Abstract
The ascorbate transporters SVCT1 and SVCT2 are crucial for maintaining intracellular ascorbate concentrations in most cell types. Although the two transporter isoforms are highly homologous, they have different physiologic functions. The SVCT1 is located primarily in epithelial cells and has its greatest effect in reabsorbing ascorbate in the renal tubules. The SVCT2 is located in most non-epithelial tissues, with the highest expression in brain and neuroendocrine tissues. These transporters are hydrophobic membrane proteins that have a high affinity and are highly selective for ascorbate. Their ability to concentrate ascorbate inside cells is driven by the sodium gradient across the plasma membrane as generated by Na+/K+ ATPase. They can concentrate ascorbate 20 to 60-fold over plasma ascorbate concentrations. Ascorbate transport on these proteins is regulated at the transcriptional, translational and post-translational levels. Available studies show that transporter function is acutely regulated by protein kinases A and C, whereas transporter expression is increased by low intracellular ascorbate and associated oxidative stress. The knockout of the SVCT2 in mice is lethal on day 1 of life, and almost half of SVCT1 knockout mice do not survive to weaning. These findings confirm the importance both of cellular ascorbate and of the two transport proteins as key to maintaining intracellular ascorbate.
Keywords: oxidative stress, anion transport, SVCT1, SVCT2
Introduction/Overview
Humans and higher primates are unable to synthesize vitamin C (ascorbic acid) and will develop scurvy if long deprived of the vitamin in their diets. Most other species can convert glucose to ascorbic acid in the liver and thus are minimally dependent upon dietary vitamin C. To preserve ascorbate, several mechanisms have evolved to efficiently recycle it from its two oxidized forms, the ascorbate free radical and dehydroascorbate (DHA), which are the one and two electron-oxidized forms respectively (May and Asard, 2004). Almost all of this recycling occurs inside cells, where ascorbate has its major functions to scavenge radical species, to recycle vitamin E in cell membranes, to serve as a co-factor for various dioxygenase enzymes, and to help generate and preserve nitric oxide that is needed for vascular regulation (May, 2000). Several of these functions require relatively high ascorbate concentrations; indeed, ascorbate concentrations in most cells and organs (0.5–4 mM) are much higher than in plasma (40–60 µM) (Evans et al., 1982). High intracellular ascorbate concentrations can be generated by transport of either ascorbate or DHA into cells, although as noted next, the latter is not usually the major route.
Ascorbate has long been known to enter cells on a sodium and energy-dependent transporter that is of high affinity and specificity, capable of generating a steep concentration gradient, but which is relatively slow compared with uptake of DHA (Rose, 1988; Wilson, 2005). Cells rapidly take up DHA by facilitated diffusion on the ubiquitous glucose transporters of the GLUT family (Wilson and Dragan, 2005). Although ascorbate itself has little or no affinity for GLUTs, DHA assumes a bicyclic hemiketal form in solution (DiLabio and Wright, 2000; Pastore et al. 2001) that is able to use the GLUT transporters even in the presence of glucose (Vera et al., 1993). Once inside cells, DHA is rapidly reduced to ascorbate by NADH and NADPH-dependent reductases, as well as by reduced glutathione (GSH) (May and Asard, 2004). GSH-dependent reduction of DHA occurs either directly or is facilitated by thiol transferase enzymes (Wells et al., 1990). The relative importance of DHA transport and reduction versus ascorbate transport to the intracellular ascorbate concentration depends on the extracellular concentration of DHA. If this is high enough to compete with glucose, such as in areas of inflammation near inflammatory cells that are releasing reactive oxygen species (Nualart et al., 2003), DHA uptake and reduction can be significant, albeit transient. On the other hand, DHA levels in plasma and probably the interstitium are very low and thus likely to contribute very little to overall uptake (Okamura, 1979; Dhariwal et al., 1991). Perhaps the strongest evidence that ascorbate rather than DHA transport is the primary means by which most cells accumulate ascorbate is that embryonic mice lacking one isoform of the ascorbate transporter (the absence of which is lethal past the embryonic stage) have very low levels of ascorbate in brain and numerous other tissues (Sotiriou et al. 2002; Harrison and May, 2009). Moreover, inflammatory macrophages from adult mice that selectively lack this ascorbate transporter in hematopoietic cells also have undetectable levels of ascorbate when placed in culture (Babaev et al., 2010). Even though ascorbate in the diet or synthesized in the liver can provide sources of ascorbate for cells and tissues, it is clear that the ascorbate transporter is necessary to prevent cellular ‘scurvy.’
Nomenclature
The transporter for ascorbate was cloned in 1999 and given the trivial name of sodium-dependent vitamin C transporter, abbreviated SVCT (Tsukaguchi et al., 1999). Two functional isoforms were identified in the rat (Tsukaguchi et al., 1999) and subsequently in the human (Daruwala et al., 1999; Rajan et al. 1999; Wang et al., 1999; 2000;). The human isoforms have 65% identity at the amino acid level, but their functions differ due to their divergent tissue distributions (Tsukaguchi et al., 1999). Initial gene names given the two isoforms differed from those ultimately assigned in 2003 in the HUGO nomenclature (Takanaga et al., 2004), such that the human SVCT1 now corresponds to the SLC23A1 gene and maps to chromosome 5q31.2–31.3 (Stratakis et al., 2000; Wang et al. 2000), and the human SVCT2 now corresponds to the SLC23A2 and maps to chromosome 20p12.2–12.3 (Hogue and Ling, 1999; Stratakis et al. 2000). There is little homology between the SVCTs and other mammalian membrane transporters (Tsukaguchi et al., 1999), although both human transporters are weakly homologous in sequence to an orphan yolk-sac permease-like protein (YSPL1, SLC23A3) and a related variant (SLC23A4), as well as to a family of nucleobase transporters in lower organisms (Tsukaguchi et al. 1999; Takanaga et al., 2004). However, no isoforms beyond the SVCT1 and SVCT2 with clear function as ascorbate transporters have been identified.
Pharmacology
Quantification
SVCT1 and SVCT2 are low abundance transporters and have not thus far been quantified by binding or coupling with radioligands or other labels. Ascorbate derivatives involving carbons 2 and 3 are poor inhibitors of ascorbate transport and also lose the reducing capacity of the molecule. Although numerous carbon-6-modified ascorbate derivatives have been prepared (Cousins et al., 1977; Raic-Malic et al., 1999; Manfredini et al. 2002; Dalpiaz et al. 2004; 2005;), affinity ligands for the SVCT transporters have not been reported.
Endogenous substrates
The SVCT's are very selective for L-ascorbic acid. As reviewed by Savini and colleagues, (Savini et al., 2008 14324/id), the apparent affinities of the SVCTs for ascorbate vary with species, cell type and assay conditions (e.g. pH). Apparent Km values for ascorbate typically range over concentrations found in plasma, or 25–100 µM. In general, the SVCT2 tends to be of higher affinity but lower capacity than the SVCT1, albeit with considerable overlap (Savini et al., 2008 14324/id). Xenopus oocyte expression studies showed that neither isoform has appreciable affinity for isoascorbate or DHA (Rumsey et al., 1999; Tsukaguchi et al. 1999).
Synthetic substrates and their selectivities
Ascorbate carbon-6 iodo, bromo and fluoro derivatives have been shown to be transported into cells with affinities similar to that of ascorbate (Osmak et al. 1990; Rumsey et al., 1999; Nishikawa et al., 2003; Corpe et al., 2005; Kim et al. 2009), which implicate influx on the SVCTs. Significantly, oocyte expression studies showed that 6-bromo-6-deoxy-L-ascorbate is transported on the SVCT2 (Corpe et al., 2005), whereas its oxidized iodo derivative is transported only on glucose transporters (Rumsey et al. 1999; Corpe et al., 2005). Although other carbon-6-modified ascorbate derivatives inhibit ascorbate transport into cells, they have not been shown to be transported (Manfredini et al., 2002; Dalpiaz et al., 2004; 2005;). A caveat in this regard is the slow uptake of ascorbate-2-phosphate on several different cell types that likely occurs on SVCTs. Although ascorbate 2-phosphate is not a substrate or inhibitor of the SVCTs (Tsukaguchi et al., 1999), in the presence of cells the phosphate group is removed by cell-surface phosphatases and the resulting ascorbate is then taken up on the SVCT (Hitomi et al. 1992; Fujiwara et al., 1997). Ascorbate 2-phosphate has been used to prolong ascorbate effects in culture, as dephosphorylation appears to be rate limiting for uptake (Nowak and Schnellmann, 1996; Fujiwara et al., 1997; Kashino et al. 2003). Fatty acid carbon-6 ascorbate derivatives such as ascorbate 6-palmitate have antioxidant activities (Nagao and Terao, 1990; Ross et al., 1999; Wang et al. 2000; Pinnell, 2002). This likely relates to intercalation into and protection of the plasma membrane due to the lipophilic fatty acid group, although ascorbate 6-palmitate did have weak inhibitory activity of the SVCT1 in oocyte expression studies (Wang et al., 2000).
Transport inhibitors of the SVCTs
For sodium- and energy-dependent transporters such as the SVCTs, transport inhibitors could inhibit the transporter, inhibit the Na+/K+ ATPase that maintains the sodium gradient or affect the driving sodium gradient itself. Indeed, Na+/K+ ATPase inhibition with agents such as ouabain has long been used to provide evidence that specific ascorbate transporters (and not the GLUT-type glucose transporters) are involved (Diliberto et al., 1983; Castro et al., 2001). Removal of the sodium gradient by replacement of extracellular sodium with lithium or choline also effectively blocks ascorbate uptake (Rajan et al. 1999; Godoy et al., 2006).
Several non-covalent inhibitors of high-affinity ascorbate transport have been described in a variety of cell types. Sulfinpyrazone and 4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid at low millimolar concentrations are well established inhibitors of sodium-dependent high-affinity ascorbate transport (Franceschi et al., 1995; Holmes et al. 2000; Daskalopoulos et al. 2002; Best et al., 2005). However, these inhibitors are not selective for the SVCTs and will inhibit anion transporters as well. For the most part, inhibitors of glucose or DHA transport (e.g. glucose derivatives, cytochalasin B) do not affect ascorbate transport. An exception to this is flavonoid compounds such as phloretin and quercetin. Phloretin concentrations of 20–100 µM substantially inhibit both glucose (LeFevre, 1961; Kotyk et al. 1965; Craik and Elliott, 1979) and ascorbate transport (Wang et al. 2000; Caprile et al., 2009). Quercetin has a similar relatively high affinity for the SVCTs as for the glucose transporters, with Ki values of 18 and 23 µM respectively (Song et al., 2002). Other agents with low micromolar Ki values for the SVCT2 include steroid hormones and non-steroidal anti-inflammatory agents (Biondi et al., 2007). Regarding the latter, diclofenamic acid, which has a Ki of 2.7 µM for inhibition of ascorbate transport in human retinal pigmented epithelial cells, when coupled to carbon-6 of ascorbate, had increased affinity for the SVCT2, with a Ki of 0.16 µM (Manfredini et al., 2002). Perhaps the highest affinity ascorbate transport inhibitor known is palytoxin, which inhibited ascorbate transport in adrenal chromaffin cells half maximally at 0.1 nM, a concentration two orders of magnitude less than its inhibition of rubidium uptake or the Na+/K+ ATPase (Morita et al., 1996).
Both cell permeant and impermeant thiol reagents have been shown to inhibit ascorbate transport, suggesting the presence of free cysteines on the protein (May and Qu, 2004). There are 16 cysteines on the rat SVCT2, although only 14 on the human SVCT2 (Figure 1). That the reactive cysteines are actually on the transporter and not on the Na+/K+ ATPase is indicated by the ability of ascorbate to protect ascorbate transport against inhibition by 5,5prime;-dithiobis(2-nitrobenzoic acid) (J.M. May, unpubl. data).
Figure 1.
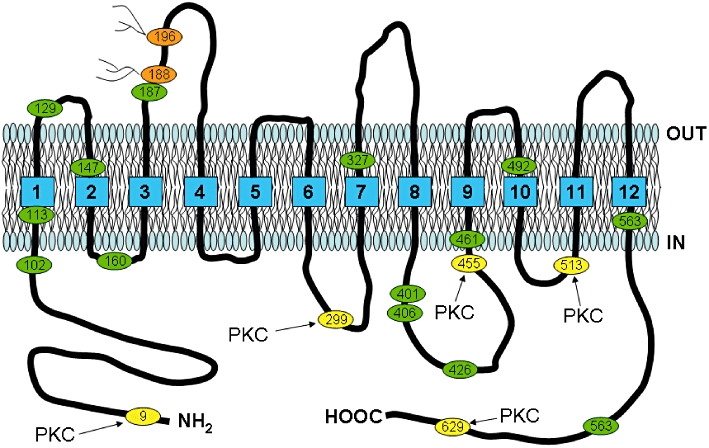
Schematic diagram based on the proposed sequence and hydropathy analysis of the human SVCT2 (Rajan et al., 1999). The human SVCT2 is predicted to have 12 membrane-spanning regions with intracellular amino (NH2-) and carboxy (HOOC-) termini. Locations of potential extracellular glycosylation sites are noted in brown, cysteine residues are noted in green and sites for protein kinase C phosphorylation are noted in yellow.
Transport stoichiometry and kinetic mechanism
The SVCTs couple the inward movement of ascorbate against its concentration gradient to the simultaneous movement of sodium down its electrochemical gradient. Studies in Xenopus oocytes (Tsukaguchi et al., 1999) and SVCT2-transfected human melanoma cells (Godoy et al., 2006) showed that ascorbate transport is driven by the sodium gradient established by the Na+/K+ ATPase. Sodium acts to initiate ascorbate transport by increasing the affinity of the transporter for ascorbate (Godoy et al., 2006). Once ascorbate has bound, it is proposed that a second sodium ion binds and drives the transport cycle (Godoy et al., 2006). This process is also regulated by the required presence of both Ca2+ and Mg2+ ions for efficient function (Godoy et al., 2006). Whereas ascorbate transport for the SVCT1 is electrogenic and associated with an inward sodium current during ascorbate transport, the SVCT2 appears to lack this property (Tsukaguchi et al. 1999; Godoy et al., 2006).
Distribution
A major feature that differentiates between the SVCTs is their distribution. The SVCT1 is primarily expressed in epithelial cells of the intestine and renal proximal tubules (Tsukaguchi et al., 1999), where it absorbs and reabsorbs ascorbate respectively. The sub-cellular distribution of the SVCT1 is primarily apical in columnar epithelial cells derived from the intestine (Caco-2) (Boyer et al., 2005) and lung (Jin et al., 2005), where it likely functions to increase intracellular ascorbate. On the other hand, the SVCT2 is found on the basolateral membrane of Caco-2 cells (Boyer et al., 2005). In the lungs, both transporters are found on the apical surfaces of columnar epithelium (Jin et al., 2005). In either case, dual expression of the transporters with their expected orientation would simply bring ascorbate into the cells. How ascorbate effluxes from intestinal cells after uptake or from hepatocytes after synthesis in most mammals has not been determined, although ascorbate efflux from hepatocytes is appreciable (Upston et al., 1999).
The highest expression of the SVCT2 is in adrenal, brain, lung and bone (Tsukaguchi et al., 1999). The SVCT2 is also functionally expressed in muscle (Savini et al., 2005), lymphoid organs (Tsukaguchi et al., 1999) and reticuloendothelial cells (Babaev et al., 2010) including platelets (Savini et al., 2007). Brain astrocytes lack the SVCT2 in vivo (Berger and Hediger, 2000), but develop it when placed in culture (Siushansian et al., 1997). Maturing erythrocytes lose the SVCT2 during maturation and extrusion of the nucleus, such that they lack it entirely when they enter the circulation (May et al., 2007). This accounts for the fact that they contain the same ascorbate concentration as does the blood plasma in which they circulate (Evans et al., 1982).
Most organs take up ascorbate directly across the endothelium from internal capillaries. Recent studies have shown that although endothelial cells express high levels of the SVCT2, this does not facilitate ascorbate entry into the tissues (May et al., 2009). Rather, ascorbate moves around endothelial cells in a paracellular manner through gaps between the cells and very likely across adherins and tight junctions. An exception to this is the brain, where the blood-brain barrier endothelium is impermeable to ascorbate (Agus et al., 1997) and also lacks the SVCT2 (García et al., 2005; Qiao & May, 2008). Rather, ascorbate enters the brain through the choroid plexus (Spector and Lorenzo, 1973), which contains the SVCT2 (Angelow et al., 2003; García et al. 2005). Through this mechanism, the ascorbate concentration is stepped up from that in plasma to 200–300 µM in the cerebrospinal fluid, and then further into neurons, where the concentration can be as high as 10 mM in rodents (Rice and Russo-Menna, 1998). Although DHA has been shown to rapidly cross the blood-brain barrier on the GLUT1 by facilitated diffusion (Agus et al., 1997), this mechanism is not likely to be of physiologic significance, given the very low circulating DHA concentrations noted previously.
The SVCT1 has been shown to translocate from the cytoplasm to the plasma membrane upon activation of protein kinase C (Liang et al., 2002) and following UVB irradiation in skin keratinocytes (Kang et al., 2007). Treatment of murine MC3T3-E1 cells with prostaglandin E2 caused translocation of the SVCT2 from the cytoplasm to the plasma membrane and activation of transport on the SVCT2 (Wu et al., 2007). The SVCT2 has also been detected in a perinuclear distribution in neurons (Mun et al., 2006), and it may be functional intracellularly. High-affinity ascorbate transport has long been known to occur in chromaffin granules and likely accounts for their high intra-vesicular ascorbate concentrations (Ingebretsen et al., 1980). The SVCT2 has also been localized to punctate structures in the axons of primary culture hippocampal neurons (Qiu et al., 2007), where it could serve to take up ascorbate from the cytoplasm into neurosecretory vesicles. The presence of ascorbate in neurosecretory vesicles is well established, as glutamate activation releases significant amounts of ascorbate into the cerebrospinal fluid (O'Neill et al. 1984; Cammack et al., 1991). This presumably derives from release of these vesicles at synaptic terminals following stimulation (Rebec and Pierce, 1994).
Regulation
Transcriptional and translational regulation of ascorbate transport
Regulation of ascorbate transport at the transcriptional level has been described for both the SVCT isoforms and generally occurs as changes in total cell and plasma membrane transport protein expression. For the SVCT1, expression increases with ascorbate deprivation in human Caco-2 intestinal cells (Maulen et al., 2003) and decreases with aging in rat hepatocytes (Michels et al., 2003). SVCT2 expression is increased during the establishment of primary culture of microcapillary brain endothelial cells (Qiao and May, 2008), following stimulation by various growth factors in human trophoblastic cells (Biondi et al., 2007), and by treatment of cultured osteoblasts with glucocorticoids (Fujita et al., 2001), zinc (Wu et al., 2003b), or calcium and phosphate ions (Wu et al., 2004). In vivo up-regulation of the SVCT2 has been demonstrated in the peri-infarct area of rodent brain following ischaemic injury (Berger et al., 2003), as well as following dietary ascorbate deprivation in the liver of mice unable to synthesize ascorbate (Amano et al., 2010).
Regulation of SVCT2 function by protein–protein interaction has also been proposed. This is based on the generation of an alternatively spliced variant of the protein that acts as a dominant-negative inhibitor of ascorbate transporter function (Lutsenko et al., 2004).
Translational regulation of the SVCT2 has been demonstrated in human platelets, where activation by thrombin and phorbol ester increases both SVCT2 expression and function (Savini et al., 2007).
Acute changes in transporter function
Post-translational regulation of the SVCTs has been proposed, based on the presence of consensus phosphorylation sites for both protein kinase A and protein kinase C on the endofacial transporter (Tsukaguchi et al., 1999). The SVCT2 is known to be stimulated by agents that increase intracellular cyclic AMP (Wilson, 1989; Korcok et al., 2000). Further, the previously noted translocation of the SVCT2 and resulting increases in ascorbate transport in murine MC3T3-E1 cells by prostaglandin E2 were blocked by inhibition of protein kinase A and by mutation of serine residues in either or both of two protein kinase A consensus sequences (Wu et al., 2007). Phorbol ester-dependent activation of protein kinase C halved ascorbate transport mediated by both SVCT1 and SVCT2 in COS-1 cells expressing the proteins (Liang et al., 2001; 2002;). For the SVCT1, this decrease was due to a decreased translocation of the protein to the plasma membrane, while for the SVCT2 it was due to decreased catalytic efficiency. On the other hand, SVCT2 message and protein were increased by phorbol ester-induced differentiation of THP-1 macrophages, an effect also blocked by inhibition of protein kinase C activity during differentiation (Qiao and May, 2009).
Biochemistry and genetics of the SVCTs
The human SVCT1 gene encodes 15 exons, while the much larger (10-fold) SVCT2 gene encodes 17 exons (Erichsen et al., 2006). Despite differences in gene size and existence on different chromosomes, the genomic organization of the two genes is quite similar (Eck et al., 2004), except for the promoter and transcription start site sequences. The genomic structure of the human SVCT1 shows that the region 100 base pairs upstream of the transcription start site to contain both CAAT and TATA boxes, as well as two AP-1 binding sites and a GATA binding site in the expected promoter region. The promoter of the SVCT2 is more complex (Rubin et al., 2005) in that it lacks classical TATA box and uses two different promoters immediately upstream of the first two exons (termed 1a and 1b), with the translation start site in exon 3. Reporter constructs showed increased activity for the exon 1b promoter compared with the 1a promoter. Key features of the two promoter variants are USF, NFY and HIF-1 sites for the exon1a promoter, and multiple SP1 and EGR1 sites for the exon 1b promoter.
The SVCTs code for proteins of different lengths. At least for the SVCT2, these vary with the species. For example, immunoblots of the mouse SVCT2 show bands typically of 65–75 kDa (corresponding to 647 amino acids) (Wu et al., 2003a; Jin et al., 2005; May et al. 2005), whereas most studies show human SVCT2 as a band about 50 kDa (corresponding to 650 amino acids) (Li et al. 2003; Godoy et al., 2006; Savini et al., 2007). Hydropathy analysis suggests that both isoforms cross the plasma membrane 12 times, with both N- and C-terminal regions intracellular (Tsukaguchi et al., 1999) (Figure 1). There are N-glycosylation sites between membrane-spanning regions 3 and 4 of the SVCTs, which may account in part for variation in band locations on immunoblot studies.
Genomic and functional analyses of the SVCT1 initially showed 22 single nucleotide polymorphisms in Caucasians and African-Americans, but no significant differences in transporter function when expressed in Xenopus oocytes (Eck et al., 2007). Subsequent studies revealed that several human polymorphic variants expressed in oocytes did in fact have substantial decreases in ascorbate transport (Corpe et al., 2010). An analysis of the larger SVCT2 gene also revealed numerous polymorphisms, but a functional analysis was not performed (Eck et al., 2004). However, an evaluation of polymorphisms in a large study of pregnancy showed that intron variants of the SVCT2 had 1.7 to 2.4-fold increased risk of preterm delivery, whereas polymorphisms of the SVCT1 had no such association (Erichsen et al., 2006). Variants of neither transporter were associated with changes in the incidence of colorectal adenoma (Erichsen et al., 2008).
Clinical significance
Changes in expression or function of the SVCTs have not yet been associated with human disease, and no drugs have been shown to affect either of the two transporters in the clinical setting. Nonetheless, the importance of the transporters for maintaining cellular ascorbate concentrations and of cellular ascorbate for maintaining the health of both cells and the organism is clear from studies in knockout mice. Targeted deletion of the SVCT1 (Corpe et al., 2010) resulted in 45% perinatal mortality (a fivefold increase) of the offspring of SVCT1–/– dams. This occurred in both the heterozygous and knockout pups and was prevented by ascorbate supplementation of the dam during pregnancy. This highlights the importance of ascorbate provided by the dam during pregnancy, even though mice can make their own ascorbate starting about day 15 of gestation (Kratzing and Kelly, 1982). Loss of the SVCT1 decreased plasma ascorbate concentrations by 50–70%, tripled ascorbate lost in the urine, but only marginally affected intestinal ascorbate absorption. As ascorbate absorption was similar in knockout mice and controls, there must be an alternative mechanism for ascorbate absorption beyond the SVCT1. Loss of up to 70% of body stores of ascorbate was ameliorated by increased hepatic synthesis of the vitamin. These results show the importance of the renal SVCT1 in maintaining ascorbate stores. Further, in a human unable to synthesize ascorbate with a dysfunctional SVCT1 polymorphism, this could lead to a significant drain of ascorbate and clinical consequences, especially during pregnancy (Corpe et al., 2010).
Deficiency of the SVCT2 causes mice to die shortly after birth, with respiratory failure and cortical brain haemorrhage in the absence of classical or biochemical signs of scurvy (Sotiriou et al., 2002). A subsequent study showed that there was also haemorrhage in lower brainstem areas and increased oxidative stress in several organs (Harrison et al., 2010). Ascorbate levels in these mice are very low in tissues served by the SVCT2, including brain, adrenal, pituitary, skeletal muscle and pancreas (Sotiriou et al. 2002; Harrison et al., 2010). Decreased placental ascorbate levels (Harrison et al., 2010) and inability to prevent death by supplementing the dam with ascorbate during pregnancy suggest that the SVCT2 is also crucial for placental ascorbate transport. Mice heterozygous for SVCT2 deficiency appear completely normal and are fertile. Nonetheless, these results show clearly the requirement for the SVCT2 during gestation.
Conclusions
Both of the two ascorbate transporter isoforms play crucial roles in maintaining plasma and tissue ascorbate levels. Key to this function is their selectivity and high affinity for ascorbate as well as their ability to move the vitamin into cells against its concentration gradient. Their tissue distributions complement their ability to retain ascorbate systemically (e.g. renal reabsorption of ascorbate by the SVCT1) and to move it into cells that require it for crucial functions (ascorbate transport into most organs and especially brain, lung and neuroendocrine tissues). These features become especially important in humans and higher primates, who cannot synthesize their own ascorbate. The clear dependence on both transporters for prenatal development in mouse models could explain why significant defects in either transporter have not been discovered in humans.
Glossary
Abbreviations
- AFR
ascorbate free radical
- DHA
dehydroascorbate
- DIDS
4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- SVCT
sodium-dependent vitamin C transporter
Conflict of Interest
The author reports no conflict of interest for the content of this review.
References
- Agus DB, Gambhir SS, Pardridge WM, Speilholz C, Baselga J, Vera JC. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A, Aigaki T, Maruyama N, Ishigami A. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch Biochem Biophys. 2010;496:38–44. doi: 10.1016/j.abb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Angelow S, Haselbach M, Galla HJ. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003;988:105–113. doi: 10.1016/s0006-8993(03)03350-x. [DOI] [PubMed] [Google Scholar]
- Babaev VR, Whitesell RR, Li L, Linton MF, Fazio S, May JM. Selective macrophage ascorbate deficiency suppresses early atherosclerosis. Free Radic Biol Med. 2010;50:27–36. doi: 10.1016/j.freeradbiomed.2010.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. The vitamin C transporter SVCT2 is expressed by astrocytes in culture but not in situ. Neuroreport. 2000;11:1395–1399. doi: 10.1097/00001756-200005150-00009. [DOI] [PubMed] [Google Scholar]
- Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, Hediger MA. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem. 2003;86:896–906. doi: 10.1046/j.1471-4159.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- Best KA, Holmes ME, Samson SE, Mwanjewe J, Wilson JX, Dixon SJ, et al. Ascorbate uptake in pig coronary artery endothelial cells. Mol Cell Biochem. 2005;271:43–49. doi: 10.1007/s11010-005-3442-0. [DOI] [PubMed] [Google Scholar]
- Biondi C, Pavan B, Dalpiaz A, Medici S, Lunghi L, Vesce F. Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: effect of steroids, flavonoids and NSAIDs. Mol Hum Reprod. 2007;13:77–83. doi: 10.1093/molehr/gal092. [DOI] [PubMed] [Google Scholar]
- Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun. 2005;334:150–156. doi: 10.1016/j.bbrc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Cammack J, Ghasemzadeh B, Adams RN. The pharmacological profile of glutamate-evoked ascorbic acid efflux measured by in vivo electrochemistry. Brain Res. 1991;565:17–22. doi: 10.1016/0006-8993(91)91731-f. [DOI] [PubMed] [Google Scholar]
- Caprile T, Salazar K, Astuya A, Cisternas P, Silva-Alvarez C, Montecinos H, et al. The Na+-dependent L-ascorbic acid transporter SVCT2 expressed in brainstem cells, neurons, and neuroblastoma cells is inhibited by flavonoids. J Neurochem. 2009;108:563–577. doi: 10.1111/j.1471-4159.2008.05788.x. [DOI] [PubMed] [Google Scholar]
- Castro M, Caprile T, Astuya A, Millán C, Reinicke K, Vera JC, et al. High-affinity sodium-vitamin C co-transporters (SVCT) expression in embryonic mouse neurons. J Neurochem. 2001;78:815–823. doi: 10.1046/j.1471-4159.2001.00461.x. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, et al. 6-bromo-6-deoxy-L-ascorbic acid – an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins RC, Seib PA, Hoseney RC, Deyoe CW, Liang YT, Lillard DW., Jr Synthesis of 6-fatty acid esters of L-ascorbic acid. J Am Oil Chem Soc. 1977;54:308–312. doi: 10.1007/BF02672431. [DOI] [PubMed] [Google Scholar]
- Craik JD, Elliott KRF. Kinetics of 3-O-methylglucose transport in isolated rat hepatocytes. Biochem J. 1979;182:503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpiaz A, Pavan B, Scaglianti M, Vitali F, Bortolotti F, Biondi C, et al. Transporter-mediated effects of diclofenamic acid and its ascorbyl pro-drug in the in vivo neurotropic activity of ascorbyl nipecotic acid conjugate. J Pharm Sci. 2004;93:78–85. doi: 10.1002/jps.10532. [DOI] [PubMed] [Google Scholar]
- Dalpiaz A, Pavan B, Vertuani S, Vitali F, Scaglianti M, Bortolotti F, et al. Ascorbic and 6-Br-ascorbic acid conjugates as a tool to increase the therapeutic effects of potentially central active drugs. Eur J Pharm Sci. 2005;24:259–269. doi: 10.1016/j.ejps.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999;460:480–484. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- Daskalopoulos R, Korcok J, Tao L, Wilson JX. Accumulation of intracellular ascorbate from dehydroascorbic acid by astrocytes is decreased after oxidative stress and restored by propofol. Glia. 2002;39:124–132. doi: 10.1002/glia.10099. [DOI] [PubMed] [Google Scholar]
- Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991;54:712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- DiLabio GA, Wright JS. Hemiketal formation of dehydroascorbic acid drives ascorbyl radical anion disproportionation. Free Radic Biol Med. 2000;29:480–485. doi: 10.1016/s0891-5849(00)00357-9. [DOI] [PubMed] [Google Scholar]
- Diliberto EJ, Jr, Heckman GD, Daniels AJ. Characterization of ascorbic acid transport by adrenomedullary chromaffin cells. Evidence for Na+-dependent co-transport. J Biol Chem. 1983;258:12886–12894. [PubMed] [Google Scholar]
- Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. 2004;115:285–294. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]
- Eck P, Erichsen HC, Taylor JG, Corpe C, Chanock SJ, Levine M. Genomic and functional analysis of the sodium-dependent vitamin C transporter SLC23A1-SVCT1. Genes Nutr. 2007;2:143–145. doi: 10.1007/s12263-007-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163:245–254. doi: 10.1093/aje/kwj035. [DOI] [PubMed] [Google Scholar]
- Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, et al. Genetic variation in sodium-dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer. 2008;60:652–659. doi: 10.1080/01635580802033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr. 1982;47:473–482. doi: 10.1079/bjn19820059. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Wilson JX, Dixon SJ. Requirement for Na+-dependent ascorbic acid transport in osteoblast function. Am J Physiol Cell Physiol. 1995;268:C1430–C1439. doi: 10.1152/ajpcell.1995.268.6.C1430. [DOI] [PubMed] [Google Scholar]
- Fujita I, Hirano J, Itoh N, Nakanishi T, Tanaka K. Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br J Nutr. 2001;86:145–149. doi: 10.1079/bjn2001406. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Nagao N, Monden K, Misumi M, Kageyama K, Yamamoto K, et al. Enhanced protection against peroxidation-induced mortality of aortic endothelial cells by ascorbic acid-2-O-phosphate abundantly accumulated in the cell as the dephosphorylated form. Free Radic Res. 1997;27:97–104. doi: 10.3109/10715769709097842. [DOI] [PubMed] [Google Scholar]
- García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, et al. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50:32–47. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- Godoy A, Ormazabal V, Moraga-Cid G, Zuniga FA, Sotomayor P, Barra V, et al. Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2. Activation by sodium and absolute dependence on bivalent cations. J Biol Chem. 2006;282:615–624. doi: 10.1074/jbc.M608300200. [DOI] [PubMed] [Google Scholar]
- Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter (SVCT2) Free Radic Biol Med. 2009;45:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Dawes SM, Meredith ME, Babaev VR, Li L, May JM. Low vitamin C and increased oxidative stress cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med. 2010;49:821–829. doi: 10.1016/j.freeradbiomed.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi K, Torii Y, Tsukagoshi N. Increase in the activity of alkaline phosphatase by L-ascorbic acid 2-phosphate in a human osteoblast cell line, HuO-3N1. J Nutr Sci Vitaminol (Tokyo) 1992;38:535–544. doi: 10.3177/jnsv.38.535. [DOI] [PubMed] [Google Scholar]
- Hogue DL, Ling V. A human nucleobase transporter-like cDNA (SLC23A1): member of a transporter family conserved from bacteria to mammals. Genomics. 1999;59:18–23. doi: 10.1006/geno.1999.5847. [DOI] [PubMed] [Google Scholar]
- Holmes ME, Samson SE, Wilson JX, Dixon SJ, Grover AK. Ascorbate transport in pig coronary artery smooth muscle: Na+ removal and oxidative stress increase loss of accumulated cellular ascorbate. J Vasc Res. 2000;37:390–398. doi: 10.1159/000025755. [DOI] [PubMed] [Google Scholar]
- Ingebretsen OC, Terland O, Flatmark T. Subcellular distribution of ascorbate in bovine adrenal medulla. Evidence for accumulation in chromaffin granules against a concentration gradient. Biochim Biophys Acta. 1980;628:182–189. doi: 10.1016/0304-4165(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Jin SN, Mun GH, Lee JH, Oh CS, Kim J, Chung YH, et al. Immunohistochemical study on the distribution of sodium-dependent vitamin C transporters in the respiratory system of adult rat. Microsc Res Tech. 2005;68:360–367. doi: 10.1002/jemt.20255. [DOI] [PubMed] [Google Scholar]
- Kang JS, Kim HN, Jung DJ, Kim JE, Mun GH, Kim YS, et al. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J Invest Dermatol. 2007;127:698–706. doi: 10.1038/sj.jid.5700572. [DOI] [PubMed] [Google Scholar]
- Kashino G, Kodama S, Nakayama Y, Suzuki K, Fukase K, Goto M, et al. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med. 2003;35:438–443. doi: 10.1016/s0891-5849(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Kim J, Yamamoto F, Gondo S, Yanase T, Mukai T, Maeda M. 6-Deoxy-6-[131I]iodo-L-ascorbic acid for the in vivo study of ascorbate: autoradiography, biodistribution in normal and hypolipidemic rats, and in tumor-bearing nude mice. Biol Pharm Bull. 2009;32:1906–1911. doi: 10.1248/bpb.32.1906. [DOI] [PubMed] [Google Scholar]
- Korcok J, Yan R, Siushansian R, Dixon SJ, Wilson JX. Sodium-ascorbate cotransport controls intracellular ascorbate concentration in primary astrocyte cultures expressing the SVCT2 transporter. Brain Res. 2000;881:144–151. doi: 10.1016/s0006-8993(00)02829-8. [DOI] [PubMed] [Google Scholar]
- Kotyk A, Kolinska J, Veres K, Szammer J. Inhibition by phloretin and phlorizin derivatives of sugar transport in different cells. Biochem Z. 1965;342:129–138. [PubMed] [Google Scholar]
- Kratzing CC, Kelly JD. Tissue levels of ascorbic acid during rat gestation. Int J Vitam Nutr Res. 1982;52:326–332. [PubMed] [Google Scholar]
- LeFevre PG. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961;13:39–70. [PubMed] [Google Scholar]
- Li X, Huang J, May JM. Ascorbic acid spares alpha-tocopherol and decreases lipid peroxidation in neuronal cells. Biochem Biophys Res Commun. 2003;305:656–661. doi: 10.1016/s0006-291x(03)00836-2. [DOI] [PubMed] [Google Scholar]
- Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- Liang WJ, Johnson D, Ma LS, Jarvis SM. Regulation of the human vitamin C transporters and expressed in COS-1 cells by protein kinase C. Am J Physiol Cell Physiol. 2002;283:C1696–C1704. doi: 10.1152/ajpcell.00461.2001. [DOI] [PubMed] [Google Scholar]
- Lutsenko EA, Carcamo JM, Golde DW. A human sodium-dependent vitamin C transporter 2 isoform acts as a dominant-negative inhibitor of ascorbic acid transport. Mol Cell Biol. 2004;24:3150–3156. doi: 10.1128/MCB.24.8.3150-3156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini S, Pavan B, Vertuani S, Scaglianti M, Compagnone D, Biondi C, et al. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J Med Chem. 2002;45:559–562. doi: 10.1021/jm015556r. [DOI] [PubMed] [Google Scholar]
- Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Smid-Kotsas A, Bachem M, et al. Upregulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem. 2003;278:9035–9041. doi: 10.1074/jbc.M205119200. [DOI] [PubMed] [Google Scholar]
- May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- May JM, Asard H. Ascorbate recycling. In: Asard H, May JM, Smirnoff N, editors. Vitamin C. Functions and Biochemistry in Animals and Plants. London: Bios Scientific Publishers; 2004. pp. 139–158. [Google Scholar]
- May JM, Qu ZC. Redox regulation of ascorbic acid transport: role of transporter and intracellular sulfhydryls. Biofactors. 2004;20:199–211. [Google Scholar]
- May JM, Huang J, Qu ZC. Macrophage uptake and recycling of ascorbic acid: response to activation by lipopolysaccharide. Free Radic Biol Med. 2005;39:1449–1459. doi: 10.1016/j.freeradbiomed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Qiao H, Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem Biophys Res Commun. 2007;360:295–298. doi: 10.1016/j.bbrc.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–C178. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels AJ, Joisher N, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys. 2003;410:112–120. doi: 10.1016/s0003-9861(02)00678-1. [DOI] [PubMed] [Google Scholar]
- Morita K, Teraoka K, Oka M, Levine M. Inhibitory action of palytoxin on ascorbic acid transport into cultured bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1996;276:996–1001. [PubMed] [Google Scholar]
- Mun GH, Kim MJ, Lee JH, Kim HJ, Chung YH, Chung YB, et al. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J Neurosci Res. 2006;83:919–928. doi: 10.1002/jnr.20751. [DOI] [PubMed] [Google Scholar]
- Nagao A, Terao J. Antioxidant activity of 6-phosphatidyl-L-ascorbic acid. Biochem Biophys Res Commun. 1990;172:385–389. doi: 10.1016/0006-291x(90)90684-f. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Dmochowska B, Madaj J, Xue J, Guo ZW, Satake M, et al. Vitamin C metabolomic mapping in experimental diabetes with 6-deoxy-6-fluoro-ascorbic acid and high resolution 19F-nuclear magnetic resonance spectroscopy. Metabolism. 2003;52:760–770. doi: 10.1016/s0026-0495(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. L-ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol. 1996;271:C2072–C2080. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, et al. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278:10128–10133. doi: 10.1074/jbc.M210686200. [DOI] [PubMed] [Google Scholar]
- Okamura M. Uptake of L-ascorbic acid and L-dehydroascorbic acid by human erythrocytes and HeLa cells. J Nutr Sci Vitaminol (Tokyo) 1979;25:269–279. doi: 10.3177/jnsv.25.269. [DOI] [PubMed] [Google Scholar]
- O'Neill RD, Fillenz M, Sundstrom L, Rawlins JN. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci Lett. 1984;52:227–233. doi: 10.1016/0304-3940(84)90166-6. [DOI] [PubMed] [Google Scholar]
- Osmak M, Eckert-Maksic M, Pavelic K, Maksic ZB, Spaventi R, Beketic L, et al. 6-Deoxy-6-bromo-ascorbic acid inhibits growth of mouse melanoma cells. Res Exp Med. 1990;190:443–449. doi: 10.1007/BF00000050. [DOI] [PubMed] [Google Scholar]
- Pastore P, Rizzetto T, Curcuruto O, Cin MD, Zaramella A, Marton D. Characterization of dehydroascorbic acid solutions by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2051–2057. doi: 10.1002/rcm.476. [DOI] [PubMed] [Google Scholar]
- Pinnell SR. Ascorbyl-6-palmitate is not ascorbic acid. J Invest Dermatol. 2002;119:991. doi: 10.1046/j.1523-1747.2002.19530.x. [DOI] [PubMed] [Google Scholar]
- Qiao H, May JM. Development of ascorbate transport in brain capillary endothelial cells in culture. Brain Res. 2008;1208:79–86. doi: 10.1016/j.brainres.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, May JM. Macrophage differentiation increases expression of the ascorbate transporter (SVCT2) Free Radic Biol Med. 2009;46:1221–1232. doi: 10.1016/j.freeradbiomed.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85:1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- Raic-Malic S, Hergold-Brundic A, Nagl A, Grdisa M, Pavelic K, De Clercq E, et al. Novel pyrimidine and purine derivatives of L-ascorbic acid: synthesis and biological evaluation. J Med Chem. 1999;42:2673–2678. doi: 10.1021/jm991017z. [DOI] [PubMed] [Google Scholar]
- Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, et al. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999;262:762–768. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988;947:335–366. doi: 10.1016/0304-4157(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Ross D, Mendiratta S, Qu ZC, Cobb CE, May JM. Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic Biol Med. 1999;26:81–89. doi: 10.1016/s0891-5849(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Rubin SA, Dey S, Reidling JC. Functional analysis of two regulatory regions of the human Na+ -dependent vitamin C transporter 2, SLC23A2, in human vascular smooth muscle cells. Biochim Biophys Acta. 2005;1732:76–81. doi: 10.1016/j.bbaexp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Welch RW, Garraffo HM, Ge P, Lu S-F, Crossman AT, et al. Specificity of ascorbate analogs for ascorbate transport – Synthesis and detection of [125I]-6-deoxy-6-iodo-L-ascorbic acid and characterization of its ascorbate-specific transport properties. J Biol Chem. 1999;274:23215–23222. doi: 10.1074/jbc.274.33.23215. [DOI] [PubMed] [Google Scholar]
- Savini I, Catani MV, Duranti G, Ceci R, Sabatini S, Avigliano L. Vitamin C homeostasis in skeletal muscle cells. Free Radic Biol Med. 2005;38:898–907. doi: 10.1016/j.freeradbiomed.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Savini I, Catani MV, Arnone R, Rossi A, Frega G, Del Principe D, et al. Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic Biol Med. 2007;42:608–616. doi: 10.1016/j.freeradbiomed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–355. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- Siushansian R, Tao L, Dixon SJ, Wilson JX. Cerebral astrocytes transport ascorbic acid and dehydroascorbic acid through distinct mechanisms regulated by cyclic AMP. J Neurochem. 1997;68:2378–2385. doi: 10.1046/j.1471-4159.1997.68062378.x. [DOI] [PubMed] [Google Scholar]
- Song J, Kwon O, Chen SL, Daruwala R, Eck P, Park JB, et al. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J Biol Chem. 2002;277:15252–15260. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- Sotiriou S, Gispert S, Cheng J, Wang YH, Chen A, Hoogstraten-Miller S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- Spector R, Lorenzo AV. Ascorbic acid homeostasis in the central nervous system. Am J Physiol. 1973;225:757–763. doi: 10.1152/ajplegacy.1973.225.4.757. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Taymans SE, Daruwala R, Song J, Levine M. Mapping of the human genes (SLC23A2 and SLC23A1) coding for vitamin C transporters 1 and 2 (SVCT1 and SVCT2) to 5q23 and 20p12, respectively. J Med Genet. 2000;37:E20. doi: 10.1136/jmg.37.9.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflugers Arch. 2004;447:677–682. doi: 10.1007/s00424-003-1104-1. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X-Z, Wang YX, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Upston JM, Karjalainen A, Bygrave FL, Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem J. 1999;342:49–56. [PMC free article] [PubMed] [Google Scholar]
- Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- Wang HP, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V, et al. Human Na+-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta Bio-Membr. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Wang YX, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000;267:488–494. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- Wells WW, Xu DP, Yang YF, Rocque PA. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990;265:15361–15364. [PubMed] [Google Scholar]
- Wilson JX. Ascorbic acid uptake by a high-affinity sodium-dependent mechanism in cultured rat astrocytes. J Neurochem. 1989;53:1064–1071. doi: 10.1111/j.1471-4159.1989.tb07396.x. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- Wilson JX, Dragan M. Sepsis inhibits recycling and glutamate-stimulated export of ascorbate by astrocytes. Free Radic Biol Med. 2005;39:990–998. doi: 10.1016/j.freeradbiomed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Nakanishi T, Tanaka K. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim Biophys Acta. 2003a;1641:65–70. doi: 10.1016/s0167-4889(03)00065-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Nakanishi T, Tatsu Y, Yumoto N, et al. Zinc-induced sodium-dependent vitamin C transporter 2 expression: potent roles in osteoblast differentiation. Arch Biochem Biophys. 2003b;420:114–120. doi: 10.1016/j.abb.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Wu XM, Itoh N, Taniguchi T, Hirano J, Nakanishi T, Tanaka K. Stimulation of differentiation in sodium-dependent vitamin C transporter 2 overexpressing MC3T3-E1 osteoblasts. Biochem Biophys Res Commun. 2004;317:1159–1164. doi: 10.1016/j.bbrc.2004.03.158. [DOI] [PubMed] [Google Scholar]
- Wu X, Zeng LH, Taniguchi T, Xie QM. Activation of PKA and phosphorylation of sodium-dependent vitamin C transporter 2 by prostaglandin E2 promote osteoblast-like differentiation in MC3T3-E1 cells. Cell Death Differ. 2007;14:1792–1801. doi: 10.1038/sj.cdd.4402190. [DOI] [PubMed] [Google Scholar]


