Abstract
Nuclear medicine bone imaging has been the optimum diagnosis for the detection of bone disorders because the lesion could be detectable before the appearance of symptomatic and radiographic changes. Over the past three decades, 99mTc-MDP and 99mTc-HMDP have been used as bone scintigraphic agents because of their superior biodistribution characteristics, although they are far from optimal from a chemical and pharmaceutical point of view. Recently, a more logical drug design has been proposed as a concept of bifunctional radiopharmaceuticals in which the carrier molecules (bisphosphonates) and radiometal chelating groups are separated within a molecule, specifically, 99mTc-mononuclear complex-conjugated bisphosphonate. Some of the 99mTc-mononuclear complex-conjugated bisphosphonate compounds showed superior biodistribution in preclinical studies. Moreover, the drug design concept could be applied to 68Ga PET bone imaging agents. These studies would provide useful information for the development of radiometal-based imaging and therapeutic agents for bone disorders such as bone metastases.
1. Background
The skeleton is one of the most common organs to be affected by metastatic cancer. Carcinomas of the breast, lung, prostate, kidney, and thyroid have a tendency to easily metastasize to bone [1]. Although there has been significant advancement in imaging technologies, such as CT and MR, nuclear medicine bone imaging has been the optimum diagnosis for the detection of bone disorders, such as bone metastases, because of its high sensitivity. Namely, bone-seeking radiopharmaceuticals usually localize in skeletal lesions before the appearance of symptomatic and radiographic changes and the resulting easy evaluation of the entire skeleton [2]. This paper reviews currently available 99mTc radiopharmaceuticals for bone scintigraphy and advances in drug design of radiometal-based bone-targeted compounds.
2. 99mTc-Bisphosphonate Complexes
Although some radiometals, such as lanthanide and rare earth, localize in bone by themselves, pertechnetate (99mTcO4 −) hardly accumulates in bone by itself. Accordingly, a carrier for bone is necessary in order to take bone images with technetium. The first bone-seeking 99mTc compound, a complex of reduced 99mTc and sodium tripolyphosphate, was reported in 1971 [3], followed by a long-chain linear polyphosphate [4] and pyrophosphate [5]. Pyrophosphate (Figure 1(a)) is composed of only two phosphate moieties and is the simplest polyphosphate. 99mTc-pyrophosphate is now seldom used for skeletal imaging because of its high soft tissue background activity but is still employed to determine myocardial infarction. Unfortunately, pyrophosphate and polyphosphate are susceptible to in vivo degradation by enzymes such as alkaline phosphatases, resulting in the release of free technetium from the complexes. Subsequently, three groups almost simultaneously reported the 99mTc complex of 1-hydroxyethyliden-1,1-diphosphonate (HEDP, Figure 1(b)) as a new bone imaging agent [6–8]. HEDP is one of the bisphosphonate (diphosphonate) compounds, which are known as compounds with high affinity for bone and inhibitors of bone resorption. Bisphosphonate compounds were synthesized with a P–C–P bonding sequence instead of the P–O–P sequence of pyrophosphate. Although these two chemical structures are similar, the P–C–P bond angles are smaller (117 degrees) than the P–O–P bond angles (128.7 degrees) and the P–C interatomic distance (1.79 Å) is longer than that of P–O (1.63 Å) [9]. Bisphosphonate bonding is very stable chemically and affords greater resistance to in vivo phosphatase hydrolysis. As a result, 99mTc-HEDP exhibited more rapid clearance from blood and higher uptake in bone. In clinical study, 99mTc-HEDP showed significantly higher lesion-to-normal bone ratios when compared with either 99mTc-pyrophosphate or 99mTc-polyphosphate [10].
Figure 1.
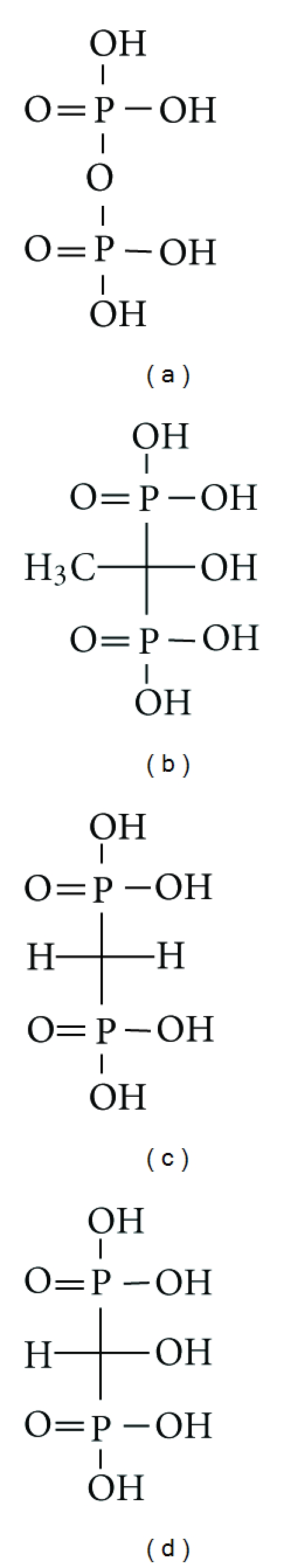
Chemical structures of bisphosphonates analogs (a) pyrophosphate, (b) HEDP, (c) MDP, and (d) HMDP.
After introduction of 99mTc-methylene diphosphonate (MDP, Figure 1(c)) by Subramanian et al. [11] in 1975 and 99mTc-hydroxymethylene diphosphonate (HMDP, Figure 1(d)) by Bevan et al. [12] in 1980, 99mTc-MDP, and 99mTc-HMDP, which showed superior biodistribution compared to 99mTc-HEDP, have been used as radiopharmaceuticals for bone scintigraphy for over thirty years [13–15]. 99mTc-MDP is postulated to form a bidentate-bidentate bridge with hydroxyapatite while the presence of the hydroxyl group in 99mTc-HMDP would convert the ligand to form a bidentate-tridentate bridge and is expected to enhance the hydroxyapatite affinity of the 99mTc complex [12, 16]. However, the lower bone accumulation of 99mTc-HEDP is not well understood because 99mTc-HEDP should also form bidentate-tridentate binding. Increasing steric hindrance associated with the methyl group at the central carbon atom of HEDP, the difference in solubility, and differences in molecular size as well as in the bisphosphonate polymeric complexes themselves have all been suggested [12, 17].
The accumulation of 99mTc-bisphoshonate complexes in bone must be derived from the coordination of bisphosphonate to calcium in the hydroxyapatite of bone, but the mechanism of high uptake to lesion sites in bone has not been completely elucidated. One factor should be the increased vascularity and regional distribution of blood flow that results from disease. However, it has been shown that regional bone blood flow alone does not account for the increased uptake of radiopharmaceuticals [18]. Other factors are involved in their binding and interaction with bone. It is generally assumed that 99mTc-bisphoshonate complexes accumulate at sites of active bone metabolism, that is to say, at areas of new bone formation or calcification [19, 20]. It has also been reported that the accumulation mechanisms might be both adsorption onto the surface of hydroxyapatite in bone and incorporation into the crystalline structure of hydroxyapatite [21]. Newly formed bone has a much larger surface area than does stable bone. That is, the crystalline structure of hydroxyapatite in newly formed bone is amorphous and has a greater surface area than that in normal bone [22]. An in vitro study demonstrated that bisphosphonate compounds have significantly higher adsorption on amorphous calcium phosphate than on crystalline calcium phosphate [17].
Bisphosphonate compounds form multiple complexes with reduced 99mTc. By using high-performance liquid chromatography (HPLC), the relative composition of 99mTc-bisphosphonate complexes in a reaction mixture has been found to vary with pH and with technetium, and with oxygen concentrations [23]. It has been postulated that 99mTc-bisphosphonate complexes would be a mixture of monomers, oxo-bridged dimers, and oligomeric clusters with varying technetium-oxo core configurations, oxidation states, and ligand coordination numbers [24]. These radiolabeled species have different biodistribution properties. It was reported that the smallest, low-charged, mononuclear 99mTc-bisphosphonate complex has the greatest uptake in bone lesions and the highest lesion-to-muscle and lesion-to-normal bone ratios in experiments using each isolated complex by HPLC [25]. Thus, the exact structures and mechanisms of the action of 99mTc-labeled bisphosphonate remain uncertain.
3. New Drug Design Concept of 99mTc-Labeled Bisphosphonate (99mTc Complex-Conjugated Bisphosphonate Compounds)
As mentioned above, despite over three decades of clinical use of 99mTc-bisphosphonate complexes, these radiopharmaceuticals are far from optimal from a chemical and pharmaceutical point of view. For example, their structures and compositions remain unknown because they cannot be obtained as a well-defined single-chemical species, but as mixtures of short-chain and long-chain oligomers. The biological behavior of this type of tracer is also affected by the different degrees of ionization and by variations in the relative amount of oligomers after preparation [23].
In addition, in clinical studies, an interval of 2 to 6 hours is required between an injection of 99mTc-labeled bisphosphonates and obtaining bone images [15]. Shortening this interval would lessen the burden to patients in terms of total examination length and radiation dose absorbed. To enable imaging at an earlier time after injection, a radiopharmaceutical with higher affinity for bone might be advantageous. Although the accumulation of bisphosphonate compounds in bone is achieved by binding the phosphonate groups with the Ca2+ of hydroxyapatite crystals [26], the phosphonate groups in 99mTc-MDP and 99mTc-HMDP serve as both coordinating ligands and Ca2+ binding functional groups [27], which might decrease the inherent accumulation of MDP and HMDP in bone.
Recently, to improve the 99mTc-labeled bisphosphonates currently used, a more logical drug design has been proposed based on the concept of bifunctional radiopharmaceuticals in which the carrier molecules (bisphosphonate) and radiometal chelating groups are separated within the molecule so that they can each function independently and effectively. In particular, 99mTc-mononuclear complex-conjugated bisphosphonate compounds have been reported [28–31]. It was hypothesized that the bone affinity of 99mTc labeled bisphosphonate would be enhanced by conjugating a stable mononuclear 99mTc chelating group with a bisphosphonate moiety so that the conjugation does not impair the inherent chemical and biological properties of the bisphosphonate compounds. 99mTc-L,L-ethylene dicysteine (EC), 99mTc-mercaptoacetylglycylglycylglycine (MAG3), 99mTc-6-hydrazinonicotinic acid (HYNIC), 99mTc-tricarbonyl anchored by pyrazolyl- (pz-) containing ligand, and 99mTc-tricarbonyl dipicolylamine (DPA) were selected as 99mTc chelating molecules, and were conjugated with bisphosphonate compounds, (99mTc-ECAMDP, 99mTc-MAG3-HBP, 99mTc-HYNIC-HBP, [99mTc(CO)3(κ 3-pz-BPOH)]+, and 99mTc(CO)3-DPA-alendronate, resp., Figure 2).
Figure 2.
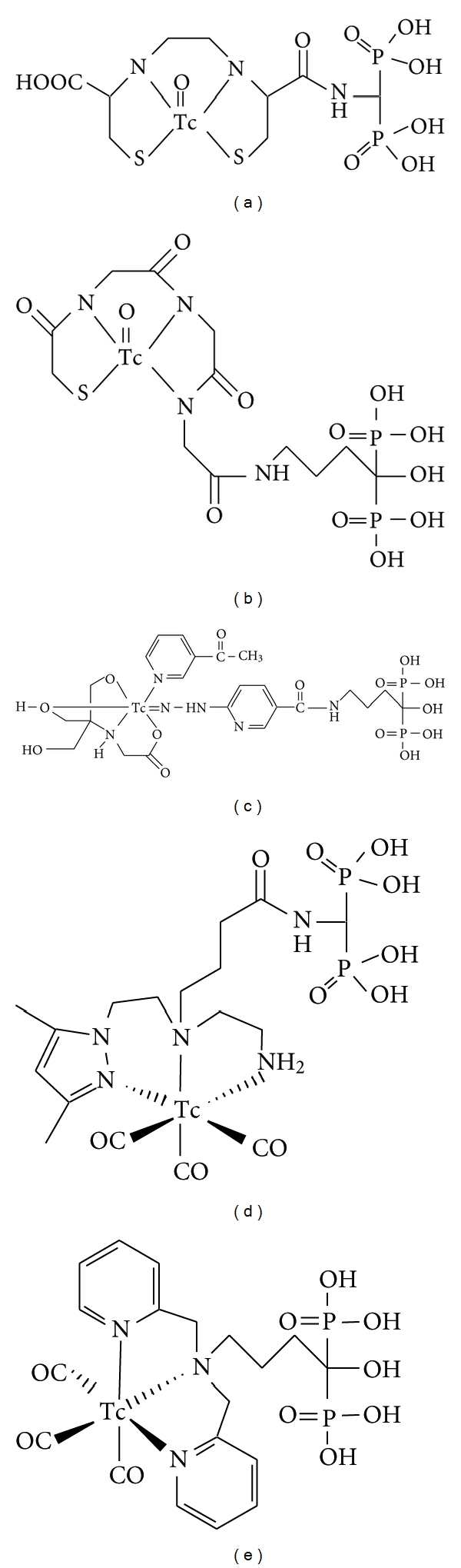
Chemical structures of Tc-complex-conjugated bisphosphonate compounds (a) Tc-ECAMDP, (b) Tc-MAG3-HBP, (c) Tc-HYNIC-HBP, (d) Tc(CO)3(κ 3-pz-BPOH)]+, and (e) Tc(CO)3-DPA-alendronate.
In the drug design of the 99mTc-mononuclear complex-conjugated bisphosphonate compounds, since these ligands contain a bisphosphonate site, there is a possibility that 99mTc coordinates not with the proposed metal coordination moiety, such as EC, MAG3, and HYNIC but with the bisphosphonate moiety. To ascertain whether 99mTc is chelated with only the proposed metal coordination moiety, some experiments were performed. For example, in the case of 99mTc-HYNIC-HBP, 99mTc-HYNIC-HBP was also prepared by the coupling of 99mTc-HYNIC previously complexed with the bisphosphonate site (prelabel method). RP-HPLC analysis revealed the 99mTc-HYNIC-HBP by the prelabel method to be identical to that obtained from the labeling of HYNIC-HBP with 99mTc. These findings exclude the possibility of complexation between technetium and the bisphosphonate structure, and indicate the chelation of 99mTc with the HYNIC moiety in HYNIC-HBP.
In these new compounds, 99mTc-MAG3-HBP, 99mTc-HYNIC-HBP, and 99mTc(CO)3-DPA-alendronate were investigated for in vitro hydroxyapatite binding as an index of bone affinity. 99mTc-MAG3-HBP and 99mTc-HYNIC-HBP showed a significantly higher rate of binding to hydroxyapatite than did 99mTc-HMDP. 99mTc(CO)3-DPA-alendronate showed a higher affinity to hydroxyapatite than did 99mTc-MDP. At the same time, all new 99mTc-mononuclear complex-conjugated bisphosphonate compounds exhibited high bone uptake in in vivo animal experiments. 99mTc-EC-AMDP and 99mTc-HYNIC-HBP showed especially superior results; 99mTc-EC-AMDP and 99mTc-HYNIC-HBP showed significantly higher bone-to-blood ratios of radioactivity than did 99mTc-MDP and 99mTc-HMDP.
4. Radiogallium-Labeled Compounds as Bone Imaging Agents for PET
68Ga is one of the greatest practical and interesting radionuclides for clinical positron emission tomography (PET) because of its radiophysical properties (T 1/2 = 68 min) [32]. 68Ga is a generator-produced nuclide and can be eluted at any time on demand. Specifically, it does not require an on-site cyclotron. In principle, the long half-life of the parent nuclide 68Ge (T 1/2 = 270.8 days) provides a long life-span generator.
Investigations of 68Ga-labeled compounds for bone imaging were previously reported in the 1970s [33, 34]. In these reports, gallium was labeled with tripolyphosphate or ethylenediamine tetramethylene phosphonate (EDTMP) or diethylenetriamine pentamethylene phosphonate (DTPMP). These complexes showed high uptakes in bone. However, since use of the PET camera generally did not spread in the 1970s and the quality of PET cameras was not high, the attention given to 68Ga PET imaging agents was not so high.
For the last decade, 68Ga as a nuclide has been considered a useful radionuclide for PET imaging. Thus, many 68Ga-labeled compounds have been developed. Recently, 68Ga-EDTMP was also reevaluated by Mitterhauser et al. [35]. However, they stated that the advantage of 68Ga-EDTMP over [18F]-fluoride was not apparent and that the future clinical prospect of 68Ga-EDTMP remained speculative.
The above-mentioned drug concept of stable mononuclear complex-conjugated bisphosphonate could be applicable to not only technetium complex radiopharmaceuticals but also to gallium radiopharmaceuticals. To develop a new PET tracer with radiogallium for imaging bone disorders such as bone metastases, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was chosen as a chelating site because it has been well known that Ga forms a stable complex with DOTA. Therefore, Ga-DOTA-conjugated bisphosphonate compounds (67Ga-DOTA-Bn-SCN-HBP and 68Ga-BPAMD, Figure 3) have been developed [36, 37]. Actually, in biodistribution experiments, 67Ga-DOTA-Bn-SCN-HBP rapidly accumulated in bone but was rarely observed in tissues other than bone. In addition, PET/CT imaging of bone metastases with 68Ga-BPAMD showed high uptake in osteoblastic metastases of human (Figure 4). The maximal standardized uptake was 77.1 and 62.1 in the 10th thoracic and L2 vertebra versus 39.1 and 39.2 for 18F-fluoride PET, respectively. These results suggest that the drug design concept of radiogallium complex-conjugated bisphosphonate could be useful for the development of 68Ga PET imaging agents for bone disorders such as bone metastases.
Figure 3.
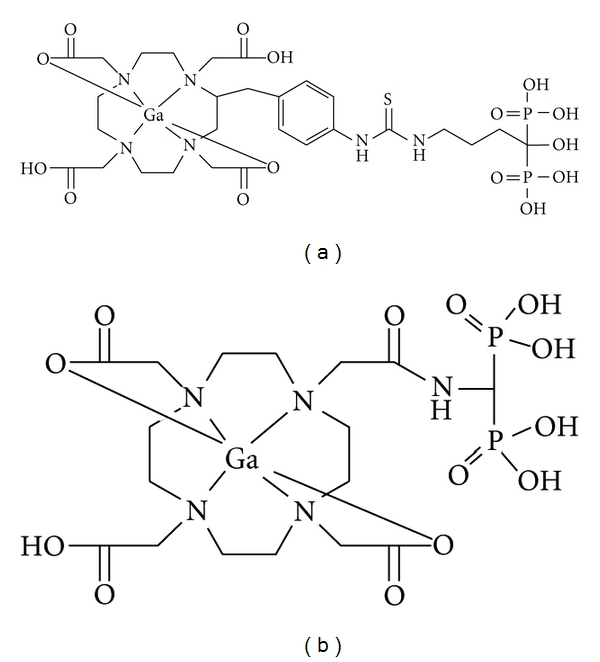
Chemical structures of radiogallium complex-conjugated bisphosphonate compounds (a) Ga-DOTA-Bn-SCN-HBP and (b) Ga-BPAMD.
Figure 4.
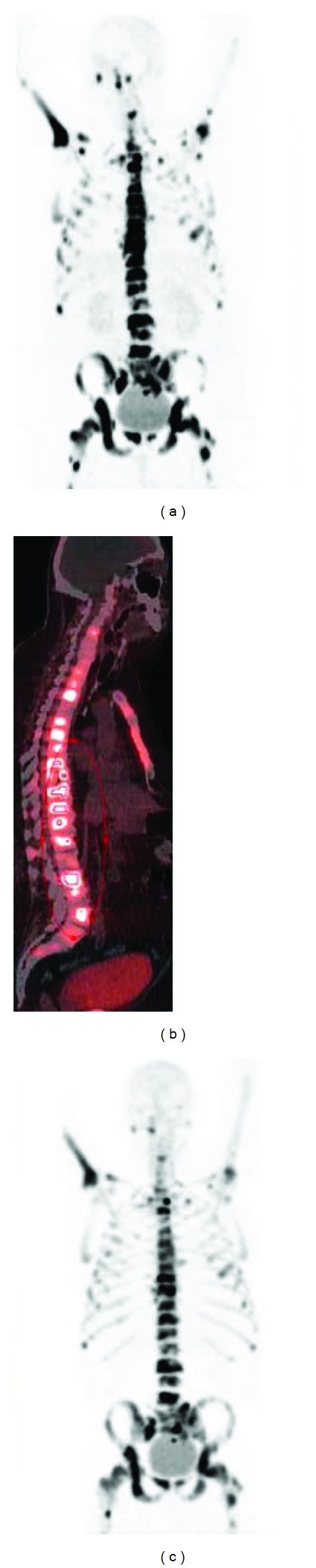
68Ga-BPAMD was injected i.v. into a patient with known extensive bone metastases of prostate cancer. 68Ga-BPAMD (maximum intensity projection (MIP) 50 min after injection (p.i.), 462 MBq) revealed intense accumulation in multiple osteoblastic lesions in the central skeleton, ribs, and proximal extremities: (a) = coronal PET, (b) = sagittal PET/CT. For comparison, (c) shows 18F-fluoride PET (sagittal, MIP 90 min p.i., 270 MBq). With kind permission from Springer Science + Business Media: [36].
5. 18F-Fluoride as Bone Imaging Agent for PET
18F-fluoride was initially reported by Blau et al. in 1962 [38]. After the development of 99mTc-labeled bone scintigraphy agents, such as 99mTc-MDP, 18F-fluoride was replaced by them because the physical characteristics of 99mTc were more convenient for imaging with conventional gamma cameras in those days. However, in the last decade, PET and PET/CT have evolved significantly and become widespread. The situation has similarly changed for 68Ga-labeled compounds. The changes caused the reemergence of 18F-fluoride bone imaging with PET because current PET cameras have higher spatial resolution and greater sensitivity than conventional gamma cameras.
Like 99mTc-MDP as mentioned above, it is known that the distribution of 18F-fluoride in bone also reflects both blood flow in bone and osteoblastic activity. Once 18F-fluoride reaches the surface of the newly formed hydroxyapatite crystals, fluoride anions are isomorphously exchanged with the hydroxyl group in hydroxyapatite (Ca10(PO4)6(OH)2) and fluorapatite (Ca10(PO4)6F2) is formed [39]. A previous paper reported that electron probe X-ray fluorescence studies on the topographical distribution of fluoride at the microscopic level in the iliac bone of an osteoporotic patient being treated with fluoride [40]. The results indicate that the distribution of 18F-fluoride in newly mineralized bone is similar to that of 99mTc-MDP.
There is an important difference between 18F-fluoride and 99mTc-MDP in terms of their protein binding rates. 18F-fluoride barely binds to serum protein [41] whereas 99mTc-MDP shows significant protein bindings. The difference in protein binding causes a difference in blood clearance between 18F-fluoride and 99mTc-MDP. Hence, an interval of 2-3 hours is needed between an injection of 99mTc-MDP and bone imaging. In contrast, bone imaging can be performed less than 1 hour after an injection of 18F-fluoride. Another difference between 18F-fluoride and 99mTc-MDP is in terms of uptake to blood cells. 18F-fluoride is taken up by red blood cells. The erythrocyte concentration of 18F-fluoride is approximately 45–50% of the plasma concentration, namely, approximately 30% of total blood concentration. However, 18F-fluoride is freely diffusible from red blood cells to the bone surface, so the uptake of 18F-fluoride to red blood cells should not interfere with the accumulation of 18F-fluoride in bone [41].
In clinical use in oncology, some papers reported on a comparison between 18F-fluoride PET and planar 99mTc-MDP scintigraphy in the detection of bone metastases [42, 43]. 18F-fluoride PET was more sensitive in detecting bone metastases than planar 99mTc-MDP scintigraphy. However, the cause of increased sensitivity—whether it was derived from 18F-fluoride itself as a tracer or because of the improved performance of the PET camera—could not be determined.
Specifically, 18F-fluoride PET has two important advantages in diagnosis imaging over planar 99mTc-MDP scintigraphy: superior sensitivity and a shorter interval between injection of a tracer and bone imaging. Thus, the use of 18F-fluoride could become common in the future.
6. Conclusion
Over the past three decades, 99mTc-MDP and 99mTc-HMDP have been used for detecting bone metastases, although their mechanisms of accumulation remain uncertain. Recent efforts of chelate-conjugated bisphosphonates and their derivatives have provided chemically well-characterized new 99mTc-labeled bone-seeking tracers. Furthermore, the drug design concept could be applied to 68Ga PET bone imaging agents. These studies would provide useful information for the development of radiometal-based imaging and therapeutic agents for bone disorders such as bone metastases.
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Krasnow AZ, Hellman RS, Timins ME, Collier BD, Anderson T, Isitman AT. Diagnostic bone scanning in oncology. Seminars in Nuclear Medicine. 1997;27(2):107–141. doi: 10.1016/s0001-2998(97)80043-8. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian G, McAfee JG. A new complex of 99mTc for skeletal imaging. Radiology. 1971;99(1):192–196. doi: 10.1148/99.1.192. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian G, McAfee JG, Bell EG, Blair RJ, O’Mara RE, Ralston PH. 99mTc-labeled polyphosphate as a skeletal imaging agent. Radiology. 1972;102(3):701–704. doi: 10.1148/102.3.701. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JW, Solaric George E, Henry RE, Donati RM. Evaluation of 99mTc pyrophosphate as a bone imaging agent. Radiology. 1973;109(2):467–469. doi: 10.1148/109.2.467. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian G, McAfee JG, Blair RJ, Mehter A, Connor T. 99mTc-EHDP: a potential radiopharmaceutical for skeletal imaging. Journal of Nuclear Medicine. 1972;13(12):947–950. [PubMed] [Google Scholar]
- 7.Castronovo FP, Callahan RJ. New bone scanning agent: 99mTc-labeled 1-hydroxy-ethylidene-1, 1-disodium phosphonate. Journal of Nuclear Medicine. 1972;13(11):823–827. [PubMed] [Google Scholar]
- 8.Yano Y, McRae J, Van Dyke DC, Anger HO. Technetium-99m-labeled stannous ethane-1-hydroxy-1 1-diphosphonate: a new bone scanning agent. Journal of Nuclear Medicine. 1973;14(2):73–78. [PubMed] [Google Scholar]
- 9.Larsen M, Willett R, Yount RG. Imidodiphosphate and pyrophosphate: possible biological significance of similar structures. Science. 1969;166(3912):1510–1511. doi: 10.1126/science.166.3912.1510. [DOI] [PubMed] [Google Scholar]
- 10.Citrin DL, Bessent RG, Tuohy JB, et al. A comparison of phosphate bone-scanning agents in normal subjects and patients with malignant disease. The British Journal of Radiology. 1975;48:118–121. doi: 10.1259/0007-1285-48-566-118. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian G, McAfee JG, Blair RJ. Technetium 99m methylene diphosphonate: a superior agent for skeletal imaging: comparison with other technetium complexes. Journal of Nuclear Medicine. 1975;16(8):744–755. [PubMed] [Google Scholar]
- 12.Bevan JA, Tofe AJ, Benedict JJ. Tc-99m HMDP (hydroxymethylene diphosphonate): a radiopharmaceutical for skeletal and acute myocardial infarct imaging. I. Synthesis and distribution in animals. Journal of Nuclear Medicine. 1980;21(10):961–966. [PubMed] [Google Scholar]
- 13.Domstad PA, Coupal JJ, Kim EE, Blake JS, DeLand FH. 99mTc-hydroxymethane diphosphonate: a new bone imaging agent with a low tin content. Radiology. 1980;136:209–211. doi: 10.1148/radiology.136.1.6446106. [DOI] [PubMed] [Google Scholar]
- 14.Mari C, Catafau A, Carrio I. Bone scintigraphy and metabolic disorders. Quarterly Journal of Nuclear Medicine. 1999;43(3):259–267. [PubMed] [Google Scholar]
- 15.Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23(2):341–358. doi: 10.1148/rg.232025103. [DOI] [PubMed] [Google Scholar]
- 16.Jurisson SS, Benedict JJ, Elder RC, Whittle R, Deutsch E. Calcium affinity of coordinated diphosphonate ligands. Single-crystal structure of [(en)2Co(O2P(OH)CH2P(OH)O2)]ClO4 · H2O. Implications for the chemistry of technetium-99m-diphosphonate skeletal imaging agents. Inorganic Chemistry. 1983;22(9):1332–1338. [Google Scholar]
- 17.Francis MD, Ferguson DL, Tofe AJ. Comparative evaluation of three diphosphonates: in vitro adsorption (C-14 labeled) and in vivo osteogenic uptake (Tc-99m complexed) Journal of Nuclear Medicine. 1980;21(12):1185–1189. [PubMed] [Google Scholar]
- 18.Lavender JP, Khan RAA, Hughes SPF. Blood flow and tracer uptake in normal and abnormal canine bone: comparisons with Sr-85 microspheres, Kr-81m, and Tc-99m MDP. Journal of Nuclear Medicine. 1979;20(5):413–418. [PubMed] [Google Scholar]
- 19.Budd RS, Hodgson GS, Hare WSC. The relation of radionuclide uptake by bone to the rate of calcium mineralization. I: experimental studies using 45Ca, 32P and 99Tcm-MDP. British Journal of Radiology. 1989;62(736):314–317. doi: 10.1259/0007-1285-62-736-314. [DOI] [PubMed] [Google Scholar]
- 20.Budd RS, Hodgson GS, Hare WSC. The relation of radionuclide uptake by bone to the rate of calcium mineralization. II: patient studies using 99Tcm-MDP. British Journal of Radiology. 1989;62(736):318–320. doi: 10.1259/0007-1285-62-736-318. [DOI] [PubMed] [Google Scholar]
- 21.Kanishi D. 99mTc-MDP accumulation mechanisms in bone. Oral Surgery Oral Medicine and Oral Pathology. 1993;75(2):239–246. doi: 10.1016/0030-4220(93)90100-i. [DOI] [PubMed] [Google Scholar]
- 22.Galasko CSB. Mechanism of uptake of bone imaging isotopes by skeletal metastases. Clinical Nuclear Medicine. 1980;5(12):565–568. [PubMed] [Google Scholar]
- 23.Tanabe S, Zodda JP, Deutsch E. Effect of pH on the formation of Tc(NaBH4)-MDP radiopharmaceutical analogues. International Journal of Applied Radiation and Isotopes. 1983;34(12):1577–1584. doi: 10.1016/0020-708x(83)90002-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilson GM, Pinkerton TC. Determination of charge and size of technetium diphosphonate complexes by anion-exchange liquid chromatography. Analytical Chemistry. 1985;57(1):246–253. [Google Scholar]
- 25.Pinkerton TC, Cheng KT, Shaw SM, Wilson GM. Influence of complex charge and size on the uptake of 99mTc-diphosphonates in osteogenic tissue. International Journal of Radiation Applications and Instrumentation. 1986;13(1):49–56. doi: 10.1016/0883-2897(86)90251-5. [DOI] [PubMed] [Google Scholar]
- 26.Meyer JL, Nancollas GH. The influence of multidentate organic phosphonates on the crystal growth of hydroxyapatite. Calcified Tissue International. 1973;13(4):295–303. doi: 10.1007/BF02015419. [DOI] [PubMed] [Google Scholar]
- 27.Libson K, Deutsch E, Barnett BL. Structural characterization of a 99Tc-diphosphonate complex. Implications for the chemistry of 99mTc skeletal imaging agents. Journal of the American Chemical Society. 1980;102(7):2476–2478. [Google Scholar]
- 28.Verbeke K, Rozenski J, Cleynhens B, et al. Development of a conjugate of 99mTc-EC with aminomethylenediphosphonate in the search for a bone tracer with fast clearance from soft tissue. Bioconjugate Chemistry. 2002;13(1):16–22. doi: 10.1021/bc0001600. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa K, Mukai T, Inoue Y, Ono M, Saji H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. Journal of Nuclear Medicine. 2006;47(12):2042–2047. [PubMed] [Google Scholar]
- 30.Palma E, Oliveira BL, Correia JDG, et al. A new bisphosphonate-containing 99mTc(I) tricarbonyl complex potentially useful as bone-seeking agent: synthesis and biological evaluation. Journal of Biological Inorganic Chemistry. 2007;12(5):667–679. doi: 10.1007/s00775-007-0215-0. [DOI] [PubMed] [Google Scholar]
- 31.Torres Martin De Rosales R, Finucane C, Mather SJ, Blower PJ. Bifunctional bisphosphonate complexes for the diagnosis and therapy of bone metastases. Chemical Communications. 2009;(32):4847–4849. doi: 10.1039/b908652h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhernosekov KP, Filosofov DV, Baum RP, et al. Processing of generator-produced 68Ga for medical application. Journal of Nuclear Medicine. 2007;48(10):1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 33.Goulet RT, Shysh A, Noujaim AA, Lentle BC. Investigation of 68Ga tripolyphosphate as a potential bone scanning agent. International Journal of Applied Radiation and Isotopes. 1975;26(4):195–199. doi: 10.1016/0020-708x(75)90116-7. [DOI] [PubMed] [Google Scholar]
- 34.Dewanjee MK, Hnatowich DJ, Beh R. New 68Ga labeled skeletal imaging agents for positron scintigraphy. Journal of Nuclear Medicine. 1976;17(11):1003–1007. [PubMed] [Google Scholar]
- 35.Mitterhauser M, Toegel S, Wadsak W, et al. Pre vivo, ex vivo and in vivo evaluations of [68Ga]-EDTMP. Nuclear Medicine and Biology. 2007;34(4):391–397. doi: 10.1016/j.nucmedbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Fellner M, Baum RP, Kubíček V, et al. PET/CT imaging of osteoblastic bone metastases with 68Ga- bisphosphonates: first human study. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(4):p. 834. doi: 10.1007/s00259-009-1355-y. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa K, Takai K, Kanbara H, et al. Preparation and evaluation of a radiogallium complex-conjugated bisphosphonate as a bone scintigraphy agent. Nuclear Medicine and Biology. 2011;38(5):631–636. doi: 10.1016/j.nucmedbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. Journal of Nuclear Medicine. 1962;3:332–334. [PubMed] [Google Scholar]
- 39.Jones AG, Francis MD, Davis MA. Bone scanning: radionuclidic reaction mechanisms. Seminars in Nuclear Medicine. 1976;6(1):3–18. doi: 10.1016/s0001-2998(76)80032-3. [DOI] [PubMed] [Google Scholar]
- 40.Bang S, Baud CA. Topographical distribution of fluoride in iliac bone of a fluoride-treated osteoporotic patient. Journal of Bone and Mineral Research. 1990;5(1):S87–S89. doi: 10.1002/jbmr.5650051313. [DOI] [PubMed] [Google Scholar]
- 41.Blake GM, Park-Holohan SJ, Cook GJR, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Seminars in Nuclear Medicine. 2001;31(1):28–49. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 42.Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. Journal of Nuclear Medicine. 1999;40(10):1623–1629. [PubMed] [Google Scholar]
- 43.Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. Journal of Clinical Oncology. 1999;17(8):2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]


