (whichever was greater) was binned into deciles (0-9, 10-19, ... 50+) and assessed using analysis of covariance (ANCOVA). Parental education and PFS were included as covariates (19). Structural equation modeling (sem in R) was used to perform simple path analyses to determine whether peer VA had different consequences depending on developmental stage of exposure (20). For these analyses we calculated the degree of exposure to male or female peer VA during elementary, middle and high school years based on maximal degree of exposure during each year and the number of years of occurrence. The model also included parental education and PFS as sociodemographic variables. Data were adjusted to control for gender differences in symptom ratings. Multiple regression analysis was used to ascertain the relative impact of exposure to peer versus parental VA in the full sample of subjects with no exposure to CSA, WDV or parental PA (n = 848). Gender, parental education and PFS were included as additional interest variables. This approach has the advantage of using exposure ratings as continuous variables without requiring cut scores to delineate significant degrees of exposure.
Image Analysis
DTI preprocessing, including skull stripping using the Brain Extraction Tool (BET) and correction for movement and eddy currents were performed using the FMRIB Software Library (FSL, Oxford, U.K.). A diffusion tensor model was fit to each voxel to derive fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) images.
The Tract-Based Spatial Statistics (TBSS) tool in FSL was used to calculate tract-based differences in FA and diffusivity. TBSS is a new approach that uses an anatomically-based carefully tuned nonlinear registration procedure to project results onto an alignment-invariant tract representation (the “mean FA skeleton”) for voxelwise analysis of multi-subject diffusion data (21). TBSS computes a group mean FA skeleton, which represents the centers of all fiber bundles that are common to the subjects involved in the study (21). Each subject's aligned FA image was projected onto the skeleton, and the resulting data fed into voxelwise statistics. Multiple regression analyses were performed to identify clusters within fiber tracts whose FA, MD, AD or RD values correlated with severity of exposure to peer VA. This analysis was adjusted for effects of gender and age. Tracts were identified in which the significance of the correlation was p < 0.05 after correction for multiple comparisons using Threshold-Free Cluster Enhancement (21).
RESULTS
Degree of Exposure and Effects of Peer VA: Subjects without abuse or parental VA (n=707)
Subjects differed widely in their degree of exposure to peer VA. Males reported a greater degree of exposure to male versus female peer VA (male peer VA: 24.4±19.3; female peer VA: 14.7±16.2; tdep = 11.29, df = 290, p < 10-24), whereas females reported the opposite pattern (male peer VA: 9.4±12.5; female peer VA: 13.0±13.6; tdep = –7.09, df = 402, p < 10-11). Within subject ratings of exposure to male versus female peer VA were well correlated (r = 0.639), indicating that individuals exposed to peer VA by one gender were often exposed to VA by the other gender. Maximal degree of exposure to either male or female peer VA was used as an overall exposure index. Males, on average, reported a substantially greater degree of exposure to peer VA than females (males: 25.8±19.6; females: 14.6±14.4; F1,705 = 76.80, p < 10-16).
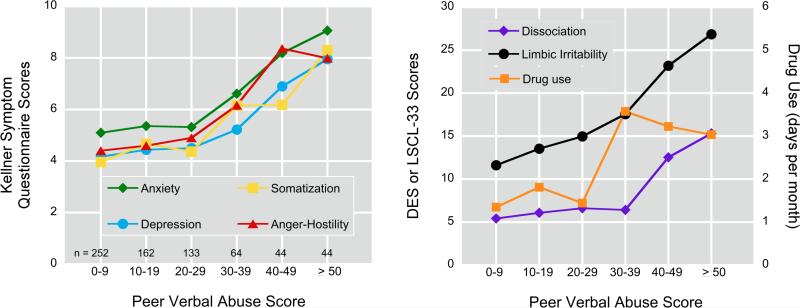
The ‘dose-dependent’ relationship between degree of exposure to peer VA and symptom ratings is illustrated in Figure 1. There were strong main effects of peer VA exposure scores on all ratings. The weakest association was on degree of drug use (F5,685 = 4.82, p < 0.0003), the strongest association was on ratings of ‘limbic irritability’ (F5,682 = 18.66, p < 10-16). The remaining ratings were effected to similar degrees, ranging from depression (F5,658 = 5.60, p < 10-4) to dissociation (F5,660 = 14.73, p < 10-12). For SQ ratings and degree of drug use there appeared to be an inflection point with peer VAQ exposure scores ≥ 30 resulting in significantly higher ratings than peer VAQ scores < 30. DES scores had an inflection point ≥ 40. LSCL-33 scores, in contrast, showed a more consistently graded response. We defined a peer VA scores ≥ 30 to be indicative of significant exposure, and a peer VA score ≥ 40 as substantial exposure.
Figure 1.
Effect of exposure to peer verbal aggression (maximal score of male or female peers) on Kellner Symptom Questionnaire, Limbic System Checklist-33, Dissociative Experience Scale, and degree of drug use in subjects with no exposure to childhood sexual abuse, witnessing domestic, parental or peer physical abuse, or parental verbal abuse (n = 707).
Substantial exposure to peer VA was associated with increased risk for clinically significant symptom ratings. Odds ratios were 2.3-fold (95% CI = 1.1-4.8) for depression, 3.7-fold (1.8-7.5) for anxiety, 4.5-fold (1.6-12.1) for ‘limbic irritability’, and 10.5-fold (2.1-51.4) for dissociation.
Males and females appeared to be similarly affected by exposure to peer VA on most measures. However, there were significant gender x peer VA interactions on ratings of dissociation (F5,673 = 4.01, p < 0.001), ‘limbic irritability’ (F5,695 = 3.56, p < 0.004) and drug use (F5,698 = 4.89, p = 0.0002). Exposure to peer VA was associated with a greater increase in DES and ‘limbic irritability’ scores in females than males. Conversely, males were more sensitive to peer VA in degree of drug use, with increased use in males emerging at lower levels of peer VA than in females.
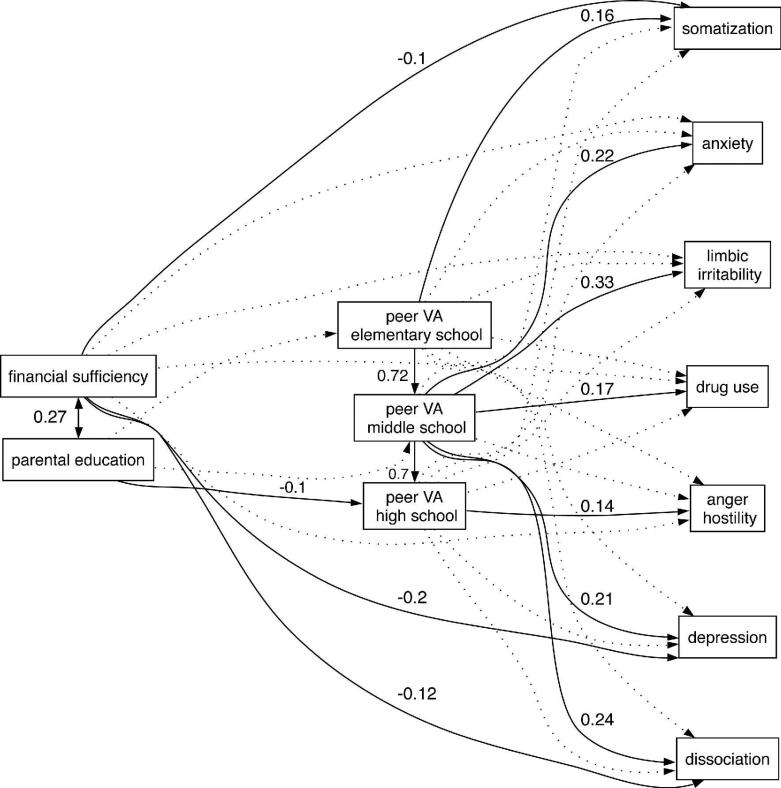
Path analyses indicated that there was a significant relationship between ages of exposure to peer VA and symptom ratings (Fig 2). The model was well fit (adjusted goodness-of-fit index = 0.989, Bentler-Bonnett NFI = 0.984, Tucker-Lewis NNFI = 1.00) and could not be rejected by chi-square criteria (χ2 = 40.88 df = 39 P = 0.82). Ratings on most of the symptom scales were associated with degree of exposure to peer VA during middle school. This was true for dissociation (P < 0.003), depression (P = 0.01), anxiety (P < 0.007), drug use (P < 0.04) and limbic irritability (P < 0.0001). Ratings of somatization were associated with degree of peer VA during elementary school (P < 0.02), while anger-hostility was significantly associated with peer VA during high school (P < 0.04). Path analyses indicated that parental education influenced the amount of exposure to peer VA during high school (P < 0.05). PFS did not directly influence degree of exposure, but low levels increased ratings of dissociation (P < 0.02), depression (P < 0.0001) and somatization (P < 0.05).
Figure 2.
Path analysis modeling the relationship between exposure to peer verbal abuse during different educational stages, perceived financial sufficiency during childhood and parental education levels on ratings of somatization, anxiety, ‘limbic irritability’, drug use, anger-hostility, depression and dissociation. Significant associations and standard weights are shown by solid lines and values. Non-significant associations included in the model are illustrated by dotted lines. The sample consists of subjects with no exposure to childhood sexual abuse, witnessing domestic, parental or peer physical abuse, or parental verbal abuse (n = 707).
Peer versus Parental VA: Subjects without abuse (n=848)
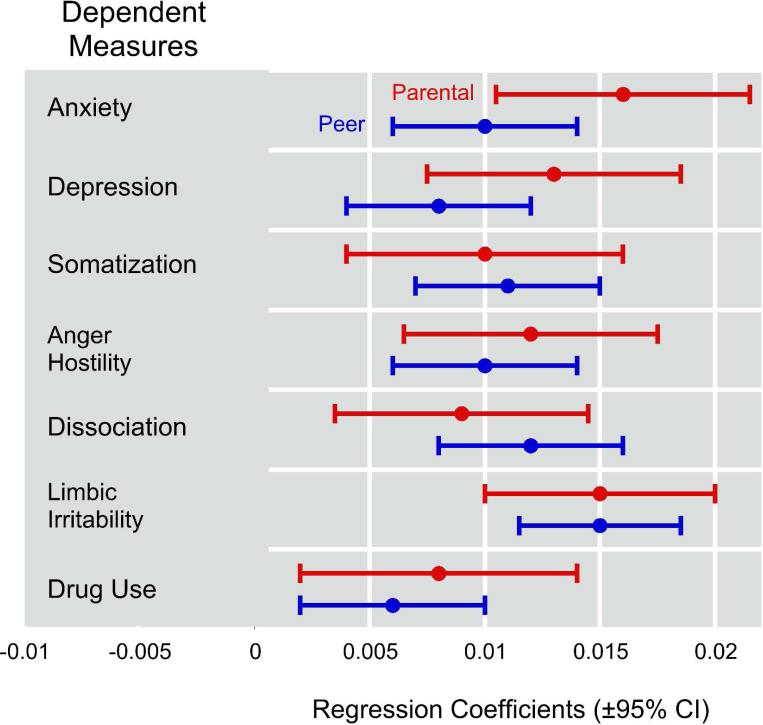
Figure 3 illustrates the least squares multiple regression coefficients predicting symptom scores by both parental and peer VA exposure. All of the regression coefficients were significantly greater than zero. There was substantial overlap between the 95% confidence intervals for parental versus peer regression coefficients across all measures, indicating that within each measure there were no significant differences between these two forms of verbal aggression in effect size. Only 2% of this sample was exposed to high levels of both peer and parental VA.
Figure 3.
Effect of exposure to parental verbal aggression and peer verbal aggression on psychiatric symptom ratings and degree of drug use. Regression coefficients for the multiple regression equation are shown, plus and minus 95% confidence intervals. Ratings on the dependent measures were standardized (mean = 0, SD =1) so that the relative impact of peer and parental VA could be compared regardless of the scoring range and scaling of the different rating instruments. Under these circumstances, a regression coefficient of 0.01 indicated that a 50-point increase in VAQ scores was associated with a 0.5 SD increase in rating scores.
Incidence and Timing of Exposure: Community sample (n=1662)
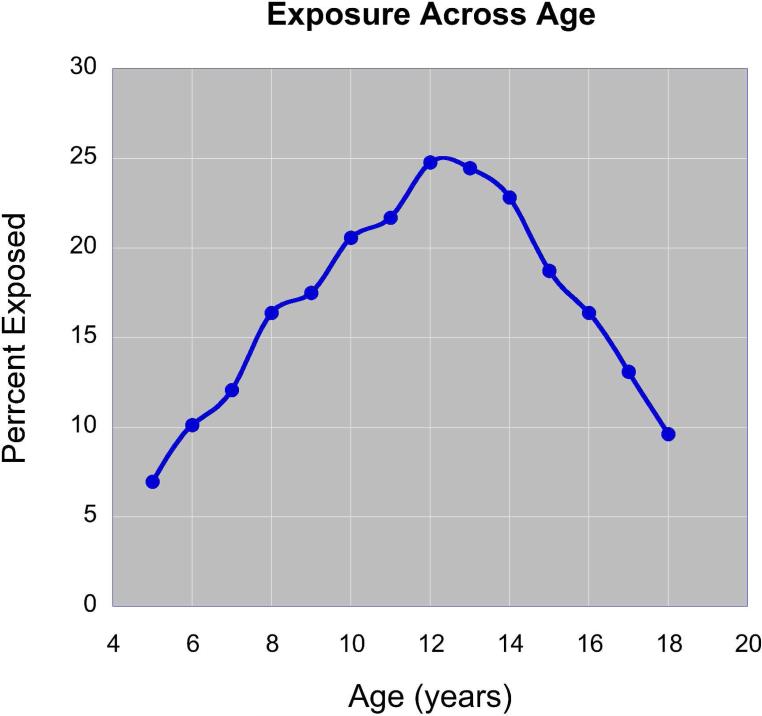
Figure 4 provides information on the frequency of exposure to significant levels of peer VA across age. Exposure peaked during middle school years (grades 6 – 8, common ages 11-14). Children exposed to peer VA during elementary school often had this exposure persist into middle school. However, 9.8% of subjects in the community sample were exposed to significant levels of peer VA during middle school but not elementary school.
Figure 4.
Percent of subjects in the on-line database (n = 1662) reporting exposure to significant peer-VA (max peer VAQ ≥ 30) between ages 5 and 18. Note that exposure peaks at ages 12-13.
Neuroimaging sample (n=63)
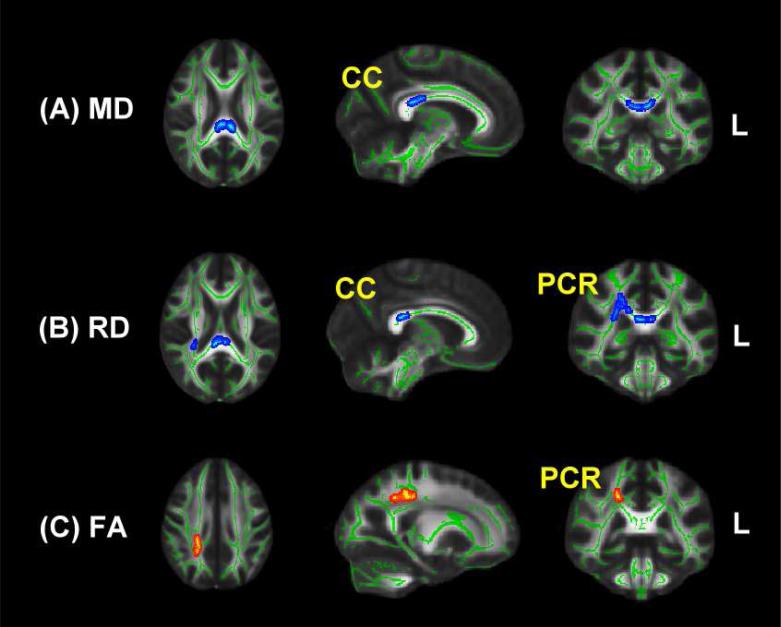
As shown in Figure 5, there was a significant correlation between degree of exposure to peer VA and MD in the splenium of the corpus callosum (CC) (MNI coordinates -7, -31, 22, p < 0.05, corrected for multiple comparisons). MD in this cluster correlated with levels of female peer VA (r = 0.617) and male peer VA (r = 0.723). The correlation between degree of exposure to peer VA and measures of MD were similar in male and females (males: r = 0.647; females: r = 0.810, Z = -1.26, P > 0.2). In this sample (selected to be free of Axis I disorders) the only noteworthy clinical correlation was between maximal peer VA scores and ratings of anxiety (r = 0.33, p = 0.01).
Figure 5.
Regions identified by TBSS in the corpus callosum (CC) and posterior corona radiata (PCR) in which there were correlations between degree of exposure to peer verbal abuse and mean diffusivity (MD), radial diffusivity (RD) and fractional anisotropy (FA), as assessed by diffusion tensor imaging and tract-based spatial statistics. Blue coloring indicates a positive correlation with diffusion measurements. Red coloring indicates an inverse correlation with measures of FA. Sample consists of 63 subjects with no exposure to childhood sexual abuse, witnessing domestic violence, parental or peer physical abuse, parental verbal abuse and are free of Axis-I and II psychiatric disorders.
Analysis of RD also showed a significant positive correlation with exposure to peer VA in the body and splenium of the CC (MNI: 4 -30 22 and -5 -34 20; P < 0.05) and the adjacent right posterior corona radiata (PCR; MNI: 28 -40 22; P < 0.05). FA values showed a negative correlation with peer VA in an overlapping portion of the right PCR (MNI: 28 -40 22), though this correlation fell short of significance after correction for multiple comparisons (P = 0.07). No positive or negative associations were detected between peer VA and AD.
DISCUSSION
We found in a community sample of late teens and young adults that exposure to peer VA was associated with increased drug use, and elevated psychiatric symptom ratings. Substantial exposure was associated with greater than 2-fold increase in clinically significant ratings of depression, 3-4-fold increase in anxiety and ‘limbic irritability’, and 10-fold increase in dissociation. This level of peer VA was reported by 9.2% of subjects with no exposure to CSA, WDV, parental PA or VA, and by 17.9% of the entire community sample. Hence, exposure to substantial levels of peer VA is a relatively common occurrence. Moreover, the effects of exposure to peer VA on risk of psychopathology in early adulthood mirror results we previously reported for parental VA (5). Thus verbal aggression from peers is an important and potent childhood stressor.
Middle school was the peak period of exposure to peer VA, with 9.8% of our community sample newly exposed. This fits with previous observations that peer physical aggression declines from 8 to 18 years of age while peer-VA increases from age 8 to 11 years, plateaus and then declines from 15 to 18 years (22, 23).
More importantly, timing of exposure appears to shape impact. Path analysis suggests that exposure during the middle school years (ages 11-14) was most consequential and associated with symptoms of anxiety, depression, dissociation, ‘limbic irritability’ and degree of drug use. Overall, there were no significant associations between these symptoms and degree of exposure during elementary or high school when degree of middle school exposure was excluded. However exposure at early and later ages amplified the association between symptom ratings and middle school exposure, more than doubling the amount of variance explained. This suggests that exposure during elementary and high school may sensitize or reinforce the effects of exposure during middle school. These findings are consistent with previous reports indicating that exposure to PV in secondary school is more serious than PV during primary school (23, 24). This may be because children in primary school predominantly engage in dyadic relationships, which can attenuate the perceived impact of bullying outside the dyad (23).
Another perspective is also possible. We recently published data indicating that there are sensitive periods when brain regions are most susceptible to the effects of CSA (20). The hippocampus was most vulnerable to CSA occurring at 3-5 and 11-13 years of age. It is possible that the hippocampus may also be susceptible to other forms of abuse occurring during these years. Anxiety, depression, dissociation and TLE-like symptoms have all been associated with aspects of hippocampal function (12, 25-29). Hippocampal volume was not assessed in this study.
DTI however revealed an association between degree of exposure to peer VA and measures of MD, RD and FA in the splenium of the CC and overlying corona radiata. The CC is a massive fiber tract interconnecting left and right hemispheres. The corona radiata contains both descending and ascending axons that carry nearly all of the neural traffic to and from the cerebral cortex. Many of these axons pass through the CC. Studies suggest that alterations in RD but not AD, as observed, result from effects on myelin rather than axon numbers (30, 31). CC alterations appear to be the most consistent finding in maltreated subjects (32-34), and it is perhaps remarkable that they emerged in a sample of control subjects with no Axis I disorders. The sensitive period for the splenium (the most caudal portion of the CC) likely occurs during middle schools years given the rostral-caudal progression of CC myelination (35), and our finding that the rostral body of the CC had a sensitive period between 9-10 years (20).
It is interesting to speculate how white matter alterations in the splenium might relate to increased risk for depression, dissociation or substance abuse. Fibers passing through the splenium interconnect right and left occipital and inferior temporal cortex. Together these regions comprise the ventral visual processing stream, which has reciprocal connections with the hippocampus. Visual cortex is a plastic structure that is extensively modified by early experience. We reported that exposure to CSA was associated with a 12-18% reduction in gray matter volume in right and left primary and secondary visual cortex (36). We have also found similar alterations in WDV. While visual cortex plays a critical role in sensory perception it may have additional functions. A reproducible finding in major depression is a substantial reduction in occipital cortex GABA, which is restored following treatment with antidepressants or ECT (37). Exposure to early stress may target GABAergic interneurons or fiber pathways of the visual cortex and increase risk for the development of mood disorders. We, and others, have also speculated that alterations in the CC may set the stage for dissociative phenomenon by diminishing intrahemispheric integration (38). It is also possible that lack of integration between right and left hemispheric processing of visual cues may lead to greater cue-induced craving in substance users and enhanced risk for abuse and dependence.
This study is unique for a number of reasons. First, this appears to be one of the only studies of PV to assess and control for exposure to other forms of maltreatment such as CSA or parental VA. Second, it focuses entirely on peer VA as a specific form of childhood trauma, distinct from PV that involves physical assaults. Third, effects of exposure during different developmental stages were assessed based on our finding of “sensitive periods” when brain regions are particularly susceptible to abuse.
However, we must acknowledge that these studies are retrospective and correlative and, as such, are subject to problems associated with faulty recall and spurious association. It is possible that individuals who show a tendency toward psychopathology in childhood are targeted as “odd” by peers and subjected to VA (39). It is also conceivable that a preexisting abnormality in the CC enhanced risk for psychopathology and peer abuse. Path analysis delineates a mathematical solution to a series of equations not a causal pathway. Causality cannot be inferred from this retrospective experimental design. Prospective studies are needed to tease out these possibilities.
Psychiatric disorders probably arise from the temporal intersection of genetic susceptibility and adverse early experience during critical developmental stages (26). Theorists have often focused on the importance of very early experience and parent-child interactions. This study and other recent reports (9, 40) should strengthen interest in the importance of peer interactions and the vulnerability of peripubertal children. These findings further enhance concern that exposure to ridicule, disdain and humiliation, from parents, partners or peers, is emotionally toxic and may adversely impact trajectories of brain development.
Disclosures and acknowledgments
None of the authors have any competing interests. Dr. Teicher has received research support and consulting fees from BioBehavioral Diagnostic Company, consulting fees from MDCI, and research support from LiteBook Inc., and CNS Response Inc. Support from these companies was associated with Dr. Teicher's work on the diagnosis of ADHD and treatment of depression. Dr. Teicher holds 13 US patents most of which are related to the diagnosis and treatment of psychiatric disorders, none of which are related to the effects of exposure to childhood abuse.
Support for this research came from NIMH award RO1 MH-66222, NIDA awards RO1 DA-016934 and RO1 DA-017846, a NARSAD Independent Investigator Award, and donations from the Simches and Rosenberg Families to Dr. Teicher.
Literature Cited
- 1.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MY, Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158(11):1878–83. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–9. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 3.Mullen PE, Martin JL, Anderson JC, Romans SE. Herbison GP: Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721–32. doi: 10.1192/bjp.163.6.721. [DOI] [PubMed] [Google Scholar]
- 4.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, R. DS, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163(6):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CM, Rabi K, Lukas SE, Teicher MH. Cerebellar lingula size and experiential risk factors associated with high levels of alcohol and drug use in young adults. Cerebellum. 2009 doi: 10.1007/s12311-009-0141-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawker DS, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: a meta-analytic review of cross-sectional studies. J Child Psychol Psychiatry. 2000;41(4):441–55. [PubMed] [Google Scholar]
- 8.Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. Jama. 2001;285(16):2094–100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreier A, Wolke D, Thomas K, Horwood J, Hollis C, Gunnell D, Lewis G, Thompson A, Zammit S, Duffy L, Salvi G, Harrison G. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66(5):527–36. doi: 10.1001/archgenpsychiatry.2009.23. [DOI] [PubMed] [Google Scholar]
- 10.Srabstein J, Piazza T. Public health, safety and educational risks associated with bullying behaviors in American adolescents. Int J Adolesc Med Health. 2008;20(2):223–33. doi: 10.1515/ijamh.2008.20.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Nansel TR, Overpeck MD, Haynie DL, Ruan WJ, Scheidt PC. Relationships between bullying and violence among US youth. Arch Pediatr Adolesc Med. 2003;157(4):348–53. doi: 10.1001/archpedi.157.4.348. [DOI] [PubMed] [Google Scholar]
- 12.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65(3):227–34. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry. 1987;48(7):268–273. [PubMed] [Google Scholar]
- 14.Bernstein EM, Putnam FW. Development, reliability and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–35. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Teicher MH, Glod CA, Surrey J, Swett C., Jr. Early childhood abuse and limbic system ratings in adult psychiatric outpatients. Journal of Neuropsychiatry & Clinical Neurosciences. 1993;5(3):301–6. doi: 10.1176/jnp.5.3.301. [DOI] [PubMed] [Google Scholar]
- 16.Tutkun H, Sar V, Yargic LI, Ozpulat T, Yanik M, Kiziltan E. Frequency of dissociative disorders among psychiatric inpatients in a Turkish University Clinic. Am J Psychiatry. 1998;155(6):800–5. doi: 10.1176/ajp.155.6.800. [DOI] [PubMed] [Google Scholar]
- 17.Spiers PA, Schomer DL, Blume HW, Mesulam MM. Temporolimbic epilepsy and behavior. In: Mesulam MM, editor. Principles of Behavioral Neurology. F.A. Davis; Philadelphia: 1985. pp. 289–326. [Google Scholar]
- 18.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49(1):177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 19.Duncan GL, Magnuson KA. Off With Hollingshead: Socioeconomic Resources, Parenting, and Child Development. In: Bornstein MH, Bradley RH, editors. Socioeconomic Status, Parenting, and Child Development. Lawrence Erlbaum; Mahwah, New Jersey: 2003. pp. 83–106. [Google Scholar]
- 20.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Björkqvist K, Österman K, Kaukiainen A. The development of direct and indirect aggressive strategies in males and females. In: Björkqvist K, Niemelä P, editors. Of mice and women. Aspects of female aggression. Academic Press; San Diego, CA: 1992. pp. 51–64. [Google Scholar]
- 23.Schäfer M, Korn S, Smith PK, Hunter SC, Mora-Merchan JA, Singer MM, van der Meulen K. Lonely in the crowd: Recollections of bullying. Br J Devel Psychol. 2004;22:379–394. [Google Scholar]
- 24.Schäfer M, Werner NE, Crick NR. A comparison of two approaches to the study of negative peer treatment: General victimization and bully/victim problems among German school children. Br J Devel Psychol. 2002;20:281–306. [Google Scholar]
- 25.Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001(1-2):60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 26.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 28.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163(4):630–6. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunewald RA, Jackson GD, Connelly A, Duncan JS. MR detection of hippocampal disease in epilepsy: factors influencing T2 relaxation time. AJNR Am J Neuroradiol. 1994;15(6):1149–56. [PMC free article] [PubMed] [Google Scholar]
- 30.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 31.Brubaker CJ, Schmithorst VJ, Haynes EN, Dietrich KN, Egelhoff JC, Lindquist DM, Lanphear BP, Cecil KM. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology. 2009;30(6):867–75. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45(10):1271–84. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 33.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56(2):80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162(3):256–61. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobel U, Sedlacik J, Gullmar D, Kaiser WA, Reichenbach JR, Mentzel HJ. Diffusion tensor imaging: the normal evolution of ADC, RA, FA, and eigenvalues studied in multiple anatomical regions of the brain. Neuroradiology. 2009;51(4):253–63. doi: 10.1007/s00234-008-0488-1. [DOI] [PubMed] [Google Scholar]
- 36.Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry. 2009;66(7):642–8. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577–9. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 38.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 39.Sourander A, Ronning J, Brunstein-Klomek A, Gyllenberg D, Kumpulainen K, Niemela S, Helenius H, Sillanmaki L, Ristkari T, Tamminen T, Moilanen I, Piha J, Almqvist F. Childhood bullying behavior and later psychiatric hospital and psychopharmacologic treatment: findings from the Finnish 1981 birth cohort study. Arch Gen Psychiatry. 2009;66(9):1005–12. doi: 10.1001/archgenpsychiatry.2009.122. [DOI] [PubMed] [Google Scholar]
- 40.Kaminski JW, Fang X. Victimization by peers and adolescent suicide in three US samples. J Pediatr. 2009;155(5):683–8. doi: 10.1016/j.jpeds.2009.04.061. [DOI] [PubMed] [Google Scholar]