Abstract
In mammals, urea is the main nitrogenous breakdown product of protein catabolism and is produced in the liver. In certain tissues, the movement of urea across cell membranes is specifically mediated by a group of proteins known as the SLC14A family of facilitative urea transporters. These proteins are derived from two distinct genes, UT-A (SLC14A2) and UT-B (SLC14A1). Facilitative urea transporters play an important role in two major physiological processes – urinary concentration and urea nitrogen salvaging. Although UT-A and UT-B transporters both have a similar basic structure and mediate the transport of urea in a facilitative manner, there are a number of significant differences between them. UT-A transporters are mainly found in the kidney, are highly specific for urea, have relatively lower transport rates and are highly regulated at both gene expression and cellular localization levels. In contrast, UT-B transporters are more widespread in their tissue location, transport both urea and water, have a relatively high transport rate, are inhibited by mercurial compounds and currently appear to be less acutely regulated. This review details the fundamental research that has so far been performed to investigate the function and physiological significance of these two types of urea transporters.
Keywords: UT-A, UT-B, urinary concentration, vasopressin, urea nitrogen salvaging, isoforms
Overview
Urea is the main breakdown product of protein catabolism in mammals. It is produced in the liver via the ornithine-urea cycle and has classically been viewed simply as a toxic nitrogenous waste product, emitted from the body in both urine and faeces. Urea (H2NCONH2) is a water soluble molecule and was originally thought to simply pass slowly across cell membranes via passive diffusion. However, the cloning of the specific proteins responsible for the enhanced trans-epithelial urea transport that is present in certain tissues has revolutionized our understanding of the physiological importance of urea.
Urea transporters (UTs) allow the rapid movement of urea molecules across cell membranes. Although this can occur in either direction through the transporters, net urea movement can only occur down a concentration gradient. This equilibrative movement of urea is also independent of ions such as sodium and chloride (You et al., 1993). These proteins are generally referred to as ‘facilitative urea transporters’, and are distinct from the undefined class of proteins responsible for the ‘active’ uptake of urea against concentration gradients.
Urea transporters are derived from two separate genes: SLC14A1 (UT-B) and SLC14A2 (UT-A). These UT-A and UT-B genes are on the same chromosome (18q12.1-q21.1), suggesting gene duplication from a common ancestor (Olives et al., 1996). The Kidd (Jk) blood group, previously known to be located on this chromosome, does in fact represent the human UT-B urea transporter (Olives et al., 1995).The human UT-A gene consists of 26 exons covering 476 kb, with similarly large genes being present in mouse and rat (Smith and Fenton, 2006). In contrast, the human UT-B gene is smaller, consisting of 11 exons spread over 30 kb (Lucien et al., 1998). Again, the UT-B gene is of a similar size in both mouse and rat (Yang and Bankir, 2005). [Note: a third urea transporter gene, UT-C, has so far only been identified in two species of fish (Mistry et al., 2005).]
The most studied physiological role for urea transporters is in the urinary concentration mechanism. Renal UT-A transporters within the kidney nephron help facilitate the reabsorption and recycling of urinary urea, hence increasing medullary urea concentration – see Figure 1. This prevents the problematic osmotic effect that urinary urea would otherwise have and hence prevents excess water loss in the urine (Fenton et al., 2004). During reasonable levels of dietary nitrogen intake, urea transporters are therefore vital in the production of concentrated urine and hence in maintaining body fluid balance. Detailed discussion of the precise renal function of urea transporters will not be presented here, as this topic has been the subject of an excellent recent review (see Fenton, 2009).
Figure 1.
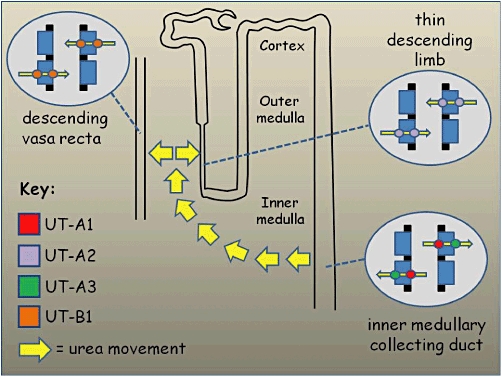
Schematic model of the kidney nephron showing role of Slc14a transporters in the renal urinary concentrating mechanism. Blood urea is freely filtered at the glomerulus and ∼40% is constitutively reabsorbed in the proximal tubule. The remaining urea can be reabsorbed across the epithelial cells of the inner medullary collecting duct, mainly via apical UT-A1 transporters and basolateral UT-A3 transporters that are both regulated by vasopressin. This has the effect of increasing urea concentration in the kidney medulla and hence preventing the excessive water loss that would otherwise occur due to the osmotic effect of the urinary urea. In order to maintain this high medullary concentration, it is necessary for the urea to be recycled and the concentration gradient not to be dissipated. This is achieved by the facilitated movement of urea across the apical and basolateral of both the thin descending limbs (via UT-A2 transporters) and the descending vasa recta blood vessels (via UT-B1 transporters).
A second important physiological role for urea transporters is now emerging in respect to its role in the process of urea nitrogen salvaging (UNS) in the mammalian intestinal tract. This process supplies intestinal bacteria with a source of nitrogen that they utilize for their growth and is hence vital in maintaining the symbiotic relationship between mammals and their bacterial populations, particularly in ruminant species (for detailed review – see Stewart and Smith, 2005). The crucial first step in UNS is the movement of urea from the blood into the intestinal tract, via UT-B urea transporters in the epithelial layers – see Figure 2. UT-B proteins have now been identified in various intestinal tissues and species, such as bovine rumen (Stewart et al., 2005), rat caecum (Collins et al., 2011) and, most interestingly, human colon (Inoue et al., 2004; Collins et al., 2010). Urea transporters may therefore indirectly play a significant role in both nutritional balance and intestinal health.
Figure 2.
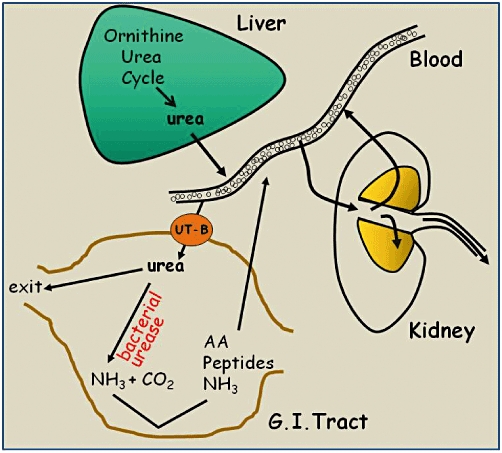
Model of the urea nitrogen salvaging process. Urea is produced in the liver via the ornithine-urea cycle and passed into the blood. Urea can then pass (a) into the kidney, where it is freely filtered and either reabsorbed or excreted, or (b) via UT-B urea transporters into specific regions of the gastrointestinal tract that contain large bacterial populations (e.g. rumen, caecum, colon). Within these regions urea is broken down by the bacterial enzyme urease into ammonia and carbon dioxide. The ammonia can either be reabsorbed directly back into the blood or be utilized as a nitrogen source by the bacteria to produce amino acids and peptides, which themselves can then be reabsorbed. The return of the nitrogen to the mammalian host in these different forms represents the ‘salvaging’ of the original urea nitrogen.
Isoforms
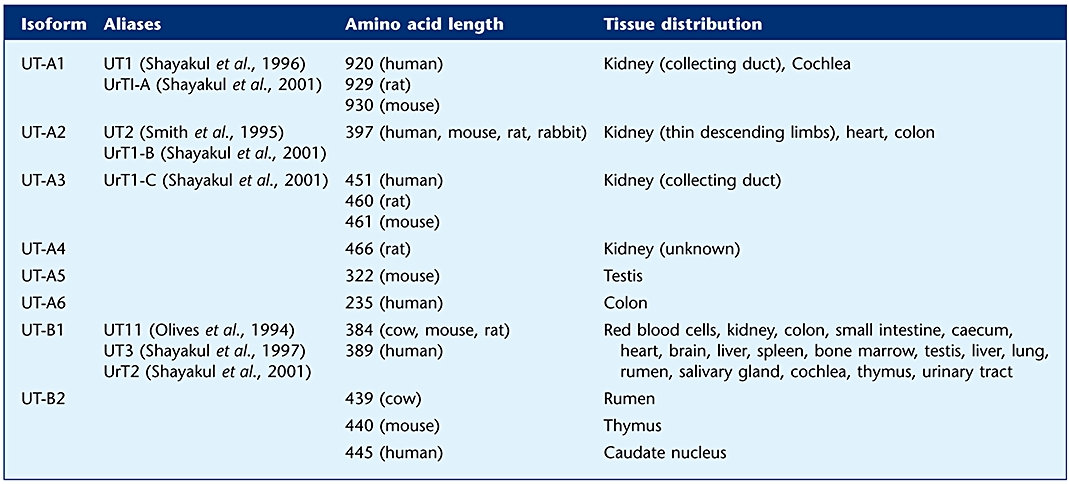
Both the Slc14a1 and Slc14a2 genes produce multiple isoforms, via the process of alternative splicing (for review of genomic organization – see Smith and Fenton, 2006). There are six known Slc14a2 (UT-A) transporters and two Slc14a1 (UT-B) transporters. Figure 3 shows a schematic representation of these eight urea transporter proteins. Some of these isoforms have yet to be fully characterized in more than one species at present. For example, cDNA sequences for UT-B2 have been reported in human caudate nucleus (GenBank Acc. No. AK091064) and mouse thymus (GenBank Acc. No. AK153891), but the proteins have yet to be investigated. Evidence is also emerging of the existence of further novel isoforms, particularly for UT-B transporters. For example, a cDNA clone from human thalamus appears to encode a novel 281-amino acid UT-B protein (GenBank Acc. No. AK127452) that has a truncated N-terminus compared with the UT-B1 transporter. Because the current nomenclature for Slc14a urea transporters was not originally utilized, the previous aliases used in the literature are listed in Table 1. This table also details the small variations in amino acid length between species that occurs for certain transporters and includes a basic guide to tissue distribution (for further details see distribution section below).
Figure 3.
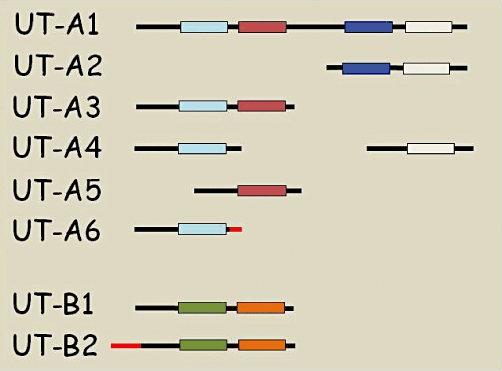
Schematic representation of the different isoforms of UT-A and UT-B urea transporters. The different boxes represent regions of hydrophobic amino acids. The black lines show coding sequences which are common, while the red lines show coding sequences that are unique to that particular isoform (i.e. derived from novel exons) (adapted from Smith, 2009).
Table 1.
The nomenclature of all the currently identified members of the SLC14A urea transporter family
 |
Biochemistry and genetics
The proposed basic structure for both UT-A and UT-B urea transporters consists of 10 transmembrane spanning domains (TMDs), a large extracellular loop containing an N-glycosylation site, plus intracellular amino and carboxy terminals (Olives et al., 1994) – see Figure 4. The main exception to this 10 TMDs structure is UT-A1, which is proposed to have 20 TMDs, and is basically UT-A2 and UT-A3 combined by a 73-amino acid central linking loop. This central loop region of UT-A1 contains serine 486, which is responsible for protein kinase A (PKA) activation of this isoform (Mistry et al., 2010). A recent paper also reports that the N-terminal 81-amino acids in rat UT-A1 are required for transport activity (Huang et al., 2010a), although further investigation is still required to fully understand its precise role. Interestingly, bacterial homologues of kidney urea transporters have recently been used to show that urea transporters function in the plasma membrane as multimers, for example, as dimers in Actinobacillus pleuropnemoniae (Raunser et al., 2009) or as trimers in Desulfovibrio vulgaris (Levin et al., 2009).
Figure 4.
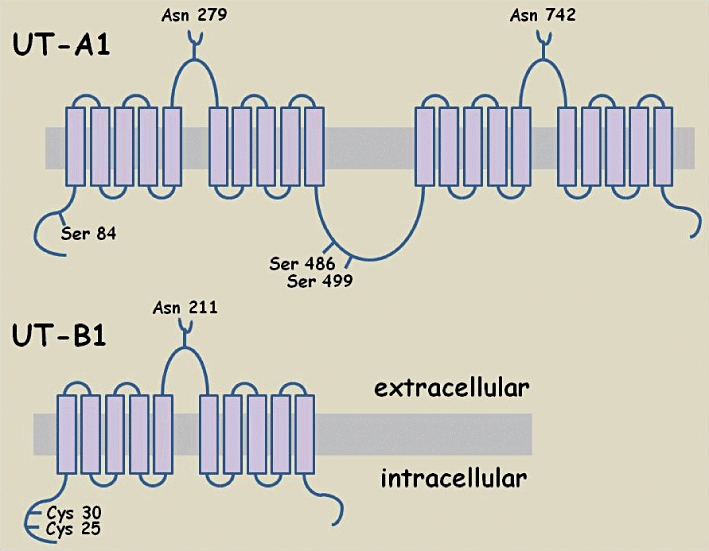
Schematic diagram representing topology of two Slc14a facilitative urea transporters: UT-A1 and UT-B1. Various important residues are highlighted in each transporter: the asparagine (Asn) residues known to be important in glycosylation; the serine residues (Ser) known to be involved in the phosphorylation events that regulate transporter function; the cysteine (Cys) residues important in targeting the protein to the plasma membrane.
As mentioned, urea transporters are N-linked glycosylated proteins that have a unique pattern of hydrophobicity, as shown originally for rabbit UT-A2 (You et al., 1993). Rat UT-A1 has two glycosylated versions (97 & 117 kDa) that are both deglycosylated to an 88 kDa core 929-amino acid protein (Bradford et al., 2001). We now know that glycosylation of UT-A1 at two sites (Asn 279 and Asn 742) is required in order for correct trafficking of the protein to the plasma membrane to occur in response to vasopressin (Chen et al., 2006). The other main UT-A isoforms are also known to have N-linked glycosylations, such as the 55 kDa glycosylated rat UT-A2 protein (Wade et al., 2000). The exact nature of the glycosylations can vary between species however. For example, rat UT-A3 has 44 & 67 kDa glycosylated forms that are deglycosylated down to 40 kDa (Terris et al., 2001), while mouse glycosylated UT-A3 is a 45–65 kDa continuous smear that again shifts to a 40 kDa core protein (Stewart et al., 2004).
The vital role of the human UT-B1 N-terminal in protein targeting to the plasma membrane has been clearly shown (Lucien et al., 2002). Double mutation of the cysteine residues Cys 25 and Cys 30 prevented successful localization of hUT-B1 to the plasma membrane (Lucien et al., 2002). UT-B urea transporters are also known to have N-linked glycosylations. Human UT-B1 gives a 46–60 kDa glycosylated protein that deglycosylates to 36 kDa in red blood cells (Olives et al., 1995), while UT-B1 in human kidney gives a 41–54 kDa signal (Timmer et al., 2001). This tissue-specific variation in the extent of glycosylation is characteristic of urea transporter proteins and occurs in other species, for example, rat glycosylated UT-B1 protein is 32 kDa in brain but 45–55 kDa in kidney (Trinh-Trang-Tan et al., 2002). The glycosylation of human UT-B1 all occurs at the Asn 211 N-linked glycosylation site in the extracellular loop (Sidoux-Walter et al., 2000). Interestingly, evidence from oocyte expression studies suggests that mutating this site surprisingly does not affect either membrane trafficking or function (Lucien et al., 2002). However, it should be noted that UT-B glycosylation function may differ in a mammalian system, hence the precise role it plays has yet to be fully determined.
Single-amino acid mutations in human UT-A2 (Val/Ile 227 or Ala/Thr 357) have been associated with a decrease in blood pressure (Ranade et al., 2001). These UT-A2 mutations have been linked with increased efficacy of the anti-hypertensive drug nifedipine (Hong et al., 2007) and, more recently, shown to be associated with metabolic syndrome (Tsai et al., 2010). Jk null individuals have mutations in UT-B1 and therefore have red blood cells that lack functional urea transporters. For example, the first recorded mutations were shown to cause the skipping of exon 6 or 7 during the transcription process, producing non-functional UT-B1 proteins that did not reach the plasma membrane (Lucien et al., 1998). However, various Jk null polymorphisms occur in different populations around the world – see Table 2. Many of these mutations have functional consequences, such as the S291P mutation, whose failure to be expressed in red blood cells explains the Finnish Jk null phenotype (Sidoux-Walter et al., 2000). However, some mutations have no functional effect. For example, the G838A mutation (Asp280 to Asn 280) is a common polymorphism (i.e. A or B Kidd allele) in human UT-B1, but does not affect transport function (Lucien et al., 2002).
Table 2.
A list of the various mutations in SLC14A1 (UT-B) urea transporters found in different populations around the world
| Population | SNP/mutation | Consequence | Reference |
|---|---|---|---|
| Polynesian | Splice site mutation | Exon 6 missing | (Irshaid et al., 2000) |
| Finnish | S291P | Prevents efficient trafficking to membrane | (Sidoux-Walter et al., 2000) |
| English | Genomic deletion | Exons 4, 5 missing | (Irshaid et al., 2002) |
| Swiss | T194Stop | Truncated exon 7 | (Irshaid et al., 2002) |
| Chinese | Splice site mutation | Exon 6 missing | (Meng et al., 2005) |
| American | |||
| Caucasians | G68Stop | Nonsense mutation | (Wester et al., 2008) |
| Spanish | I262Stop | Nonsense mutation | |
| African | T319M | Missense mutation |
SNP, single-nucleotide polymorphism.
Pharmacology
Using a fluorescent based enzyme-linked immunosorbent assay, it was estimated that UT-A1 transporters in the rat renal inner medullary collecting duct (IMCD) had a turnover number of 100 000 urea molecules per second and that there were ∼5 million copies of UT-A1 per IMCD cell (Kishore et al., 1997). More recently, stopped flow fluorometry measurements of mouse UT-A transporters expressed in Xenopus oocyte plasma membranes showed transport rates of 46 000 and 59 000 urea molecules per second per protein for UT-A2 and UT-A3 respectively (MacIver et al., 2008).
Two reports have independently estimated that there are 14 000 copies of UT-B1 per human red blood cell (Masouredis et al., 1980; Mannuzzu et al., 1993). It has therefore been calculated that human UT-B1 transporters have a turnover of up to 6 000 000 urea molecules per second (i.e. at least ∼60-fold greater than UT-A transporters) (Mannuzzu et al., 1993). Although not as impressive as the Aquaporin-1 (AQP1) water channel, which transports 3 billion water molecules per second (Gade and Robinson, 2006), one might argue that with this channel-like transport rate the correct terminology for these proteins is ‘UT-B channels’. Interestingly, the structure of a bacterial homologue urea transporter has recently been shown to consist of two oppositely orientated homologous halves, known as ‘an inverted repeat motif’ and operate by a channel-like mechanism (Levin et al., 2009). Furthermore, this ‘inverted repeat motif’ is also found in channels that transport other neutral solutes, such as ammonia and water, for example, AQP1 itself (Murata et al., 2000).
Urea transporters have a low affinity for urea, for example, rabbit UT-A2 Km > 200 mM (You et al., 1993), and so are not saturated by the levels of between 2 and 10 mM of urea generally found in mammalian blood. Initial reports suggested that UT-A proteins are highly selective for urea and do not transport water (Hill et al., 2005). Recent stopped flow fluorometry measurements of transporters expressed in Xenopus oocyte plasma membranes confirmed that mouse UT-A2 and UT-A3 did not transport water, ammonia or urea analogues, such as formamide, acetamide, methylurea and dimethylurea (MacIver et al., 2008).
The observation that rat UT-B1, as well as facilitating the movement of urea, also transports water (1.4 × 10−14 cm3·s−1 per channel cf. 0.1 × 10−14 cm3·s−1 per channel for rat UT-A2) (Yang and Verkman, 1998) was initially controversial (Sidoux-Walter et al., 1999). However, stopped flow light scattering measurements using mouse erythrocytes from knockout (KO) models confirmed UT-B1 has a water permeability (Pf) of 7.5 × 10−14 cm3·s−1 per channel (Yang and Verkman, 2002). This promiscuous nature of the UT-B channel has now been confirmed by the fact it also transports formamide, acetamide, methylurea, methylformamide, ammonium carbonate and acrylamide (Zhao et al., 2007).
In humans, this relatively unselective UT-B mediated transport is shown by the fact that red blood cells from Jk null individuals (i.e. ones lacking UT-B1) have a decreased permeability to both urea and water (Meng et al., 2005). Indeed, it has been reported that a known urea transporter inhibitor, the analogue thiourea, can actually go through human UT-B1 (Km = 40 mM), but not through human UT-A2 (Martial et al., 1996).
Urea transport in perfused rat IMCD was originally shown to be inhibited by 250 µM phloretin and 200 mM of the urea analogues thiourea, methylurea and acetamide (Chou and Knepper, 1989). The K1/2-value for thiourea inhibition was 27 mM, which was unaffected by vasopressin (see regulation section below) even though transport increased fourfold (Chou and Knepper, 1989). Rat IMCD urea transport was also inhibited by dimethylurea and phenylurea (Zhang and Verkman, 1990). Rat UT-A1, located in the IMCD, was later confirmed to indeed be inhibited by phloretin and urea analogues (Shayakul et al., 1996), as well as by thionicotinamide (Frohlich et al., 2004). Importantly, all other known UT-A isoforms are also inhibited by phloretin – including UT-A2 (You et al., 1993), UT-A3 and UT-A4 (Karakashian et al., 1999), UT-A5 (Fenton et al., 2000) and UT-A6 (Smith et al., 2004).
Human UT-B1 expressed in oocytes was initially confirmed to be inhibited by phloretin and thiourea (Olives et al., 1994). In agreement with this, rat UT-B1 function in vasa recta was also inhibited by thiourea, methylurea, acetamide and phloretin (Pallone, 1994), with a K1/2-value for thiourea of 19 mM, as well as dimethylurea (Yang and Verkman, 1998). More recently, mouse UT-B1 mediated transport in red blood cells has been shown to be inhibited by more than 60% by dimethylurea, acrylamide, thiourea and methylformamide (Zhao et al., 2007). As expected, both bovine UT-B1 and UT-B2 isoforms were also inhibited by both phloretin and thionicotinamide (Stewart et al., 2005). Interestingly, it has been suggested that UT-B proteins are more sensitive to phloretin inhibition than UT-A transporters – for example, IC50 phloretin: human UT-B1 = 75 µM versus human UT-A2 230 µM (Martial et al., 1996). In addition, human red blood cell urea transport is also inhibited by mercurial compounds, such as p-chloromercuribenzenesulphonate (pCMBS) (Mannuzzu et al., 1993), because human UT-B1 is pCMBS-sensitive (Olives et al., 1994; Lucien et al., 2002) with an IC50 of 150 µM (Martial et al., 1996). In direct contrast, human UT-A2 is not inhibited by pCMBS (Olives et al., 1996), while rat kidney UT-B1 is indeed pCMBS-sensitive (Pallone, 1994).
Finally, a human red blood lysis assay has been used to investigate the potential inhibitory effects of over 50 000 compounds on UT-B1 facilitated acetamide transport (Levin et al., 2007). This research discovered 30 specific inhibitors that selectively inhibited UT-B but not UT-A transporters, while also having no effect on AQP1 (Levin et al., 2007). These compounds were from the phenylsulphoxyoxazole, benzensulphonanilide, phthalazinamine and aminobenzimidazole classes (Levin et al., 2007).
Some members of the aquaporin water channel group are also permeable to urea. For example, AQP7 is urea permeable and was originally localized in the testis (Ishibashi et al., 1997). AQP7 has also been localized to the proximal tubule in rat kidney (Ishibashi et al., 2000) and is hence located in a different nephron segment to the UT-A urea transporters. Although this implies that AQP7 could play a role in renal urea transport, a mouse AQP7 KO model does not actually display a urinary concentrating defect and shows the renal function of AQP7 to be the reabsorption of water and glycerol (Sohara et al., 2005). AQP9 is another urea permeable aquaporin, which was originally located in human leukocytes (Ishibashi et al., 1998). However, AQP9 KO mice have no change in their plasma urea levels and results showed AQP9 to be involved in glycerol metabolism in the liver (Rojek et al., 2007). These results strongly suggest aquaporins do not play a significant physiological role in urea transport.
Urea has also been reported to pass through other co-transporters, such as the sodium-dependent glucose transporter (SGLT1) (Leung et al., 2000). However, the actual urea transport rate through SGLT1 was Purea= 1.2 × 10−7 cm·s−1 (Leung et al., 2000), which is several orders of magnitude lower than Purea values for urea transporters, for example, UT-A2 = 4.5 × 10−5 cm·s−1 (You et al., 1993). It therefore again seems unlikely that these co-transporters have a significant physiological role in transporting urea.
Distribution
In the original research that cloned the first urea transporter, rabbit UT-A2 was discovered in the kidney, but also detected in the colon (You et al., 1993). Human UT-A1 was detected in the kidney, as it was for other species such as rat (Nielsen et al., 1996) and mouse (Fenton et al., 2002b). Similarly, UT-A2 (Olives et al., 1996), UT-A3 (Stewart et al., 2004) and UT-A4 (Karakashian et al., 1999) are also all found in the kidney of various species. Human UT-A3 protein has yet to be investigated, although a cDNA clone has been identified (Smith and Fenton, 2006) and an appropriately sized 2.4 kb transcript detected in human kidney medullary RNA (Bagnasco et al., 2001). In contrast to these renal locations, mouse UT-A5 is found in the testis (Fenton et al., 2000) and human UT-A6, a 235-amino acid protein including a novel 5a exon and unique 19-amino acid C-terminal, has been located in the colon (Smith et al., 2004). Other tissues in which UT-A proteins have been found include heart (Duchesne et al., 2001), cochlea (Kwun et al., 2003), placenta (Damiano et al., 2006), brain and liver (Fenton et al., 2002b).
Human UT-A1 was located in the renal IMCD (Bagnasco et al., 2001). In rats, UT-A1 was present in the terminal portions of the IMCD (Nielsen et al., 1996), while UT-A2 was found in the late part of descending thin limbs of short loops of Henle and the inner medullary part of descending thin limbs of long loops of Henle (Shayakul et al., 1997). In mice, UT-A1 was again found in terminal IMCD, and UT-A2 located in short (type I) and long (type 3) thin descending limbs of the loops of Henle (Fenton et al., 2002b). UT-A3 was also only located in the IMCD (Stewart et al., 2004). Interestingly, mouse UT-A1 and UT-A3 co-localized with AQP2 in principal cells in IMCD (Fenton et al., 2006). Mouse UT-A5 protein is localized to the peritubular myoid cells of the seminiferous tubules in the testis (Fenton et al., 2000).
In IMCD cells, UT-A1 was mainly located at the apical membrane region (Nielsen et al., 1996), while UT-A3 was mainly located at the basolateral membrane region (Shayakul et al., 2001; Stewart et al., 2004). However, it should be noted that UT-A1 is capable of going to the basolateral membrane (Frohlich et al., 2004) and UT-A3 has been reported in one study at the apical membrane after vasopressin treatment (Blount et al., 2007). UT-A2 was located on both apical and basolateral membranes in rats (Lim et al., 2006) and mice (Fenton et al., 2002b; Potter et al., 2006). The subcellular location of UT-A4, UT-A5 and UT-A6 remain unclear at present.
In contrast to the mainly renal location of UT-A transporters, UT-B protein has been detected in numerous tissues. Initially, a triated urea analogue 1-(3-azido-4-chlorophenyl)-3methyl-2-thiourea ([3H]MeACPTU) was used as a probe to photolabel human red blood cell urea transporters (Neau et al., 1993). This technique detected a 40 kDa polypeptide in red blood cells, later called UT-B1, which was absent in Jk null red blood cells (i.e. showing that the Kidd antigen was a urea transporter) (Neau et al., 1993). This UT-B1 transporter was initially detected in human bone marrow, erythrocytes and also the kidney, as 2.5 kb and 4.7 kb transcripts (Olives et al., 1994). Rat UT-B1 was found in brain, spleen, kidney and testis, compared with rat UT-A2 which was only located in the kidney (Promeneur et al., 1996). Rat UT-B1 was also found in urinary tract epithelia, thymus and lung (Tsukaguchi et al., 1997). UT-B1 proteins were also detected in the heart (Meng et al., 2009), bladder and gastrointestinal tract in mice (Lucien et al., 2005), human colon (Inoue et al., 2004), rat colon (Inoue et al., 2005) and throughout sheep gastrointestinal tract, including the salivary glands (Ludden et al., 2009). Bovine UT-B1 and UT-B2 isoforms are both present in the bovine rumen (Stewart et al., 2005), while UT-B1 has also been reported in the cochlea of rats (Kwun et al., 2003).
UT-B1 localized to non-fenestrated endothelial cells in descending vasa recta of human kidney (Xu et al., 1997; Timmer et al., 2001) and rat kidney (Pallone, 1994). In addition to its location in descending vasa recta, mouse UT-B1 has been reported in the renal proximal tubule and papillary surface epithelium (Jung et al., 2003). UT-B1 was prevalent in the colonic epithelial cells in the human ascending colon (Collins et al., 2010), while bovine UT-B protein was present in rumen epithelial layers (Stewart et al., 2005). UT-B1 was also found in the Sertoli cells of the seminiferous tubules in the testis (Tsukaguchi et al., 1997).
UT-B1 was located on both apical and basolateral membranes of descending vasa recta in both rats (Lim et al., 2006) and mice (Jung et al., 2003). UT-B1 was also located on both apical and basolateral membranes in rat testis Sertoli cells (Fenton et al., 2002c). In agreement with these findings, bovine ruminal UT-B protein has been localized to both apical and basolateral membranes of epithelial cells (Stewart et al., 2005; Simmons et al., 2009). In addition, functional evidence for bovine UT-B2, when over-expressed in a Madin–Darby canine kidney (MDCK) cell line, also showed that this isoform is capable to going to both apical and basolateral membranes (Tickle et al., 2009).
Regulation
IMCD plasma membranes limit the rate of trans-epithelial urea transport (Star, 1990) and it is known that the antidiuretic hormone vasopressin stimulates an increase in membrane urea permeability (Nielsen and Knepper, 1993). Vasopressin binds to V2 receptors on the basolateral membrane of IMCD cells, increasing cAMP levels and stimulating PKA – hence vasopressin stimulation of urea transport was prevented by PKA inhibition (Zhang and Verkman, 1990).
Vasopressin regulates rat UT-A1 function in the short term (Terris et al., 1998), and its action is prevented by PKA inhibition (Frohlich et al., 2006). Vasopressin activates PKA rapidly (within 5 to 10 min.) and stimulates phosphorylation of UT-A1 protein (Zhang et al., 2002). This causes an increase in urea transport by increasing UT-A1 abundance at the apical plasma membrane in IMCD cells (Klein et al., 2006a). This regulation of UT-A1 activity and membrane accumulation involves rapid phosphorylation of serine 486 (Klein et al., 2010). Other UT-A1 amino acids reported to be phosphorylated in response to vasopressin include serine 84 (Hwang et al., 2010) and serine 499 (Blount et al., 2008) (see Figure 4).
Vasopressin also stimulates UT-A3 function (Stewart et al., 2007), via a process again involving the stimulation of both phosphorylation and membrane accumulation (Blount et al., 2007). Vasopressin firstly produces PKA-dependent stimulation of UT-A3 transporters in the basolateral membrane (Stewart et al., 2009). Secondly, vasopressin also then stimulates casein kinase II-dependent trafficking of additional UT-A3 transporters to the basolateral membrane, via a process that is dependent on both protein kinase C and calmodulin (Stewart et al., 2009).
Both UT-A1 (Shayakul et al., 1996) and UT-A3 (Karakashian et al., 1999; Stewart et al., 2007) are stimulated by increased levels of cAMP, although the increase in human UT-A1 function is modest (Bagnasco et al., 2001). PKA also stimulates the transport function of human UT-A6 (Smith et al., 2004), but not UT-A2 (Fenton et al., 2002b). In contrast, when mouse UT-A2 was expressed in a stable MDCK cell line it was acutely regulated by vasopressin, cAMP and calcium (Potter et al., 2006). Increased tonicity in rat IMCD suspensions increased plasma membrane localization of UT-A1 and UT-A3 (Blessing et al., 2008). Indeed, hypertonicity stimulates urea transport through a protein kinase C-mediated phosphorylation event (Wang et al., 2010). Angiotensin II also increases vasopressin-stimulated urea transport in rat IMCD via a protein kinase C-dependent effect on UT-A1 (Kato et al., 2000).
As well as insertion, regulation of urea transporter removal from the plasma membrane also occurs. For example, ubiquitination regulates the plasma membrane expression of the three main renal UT-A urea transporters: UT-A1, UT-A2 and UT-A3 (Stewart et al., 2008). Ubiquitination and subsequent degradation of UT-A1 by the proteasome pathway involves MDM2 (murine double minute) E3 ubiquitin ligase (Chen et al., 2008). UT-A1 is internalized by a dynamin-dependent mechanism, which is mediated by both caveolae and clathrin coated pit pathways (Huang et al., 2010b). In contrast to all this, UT-B proteins have not been reported to be acutely regulated. For example, bovine bUT-B2 is constitutively activated when over-expressed in an MDCK cell line and is not stimulated by cAMP, calcium, vasopressin or protein kinases (Tickle et al., 2009).
A number of factors have been shown to influence UT-A gene expression – including hydration state, dietary protein, glucocorticoids and mineralocorticoids. There are two promoters in the UT-A gene: one controlling UT-A1/UT-A3 expression (called UT-Aα or UT-A promoter I) and one controlling UT-A2 (called UT-Aβ or UT-A promoter II). In mice, the UT-Aα promoter is cAMP and tonicity sensitive, while the UT-Aβ promoter is only cAMP sensitive (Fenton et al., 2002a), hence both UT-A2 and UT-A3 mRNA levels are increased in water-deprived mice (Fenton et al., 2002a). Glucocorticoids have been reported to inhibit transcription and expression of UT-A1 and UT-A3 by decreasing UT-A promoter I activity in rats (Peng et al., 2002), with similar findings for the UT-Aα promoter in mice (Fenton et al., 2006). Interestingly, rat kidney UT-A1 has also been shown to be down-regulated by aldosterone via the mineralocorticoid receptor (Gertner et al., 2004). In contrast, the down-regulation of UT-A1 by glucocorticoids in the same study was not via the mineralocorticoid receptor, but most likely through the glucocorticoid receptor (Gertner et al., 2004). The implications of these findings are that both glucocorticoid agonists used for inflammatory conditions and mineralocorticoid antagonists used for diuresis are likely to alter SLC14A2 transporter expression in the human kidney.
Glucocorticoids have been shown to have no effect on the UT-A promoter II (i.e. UT-Aβ) and so did not alter UT-A2 expression levels (Peng et al., 2002). However, UT-A2 expression in the rat kidney can be influenced by other factors. UT-A2 has a 4.0 kb RNA transcript that is regulated by dietary protein content, while a 2.9 kb transcript is responsive to hydration state (Smith et al., 1995), thus meaning fluid and nitrogen balance can be regulated independently. It has also been reported that tonicity responsive regulation of rat UT-A1 and UT-A3 is mediated by the TonE/TonEBP pathway (Nakayama et al., 2000).
Investigation of the regulation of UT-A protein level expression also confirms many of these findings. For example, osmolality and urea concentration regulate UT-A1 expression (Terris et al., 1998), glucocorticoids down-regulate UT-A1 in rat terminal IMCD (Naruse et al., 1997) and rat UT-A2 abundance is regulated by the antidiuretic hormone vasopressin (Wade et al., 2000). Interestingly, one study has shown that water deprivation increased UT-A3 expression, but decreased UT-A1, in the rat IMCD (Lim et al., 2006). The mechanisms for this differential effect on UT-A1 and UT-A3 are not yet clearly understood.
To date, little is known about the factors that influence expression of the UT-B gene. However, research at the protein level suggests a number of factors may be involved. Long-term vasopressin infusion has been shown to greatly decrease UT-B1 in rat kidney (Trinh-Trang-Tan et al., 2002), while water derivation increased UT-B staining intensity of descending vasa recta (Lim et al., 2006). Rat UT-B1 renal and intestinal protein expression is also regulated by low protein and urea diets (Inoue et al., 2005). Furthermore, dietary intake has shown to alter abundance and localization of UT-B protein in the rumen of two species – cows (Simmons et al., 2009) and sheep (Ludden et al., 2009). Further research is now required to clarify how these dietary effects on UT-B proteins are actually regulated.
Pathology and clinical significance
Major dysfunction of UT-A protein has not been reported in humans. However, specific KO mouse models have been investigated and the lack of major dysfunction may well be due to the compensatory effects of up-regulating other urea transporter isoforms. For example, it has been reported that there are increased levels of UT-A2 in mice lacking UT-B (Klein et al., 2004). The UT-A1/3 KO mice, lacking both the collecting duct urea transporters UT-A1 and UT-A3, have a severe concentrating defect when fed a normal 20% protein diet (Fenton et al., 2004) – with increased fluid consumption, increased urine flow and decreased urine osmolality. More recently, these UT-A1/3 KO mice have also been shown to have increased blood pressure, plus increased chance of hydronephrosis and renal pelvic reflux compared with wild-type controls (Jacob et al., 2008). UT-A2 KO mice have a much milder concentrating defect that is only detectable when on a low protein diet – suggesting UT-A2 mainly plays a role in maintaining inner medulla urea concentration when urea production is low (Uchida et al., 2005). While these findings confirm that UT-A function is predominantly concerned with the renal urinary concentrating mechanism, the implications of UT-A mutations to the health of the human population is not yet fully understood.
As previously mentioned, humans lacking red blood cell UT-B1 protein do exist (i.e. Jk null individuals). These patients displayed moderate maximal concentrating ability (∼800 mOsm vs. ∼1000–1100 mOsm in controls), that is, they have only a mild concentrating defect (Sands et al., 1992). As expected, Jk null individuals have red blood cells that have a selective defect in urea transport (Olives et al., 1995). Jk null individuals are rare within a population, for example, five out of 20 163 Thai individuals (i.e. 0.0002%) (Deelert et al., 2010). However, after immunization anti-Jk3 forms and therefore it can be difficult to find donors suitable for these Jk null individuals (Deelert et al., 2010).
UT-B KO mice have a 45-fold reduction in red blood cell urea permeability, 50% increase in urine output and 30% decrease in urine osmolality (i.e. mild, urea-selective concentrating defect (Yang et al., 2002). Interestingly, the UT-B KO mice gain less weight than wild-type littermates, suggesting intestinal UT-B plays important role in weight gain (Yang and Bankir, 2005). Although it cannot be ruled out that this reduced weight gain was simply due to adverse effects on general health, the KO mice displayed no obvious signs of ill-health compared with the wild-type littermates. This proposed nutritional role for intestinal UT-B protein is linked to its involvement in the UNS process that helps maintain the symbiotic relationship between mammals and their intestinal bacteria (Stewart and Smith, 2005) – see Figure 2. Recent advances in our understanding of the importance of colonic bacteria populations to human health and nutrition strongly indicate that the UNS process may play a vital role in our well-being. Urea hydrolysis in the human gastrointestinal tract is regulated by diet (Fouillet et al., 2008) and one may predict that this involves regulation of the UT-B1 proteins mediating trans-epithelial urea transport in the human ascending colon (Collins et al., 2010) – in a manner similar to the dietary effects observed on ruminal UT-B transporters (Simmons et al., 2009). Although the precise role of human colonic urea transporters still remain to be determined, it is intriguing to speculate that alteration of their normal function could alter bacterial populations and potentially contribute to disease states of the human colon.
Numerous factors have been shown to influence renal UT-A transporter expression. Excess of glucocorticoids produces a decrease in rat renal UT-A1 and UT-A3 abundance, and may explain impaired urinary concentrating capacity in human patients suffering from Cushing syndrome (Li et al., 2008). Renal urea transporters are down-regulated by severe inflammation, such as occurs with sepsis-induced acute renal failure (Schmidt et al., 2007), while UT-A1 is down-regulated in adriamycin-induced nephritic syndrome (Fernandez-Llama et al., 1998). It has also been suggested that expressional changes in kidney UT-A protein may be responsible for reduced concentrating ability of mammalian kidney as ageing occurs (Combet et al., 2003).
Rat UT-A1 in kidney medulla is down-regulated in angiotensin II-induced hypertension (Klein et al., 2006b), while UT-A protein was increased in kidney of streptozotocin-induced diabetic rats compared with control rats (Bardoux et al., 2001). Importantly, increases in UT-A1 (but not UT-B1) in renal medulla during diabetes mellitus may help limit fluid loss during this disease (Kim et al., 2003). Lastly, there is increased UT-A1 and UT-A3 expression, and resulting function, in the IMCD of salt-sensitive rats (cf. salt-resistant rat) (Fenton et al., 2003). There are also disease and environmental-related changes in non-renal UT-A transporters. For example, pre-eclampsia appears to increase phloretin-sensitive urea transport and UT-A urea transporter abundance in the placenta (Damiano et al., 2006), while UT-A protein abundance in the heart is increased during uraemia, hypertension and heart failure (Duchesne et al., 2001).
There are a limited number of reports concerning changes in both UT-A and UT-B proteins. Decreases in UT-A1, UT-A3 and UT-B1 in rats with ureteral obstruction may explain reduction in the urinary concentrating ability in these animals (Li et al., 2004). Chronic renal failure significantly decreases UT-A1, UT-A2 and UT-B1 in the rat kidney, and also decreased UT-B1 in rat brain (Hu et al., 2000). A decrease in UT-A1 and UT-B1 abundance in the renal inner medulla has been reported in lithium-fed rats (Klein et al., 2002). However, UT-A1 recovered to normal levels 14 days after cessation of lithium administration (Blount et al., 2010). Treatment with the immunosuppressant drug cyclosporine reduces UT-A2, UT-A3 and UT-B1 levels in the kidney, explaining the impaired urine concentrating ability that is a main feature of cyclosporine-induced nephropathy (Lim et al., 2004). The diuretic furosemide also moderately decreases UT-B1 abundance in rat kidney (Trinh-Trang-Tan et al., 2002), which may have implications for its long-term use. Finally, there has also been a reported increase in UT-B1 abundance in the ageing rat brain (Trinh-Trang-Tan et al., 2003). It is not yet known what might cause this change or whether it leads to dysregulation in cerebral function (Trinh-Trang-Tan et al., 2003).
Conclusion
The family of SLC14A facilitative urea transporters play an important role in two major physiological processes, namely the urinary concentration mechanism and UNS. These facilitative transporters are found in specific locations within different tissues and are derived from two distinct genes: UT-A and UT-B. Generally, the UT-A and UT-B classes of urea transporters have a similar function, topology and basic structure. However, they do display marked differences in terms of substrate specificity, transport rates, inhibition, gene regulation, functional regulation and tissue localization. Although there are no major pathologies linked with urea transporter dysfunction, understanding these proteins has important clinical implications, especially within the context of renal disease.
Acknowledgments
The author acknowledges the funding support provided by University College Dublin seed funding grant SF376.
Glossary
Abbreviations
- AQP
aquaporin
- IMCD
inner medullary collecting duct
- KO
knockout
- MDM2
murine double minute
- MeACPTU
1-(3-azido-4-chlorophenyl)-3 methyl-2-thiourea
- pCMBS
p-chloromercuribenzenesulphonate
- PKA
protein kinase A
- SGLT1
sodium-dependent glucose transporter
- tDL
thin descending limbs
- TMD
transmembrane spanning domain
- UNS
urea nitrogen salvaging
- UT
urea transporter
Conflicts of interest
There are no conflicts of interest to report regarding this article.
References
- Bagnasco SM, Peng T, Janech G, Karakashian A, Sands JM. Cloning and characterization of the human urea transporter UT-A1 and mapping of the human Slc14a2 gene. Am J Physiol Renal Physiol. 2001;281:F400–F406. doi: 10.1152/ajprenal.2001.281.3.F400. [DOI] [PubMed] [Google Scholar]
- Bardoux P, Ahloulay M, Le Maout S, Bankir L, Trinh-Trang-Tan MM. Aquaporin-2 and urea transporter-A1 are up-regulated in rats with type diabetes mellitus. Diabetologia. 2001;44:637–645. doi: 10.1007/s001250051671. [DOI] [PubMed] [Google Scholar]
- Blessing NW, Blount MA, Sands JM, Martin CF, Klein JD. Urea transporters UT-A1 and UT-A3 accumulate in the plasma membrane in response to increased hypertonicity. Am J Physiol Renal Physiol. 2008;295:F1336–F1341. doi: 10.1152/ajprenal.90228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol. 2007;293:F1308–F1313. doi: 10.1152/ajprenal.00197.2007. [DOI] [PubMed] [Google Scholar]
- Blount MA, Mistry AC, Frohlich O, Price SR, Chen G, Sands JM, et al. Phosphorylation of UT-A1 urea transporter at serine 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol. 2008;295:F295–F299. doi: 10.1152/ajprenal.00102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount MA, Sim JH, Zhou R, Martin CF, Lu W, Sands JM, et al. Expression of transporters involved in urine concentration recovers differently after cessation of lithium treatment. Am J Physiol Renal Physiol. 2010;298:F601–F608. doi: 10.1152/ajprenal.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AD, Terris JM, Ecelbarger CA, Klein JD, Sands JM, Chou CL, et al. 97 and 117 kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol. 2001;281:F133–F143. doi: 10.1152/ajprenal.2001.281.1.F133. [DOI] [PubMed] [Google Scholar]
- Chen G, Frohlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem. 2006;281:27436–27442. doi: 10.1074/jbc.M605525200. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang H, Frohlich O, Yang Y, Klein JD, Price SR, et al. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol. 2008;295:F1528–F1534. doi: 10.1152/ajprenal.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CL, Knepper MA. Inhibition of urea transport in inner medullary collecting duct by phloretin and urea analogues. Am J Physiol. 1989;257:F359–F365. doi: 10.1152/ajprenal.1989.257.3.F359. [DOI] [PubMed] [Google Scholar]
- Collins D, Winter DC, Hogan AM, Schirmer L, Baird AW, Stewart GS. Differential protein abundance and function of UT-B transporters in human colon. Am J Physiol Gastro Physiol. 2010;298:G345–G351. doi: 10.1152/ajpgi.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Walpole C, Ryan E, Winter D, Baird A, Stewart G. UT-B1 mediates transepithelial urea flux in the rat gastrointestinal tract. J Membr Biol. 2011;239:123–130. doi: 10.1007/s00232-010-9331-9. [DOI] [PubMed] [Google Scholar]
- Combet S, Geffrey N, Berthonaud V, Dick B, Teillet L, Verbavatz JM, et al. Correction of age-related polyuria by ddAVP: molecular analysis of aquaporins and urea transporters. Am J Physiol Renal Physiol. 2003;284:F199–F208. doi: 10.1152/ajprenal.00167.2002. [DOI] [PubMed] [Google Scholar]
- Damiano AE, Zotta E, Ibarra C. Functional and molecular expression of AQP9 channel and UT-A transporter in normal and preeclamptic human placentas. Placenta. 2006;27:1073–1081. doi: 10.1016/j.placenta.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Deelert S, Thippayaboon P, Sriwai W, Sriwanitchrak P, Tubrod J, Kupatawintu P, et al. Jk(a-b-) phenotype screening by the urea lysis test in thai blood donors. Blood Transfus. 2010;8:17–20. doi: 10.2450/2009.0104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne R, Klein JD, Velotta JB, Doran JJ, Rouillard P, Roberts BR, et al. UT-A urea transporter protein in heart: increased abundance during uremia, hypertension and heart failure. Circ Res. 2001;89:139–145. doi: 10.1161/hh1401.093293. [DOI] [PubMed] [Google Scholar]
- Fenton RA. Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflugers Arch. 2009;458:169–177. doi: 10.1007/s00424-008-0612-4. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Howorth A, Cooper GJ, Meccariello R, Morris ID, Smith CP. Molecular characterization of a novel UT-A urea transporter isoform (UT-A5) in testis. Am J Physiol Cell Physiol. 2000;279:C1425–C1431. doi: 10.1152/ajpcell.2000.279.5.C1425. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Cottingham CA, Stewart GS, Howorth A, Hewitt JA, Smith CP. Structure and characterization of the mouse UT-A gene (Slc14a2) Am J Physiol Renal Physiol. 2002a;282:F630–F638. doi: 10.1152/ajprenal.00264.2001. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Stewart GS, Carpenter B, Howorth A, Potter EA, Cooper GJ, et al. Characterization of mouse urea transporters UT-A1 and UT-A2. Am J Physiol Renal Physiol. 2002b;283:F817–F825. doi: 10.1152/ajprenal.00263.2001. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Cooper GJ, Morris ID, Smith CP. Co-ordinated expression of UT-A and UT-B urea transporters in rat testis. Am J Physiol Cell Physiol. 2002c;282:C1492–C1501. doi: 10.1152/ajpcell.00567.2001. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Chou CL, Ageloff A, Brandt W, Stokes JB, Knepper MA. Increased collecting duct urea transporter expression in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2003;285:F143–F151. doi: 10.1152/ajprenal.00073.2003. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci U S A. 2004;101:7469–7474. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, Shodeinde A, Knepper MA. UT-A urea transporter promoter, UT-A alpha, targets principal cells of the renal inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;290:F188–F195. doi: 10.1152/ajprenal.00285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Llama P, Andrews P, Nielsen S, Ecelbarger CA, Knepper MA. Impaired aquaporin and urea transporter expression in rats with adriamycin-induced nephritic syndrome. Kidney Int. 1998;53:1244–1253. doi: 10.1046/j.1523-1755.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- Fouillet H, Juillet B, Bos C, Mariotti F, Gaudichon C, Benamouzig R, et al. Urea-nitrogen production and salvage are modulated by protein intake in fed humans: results of an oral stable-isotope-tracer protocol and compartmental modelling. Am J Clin Nutr. 2008;87:1702–1714. doi: 10.1093/ajcn/87.6.1702. [DOI] [PubMed] [Google Scholar]
- Frohlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol. 2004;286:C1264–C1270. doi: 10.1152/ajpcell.00499.2003. [DOI] [PubMed] [Google Scholar]
- Frohlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1 mediated transepithelial urea flux in MDCK cells. Am J Physiol Renal Physiol. 2006;291:C600–C606. doi: 10.1152/ajpcell.00413.2005. [DOI] [PubMed] [Google Scholar]
- Gade W, Robinson B. CLS meets the aquaporin family: clinical cases including aquaporin systems. Clin Lab Sci. 2006;19:80–89. [PubMed] [Google Scholar]
- Gertner RA, Klein JD, Bailey JL, Kim DU, Luo XH, Bagnasco SM, et al. Aldosterone decreases UT-A1 urea transporter expression via the mineralocorticoid receptor. J Am Soc Nephrol. 2004;15:558–565. doi: 10.1097/01.asn.0000113244.37857.ac. [DOI] [PubMed] [Google Scholar]
- Hill WG, Southern NM, MacIver B, Potter E, Apodaca G, Smith CP, et al. Isolation and characterization of the Xenopus oocyte plasma membrane: a new method for studying activity of water and solute transporters. Am J Physiol Renal Physiol. 2005;289:F217–F224. doi: 10.1152/ajprenal.00022.2005. [DOI] [PubMed] [Google Scholar]
- Hong X, Xing H, Yu Y, Wen Y, Zhang Y, Zhang S, et al. Genetic polymorphisms of the urea transporter gene are associated with antihypertensive response to nifedipine. Methods Find Exp Clin Pharmacol. 2007;29:3–10. doi: 10.1358/mf.2007.29.1.1063490. [DOI] [PubMed] [Google Scholar]
- Hu MC, Bankir L, Michelet S, Rousselet G, Trinh-Trang-Tan MM. Massive reduction of urea transporters in remnant kidney and brain of uremic rats. Kidney Int. 2000;58:1202–1210. doi: 10.1046/j.1523-1755.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Yang Y, Eaton DC, Sands JM, Chen G. The N-terminal 81-aa fragment is critical for UT-A1 urea transporter bioactivity. J Epithelial Biol Pharmacol. 2010a;3:34–39. doi: 10.2174/1875044301003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng X, Zhuang J, Frohlich O, Klein JD, Cai H, et al. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae and clathrin coated pit pathways. Am J Physiol Renal Physiol. 2010b;299:F1389–F1395. doi: 10.1152/ajprenal.00718.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Gunaratne R, Rinschen MM, Yu MJ, Pisitkun T, Hoffert JD, et al. Vasopressin increases phosporylation of Ser 84 and Ser 486 in Slc14a2 collecting duct urea transporters. Am J Physiol Renal Physiol. 2010;299:F559–F567. doi: 10.1152/ajprenal.00617.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Jackson SD, Vikulina T, Klein JD, Tomita K, Bagnasco SM. Identification and characterization of a Kidd antigen/UT-B urea transporter expressed in human colon. Am J Physiol Cell Physiol. 2004;287:C30–C35. doi: 10.1152/ajpcell.00443.2003. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kozlowski SD, Klein JD, Bailey JL, Sands JM, Bagnasco SM. Regulated expression of renal and intestinal UT-B urea transporter in response to varying urea load. Am J Physiol Renal Physiol. 2005;289:F451–F458. doi: 10.1152/ajprenal.00376.2004. [DOI] [PubMed] [Google Scholar]
- Irshaid NM, Henry SM, Olsson ML. Genomic characterization of the kidd blood group gene: different molecular basis of the Jk(a-b-) phenotype in Polynesians and Finns. Transfusion. 2000;40:69–74. doi: 10.1046/j.1537-2995.2000.40010069.x. [DOI] [PubMed] [Google Scholar]
- Irshaid NM, Eicher NI, Hustinx H, Poole J, Olsson ML. Novel alleles at the Jk blood group locus explain the absence of the erythrocyte urea transporters in European families. Br J Haematol. 2002;116:445–453. doi: 10.1046/j.1365-2141.2002.03238.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, et al. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol and urea. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Kuwuhara M, Gu Y, Tanaka Y, Marumo F, Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not glycerol. Biochem Biophys Res Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Imai M, Sasaki S. Cellular localization of aquaporin 7 in the rat kidney. Exp Nephrol. 2000;8:252–257. doi: 10.1159/000020676. [DOI] [PubMed] [Google Scholar]
- Jacob V, Harbaugh C, Dietz JR, Fenton RA, Kim SM, Castrop H, et al. Magnetic resonance imaging of urea transporter knockout mice shows renal pelvic abnormalities. Kidney Int. 2008;74:1202–1208. doi: 10.1038/ki.2008.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JY, Madsen KM, Han KH, Yang CW, Knepper MA, Sands JM, et al. Expression of urea transporters in potassium-depleted mouse kidney. Am J Physiol Renal Physiol. 2003;285:F1210–F1224. doi: 10.1152/ajprenal.00111.2003. [DOI] [PubMed] [Google Scholar]
- Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol. 1999;10:230–237. doi: 10.1681/ASN.V102230. [DOI] [PubMed] [Google Scholar]
- Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopress-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol. 2000;279:F835–F840. doi: 10.1152/ajprenal.2000.279.5.F835. [DOI] [PubMed] [Google Scholar]
- Kim D, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol. 2003;285:F303–F309. doi: 10.1152/ajprenal.00438.2002. [DOI] [PubMed] [Google Scholar]
- Kishore BK, Terris J, Fernandez-Llama P, Knepper MA. Ultramicrodetermination of vasopressin-regulated urea transporter protein in microdissected renal tubules. Am J Physiol. 1997;272:F531–F537. doi: 10.1152/ajprenal.1997.272.4.F531. [DOI] [PubMed] [Google Scholar]
- Klein JD, Gunn RB, Roberts BR, Sands JM. Down regulation of urea transporters in the renal inner medulla of lithium-fed rats. Kidney Int. 2002;61:995–1002. doi: 10.1046/j.1523-1755.2002.00210.x. [DOI] [PubMed] [Google Scholar]
- Klein JD, Sands JM, Qian L, Wang X, Yang B. Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol. 2004;15:1161–1167. doi: 10.1097/01.asn.0000125617.19799.72. [DOI] [PubMed] [Google Scholar]
- Klein JD, Frohlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol. 2006a;17:2680–2686. doi: 10.1681/ASN.2006030246. [DOI] [PubMed] [Google Scholar]
- Klein JD, Murrell BP, Tucker S, Kim YH, Sands JM. Urea transporter UT-A1 and aquaporin-2 proteins decrease in response to angiotensin II or norepinephrine-induced acute hypertension. Am J Physiol Renal Physiol. 2006b;291:F952–F959. doi: 10.1152/ajprenal.00173.2006. [DOI] [PubMed] [Google Scholar]
- Klein JD, Blount MA, Frohlich O, Denson CE, Tan X, Sim JH, et al. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol. 2010;298:F935–F940. doi: 10.1152/ajprenal.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwun YS, Yeo SW, Ahn YH, Lim SW, Jung JY, Kim WY, et al. Immunohistochemical localization of urea transporters A and B in the rat cochlea. Hear Res. 2003;183:84–96. doi: 10.1016/s0378-5955(03)00218-1. [DOI] [PubMed] [Google Scholar]
- Leung DW, Loo DD, Hirayama BA, Zeuthen T, Wright EM. Urea transport by cotransporters. J Physiol. 2000;528:251–257. doi: 10.1111/j.1469-7793.2000.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MH, Fuente R, Verkman AS. Ureatics: a small molecule screen yields nanomolar potency inhibitors of urea transporter UT-B. FASEB J. 2007;21:551–563. doi: 10.1096/fj.06-6979com. [DOI] [PubMed] [Google Scholar]
- Levin EJ, Quick M, Zhou M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature. 2009;462:757–761. doi: 10.1038/nature08558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Klein JD, Wang W, Knepper MA, Nielsen S, Sands JM, et al. Altered expression of urea transporters in response to ureteral obstruction. Am J Physiol Renal Physiol. 2004;286:F1154–F1162. doi: 10.1152/ajprenal.00453.2003. [DOI] [PubMed] [Google Scholar]
- Li C, Wang W, Summer SN, Falk S, Schrier RW. Downregulation of UT-A1/UT-A3 is associated with urinary concentrating defect in glucorticoid-excess state. J Am Soc Nephrol. 2008;19:1975–1981. doi: 10.1681/ASN.2008010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SW, Li C, Sun BK, Han KH, Kim WY, Oh YW, et al. Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat kidney. Am J Physiol Renal Physiol. 2004;287:F139–F151. doi: 10.1152/ajprenal.00240.2003. [DOI] [PubMed] [Google Scholar]
- Lim SW, Han KH, Jung JY, Kim WY, Yang CW, Sands JM, et al. Ultrastructural localization of UT-A and UT-B in rat kidneys with different hydration status. Am J Physiol Regul Integr Comp Physiol. 2006;290:R479–R492. doi: 10.1152/ajpregu.00512.2005. [DOI] [PubMed] [Google Scholar]
- Lucien N, Sidoux-Walter F, Olives B, Moulds J, Le Pennec PY, Cartron JP, et al. Characterization of the gene encoding the human Kidd blood group/urea transporter protein. Evidence for splice site mutations in Jknull individuals. J Biol Chem. 1998;273:12973–12980. doi: 10.1074/jbc.273.21.12973. [DOI] [PubMed] [Google Scholar]
- Lucien N, Sidoux-Walter F, Roudier N, Ripoche P, Huet M, Trinh-Trang-Tan MM, et al. Antigenic and functional properties of the human red blood cell urea transporter hUT-B1. J Biol Chem. 2002;277:34101–34108. doi: 10.1074/jbc.M205073200. [DOI] [PubMed] [Google Scholar]
- Lucien N, Bruneval P, Lasbennes F, Belair MF, Mandet C, Cartron JP, et al. UT-B1 urea transporter is expressed along the urinary and gastrointestinal tracts of the mouse. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1046–R1056. doi: 10.1152/ajpregu.00286.2004. [DOI] [PubMed] [Google Scholar]
- Ludden PA, Stohrer RM, Austin KJ, Atkinson RL, Belden EL, Harlow HJ. Effect of protein supplementation on expression and distribution of urea transporter B in lambs fed low-quality forage. J Anim Sci. 2009;87:1354–1366. doi: 10.2527/jas.2008-1399. [DOI] [PubMed] [Google Scholar]
- MacIver B, Smith CP, Hill WG, Zeidel ML. Functional characterization of mouse urea transporters UT-A2 and UT-A3 expressed in purified Xenopus laevis oocyte plasma membranes. Am J Physiol Renal Physiol. 2008;294:F956–F964. doi: 10.1152/ajprenal.00229.2007. [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Macey RI. Estimate of the number of urea transport sites in erythrocyte ghosts using a hydrophobic mercurial. J Membr Biol. 1993;133:85–97. doi: 10.1007/BF00231880. [DOI] [PubMed] [Google Scholar]
- Martial S, Olives B, Abrami L, Couriaud C, Bailly P, You G, et al. Functional differentiation of the human red blood cell and kidney urea transporters. Am J Physiol. 1996;271:F1264–F1268. doi: 10.1152/ajprenal.1996.271.6.F1264. [DOI] [PubMed] [Google Scholar]
- Masouredis SP, Sudora E, Mahan L, Victoria EJ. Quantitaive immunoferrin microscopy of Fya, Fyb, Jka, U and Dib antigen site numbers of human red cells. Blood. 1980;56:969–977. [PubMed] [Google Scholar]
- Meng Y, Zhou X, Li Y, Zhao D, Liang S, Zhao X, et al. A novel allele mutation at the Jk locus causing Jk null phenotype in a Chinese family. Sci China C Life Sci. 2005;48:636–640. doi: 10.1360/062005-127. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhao C, Zhang X, Zhao H, Guo L, Lu B, et al. Surface electrocardiogram and action potentital in mice lacking urea transporter B. Sci China C Life Sci. 2009;52:474–478. doi: 10.1007/s11427-009-0047-y. [DOI] [PubMed] [Google Scholar]
- Mistry AC, Chen G, Kato A, Nag K, Sands JM, Hirose S. A novel type of urea transporter, UT-C, is highly expressed in proximal tubule of seawater eel kidney. Am J Physiol Renal Physiol. 2005;288:F455–F465. doi: 10.1152/ajprenal.00296.2004. [DOI] [PubMed] [Google Scholar]
- Mistry AC, Mallick R, Klein JD, Sands JM, Frohlich O. Functional characterization of the central hydrophilic linker region of the urea transporter UT-A1: cAMP activation and snapin binding. Am J Physiol Cell Physiol. 2010;298:C1441–C1437. doi: 10.1152/ajpcell.00497.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, et al. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Peng T, Sands JM, Bagnasco SM. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J Biol Chem. 2000;275:38275–38280. doi: 10.1074/jbc.M004678200. [DOI] [PubMed] [Google Scholar]
- Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM. Glucocorticoids downregulate the vasopressin-regulated urea transporter in tIMCD. J Am Soc Nephrol. 1997;8:517–523. doi: 10.1681/ASN.V84517. [DOI] [PubMed] [Google Scholar]
- Neau P, Degeilh F, Lamotte H, Rousseau B, Ripoche P. Photoaffinity labelling of the human red blood cell urea transporter polypeptide components – possible homology with the Kidd blood group antigen. Eur J Biochem. 1993;218:447–455. doi: 10.1111/j.1432-1033.1993.tb18395.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Knepper MA. Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am J Physiol. 1993;265:F204–F213. doi: 10.1152/ajprenal.1993.265.2.F204. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Celullar and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci U S A. 1996;93:5496–5500. doi: 10.1073/pnas.93.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olives B, Neau P, Bailly P, Hediger MA, Rousselet G, Catron JP, et al. Cloning and functional expression of a urea transporter from human bone marrow cells. J Biol Chem. 1994;269:31649–31652. [PubMed] [Google Scholar]
- Olives B, Mattei MG, Huet M, Neau P, Martial S, Cartron JP, et al. Kidd blood group and urea transport function of human erythrocytes are carried by the same protein. J Biol Chem. 1995;270:15607–15610. doi: 10.1074/jbc.270.26.15607. [DOI] [PubMed] [Google Scholar]
- Olives B, Martial S, Mattei MG, Matassi G, Rousselet G, Ripoche P, et al. Molecular characterization of a new urea transporter in the human kidney. FEBS Lett. 1996;386:156–160. doi: 10.1016/0014-5793(96)00425-5. [DOI] [PubMed] [Google Scholar]
- Pallone TL. Characterization of the urea transporter in outer medulla descending vasa recta. Am J Physiol. 1994;267:R260–R267. doi: 10.1152/ajpregu.1994.267.1.R260. [DOI] [PubMed] [Google Scholar]
- Peng T, Sands JM, Bagnasco SM. Glucocorticoids inhibit transcription and expression of the UT-A urea transporter gene. Am J Physiol Renal Physiol. 2002;282:F853–F858. doi: 10.1152/ajprenal.00262.2001. [DOI] [PubMed] [Google Scholar]
- Potter EA, Stewart G, Smith CP. Urea flux across MDCK-mUT-A2 monolayers is acutely sensitive to AVP, cAMP and [Ca2+]i. Am J Physiol Renal Physiol. 2006;291:F122–F128. doi: 10.1152/ajprenal.00423.2005. [DOI] [PubMed] [Google Scholar]
- Promeneur D, Rousselet G, Bankir L, Bailly P, Cartron JP, Ripoche P, et al. Evidence for distinct vascular and tubular urea transporters in the rat kidney. J Am Soc Nephrol. 1996;7:852–860. doi: 10.1681/ASN.V76852. [DOI] [PubMed] [Google Scholar]
- Ranade K, Wu WD, Hwu CM, Ting CT, Pei D, Pesich R, et al. Genetic variation in the human urea transporter-2 is associated with variation in blood pressure. Hum Mol Genet. 2001;10:2157–2164. doi: 10.1093/hmg/10.19.2157. [DOI] [PubMed] [Google Scholar]
- Raunser S, Mathai JC, Abergrathne PD, Rice AJ, Zeidel ML, Walz T. Oligomeric structure and functional characterization of the urea transporter from Actinobacillus pleuropneumoniae. J Mol Biol. 2009;387:619–627. doi: 10.1016/j.jmb.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, et al. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci U S A. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands JM, Gargus JJ, Frohlich O, Gunn RB, Kokko JP. Urinary concentrating ability in patients with Jk (a-b-) blood type who lack carrier-mediated urea transport. J Am Soc Nephrol. 1992;2:1689–1696. doi: 10.1681/ASN.V2121689. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Hocherl K, Bucher M. Cytokine-mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292:F1479–F1489. doi: 10.1152/ajprenal.00460.2006. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Steel A, Hediger MA. Molecular cloning and characterization of the vasopressin-regulated urea transporter of rat kidney collecting ducts. J Clin Invest. 1996;98:2580–2587. doi: 10.1172/JCI119077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakul C, Knepper MA, Smith CP, Di Giovanni SR, Hediger MA. Segmental localization of urea transporter mRNAs in rat kidney. Am J Physiol. 1997;272:F654–F660. doi: 10.1152/ajprenal.1997.272.5.F654. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Tsukaguchi H, Berger UV, Hediger MA. Molecular characterization of a novel urea transporter from kidney inner medullary collecting ducts. Am J Physiol Renal Physiol. 2001;280:F487–F494. doi: 10.1152/ajprenal.2001.280.3.F487. [DOI] [PubMed] [Google Scholar]
- Sidoux-Walter F, Lucien N, Olives B, Gobin R, Rousselet G, Kamsteeg EJ, et al. At physiological expression levels the Kidd blood group/urea transporter protein is not a water channel. J Biol Chem. 1999;274:30228–30235. doi: 10.1074/jbc.274.42.30228. [DOI] [PubMed] [Google Scholar]
- Sidoux-Walter F, Lucien N, Nissinen R, Sistinen P, Henry S, Moulds J, et al. Molecular heterogeneity of the Jk(null) phenotype: expression analysis of the Jk(S291P) mutation found in Finns. Blood. 2000;96:1566–1573. [PubMed] [Google Scholar]
- Simmons NL, Chaudhry AS, Graham C, Scriven ES, Thistlethwaite A, Smith CP, et al. Dietary regulation of ruminal bovine UT-B urea transporter expression and localization. J Ani Sci. 2009;87:3288–3299. doi: 10.2527/jas.2008-1710. [DOI] [PubMed] [Google Scholar]
- Smith CP. Mammalian urea transporters. Exp Physiol. 2009;94:180–185. doi: 10.1113/expphysiol.2008.043042. [DOI] [PubMed] [Google Scholar]
- Smith CP, Fenton RA. Genomic organization of the mammalian Slc14a2 urea transporter genes. J Memb Biol. 2006;212:109–117. doi: 10.1007/s00232-006-0870-z. [DOI] [PubMed] [Google Scholar]
- Smith CP, Lee WS, Martial S, Knepper MA, You G, Sands JM, et al. Cloning and regulation of expression of the rat kidney urea transporter (rUT2) J Clin Invest. 1995;96:1556–1563. doi: 10.1172/JCI118194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CP, Potter EA, Fenton RA, Stewart GS. Characterization of a human colonic cDNA encoding a structurally novel urea transporter, hUT-A6. Am J Physiol Cell Physiol. 2004;287:C1087–C1093. doi: 10.1152/ajpcell.00363.2003. [DOI] [PubMed] [Google Scholar]
- Sohara E, Rai T, Miyazaki J, Verkman AS, Sasaki S, Uchida S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am J Physiol Renal Physiol. 2005;289:F1195–F1200. doi: 10.1152/ajprenal.00133.2005. [DOI] [PubMed] [Google Scholar]
- Star RA. Apical membrane limits urea permeation across the rat inner medullary collecting duct. J Clin Invest. 1990;86:1172–1178. doi: 10.1172/JCI114823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GS, Smith CP. Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr Res Rev. 2005;18:49–62. doi: 10.1079/NRR200498. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Fenton RA, Wang W, Kwon TH, White SJ, Collins VM, et al. The basolateral expression of mUT-A3 in the mouse kidney. Am J Physiol Renal Physiol. 2004;286:F979–F987. doi: 10.1152/ajprenal.00334.2003. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Graham C, Cattell S, Smith TP, Simmons NL, Smith CP. UT-B is expressed in bovine rumen: potential role in ruminal urea transport. Am J Physiol Regul Integr Comp Physiol. 2005;289:R605–R612. doi: 10.1152/ajpregu.00127.2005. [DOI] [PubMed] [Google Scholar]
- Stewart GS, King SL, Potter EA, Smith CP. Acute regulation of mUT-A3 urea transporter expressed in a MDCK cell line. Am J Physiol Renal Physiol. 2007;292:F1157–F1163. doi: 10.1152/ajprenal.00183.2006. [DOI] [PubMed] [Google Scholar]
- Stewart GS, O'Brien JH, Smith CP. Ubiquitination regulates the plasma membrane expression of renal UT-A urea transporters. Am J Physiol Cell Physiol. 2008;295:C121–C129. doi: 10.1152/ajpcell.00444.2007. [DOI] [PubMed] [Google Scholar]
- Stewart GS, Thistlethwaite A, Lees H, Cooper GJ, Smith C. Vasopressin regulation of the renal UT-A3 urea transporter. Am J Physiol Renal Physiol. 2009;296:F642–F648. doi: 10.1152/ajprenal.90660.2008. [DOI] [PubMed] [Google Scholar]
- Terris J, Ecelbarger CA, Sands JM, Knepper MA. Long term regulation of renal urea transporter protein expression in rat. J Am Soc Nephrol. 1998;9:729–736. doi: 10.1681/ASN.V95729. [DOI] [PubMed] [Google Scholar]
- Terris JM, Knepper MA, Wade JB. UT-A3 localization and characterization of an additional urea transporter isoform in the IMCD. Am J Physiol Renal Physiol. 2001;280:F325–F332. doi: 10.1152/ajprenal.2001.280.2.F325. [DOI] [PubMed] [Google Scholar]
- Tickle P, Thistlethwaite A, Smith CP, Stewart GS. Novel bUT-B2 urea transporter isoform is constitutively activated. Am J Physiol Regul Integr Comp Physiol. 2009;297:R323–R329. doi: 10.1152/ajpregu.00199.2009. [DOI] [PubMed] [Google Scholar]
- Timmer RT, Klein JD, Bagnasco SM, Doran JJ, Verlander JW, Gunn RB, et al. Localization of the urea transporter UT-B protein in human and rat erythrocytes and tissues. Am J Physiol Cell Physiol. 2001;281:C1318–C1325. doi: 10.1152/ajpcell.2001.281.4.C1318. [DOI] [PubMed] [Google Scholar]
- Trinh-Trang-Tan MM, Lasbennes F, Gane P, Roudier N, Ripoche P, Cartron JP, et al. UT-B1 proteins in rat: tissue distribution and regulation by antidiuretic hormone in kidney. Am J Physiol Renal Physiol. 2002;283:F912–F922. doi: 10.1152/ajprenal.00359.2001. [DOI] [PubMed] [Google Scholar]
- Trinh-Trang-Tan MM, Geelen G, Teillet L, Corman B. Urea transporter expression in aging kidney and brain during dehydration. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1355–R1365. doi: 10.1152/ajpregu.00207.2003. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Hsiao CF, Ho LT, Chuang LM, He CT, Curb JD, et al. Genetic variants of human urea transporter 2 are associated with metabolic syndrome in Asian population. Clin Chim Acta. 2010;411:2009–2013. doi: 10.1016/j.cca.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Tokui T, Brown D, Hediger MA. Cloning and characterization of the urea transporter UT3: localization in rat kidney and testis. J Clin Invest. 1997;99:1506–1515. doi: 10.1172/JCI119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol. 2005;25:7357–7363. doi: 10.1128/MCB.25.16.7357-7363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JB, Lee AJ, Liu J, Ecelbarger CA, Mitchell C, Bradford AD, et al. UT-A2, a 55 kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol. 2000;278:F52–F62. doi: 10.1152/ajprenal.2000.278.1.F52. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klein JD, Liedtke CM, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2010;299:F1401–F1406. doi: 10.1152/ajprenal.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester ES, Johnson ST, Copeland T, Malde R, Lee E, Storry JR, et al. Erythroid urea transporter deficiency due to novel Jk null alleles. Transfusion. 2008;48:365–372. doi: 10.1111/j.1537-2995.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Olives B, Bailly P, Fischer E, Ripoche P, Ronco P, et al. Endothelial cells of the kidney vasa recta express the urea transporter HUT11. Kidney Int. 1997;51:138–146. doi: 10.1038/ki.1997.17. [DOI] [PubMed] [Google Scholar]
- Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–F896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Urea transporter UT3 functions as an efficient water channel. Direct evidence for a common water/urea pathway. J Biol Chem. 1998;273:9369–9372. doi: 10.1074/jbc.273.16.9369. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Analysis of double knockout mice lacking AQP1 and urea transporter UT-B. Evidence for UT-B facilitated water transport in erthyrocytes. J Biol Chem. 2002;277:36782–36786. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter B. J Biol Chem. 2002;277:10633–10637. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993;365:844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCD through PKA. Am J Physiol Renal Physiol. 2002;282:F85–F90. doi: 10.1152/ajprenal.0054.2001. [DOI] [PubMed] [Google Scholar]
- Zhang RB, Verkman AS. Urea transport in freshly isolated and cultured cells from rat inner medullary collecting duct. J Memb Biol. 1990;117:253–261. doi: 10.1007/BF01868455. [DOI] [PubMed] [Google Scholar]
- Zhao D, Sonawane ND, Levin MH, Yang B. Comparative transport efficiencies of urea analogues through urea transporter UT-B. Biochim Biophys Acta. 2007;1768:1815–1821. doi: 10.1016/j.bbamem.2007.04.010. [DOI] [PubMed] [Google Scholar]


