Abstract
Metabolic syndrome (MetS) is a constellation of risk factors including insulin resistance, central obesity, dyslipidemia and hypertension that markedly increase the risk of Type 2 diabetes (T2DM) and cardiovascular disease (CVD). The peroxisome proliferators-activated receptor (PPAR) isotypes, PPARα, PPARδ/β and PPARγ are ligand-activated nuclear transcription factors, which modulate the expression of an array of genes that play a central role in regulating glucose, lipid and cholesterol metabolism, where imbalance can lead to obesity, T2DM and CVD. They are also drug targets, and currently, PPARα (fibrates) and PPARγ (thiazolodinediones) agonists are in clinical use for treating dyslipidemia and T2DM, respectively. These metabolic characteristics of the PPARs, coupled with their involvement in metabolic diseases, mean extensive efforts are underway worldwide to develop new and efficacious PPAR-based therapies for the treatment of additional maladies associated with the MetS. This article presents an overview of the functional characteristics of three PPAR isotypes, discusses recent advances in our understanding of the diverse biological actions of PPARs, particularly in the vascular system, and summarizes the developmental status of new single, dual, pan (multiple) and partial PPAR agonists for the clinical management of key components of MetS, T2DM and CVD. It also summarizes the clinical outcomes from various clinical trials aimed at evaluating the atheroprotective actions of currently used fibrates and thiazolodinediones.
Keywords: atherosclerosis, diabetes, dyslipidemia, fibrates, inflammation, insulin resistance, metabolic diseases, PPAR agonists, thiazolodinediones
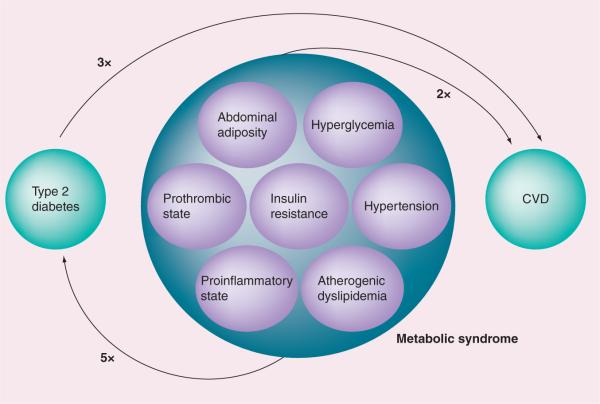
Despite significant advances in the therapeutic management of cardiovascular disease (CVD) [1,2], it still remains the leading cause of morbidity and mortality both in the Western world and developing countries [3,4,301,302]. Obesity [5–8] and Type 2 diabetes mellitus (T2DM) [8–11], are considered significant risk factors for the development of CVD. The recent escalation of obesity [8,12,13] and the surge in the prevalence of diabetes [8,12,13,303], both reaching to epidemic proportions around the globe, have the potential to further fuel the epidemic of CVD and raise the cardiovascular toll in the future. Over-nutrition and decreasing physical activity have contributed significantly to the increasing prevalence of these diseases [14,15]. During the past two decades, considerable attention has been given to the clustering of cardiovascular risk factors and metabolic abnormalities that markedly increase the risk for development of T2DM and CVD [16,17]. The clustering has been referred to by a variety of names including `syndrome X', `insulin resistance syndrome', `the deadly quartet', `syndrome X plus', `metabolic cardiovascular syndrome' and in recent years, it is commonly referred to as `metabolic syndrome' (MetS) [18]. MetS is now considered as one of the most common contributors to the pathogenesis of diabetes and CVD [19]. In fact, MetS is associated with an approximate doubling of cardiovascular risk and a fivefold increased risk for T2DM (Figure 1) [20].
Figure 1. Individual risk components of metabolic syndrome.
CVD: Cardiovascular disease.
The clinical features of MetS include insulin resistance/hyperinsulinemia (with or without hyperglycemia), central obesity, atherogenic dyslipidemia (increased triglycerides [TGs], decreased HDL cholesterol [HDL-C], increased small dense LDL cholesterol [LDL-C]), hypertension, elevation of inflammatory markers and, increased prothrombotic and antifibrinolytic factors [18–26]. MetS is often associated with a variety of other conditions such as nonalcoholic fatty liver disease [27], polycystic ovary syndrome [28], cholesterol gallstone [20], protease-inhibitor therapy for HIV [29] and cancer [30]. Over the years, several definitions for the MetS have been proposed by different health organizations over the past decade (Table 1) [31–37]. While these definitions may differ in their criteria and threshold values, they uniformally emphasize key pathophysiologic processes:
-
■
Visceral obesity
-
■
Dyslipidemia
-
■
Insulin resistance
-
■
Hypertension
Table 1.
Current definitions of the metabolic syndrome.
| Definition | Central obesity | Dyslipidemia | Blood pressure | Renal dysfunction | Fasting plasma glucose | Ref. | |
|---|---|---|---|---|---|---|---|
| WHO (1998) | High insulin levels, IFG or IGT and two or more of the following: | WHR>0.9 (men), >0.85 (women) or BMI >30 kg/m2 | Triglycerides ≥150 mg/dl HDL <35 mg/dl (men) HDL <39 mg/dl (women) | ≥140/90 mmHg | Urinary albumin excretion rate ≥20 μg/min or albumin:creatine ratio ≥30 mg/kg | - | [31] |
| EGIR (1999) | Top 25% of the fasting insulin values among nondiabetic individuals and two or more of thefollowing: | WC: ≥94 cm (men), ≥80 cm (women) | Triglycerides ≥2.0 mmol/l and HDL-C <1.0 mg/dl | ≥140/90 mmHg or antihypertensive medication | - | ≥6.1 mmol/l | [32] |
| NCEP-ATPIII (2001, 2004 and 2005) | Three or more of the following: | WC: ≥102 cm(40″ men), ≥88 cm(35″ women) | Triglycerides ≥150 mg/dl or HDL >40 mg/dl (men), HDL >50 mg/dl (women) | ≥130/85 mmHg | - | ≥110 mg/dl† | [33–35] |
| AACE-ACE (2003) | IGT and two or more of the following: | - | Triglycerides ≥150 mg/dl or HDL <40 mg/dl (men), HDL <50 mg/dl (women) | ≥130/85 mmHg | - | - | [36] |
| IDF (2005) | Central obesity as defined by ethnic/racial, specific WC and two or more of the following: | Triglycerides ≥150 mg/dl or specific treatment for this lipid abnormality HDL <40 mg/dl (men) HDL <50 mg/dl (women) or specific treatment for this lipid abnormality | ≥130/85 mmHg or treatment of previously diagnosed hypertension | - | ≥100 mg/dl | [37] |
In 2003, the American Diabetic Association changed the criteria for IFG from ≥110 to <100 mg/dl.
AACE-ACE: The American Association of Clinial Endocrinologists-American College of Endocrinology; EGIR: The European Group for the Study of Insulin Resistance; IDF: The International Diabetes Federation; IFG: Impaired fasting glucose; IGT: Impaired glucose tolerance; NCEP-ATPIII: The National Cholesterol Education Program Adult Treatment Panel III; WC: Waist circumference; WHR: Waist:hip ratio.
The underlying goal of these proposed definitions is to identify individuals at increased long-term risk of CVD, who could benefit from early prevention. Recently, several major organizations held a meeting in an attempt to unify criteria [21]. Their joint interim statement has identified five risk factors out of which three abnormal findings would qualify a person for the MetS (Table 2) [21]. A single set of cut points is indicated for all components except waist circumference where the recommendation is to use the International Diabetes Federation cut points for non-Europeans and either the International Diabetes Federation or the American Heart Association and the National Heart, Lung and Blood Institute cut points for people of European origin until more relevant data become available. Clinical management of MetS focuses on ways in reducing the individual components of the risk factors and thus, current therapies target these risk factors as well as controlling inflammation and the prothrombotic state.
Table 2.
Criteria for the clinical diagnosis of metabolic syndrome according to a joint interim statement of the AHA, IAS, IASO, IDF, NHLBI and WHF.
| Measure | Categorical cut points |
|---|---|
| Elevated waist circumference | Population- and country-specific definitions |
| Elevated triglycerides (drug treatment for elevated triglycerides is an alternate indicator) | >150 mg/dl (1.7 mmol/l) |
| Reduced HDL-cholesterol (drug treatment for reduced HDL-cholesterol is an alternative indicator) | <40 mg/dl (1.0 mmol/l) in males <50 mg/dl (1.3 mmol/l) in females |
| Elevated blood pressure (antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator) | Systolic >130 and/or diastolic >85 mmHg |
| Elevated fasting glucose treatment (drug treatment of elevated glucose is an alternate indicator) | >100 mg/dl |
AHA: American Heart Association; IAS: International Atherosclerosis Society; IASO: International Association for the Study of Obesity; IDF: International Diabetes Federation Task Force on Epidemiology and Prevention; NHLBI: National Heart, Lung and Blood Institute; WHF: World Heart Federation. Modified from [21].
Metabolic syndrome is highly prevalent in the USA [20,24,26] and other parts of the world [16,20,24,26,38,39]. Approximately 47 million adult Americans (24%) have MetS [18,40]. Moreover, approximately 44% of Americans above the age of 50 years have MetS [18]. Surprisingly, a significant percentage (~9%) of the adolescent population is afflicted with this condition [18,20,41–43]. The epidemiological data from countries including Brazil, China, Ecuador, Finland, France, Greece, India, Iran, Ireland, Korea, Latin America, Mauritius, Mexico, New Zealand, Oman, Singapore and Turkey indicate that overall 16–41% of the adult population has MetS [38,39]. The highest prevalence of MetS was reported for the urban Asian Indian adult males (47%) and Turkish adult females (45%) whereas the French population was found to have the lowest incidence of MetS (male 10%, female 7%) [39]. Owing to the relatively high prevalence of MetS, it accounts for a greater proportion of cardiovascular risk worldwide. Moreover, given that the prevalence of obesity and diabetes is accelerating at an alarming rate and reaching epidemic proportions, these trends can be expected to translate into even greater prevalence of MetS and consequently CVD in the future. Currently, two principal approaches with a goal to effectively control cardiometabolic risk factors associated with MetS are lifestyle management (e.g., weight loss, increased physical activity and consumption of an antiatherogenic or low-calorie diet) and medications. The therapeutic management of cardiovascular risk factors associated with MetS and T2DM is being achieved through the use of combination therapy, but even so, core risk factors, particularly dyslipidemia and insulin resistance, are often poorly controlled. Thus, to cope with the relatively high prevalence of MetS, there is a greater need for the development of new safe and effective combinations of drugs, and more efficacious drugs, as well as multifunctional drugs that can be used as valuable clinical tools in the management of individual components of this syndrome.
The peroxisome proliferator-activated receptors (PPARs) -α, -β/δ and -γ are the ligand-activated transcription factors [44–50] that function as the master regulators of glucose, fatty acid and lipoprotein metabolism, energy balance, cell proliferation and differentiation, inflammation and atherosclerosis [18,44–54]. Considering this, any dysregulation of these metabolic pathways can lead to obesity, diabetes and CVD. Given this, PPARs represent important molecular targets for developing new and more effective PPAR-modulating drugs in the clinical management of T2DM, obesity, MetS and CVD [55–57]. Drugs that activate PPARα and PPARγ have already been marketed. Among these, PPARα agonists, such as fenofibrate, clofibrate and gemfibrozil, act as hypolipidemic agents and are clinically used for the treatment of hyperlipidemia, particularly hypertriglyceridemia associated with MetS, diabetes and diabetes-linked athero sclerotic disease [58,59]. Likewise, thiazolidinediones (TZDs), such as pioglitazone and rosiglitazone, which are specific ligands for PPARγ, function as insulin sensitizers and are currently marketed for the treatment of hyperglycemia in patients with T2DM [60,61]. While currently there are no marketed drugs that target PPARβ/δ, pharmacological activation of PPARβ/δ with a potent PPARβ/δ agonist, GW501516, appears to improve several metabolic parameters in humans [61–63]. Further exploration of the mode of action of GW501516 or its derivatives may lead to development of PPARβ/δ-specific drugs that can be employed either individually or in combination with the existing PPARα-and/or PPARγ-specific drugs in the therapeutic management of MetS, diabetes and associated cardiovascular complications. In addition, extensive studies are underway in various pharmaceutical companies and academic institutions to develop agonists with multiple or partial receptor activity in an effort to improve treatment strategies in the management of diabetes, MetS and associated cardiovascular complications. The underlying theme of this approach is that drugs with specificity for at least two PPAR isoforms (e.g., PPARα/PPARγ, PPARα/PPARβ/δ and PPARγ/PPARβ/δ), or that exhibit cell or tissue specificity would be more efficacious and have relatively less undesirable side effects compared with currently used agonists with specificity towards a single PPAR isoform. Finally, development and availability of specific agonists targeting all three PPAR isoforms (pan agonists) would further expand the treatment option.
This article presents an overview of the molecular and cellular events connected with the expression, function and regulation of PPARs, discuss recent advances in our standing of the diverse biological actions of PPARs in the vascular system, and the current developmental status of new single, dual, pan (multiple) and partial PPAR agonists for the clinical management of key components of MetS such as insulin resistance/hyperglycemia, dyslipidemia, T2DM and CVD. It also summarizes the clinical outcomes from various clinical trials aimed at evaluating the atheroprotective actions of currently used fibrates and TZDs.
PPARs
PPAR structure & activation
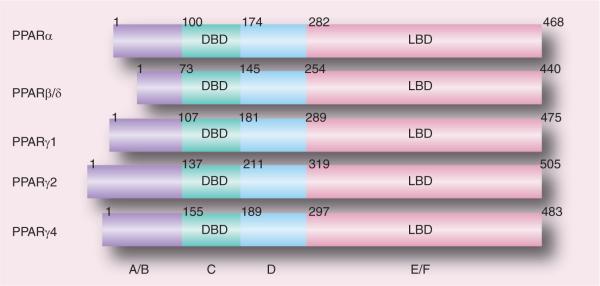
The PPARs are orphan nuclear receptors belonging to the steroid, retinoid and thyroid hormone receptor superfamily of ligand-activated transcription factors [64,65]. Three distinct receptor types, PPARα (NR1C1), PPARβ/δ (NR1C2) and PPARγ (NR1C3) have been cloned and characterized. Similar to other nuclear receptors, all three PPAR isoforms have five or six structural regions within four functional domains, termed A/B, C, D and E/F (Figure 2) [66]. The variable NH2-terminal end, ligand-independent transactivation domain (or A/B domain) contains activation function (AF)-1 which is a target of phosphorylation by kinases. The 70 amino acid-long PPAR DNA-binding domain or C domain contains two highly conserved zinc finger motifs and promoted the binding of receptor to a DNA sequence in the promoter region of target genes known as the peroxisome proliferator response element (PPRE). The hinge region or D domain acts as a docking site for cofactors. The C-terminal, E/F domain or ligand-binding domain (LBD) is responsible for ligand specificity and activation of PPAR binding to the PPRE, which increases the expression of target genes; it recruits cofactors to assist the transactivation process via the ligand-dependent transactivation function (AF-2). Upon activation by endogenous or synthetic ligands, similar to other nuclear hormone receptors, PPARs hetero dimerize with the 9-cis-retinoic acid receptor (retinoid × receptor). The PPAR–retinoid × receptor heterodimer undergoes conformational changes, binds to PPRE in the promoter region of the target genes and alters coactivator/corepressor dynamics to modulate transcription machinery, which in turn affects (upregulation or downregulation) the transcription initiation and mRNA abundance of the target genes [67,68].
Figure 2. Functional domains of human peroxisome proliferator-activated receptors PPARα, PPARδ/β, PPARγ1, PPARγ2 and PPARγ4.
A/B, C, D and E/F indicate N-terminal A/B domain containing a ligand-independent activation function (AF)-1, DBD, hinge region and C-terminal LBD containing AF-2, respectively. AF-1 is responsible for phosphorylation, while AF-2 promotes the recruitment of coactivators for the gene transcription.
DBD: DNA-binding domain; LBD: Ligand-binding domain; PPAR: Peroxisome proliferator-activated receptor.
PPARα
PPARα was the first member of the PPAR iso-types to be cloned and was named based on its ability to be activated by peroxisome proliferator chemicals [69,70]. Its expression is most abundant in tissues characterized by a high rate of fatty acid oxidation such as the liver, heart and skeletal muscle, where it functions as a major regulator of fatty acid homeostasis [18,44,47,70]. Significant expression of PPARα is also detected in kidney, adrenal and adipose tissues (especially brown adipose tissue) and most cell types present in the vasculature including endothelial cells, smooth muscle cells and macrophages (Table 3) [44,47–54,71]. Treatment of mice and rats with peroxisome proliferators including fibrate drugs results in enhanced expression of genes encoding proteins involved in fatty acid transport and β-oxidation along with peroxi some proliferation [69,70]. However, these two rodent types also show enhanced susceptibility to hepatocellular carcinogenesis when chronically treated with fibrate drugs or other peroxisome proliferators [69]. Although hypolipidemic fibrate drugs such as clofibrate (Atromid-S®), fenofibrate (TriCor®) and gemfibrozil (Gemcor®, Lopid®) are widely used to treat hypertriglyceridemia, no significant effect on peroxisome proliferation or hepatocellular proliferation has been reported for patients receiving these drugs [69,72]. The under lying mechanism for this species difference in toxic and carcinogenic effects of peroxisome proliferators is currently known, but may be due to differences in the levels of expression of PPARα, differences in cellular context of PPARα expression, and species differences in PPREs found upstream of critical target genes [69]. Interestingly, it has been reported that human liver expresses only 10% of the levels of PPARα, mRNA and functional DNA-binding activity present in mouse liver, suggesting that PPARα signaling is much more robust in mice relative to humans [69].
Table 3.
Tissue distribution of peroxisome proliferator-activated receptor isoforms.
| PPAR isoform | Tissue distribution |
|---|---|
| PPAR α | Liver, heart, kidney, adrenal, skeletal muscle, adipose tissue, endothelial cells, smooth muscle cells, lymphocytes, macrophages and monocytes |
| PPARγ1 → PPARγ1 | Cardiac muscle, skeletal muscle, kidney, adrenal, spleen, intestine, pancreatic β-cells and vascular smooth muscle cells |
| PPARγ2 → PPARγ2 | Adipose tissue |
| PPARγ3 → PPARγ1 | Adipose tissue, colon and macrophages |
| PPARγ4 → PPARγ4† | Macrophages |
| PPARγ5 → PPARγ1† | Macrophages |
| PPARγ6 → PPARγ4† | Macrophages and adipose tissue |
| PPARγ7 → PPARγ1† | Macrophages and adipose tissue |
| PPARδ/β | Widely expressed in many tissues and cell types |
Italics: mRNA transcripts; Bold: protein products.
mRNA analysis was carried out only on adipose tissue and/or macrophage samples.
PPAR: Peroxisome proliferator-activated receptor.
PPARα & hepatic lipid metabolism
PPARα controls the expression of a wide range of hepatic genes encoding for proteins involved in fatty acid catabolism and lipoprotein metabolism (Table 4). Ligand activation of PPARα is shown to enhance fatty acid catabolism as a result of upregulated transcriptional expression of genes coding proteins involved in lipid transport, fatty acid β- and ω-oxidation and ketogenesis [18,44,47]. PPARα activation also modulates the expression of key genes involved in very low-density lipoprotein (VLDL)-TG turnover as well as apolipoproteins associated with HDL, such as ApoA-I and ApoA-II [48]. Definitive evidence about the pivotal role of PPARα in hepatic fatty acid oxidation and lipo-protein metabolism has been provided by studies involving the use of PPARα ligands [69,70], PPARα-deficient (PPARα−/−) and transgenic mice (PPARαTg) (Table 5), as well as from studies in human subjects harboring natural mutations and polymorphisms within the receptor [70,73]. It is widely believed that PPARα acts as a general sensor of overall fatty acid load. Any increases in the circulating levels of free fatty acids (FFA), or in their metabolites or intermediates, transcriptionally activate PPARα, which, in turn, upregulates the expression of critical catabolic enzymes that are involved in mitochondrial and peroxisomal β-oxidation and microsomal ω-oxidation with resultant enhanced hepatic fatty acid catabolism and secondarily prevent the patho logical accumulation of lipids in liver [18,44,47]. More importantly, such PPARα-mediated increased fatty acid channeling for oxidation also restricts the availability of fatty acids for the VLDL-TG assembly, and consequently, a reduction in the circulating levels of TGs. In addition, activated PPARα attenuates hyper triglyceridemia by directly modulating the expression of certain apolipoproteins and the critical steps involved in VLDL-TG assembly and secretion [48,70,73]. Given this, dysregulation of these various relatively well-coordinated metabolic pathways are now believed to be one of the major contributors to inappropriate lipid accumulation in the liver (steatosis) and skeletal muscle, increased hepatic VLDL production and subsequent development of insulin resistance, MetS and T2DM [74].
Table 4.
Metabolic, anti-inflammatory and vascular actions of activated peroxisome proliferator-activated receptors.
| PPARs | Metabolic actions | Vascular and anti-inflammatory actions |
|---|---|---|
| PPARα | Liver: ↑FFA oxidation, ↑HDL, ↑TG clearance (LPL); ↑cholesterol catabolism, ↑ketogenesis, ↑gluconeogenesis, ↓VLDL-TG, ↓sdLDL, ↓HL | ↓Inflammation Plasma: ↑HDL, ↓dyslipidemia, ↓TG, ↓apoCIII, ↓sdLDL Blood vessels: ↑RCT, ↓inflammatory response VSMCs: ↑HO-1, ↑p16INK4a, ↓proliferation and migration, ↓IL-6, ↓NF-κB, ↓COX-2, ↓p38 MAPK, ↓6-keto-PGF1α, ↓β5 integrin, ↓sPLA2-IIA EC: ↑eNOS, ↑FATP-1, ↑Cu,Zn-SOD, ↓VCAM-1, ↓TF, ↓MCP-1, ↓ET-1, ↓ICAM-1, ↓AP-1, ↓NF-κB, ↓VEGER2, ↓IL-8, ↓leukocyte recruitment Monocyte/macrophages: ↑HO-1, ↑CPT-1, ↑CLA-1, ↑ABCA1, ↑NPC1, ↑NPC2, ↑RCT, ↓iNOS, ↓MMP9, ↓TF, ↓glycated LDL uptake, ↓osteopontin, ↓JNF-α, ↓LPL, ↓IL-2, ↓IFNγ, ↓inflammatory signals, ↓lipid accumulation |
| PPARβ/δ | Muscle: ↑FFA oxidation, ↓TG storage, switching fuel preference from glucose to FFA, alteration of oxidative capacity in skeletal muscle Adipose tissue: ↑FFA oxidation ↓Body weight ↓PPARα and PPARγ target genes |
↓lnflammation Plasma: ↑HDL-C, ↓hyperglycemia, ↓TG, ↓LDL-C, ↓apoB, ↓MCP-1, ↓ICAM-1, ↓VCAM-1, ↓TNF-α, ↓Angll-stimulated collagen synthesis, ↓macrophage inflammatory responses to atherogenic cytokines, ↓monocyte transmigration, ↓chemotractant signaling in the vascular wall, ↓inflammatory genes, ↑survival of endothelial cells, ↓VSMC proliferation, ↑ABAC1 |
| PPARγ | Adipose tissue: ↑adiponectin, ↑adipogenesis, ↑resistin, ↓TNF-α, ↑fatty acid storage Liver: ↑fatty acid storage | ↓Inflammation, ↑RCT VSMCs: ↓proliferation and migration, ↓Angll receptor, ↓apoptosis EC: ↓endothelial dysfunction, ↓endothelin-1, ↓chemokines (IL-8 and MCP-1), ↓adhesion molecules (VCAM-1 and ICAM-1), ↓NF-κB, ↓AP-1 Monocyte/macrophages: ↓inflamatory cytokines (IL-1β, IL-6,iNOS and TNF-α), ↓M1/M2 macrophages, ↓lipid efflux |
↓ Decreased; ↑: Increased; COX: Cyclooxygenase; EC: Endothelial cell; eNOS: Endothelial nitric oxidase synthase; ET: Endothelin; FATP: Fatty acid transport protein; FFA: Free fatty acid; HL: Hepatic lipase; HO: Heme oxygenase; iNOS: Inducible nitric oxide synthase; LPL: Lipoprotein lipase; M1: Classically activated macrophages; M2: Alternatively activated macrophages; MCP: Monocyte chemotactic protein; MMP: Matrix metallopeptidase; PPAR: Peroxisome proliferator-activated receptor; RCT: Reverse cholesterol transport; sdLDL: Small dense LDL; SOD: Superoxide dismutase; TF: Tissue factor; TG: Triglycerides; VSMC: Vascular smooth muscle cell.
Table 5.
Phenotypes of PPARα-null mice and tissue-restricted (heart, liver, adipose tissue or skeletal muscle) PPARα-deficient or -overexpressing transgenic mice.
| Genetically modified mice | Phenotypes | Ref. |
|---|---|---|
| PPARα-null, liver | No hepatic response to peroxisome proliferators or induction of lipid-metabolizing enzymes by fibrates Pronounced hepatic steatosis, elevated levels of plasma triglycerides and increased gonadal fat mass Increased obesity with advancing age No change in body weight in congenic 129/SvJae or C57BL/6N PPAR Elevated circulating levels of fatty acids, increased hepatic and cardiac lipid accumulation, loss of ketogenic response, exaggerated hyperglycemia and hepatic glycogen depletion Show susceptibility (similar to wild-type mice) to HFD-induced weight gain, and hepatic steatosis but are resistant to glucocorticoid-induced hypertension One study reported blunted hyperinsulinemia and improved glucose and glucose tolerance following 2 h fasting whereas another study found no significant differences in hyperinsulinemia and peripheral glucose utilization during euglycemic clamp of HFD-fed wild-type versus knockout mice Fat-pad weight reduction and increased lipogenesis in response to feeding a chow diet supplemented with 2% cholesterol |
[67,70,88] |
| PPARα-null, heart | Blunted expression of constitutive and inducible PPARα target genes Lack of induction of PPARα target genes in response to fasting or diabetes Increased trigylceride accumulation during fasting Increased glucose transporter-4 expression, glucose uptake and reliance on glucose for cardiac ATP production Mild aging-associated fibrosis |
[88,85,83,94] |
| Liver-specific overexpression of human PPARα (LAP1[C/EBPβ] hPPARαTet-off transgenic mice) | A PPARα-humanized mouse line that expresses human receptor in a PPARα-null background under the control of the Tet-Off system of deoxycycline control with liver-specific LAP1(C/EBPβ) promoter High constitutive expression of hPPARα in the absence deoxycycline Increased transcriptional activation of hepatic PPARα target genes in response to PPARα agonist treatment Decreased fasting plasma triglyceride levels No significant hepatocellular proliferation |
[238] |
| Liver-specific overexpression of human PPARα (hPPARαPAC transgenic mice) | This mouse line was generated using a P1 phage artificial chromosome containing the complete human PPARα gene Fibrate drug (fenofibrate)-induced peroxisome proliferation, lowering of plasma triglycerides and induction of PPARα target genes encoding enzymes involved in fatty acid metabolism in liver, kidney and heart No significant hepatomegaly and hepatocyte proliferation |
[239] |
| Heart-specific overexpression of PPARα (MHC-PPARα transgenic mice) | Increased expression of target genes encoding enzymes involved in fatty acid uptake and metabolism Impaired glucose uptake and utilization as a result of markedly diminished expression of multiple genes involved in glucose metabolism Excerbation of metabolic and functional abnormalities in animals rendered diabetic or maintained on a HFD Ventricular hypertrophy and moderate systolic dysfunction Striking steatosis and reactive oxygen species accumulation in the myocardium in response to diabetes or feeding a HFD Detrimental to cardiac recovery after ischemia Diabetic cardiac myopathy |
[88,85,89] |
HFD: High-fat diet; PPAR: Peroxisome proliferator-activated receptor.
The locus encoding PPARα (PPARA) is polymorphic in humans and over a dozen missence polymorphisms resulting in amino acid changes have been identified including L162V and V227A, which are the most common PPARα polymorphisms reported to date and associated with variations in lipid metabolism [75,76]. The L162V polymorphism in the PPARα LBD occurs more commonly in European and North American Caucasian populations as well as in East Indians [75–77], whereas V227A, a non-synonymous variant at the PPAR locus encoding a substitution of valine for alanine at amino acid residue 227 in the hinge region of the receptor occurs with relatively high allelic frequency in oriental populations including Singaporean Chinese [76,78] and Japanese [75,77]. Of particular interest, the PPARα L162V HDL-C, LDL-C apolipoprotein (ApoB), postprandial lipemia and potentially the progression of atherosclerosis [73,78–82], but not with obesity, T2DM or body fat composition [83]. In addition, there is an interaction between the PPARα polymorphism and fat intake [82], and that high-intake of polyunsaturated fatty acid is associated with lowering of TG levels and alterations in total plasma cholesterol, small LDL and ApoA-I, in carriers of the V162-PPARα variant ([76,82] and references therein). By contrast, carriers of the V227A allele appear to have lower levels of total cholesterol and TGs [75,76,78]. Recently, several lipid-association studies on the noncoding regions of the PPARα gene with a major emphasis on the T/C transactivation of intron 2 and 7 have been reported [76]. For example, the carriers of the interon 7G/C transversion show lower levels of TGs, especially in diabetics, but mutation also reduces the age of onset of diabetes and increases the risk for nonfatal myocardial infarction, total cholesterol and LDL-C [84]. A recent study has also reported an association of the T/C transversion at intron 2 with postprandial TGs and cholesterol [80].
PPARα & cardiac metabolism
In recent years, the role played by PPARα in regulating cardiac metabolic homeostasis has also been evaluated particularly by using both gain-of-function and loss-of-function genetic approaches. Similar to in the liver, PPARα is expressed at a relatively high level in the parenchymal cells of the adult heart. Treatment of cultured myocytes with PPARα agonists or adenoviral-mediated PPARα overexpression results in the induction of many genes involved in the fatty acid catabolic pathway, including fatty acid transport, esterification and β-oxidation [85,86]. By contrast, administration of PPARα agonist to rodent models is reported to have very little stimulatory effect on the expression of cardiac PPARα target genes [87], suggesting that at least in rodents, most of the in vivo actions of PPARα ligands are directed towards hepatic PPARα [85]. To further explore the cardiac-specific effects of PPARα, transgenic mice with cardiac-specific overexpression of PPARα under the control of the myosin heavy light chain (MHC) promoter (MHC-PPARα mice; use of MHC promoter leads to cardiac-specific expression of the protein of interest) and PPARα-knockout mouse models have been evaluated [85,87,88].
Constitutive transgenic overexpression of PPARα in cardiac muscle of mice via the MHC promoter results with an increase in the expression of genes encoding for key proteins/enzymes involved in myocyte fatty acid uptake and β-oxidation, and a reciprocal decrease in the expression of multiple genes involved in glucose metabolism, which result in impaired glucose uptake and utilization, and show signs of cardiac steatosis (increased TG accumulation in cardiac muscle), especially in response to fasting or feeding a high-fat diet (Table 5) [86–90]. MHC-PPARα mice show symptoms of ventricular hypertrophy, exhibit impaired recovery of cardiac function when subjected to ischemic-reperfusion injury and also signs of dysregulated mitochondrial biogenesis [85,87,90–92]. The impact of whole-body PPARα gene ablation on cardiac energy metabolism and function has also been evaluated. The PPAR-null mice exhibit impaired cardiac mitochondrial fatty acid oxidation gene and reduced constitutive expression of fatty acid oxidation genes, particularly genes involved in mitochondrial β-oxidation [85,88]. The PPARα-deficient mice also show an increased glucose transporter (GLUT)4 expression, glucose uptake and a greater dependence on glucose for energy production [93]. The PPARα-null hearts exhibit abnormal TG accumulation during fasting, progressive deterioration of myofibrillar and mitochondrial integrity in response to aging and also develop cardiac fibrosis with advancing age [94]. In addition, PPARα-deficient mice show impaired response to several physiologic stressors [94–96].
PPARα: the vasculature, inflammation, hypertension & atherosclerosis
PPARα is expressed in the vasculature [54,71,97–99]. Its expression is detected in endothelial cells, monocytes/macrophages and vascular smooth muscle cells (VSMCs) [54,71,97–99]. In endothelial cells, PPARα activation reduces inflammation by interfering with the recruitment of inflammatory cells. PPARα ligands reduce the expression adhesion molecules, ICAM-1, VCAM-1 and MCP-1 [54,71,98], PPARα and thus, interfere with the binding of leukocytes (inflammatory cells) to endothelial cells. Mechanistically, attenuation of expression of adhesion molecules by PPARα agonists is probably through inhibition of the proinflammatory mediator, the master transcription factor, NF-κB [54,71,100]. In addition, PPARα ligands inhibit synthesis and the section of endothelin (ET)-1 in endothelial cells through repression of AP-1 transcription factor [54,71]; ET-1 is a potent vasoconstrictor peptide and inducer of smooth muscle cell proliferator. PPARα ligands have also been shown to interfere with the endothelial cell signaling including attenuation of expression of VEGFR2, MCP-1, IL-8 and NAD(P)H oxidase, and induction of fatty acid transport protein, antioxidant enzyme Cu, Zn-superoxide dismutase and endothelial nitric oxide (NO) synthase [54,71].
PPARα is expressed in inflammatory cells with relevance to atherosclerosis such as monocytes, lymphocytes, differentiated macrophages and macrophages present in atherosclerotic lesions [54,71,98,100]. Ligand activation of PPARα in macrophages inhibits the expression of inducible NO synthase and the synthesis of tissue factor, matrix metallopeptidase-9 (MMP-9; also termed gelatinase B) and TNF-α secretion [54,70,98]. PPARα activation also has been shown to attenuate the expression of platelet-activating factor receptor in monocyte and macrophage, inhibit the inflammatory signaling including the expression of INF-γ and TNF-α in T lymphocytes and downregulate osteopontin expression in human macrophages via inhibition of AP-1 [54,71,100]. Likewise, PPARα ligands cause the accelerated degradation of the neutrophil chemoattractant LTB4 expression in granulocytes and macrophages [98]. In addition, PPARα also regulates the macrophage cholesterol homeostasis by promoting cholesterol efflux through modulation of the expression of key proteins involved in this process [54,71,100,101] as well as reducing the intracellular lipid accumulation in macrophages [101]. For example, treatment of human macrophages with PPAR agonists upregulates the expression of cholesterol efflux proteins, ABC transporter ABCA1, HDL receptor CLA-1/SR-BI and Niemann–Pick type C1 and C2 (NPC1 and NPC2), which facilitate the mobilization of intracellular cholesterol to the plasma membrane for efflux [54,71,100,102]. Similarly, PPARα ligands downregulate the expression of the apoB48-remnant type of receptor in differentiated macrophages and interfere with the uptake of glycated LDL and TG-rich remnant lipoprotein particles [101]. PPARα has also been shown to upregulate the expression of TG-hydrolyzing enzyme lipoprotein lipase (LPL) and downregulate activity of ACAT-1 activity, an enzyme involved in intracellular cholesterol storage [101].
Similar to other vascular cells, PPARα is expressed in appreciable amounts in human VSMCs and plays an anti-inflammatory role [54,71,98,101,102]. PPARα agonists inhibit the synthesis of proinflammatory agents such as IL-6 and prostaglandin along with cyclo-oxygenase (COX)-2 through suppression of NF-κB signaling in VSMCs [54,78,100]. The expression of hemeoxygenase (HO)-1, a mediator of the anti-inflammatory effects of PPARα inhibitor of VSMC proliferation, is upregulated in VSMCs in response to treatment with PPARα ligands [54,71]. PPARα agonists have also been shown to abolish the IL-1β-induced expression of group IIA secretory phospholipase A2 (sPLA2-IIA), a proinflammatory mediator of atherosclerosis [54,71]. In addition, PPARα has been implicated in the negative regulation of VSMC proliferation and migration [100].
The anti-inflammatory actions of PPARα has also been examined in vivo [54,71,100]. Basal expression of endothelial VCAM-1 is increased in PPARα-null mice and these mice exhibit a considerably longer inflammatory response when challenged with LTB4 or arachidonic acid, compared with the normal controls. Increasing evidence now indicates that PPARα may exert its anti-inflammatory actions through reduction in the production of inflammatory cytokines [99,100] via the inhibition of NF-κB and inducible COX-2 activities [54,71]. Although a majority of in vivo animal studies suggest that PPARα exerts anti-inflammatory actions, there are also indications that these anti-inflammatory effects may be cell- or tissue-type specific [99]. For example, PPARα agonists increase TNF-α levels and decrease survival of lipopolysaccharide-primed mice, despite a significant reduction in the release of TNF-α by macrophages [99].
The evidence presented previously strongly suggests that PPARα plays a crucial role in the development and progression of atherosclerotic lesion formation. Indeed, much of the existing clinical evidence suggests that PPARα ligands may decrease the risk and protect against coronary heart disease (addressed later). However, the use of the genetic mouse model of atherosclerosis has yielded conflicting data [54,71,100]. It is shown that genetic deficiency of PPARα (PPARα−/−) protects against atherosclerosis in apoE-null mice (apoE−/−) [54,71,100]. By contrast, PPARα ligand fenofibrate is shown to retard the development of atherosclerotic lesions, with more pronounced effect noted in apoE−/− mice overexpressing human apoA-I [54,71,100]. In another study, fenofibrate, but not PPARγ agonist, decreased atherosclerotic lesions in a nondiabetic dyslipidemic mouse model in which human apoE2 had been introduced (human apoE2 knock-in mice) [103]. In another study, PPARα agonist treatment of LDL receptor null (LDL-R−/−) mice significantly decreased the extent of atherosclerosis [104]. A more recent study provides evidence that male and female LDL-R−/− transplanted with bone marrow from PPARα−/− mice develop more pronounced atherosclerosis coupled with decreased cholesterol efflux from peritoneal macrophages [105]. Based on these various studies, the potential involvement of PPARα in the development and progression of atherosclerotic lesion formation remains inconclusive; however, much of the existing literature tends to favor an atheroprotective action of PPARα.
PPARβ/δ
PPARβ/δ (referred to as PPARδ from here on) is ubiquitously expressed with relatively high levels as found in the liver, kidneys, cardiac and skeletal muscle, adipose tissue, brain, colon and vasculature [54,71,106,107]. In contrast with PPARα and PPARγ, PPATd does not appear to be a target of any of the currently available drugs. Because of the lack of availability, PPARδ-targeted drugs are coupled with its ubiquitous expression; the physiological function of PPARδ is much less studied and understood. However, in recent years the availability of potent synthetic high-affinity ligands (agonists) such as GW501516, GW610742 and L165041 (Table 6) [108], and various types of PPARδ knockout and transgenic mice have sparked considerable interest in understanding the metabolic actions of PPARδ. The following is a summary of the effects of loss-of-function and gain-of-function of PPARδ and in vivo treatment of experimental animals with synthetic PPARδ ligands on tissue-specific regulation of glucose and lipid metabolism, insulin sensitivity, obesity, inflammation and atherosclerosis. Table 7 lists the phenotypes of PPARδ-null and tissue-specific (heart, adipose tissue or skeletal muscle) PPARδ transgenic mice.
Table 6.
Selected peroxisome proliferator-activated receptor agonists used as medication or in development.
| Agonists | Company/publication | Developmental phase |
|---|---|---|
| PPARα agonists | ||
|
| ||
| Clofibrate | Used as medication | |
| Fenofibrate | Used as medication | |
| Benzafibrate | Used as medication | |
| Gemfibrozil | Used as medication | |
| WY14643 | Wyeth Pharmaceuticals | Investigational agonist |
| LY518674 | Ligand/Eli Lilly | Phase II |
| AVE8134 | Sano-Aventis | Phase II |
| GW590735 | GlaxoSmithKline | Phase II |
| DRF-10945 | Perlecan Pharma | Phase II |
| GW7647 | GlaxoSmithKline | Investigational agonist |
| GW9578 | GlaxoSmithKline | Investigational agonist |
|
| ||
| PPARδ/β agonists | ||
|
| ||
| GW501516 | GlaxoSmithKline | Phase II |
| GW0742 | GlaxoSmithKline | Experimental agonist |
| L-165041 | Merck | Experimental agonist |
|
| ||
| PPARγ agonists | ||
|
| ||
| Rosiglitazone (Avandia) | GlaxoSmithKline | Used as medication |
| Pioglitazone (Actos) | Pharmaceuticals North America | Used as medication |
| Troglitazone (Rezulin) | Daiichi Sankyo Inc./Parke-Davis | Withdrawn (2000) |
| Ciglitazone | Takeda Pharmaceuticals | Experimental agonist |
| Balaglitazone | Rheoscience/Dr Reddy's Laboratories | Phase III |
| Rivoglitazone (CS-011) | Daiichi Sankyo Inc. | Phase II/III |
| Non-TZD PPARγ agonists | Preclinical | |
| RWJ-348260 | Preclinical | |
| Indone derivatives | Preclinical | |
| Compound (14c) S 26948 | Preclinical | |
TZD: Thiazolidinedione.
Table 7.
Phenotypes of PPAR8/p-null mice and tissue-restricted (heart, adipose tissue or skeletal muscle) PPAR8/p-deficient or -8/p-overexpressing mice.
| Genetically modified mice | Phenotypes | Ref. |
|---|---|---|
| PPARδ/β-null mice | Significant embryonic lethality, reduced adiposity, placental defects, growth retardation Pronounced hypertriglyceridemia Decreased metabolic rate and glucose intolerance |
[88,86,91] |
|
| ||
| Conditional cardiac-specific deletion of PPAδ/β gene | Severe impairments in mitochondrial FAO gene expression,reduced rates of FAO, enhanced myocardial lipid accumulation, severe cardiomyopathy and congestive heart failure | [95] |
| Cardiac-specific overexpression of PPARδ (MHC-PPARβ/δ transgenic mice) | Increased expression of genes encoding key FAO enzymes in response to fasting Enhanced expression of genes encoding key FAO enzymes (but decreased expression of corresponding protein), PGC1α, PPARα, and deactivation of protein kinase B and p42/44 MAPK signaling but no improvement in cardiac pathology in response to high-fat feeding Increased cardiac glucose uptake and oxidation rates concomitant with increased GLUT4 and PFK (glycolysis) gene expression. Attenuation of ischemia and reperfusion-induced myocardial injury |
[96,97] |
|
| ||
| Skeletal muscle-specific overexpression of PPARδ/β | Increased endurance activity and oxidative fiber content in skeletal muscle, elevated activities of oxidative enzymes, citrate synthase and β-hydroxacyl-CoA dehydrogenase, increased mRNA expression of UCP2 and h-FABP proteins thought to be involved in fatty acid catabolism and decreased body-fat content | [88] |
| Skeletal muscle-specific overexpression of PPARδ/β (VP16-PPARδ/β) | Reduced body weight, fat mass and skeletal muscle TG content. Enhanced endurance capacity and greatly increased levels of endurance type I oxidative/slow twitch muscle fibers | |
|
| ||
| Adipose tissue-specific PPARδ/β-null mice | No change in fat mass | [73] |
| Adipose tissue-specific overexpression of PPARδ/β (VP16-PPARδ/β) | Attenuation of high-fat diet or genetically induced obesity, steatosis and dyslipidemia Upregulation of genes involved in FAO and adaptive thermogenesis | [88] |
|
| ||
| Conditional myeloid-specific deletion of PPARδ/β gene | In response to high-fat feeding, mice develop insulin resistance and show increased adipocyte lipolysis and severe hepatic steatosis | [93] |
CoA: Coenzyme A; FAO: Fatty acid oxidation; GLUT: Glucose transporter; h-FABP: Heart-type fatty acid-binding protein; PFK:Phosphofructokinase; PGC: PPARγ coactivator; TG: Triglycerlde; UCP: Uncoupling protein.
Regulatory roles of PPARδ in metabolism
Many recent functional studies suggest that PPARδ is critically involved in the regulation of lipid, lipoprotein and glucose metabolism in multiple tissues including adipose tissue, skeletal muscle and the heart (Table 4). These conclusions have been drawn mainly from the results obtained with the use of potent PPARδ agonists, particularly GW501516 and various metabolic and genetic animal models (some of which are described in Table 7). Treatment of insulin-resistant obese rhesus monkeys (a nonhuman primate model of obesity and T2DM) with a potent synthetic PPARδ agonist, GW50516, for 4 weeks greatly improved the plasma lipid profiles, causing a 79% increase in HDL-C levels, while significantly lowering the plasma levels of small dense LDL, TGs and insulin [109]. No changes in plasma glucose levels were detected. A similar type of study reported that treatment of St Kitts vervet monkeys (a nonhuman primate model of atherosclerosis) with GW50516 increased the circulating levels of HDL-C, apoA-I and apoA-II and increased HDL particle size [110]. By contrast, such treatment had no significant effect on the plasma levels of total cholesterol, TGs, VLDL-C, LDL-C or ApoB [110]. Likewise, obese and nonobese mice treated with PPARδ agonist showed elevated levels of HDL-C but at the same time also exhibited elevated levels of total plasma cholesterol [111–114]. In addition, GW501516 treatment attenuated weight gain, insulin resistance and intracellular accumulation of lipid TG in skeletal muscle, liver and adipose tissue [111,113,114]. These changes appear to result from increased expression of skeletal muscle genes that promote lipid catabolism and mitochondrial uncoupling and enhanced channeling of fatty acids for β-oxidation in skeletal muscle [112,115]. PPARδ agonist treatment also resulted in a robust induction of ABCA1 mRNA expression in many cell types raising the possibility that PPARδ may also be involved in the regulation of the HDL-mediated reverse cholesterol transport process. Indeed, van der Veen et al. have provided experimental evidence confirming that PPARδ agonists upregulate HDL-C levels via increased expression of ABCA1; they showed that treatment of control mice with PPARδ agonist, GW610742, resulted in approximately 50% increase in plasma HDL-C, whereas HDL-associated cholesterol levels did not rise and remained low in ABCA1 null mice [116]. In addition, the authors reported that intestinal cholesterol absorption efficiency was reduced following GW610742 activation of PPARδ along with the simultaneous downregulation of the expression of the intestinal cholesterol absorption protein, NPC1L1. In another study, it was demonstrated that treatment of obese and diabetic db/db mice with a week nonselective PPARδ agonist, L-165041, at a relatively low dose raised plasma HDL-C levels without impacting either plasma glucose or TG levels [113].
In addition to pharmacological approaches, genetic approaches involving the use of total and tissue-specific PPARδ-null mice as well as tissue-restricted transgenic mice overexpressing PPARδ are being increasingly employed to further define the role of PPARδ in the regulation of whole-body lipid and glucose metabolism and energy homeostasis. Unfortunately, some of these approaches have not been met with great success. For example, most of the PPARδ-deficient mice (PPARδ−/−) die in utero as a result of placental malformation [113,114]. The mice that do survive are smaller in size, weigh less and demonstrate reduced adiposity, although mice with adipocyte-specific PPARγ deletion do not show any reduction in their fat mass [113,114]. The first-generation PPARδ-null mice (lacking the ligand-binding domain of the murine PPARδ gene) exhibited hyperlidemia with elevated plasma TGs and FFA, but showed no changes in total plasma cholesterol, free cholesterol, or phospholipid levels when maintained on a standard chow diet [113,117]. In response to chronic high-fat feeding, the PPAR-null mice not only maintained high plasma TG levels, but also showed an increased rate of hepatic VLDL production and simultaneous reduction in the activity of lipoprotein lipase [114–117]. The loss of LPL activity in PPARδ−/− mice was closely correlated with the increases in the production of endogenous LPL inhibitors, hepatic angiopoietin-like proteins (Angptl) 3 and 4 [114–117]. In contrast to these results, Barat et al, using second-generation PPARδ−/− mice (deletion of DNA binding domain of PPARδ gene) reported no changes in total plasma cholesterol, TGs, HDL-C and FFA in mice maintained on a chow diet [113,117]. However, the authors neither discussed nor provided any data with regard to changes in plasma lipid metabolites in response to high-fat feeding. The third-generation mice were not evaluated for any changes in plasma lipid metabolites [118]. Besides the significant impact of PPARδ on lipid metabolism, current evidence suggests that it also regulates glucose metabolism and insulin sensitivity. PPARδ-deficient mice have been reported to be glucose intolerant, whereas pharmacological activation of PPARδ in diabetic db/db mice improves insulin sensitivity [119]. Moreover, it is shown that PPARδ agonist, GW501516 improves hyperglycemia by attenuating hepatic glucose production, promoting glucose disposal and preventing fatty acid release from adipose tissue deposits. Gene array analysis suggested that increased glucose metabolism through the pentose phosphate pathway, which is to enhance de novo fatty acid synthesis, may be one potential mechanism by which PPARδ ameliorates hyperglycemia [119]. Two more recent reports [120,121] have led to the demonstration that alternative activation of the anti-inflammatory M2 phenotype of resident macrophages in the liver and adipose tissue is critically dependent on PPARδ activity, resulting in improved fatty acid metabolism and insulin sensitivity [120–122].
The impact of tissue-specific genetic alterations in PPARδ expression on lipid and energy metabolism has also been evaluated. Mice that are gene ablated for cardiac PPARδ (cardiac-specific deletion of PPARδ) display severe impairments in mitochondrial fatty acid gene expression, reduced rates of fatty acid oxidation, increased myocardial lipid accumulation, severe cardiomyopathy and congestive heart failure [123]. Very recently, it has been demonstrated that high-fat feeding in cardiac PPARδ-null mice leads to robust induction of genes encoding key fatty acid oxidation enzymes (but decreased expression of the corresponding protein), PGC-1α and PPARα, but does not improve cardiac pathology [124]. On the other hand, it has been shown that cardiac overexpression of PPARδ results in increased cardiac glucose uptake and oxidation along with increased GLUT4 and PFK (glycolysis) gene expression and also attenuation of ischemia and reperfusion-induced myocardial injury [125]. Likewise, transgenic overexpression of PPARδ in adipose tissue protects against high-fat diet or genetically induced obesity, steatosis and dyslipidemia primarily through upregulation of genes involved in fatty acid oxidation and adaptive thermogenesis [114]. Overexpression of constitutively active PPARδ in skeletal muscle decreases weight gain, fat mass and skeletal muscle TG content, improves glycemic control in mice maintained on a high-fat diet [115,126–128]. Interestingly, mice overexpressing skeletal muscle PPARδ exhibit an enhanced endurance capacity and greatly increased levels of endurance of type I oxidative/slow twitch muscle fibers [115,126,127].
Genetic variants of PPARβ/δ have also been reported in humans [76]. Although, screening of the PPARβ/δ gene has thus far not identified any missence mutations, but several single nucleotide polymorphism have been documented, which variably impact metabolic diseases (e.g., MetS, diabetes and CVD), lipid profiles, BMI, leptin, TNF-α, gender, hepatic fat storage and relative muscle mass [76,129–134]. Moreover, single nucleotide polymorphisms of PPARβ/δ in combination with PGC-1α or PPARγ2P12A variants have been reported to have an additive effect on aerobic physical fitness, endurance performance and insulin sensitivity [132,135,136]. In addition, combined polymorphisms of PPARβ/δ and PPARα are also shown to affect body weight [137], insulin sensitivity in the skeletal muscle [138], fasting plasma glucose levels and BMI in nondiabetic subjects [139,140]. Interestingly, Alzheimer's disease patients with PPARβ/δ variations in exon 4 (rs2016520] and 9 (rs9794) are reported to have elevated levels of cholesterol metabolites compared with control subjects [141].
PPARδ: the vasculature, inflammation, hypertension & atherosclerosis
The roles of PPARδ in inflammation, vascular cells and atherosclerosis are just beginning to emerge (Table 4). The readers are encouraged to consult several excellent recent reviews on these topics [54,71,108,142,143]. In brief, PPARδ expressed in the vasculature [54,71,100,142] with significant expression is detected in endothelial cells, monocytes/macrophages and VSMCs [54,71,108]. A number of studies conducted in recent years have shown that PPARδ ligands (L-165041, GW0742 and GW501516) exert potent anti-inflammatory effects in endothelial cells, inhibiting the inflammatory cytokine-induced expression of adhesion molecules that promote recruitment of leukocytes to endothelial cells both in vitro [54,71,108,144–147] and in vivo [108,145]. The potential mechanisms that have been suggested for the anti-inflammatory effects of PPARδ ligands in endothelial cells include attenuation of oxidative stress through upregulation of antioxidant enzymes as well as modulation of events connected with the BCL-6 corepressor translocation to proinflammatory genes [144]. In addition to its anti-inflammatory actions on endothelial cells, ligand-activated PPARδ have also been shown to directly stimulate proliferation of human endothelial cell proliferation and angiogenesis [54,71,108].
PPARδ is expressed in VSMCs and is induced in these cells in response to PDGF, a neointimal stimulator, through increased activation of PI3K/Akt signaling pathway [54,71]. Expression of PPARδ is also upregulated in vivo during the development of vascular lesion formation [54,71]. These results correlate well with the demonstration that PPARδ overexpression in VSMCs results in enhanced cell proliferation [54,71]. By contrast, selective PPARδ agonists effectively suppress VSMC proliferation both under basal condition [148], and in response to TNF-α [54,71]. Likewise, PPARδ agonist L-16501 interferes with neointima formation in the carotid-artery balloon injury model [146]. In addition, it has been demonstrated that PPARδ ligand inhibits VSMC proliferation via simultaneous induction of TGF-β1 and inhibition of MCP-1 and IL-1β production [118,149]. When considering these variable observations together, it appears that PPARδ receptor and PPARδ agonists may play opposing roles in the proliferation of VSMCs.
Monocytes and macrophages play critical roles in the pathophysiology and the development of atherosclerosis. PPARδ is expressed in myeloid cells and its expression is induced during the differentiation of human macrophages [142]. PPARδ plays a significant role in the regulation of macrophage lipid metabolism. It has been reported that PPARδ acts as a sensor for VLDL, activated by VLDL and may be potentially involved in VLDL-supported TG accumulation in macrophages [142,148]. The PPARδ agonist, GW501516, in a human monocytic cell line increases ABCA1 expression and promotes apoA-I-dependent cholesterol efflux [54,71,148,150]. However, somewhat opposite results are reported using primary macrophages, THP-1 human monocytes and a different PPARδ agonist, termed compound F; evidence is presented showing that compound F upregulates genes involved in lipid uptake and storage such as SAR and CD36 (class A and B scavenger receptors) but repressed key genes involved in lipid meta bolism and efflux (e.g., apoE and cholesterol 27-hydroxylase) [54,71,150]. Treatment with compound F or overexpression of PPARδ induces lipid accumulation in macrophages [54,71,150]. Interestingly, in macrophages neither PPARδ deficiency nor agonists exhibit any significant effect on either cholesterol efflux or its cellular accumulation [104,150]. Two recent studies indicate that adipocyte-derived Th2 cytokines IL-13 and Il-4 induce expression of macrophage PPARδ [120–122]. Both adipose tissue and liver-resident macrophages are activated to an alternative anti-inflammatory M2 phenotype by PPARδ, resulting in improved fatty acid metabolism and insulin sensitivity [120–122]. Besides the effects on lipid metabolism, PPARδ appears to regulate macrophage inflammatory responses, some of which are mediated by its association or dissociation with transcriptional corepressor B-cell lymphoma-(BCL)-6 protein [108]. In an unliganded state, PPARδ associates with BCL-6 (protein–protein interaction) and, as a result, inhibits its anti-inflammatory actions leading to increased levels of anti-inflammatory agents such as MCP-1, -3 and IL-1β. In response to PPARδ agonist, PPARδ deficiency or its lack of activity, BCL-6 is released and in turn inhibits proinflammatory responses [108,111,112]. In addition, in murine macrophages, PPARδ agonists have been shown to inhibit expression of a TNF-α, IL-6, the MCP-1 receptor CC-chemokine receptor-2, osteopontin and VCAM-1 [108,111,112].
In recent years, several studies have been conducted that directly evaluate the anti-atherosclerotic actions of PPARδ and its potent agonists using mouse models of atherosclerosis. It is reported that deletion of PPARδ via the transplantation of PPARδ−/− bone marrow into γ-irradiated LDL-R−/− mice significantly reduced (~50%) the atherosclerotic lesion area in mice chronically fed a high-fat diet, presumably as a result of attenuation of the proinflammatory status of the macrophages [113,128,148,150,151]. These anti atherosclerotic actions of PPARδ were confirmed by Graham et al., who demonstrated that treatment of high-cholesterol/high-fat fed female LDL-R−/− mice with a PPARδ specific agonist, GW0742 (5 or 60 mg/kg/day), attenuates atherosclerosis by at least 30% and also reduces the expression of MCP-1 and ICAM-1 expression in the aorta [152]. In contrast to these observations, Li et al. reported that a low dose of GW0742 (5 mg/kg/day similar to that used by Graham et al. [152]) has no effect on the extent of atherosclerosis, whereas use of PPARα- and PPARγ-specific agonists effectively reduce atherosclerotic lesion formation in LDL-R−/− mice maintained on a high-cholesterol/high-fat diet [104]. It should be noted, however, that in both studies, GW0742 treatment inhibited the expression of inflammatory genes associated with atherosclerosis, such as IFN-γ, TNF-α, MCP-1, VCAM-1 and ICAM-1 in aortic lesions, suggesting that it possesses anti-inflammatory properties in vivo [104,128,150–152]. In another mouse model of atherosclerosis, apoE−/−, treatment with a potent PPARδ agonist, GW501516, attenuates atherosclerotic lesion formation [148]; this anti atherosclerotic action of GW501516 appears to be associated with the modulation of several pathways including elevation of HDL levels, inhibition of chemoattractant signaling in the vessel wall, potent anti-inflammatory effects on the macrophage responses to inflammatory atherogenic cytokines and suppression of monocyte transmigration. It is also shown that PPARδ agonist, GW0742, attenuates AngII-induced atherosclerotic lesion formation in an AngII-accelerated atherosclerotic model [112]. Furthermore, in vivo use of GW0742 induces the expression of BCL-6, RGS4 and RGS5 in the vessel wall, which negatively impacts the expression of key genes involved in inflammation and atherosclerosis [112], reduces AngII-stimulated collagen type I expression and collagen synthesis in cardiac fibroblasts [152], promotes survival of endothelial cells [151], upregulates the ABCA1 expression [153,154], causes reduction in the levels of inflammatory cytokines (e.g., TNF-α) [151,152] and inhibits AngII-mediated proatherogenic MAP kinases (see later) and ERK1/2 [112,154]. Overall, these various studies strongly suggest the possibility that activated PPARδ attenuates atherosclerosis through modulation of multiple but relevant inflammatory pathways.
PPARγ
PARγ is highly expressed in adipose tissue, where it plays an indispensible role in the regulation of adipocyte differentiation, lipid storage, glucose metabolism and the transcriptional regulation of a number of genes involved in these metabolic processes [46,47,49,52]. Some of the key target genes of PPARγ include the fat-specific ap2 gene, LPL, fatty acid transport, fatty acid-binding protein, FAT/CD36, acyl-CoA synthase, GLUT4, glucokinase, phosphoenolpyruvate carboxykinase, uncoupling proteins 1, 2 and 3, and LXRα [46,49,155]. PPARγ also regulates genes involved in insulin signaling, as well as the expression of the proinflammatory cytokines such as TNF-α [52,155]. It also exerts significant anti-inflammatory effects [46,49,155]. Most importantly, PPARγ is a well-recognized cellular target for the anti-diabetic drugs thiazolidinediones, which sensitize cells to insulin and improve insulin sensitivity and action [60,61,156]. PPARγ protein is expressed in four isomeric forms: PPARγ1, -γ2, and -γ4 (see later). The highest expression of PPARγ1 occurs in brown and white adipose tissue (WAT) from which it was first cloned [46,49]. Its expression is also detected in skeletal muscle, liver, pancreatic β-cells, heart, colon, placenta and in cells of vascular and immune systems [155,157]. Under normal physiological conditions, the expression of PPARγ2 isoform is restricted to brown and WAT only [157], but its expression is ectopically induced in the liver and skeletal muscle in response to excess calorie intake or genetic obesity [157–160]. The least studied, PPARγ4 is expressed in macrophage and adipose tissue [156,161].
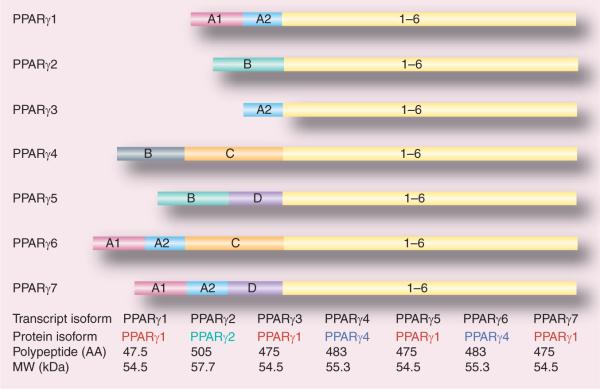
Until now seven mRNA transcripts generated through different initiation and alternative splicing of five exons at the 5′-terminal region (A1, A2, B, C and D) have been identified (Figure 3) [156,162,163]. These are designated as PPARγ1, -γ2, -γ3, -γ4, -γ5, -γ6 and -γ7. PPARγ1, -γ3, -γ5 and -γ7 mRNA transcripts translate into the identical PPARγ1 protein. PPARγ2 mRNA yields PPARγ2 protein, while PPARγ4 and -γ6 mRNA transcripts produce identical PPARγ4 protein (Figure 2) [161–163]. PPARγ1 mRNA isoform is expressed in a broad range of tissues including cardiac and skeletal muscle, pancreatic β-cells, spleen, intestine and vascular cells such as endothelial cells, smooth muscle cells and macrophages [163]. Expression of PPARγ2 mRNA is mostly restricted to adipose tissue [156,163, 164], whereas PPARγ3 mRNA is abundant in macrophages, the large intestine (colon), and adipocytes [156,163, 164]. High levels of PPARγ4, -γ5, -γ6 and -γ7 mRNA transcripts are expressed in macrophages [156,161,162,164], while PPARγ6 and -γ7 mRNAs are also detected in adipose tissue [156,161].
Figure 3. cDNA structures of seven PPARγ isoforms and major features of their protein products.
AA: Amino acids; MW: Molecular weight.
Regulatory roles of PPARγ in metabolism
A mechanistic understanding about the transcriptional role of PPARγ in the regulation of adipogenesis, lipid metabolism and glucose homeostasis and other metabolic processes has greatly improved with the identity of PPARγ as the molecular target for the antidiabetic and insulin-sensitizing agents, the TZDs and the availability of various genetically altered PPARγ mouse models. TZDs have been widely used as a pharmacological tool for defining the metabolic actions of PPARγ whereas the use of total or tissue-specific PPARγ-null or transgenic have provided vital information about the tissue-specific role of PPARγ in the regulation of lipid and glucose metabolism, insulin sensitivity and inflammatory responses. TZDs such as rosiglitazone and pioglitazone are also currently in use in clinical practice for the treatment of T2DM and other associated metabolic complications [60,61].
Earlier in vitro studies showed that TZDs specifically bind to the LBD of recombinant PPARγ, but not PPARα or -β/δ. TZDs also selectively stimulate PPARγ gene promoter activity and modulate the expression of a number of PPARγ target genes [49]. Studies in both humans and animals indicate that TZDs, rosiglitazone and pioglitazone (PPARγ agonists) ameliorate hyperglycemia by reversing insulin resistance and improving insulin sensitivity [49,165–170]. Likewise, PPARγ agonists attenuate hyperlipidemia-induced elevation by circulating FFAs, lipotoxic accumulation of lipid in peripheral tissues and insulin resistance. They do so by promoting increased channeling of FFAs for storage in adipose tissue, reducing the lipid content especially of the liver and regulating the expression of adipokines and inflammatory cytokines that impact hepatic and muscle glucose metabolism and whole-body insulin sensitivity [49,163–168].
Besides improving hyperglycemia and insulin action, both pioglitazone and rosiglitazone also show significant effects on plasma lipo-protein lipids in humans, although the use of pioglitazone results in a relatively better lipid profile as compared with rosiglitazone [168–170], for example, pioglitazone appears to be more effective than rosiglitazone in lowering plasma TGs and LDL-C levels; however, both drugs increase HDL-C to similar levels. Although the exact reason for this differential effect is not known at present, it may be related to the fact that pioglitazone is known to possess weak PPARα-activating activity [168]. Finally, in humans, use of TZDs also leads to increased adiposity along with the redistribution of WAT [162,171]. Specifically, treatment with TZDs causes a beneficial redistribution of fat from visceral deposits (which are more strongly linked to insulin resistance) to WAT with gradual buildup of increased WAT mass [171]. It is suggested that visceral fat drainage of FFA, adipokines and cortisol directly into the liver via the portal vein facilitate WAT redistribution [172]. However, one cannot rule out other possibilities given that various WAT deposits may be derived from distinct precursors with variable metabolic characteristics [161,171–173].
Thus as eluded above, although extensive, pharmacological studies conducted with the use of PPARγ agonists, such as TZDs, during the past decade or so have yielded a wealth of information about the role of PPARγ in the regulation of gene expression and basic metabolic pathways, however, the effects of agonists are often indirect or systemic. Thus, understanding the molecular and cellular actions of PPARγ on specific target tissues independently of the systemic effects of agonists have created the need for and led to the development of many types of genetically altered mice with tissue-specific PPARγ loss-of-function and gain-of-function phenotypes [88,174]. The impact of constitutive whole-body PPARγ deficiency cannot be studied in adult mice because such deficiency causes embryonic lethality due to placental and cardiac defects [88,174]. However, very recently, Duan et al. reported the generation of a generalized PPARγ-knockout (both PPARγ1 and -γ2) mouse line, by rescuing embryonic lethality through the preservation of PPARγ expression in the trophoblasts (Table 8) [175]. Thus, PPARγ deletion caused severe lipodystrophy and impaired insulin action, but surprisingly, also caused hypotension [175]. To examine the physiological functions of PPARγ in individual tissues, many types of conditional knockout mice with cell-specific deletion of PPARγ, such as adipocytes, hepatocytes, myocytes, pancreatic β-cells, macrophages and endothelial cells, have been generated and characterized [88,174,176]. In addition, several tissue-specific PPARγ overexpressing transgenic have been introduced which further aided in understanding the metabolic functions of PPARγ [88,174]. The phenotypic characteristics of some of these PPARγ genetic mouse models are summarized in Table 8.
Table 8.
Phenotypes of PPARγ-haploinsufficient mice and tissue-restricted (heart, adipose tissue, skeletal muscle or liver) PPARγ-deficient or knock-in of dominant-negative mutations from human patients.
| Genetically modified mice | Phenotypes | Ref. |
|---|---|---|
| Heterozygous PPARγ-knockout mice | Reduced adiposity Exhibit an enhanced insulin sensitivity compared with wild-type in response to feeding of a high-fat diet or aging Treatment with PPARγ agonists reverse the effects and cause lipodystrophy and insulin resistance |
[69,88] |
|
| ||
| Generalized PPARγ-deficient mice | Generalized PPARγ-deficient mice have been created by rescuing embryonic lethality of global PPARγ knockout by breeding Mox2-Cre mice with floxed PPARγ mice to inactivate PPARγ in the embryo but not in trophoblasts The mice display severe lipodystrophy and insulin resistance along with hypotension | [174,175] |
|
| ||
| PPARγP465L/+ knock-in mice and and PPARγP465L/+ knock-in ob/ob mice | Homozygous mice for P465L mutation die in utero Heterozygous knock-in mice carrying one copy of PPARγ containing the dominant-negative P465L mutation (equivalent to human P467L, PPARγP465L/+) exhibit abnormal fat distribution (decreased brown adipocyte requirement and thermogenic capacity) and elevated blood pressure PPARγP465L/+ mice are more glucose tolerant than control (wild-type) mice When expressed on ob/ob background, the P465L PPARγ mutant grossly exacerbates insulin resistance and metabolic disturbances associated with the leptin deficiency, yet reduce whole body adiposity and adipose size |
[180,183] |
|
| ||
| PPARγ2Pro12Ala (P/P→A/A) knock-in mice | Reduced body weight, enhanced insulin sensitivity, favorable plasma lipid profile and increased longevity Beneficial metabolic responses are lost in response to feeding a high-fat diet | [179] |
|
| ||
| Tamoxifen-inducible adipocyte PPARγ-knockout mice (aP2-Cre-ERT2 transgenic mice) | Induction of Cre activity in these mice results in simultaneous and near complete loss of brown and white adipocytes within 7 days Adipocyte number and adipose tissue integrity is restored within 6 weeks of the initial insult |
[88,240] |
|
| ||
| Adipocyte-specific PPARγ-knockout mice | Marked adipocyte hypocellularity and hypertrophy Elevated levels of plasma FFAs and triglycerides and decreased levels of plasma leptin and ACR30. Exhibit increased hepatic gluconeogenesis and insulin resistance Normal blood glucose levels, glucose and insulin tolerance, and insulin-stimulated muscle glucose uptake |
[88,241,242] |
|
| ||
| Adipocyte-specific PPARγ-knockout mice | Diminished weight gain and serum concentrations of both leptin and adiponectin in response to high-fat feeding Resistant to the development of high-fat diet-induced glucose intolerance or hyperinsulinemia (insulin resistance) |
[88,243] |
|
| ||
| Hepatocyte-specific PPARγ-knockout mice on wild-type and steatosis prone–AZIP/F lipoatrophic background | On a wild-type background, hepatic PPARγ deficiency leads to a significant defect in TG clearance, increased adiposity with age, hyperlipidemia and insulin resistance In AZIP mice, liver-specific gene ablation of PPARγ reduces the hepatic steatosis, but exaggerates hyperlipidemia, triglyceride clearance and muscle insulin resistance Hepatic deletion of PPARγ in AZIP mice also abolishes hypoglycemic and hypolipidemic actions effects of rosiglitazone suggesting that in the absence of adipose tissue, liver is a primary and major site of TZDs |
[88,244] |
|
| ||
| Hepatocyte-specific PPARγ-knockout mice on leptin-deficient (ob/ob) background | Marked attenuation of hepatic steatosis and improvement in the pathogenesis of fatty liver condition Decreased expression of lipogenic genes Elevated serum levels of TGs and FFAs Exerbation of hyperglycemia and muscle insulin resistance These traits are reversed by rosiglitazone treatment |
[88,245] |
|
| ||
| Myocyte-specific PPARγ-knockout (MCK promoter-driven Cre recombinase) mice | Mice develop excess adiposity as well as whole-body insulin resistance Normal sensitivity to TZD rosiglitazone in ameliorating high-fat diet-induced hyperinsulinemia and impaired glucose homeostasis |
[88,246] |
|
| ||
| Myocyte-specific PPARγ-knockout (MCK promoter-driven Cre recombinase) mice | Mice develop age-related insulin and glucose intolerance Exhibit-impaired insulin-stimulated muscle glucose uptake, as well as liver and adipose tissue insulin resistance Loss of sensitivity to TZDs |
[88,247] |
|
| ||
| Cardiomyocyte-specific PPARγ-knockout (CM-PGKO) mice | PPARγ deficiency in cardiomyocytes causes cardiac hypertrophy Increased expression of cardiac embryonic genes and enhanced expression of cardiac NF-κB activity |
[248] |
|
| ||
| Vascular smooth muscle cell-specific PPARγ-knockout (SM22Cre/flox) mice | Impaired vasoactivity and hypotension Reduced aortic contraction and increased vasorelaxation, respectively, in response to norepinephrine and agonists of β-adrenergic receptor Increased β-adrenergic receptor expression Blunted sensitivity to α1-adrenergic receptor agonists |
[249] |
|
| ||
| Transgenic mice expressing vascular smooth muscle cell-specific dominant negative P467L or V290M PPARγ human mutations | Transgenic mice expressing dominant-negative mutations, P467L or V290M under the control of a smooth muscle-specific promoter exhibit a loss of responsiveness to NO and significant alterations in contractility in the aorta, hypertrophy and inward remodeling in the cerebral microcirculation The mice also develop systolic hypertension |
[250] |
|
| ||
| Vascular endothelial cell-specific PPARγ-knockout (Tie2Cre/flox) mice | Reduced vascular NO production Elevated baseline blood pressure, but no change in sensitivity of angiotensin II stimulation of cystolic blood pressure Increased reactivity of femoral arteries to various vasoconstrictors without any significant alteration in acetylcholine-induced relaxation Nonresponsive to TZD's blood pressure-lowering effect |
[251,252] |
|
| ||
| Vascular endothelial cell-specific PPARγ-knockout (Tie2Cre/flox)/apoE-null mice | Elevated blood pressure in response to feeding a high-fat diet | [253] |
|
| ||
| Macrophage-specific PPARγ-knockout mice | Impaired activation of alternatively activated macrophages Mice show increased susceptibility to the development of diet-induced obesity, insulin resistance and glucose intolerance Downregulation of oxidative phosphorylation gene expression in skeletal muscle and liver leads to decreased insulin sensitivity in these tissues |
[254] |
|
| ||
| Heart-specific overexpression of PPARγ1 in mice (cardiomyocyte-specific PPARγ1 transgenic mice) | Increased cardiac expression of fatty acid oxidation genes and enhanced uptake of VLDL-TG Mice develop dilated cardiomyopathy along with increased accumulation of lipid and glycogen Pathological architectural changes in the inner matrix of the mitochondria and disrupted cristae |
[255] |
|
| ||
| Skeletal muscle-specific overexpression of a constitutively active version of PPARγ1 (myocyte-specific CA-PPARγ transgenic mice) | Upregulation of circulating levels of an adipose tissue-secreted endogenous insulin sensitizer, adiponectin, in CA-PPARγ transgenic mice Such mice are also insensitive to high-fat diet-induced insulin resistance |
[256] |
|
| ||
| PPARγ2-null mice | ||
| – Study 1 | Created by knock-in of red fluorescent protein into the PPARγ2-specific B exon Normal PPARγ1 expression Significant reduction in white adipose tissue mass, decreased lipid accumulation and blunted expression of adipogenic genes in adipose tissue Impaired insulin sensitivity in male PPARγ2-null mice |
[257] |
| –Study 2 | Created by replacing the entire B exon and flanking intronic sequences with a lacZ-neo cassette Normal PPARγ1 expression Male PPARγ2-null mice are significantly more insulin resistant than wild-type mice on chow diet; however, they do not become more insulin resistant when maintained on a high-fat diet |
[258] |
| –Study 3 | Created by introduction of an intronic neo cassette downstream of exon B Altered PPARγ1 expression Homozygous (PPARγhyp/hyp) mice show high mortality, growth retardation and develop severe lipodystrophy and hyperlipidemia during infancy; surviving PPARγhyp/hyp animals overcome the growth retardation, yet remain lipodystrophic The adult PPARγhyp/hyp mice are mildly glucose intolerant but do not develop hepatic steatosis |
[259] |
| – Study 4 | PPARγ2-null mice on ob/ob (Lepob/Lepob) background show a decreased fat mass, severe insulin resistance, β-cell failure and dyslipidemia Increased hepatic deposition of lipids, but decreased liver steatosis as measured by hematoxylin and eosin staining |
[176] |
FFA: Free fatty acid; NO: Nitric oxide; TG: Triglyceride; TZD: Thiazolidinedione; VLDL: Very-low-density lipoprotein.
Like PPARα and PPARβ/δ, genetic variants of PPARγ (-γ1 and -γ2) have also been reported [76,177]. To date, one common variant (P12A) and 16 rare missence and nonsense mutations in the coding region (AF-1, DNA-binding domain and LBD) have been described, and all but one (P113Q) lead to a loss of function [46,76,177]. The P12A polymorphism within the AF-1 region of the PPARγ2 is the most frequently found genetic variant of the PPARγ gene (2–25% depending on ethnicity) and this polymorphism is associated with a reduction in T2DM risk [178]. Animal studies further indicated that homozygous P12A mice are also reported to be more insulin sensitive than P12 animals [179]. However, this increased insulin sensitivity effect is lost in response to feeding a high-fat diet [179]. The majority of rare, heterozygous loss-of-function mutations reported so far are associated with a an inherited distinct clinical phenotype of familial partial lipodystrophic type 3 (FPLD3) characterized by altered fat distribution of subcutaneous fat, insulin resistance diabetes, elevated TGs, decreased HDL-C levels, hypertension, and polycystic ovary syndrome [46]. Among these, two missence mutants, V290M and P467L in PPARγ LBD, are associated with severe insulin resistance [76]. Surprisingly, transgenic mice expressing P465L (human equivalent of P467L) do not show signs of lipodystrophy and are not insulin resistant [174,172]. However, when expressed in the ob/ob (leptin deficient) mice, the P465L exacerbated insulin resistance and other metabolic abnormalities and alterations in the relative distribution of brown and WAT [180–183]. Besides, many additional mutants have been identified in FPLD3 patients, which either function as a dominant negative and inhibit normal receptor function or cause haploinsufficiency [90,177].
PPARγ: the vasculature, inflammation, hypertension & atherosclerosis
PPARγ expression is reported in cells within the vascular and immune systems including endothelial cells, VSMCs and monocytes/macrophages (Table 4) [54,71,97,98]. PPARγ ligands repress the activation and inflammation of endothelial cells. PPARγ activation by its ligands has been shown to inhibit the expression of cellular adhesion molecules including VCAM-1, ICAM-1, PECAM-1 and E-selectin, and MHC-II leading to decreased binding of monocytes to the activated endothelial cells [49,54,71]. PPARγ agonists inhibit inflammatory transcription factor signaling (NF-κB and AP-1), expression of their target genes [54,71], inflammatory signaling (protein kinase C) [54,71,184,185], and TNF-α, IL-6 and IL-1β production [54,71,184,185]. PPARγ ligands ameliorate endothelial cell oxidative stress and inflammation [54,71,186] and induce the expression of a PPARγ target gene, the anti-inflammatory HO-1 [187]. PPARγ agonists attenuate the expression of chemokine genes (e.g., interferon-inducible protein of 10 kDa a monokine induced by interferon-γ (MIG) and interferon-inducible T-cell α-chemoattractant in endothelial cells. [188]. A role for PPARγ has also been reported in the regulation of vascular tone, with PPARγ ligands inhibiting ET-1 and AT-1R expression while reducing the impact of excessive oxidative and nitrative stress [54,71]. PPARγ agonists increase AT-2R expression [54,71], and promote NO production and release [54,71,189,190], which, in turn, can activate PPARγ activity via MAPK [190]. Other studies suggest that PPARγ agonists reduce VEGF secretion, modulate VEGF-induced angiogenesis and inhibit VEGF-induced migration of endothelial cells. These effects appear to be mediated by the inhibition of Akt phosphorylation through upregulation of PTEN, a modulator of the PI3K/Akt signaling pathway [54,71]. Finally, there is some evidence that PPARγ promotes apoptosis and induces the expression of apoptotic genes in endothelial cells [54,71].
Many studies have shown that PPARγ ligands attenuate mitogen-induced VSMC proliferation, and this inhibition of cell proliferation appears to involve cell cycle arrest at the G1 phase [191]. MAPK signaling plays a key role in growth factor-induced VSMC cell proliferation, and TZDs appear to suppress VSMC proliferation primarily by inhibiting the MAPK signaling [54,71]. Besides growth factors, AngII–MAPK signaling cascade also plays a critical role in controlling the proliferation and migration of VSMCs. Ligand activation of PPARγ inhibits VSMC proliferation, migration and MMP-9 expression by blocking MAPK activation, possibly through the attenuation of PKC nuclear activity and PKC-dependent trans-location of ERK1/2 to the nucleus [54,71,191,192]. Another mechanism by which angiotensin II promotes VSMC proliferation is the upregulation of AT-1 receptors [54,71]. PPARγ ligands are known to downregulate AT-1 receptor expression in VSMCs. A number of PPARγ ligands such as troglitazone, rosiglitazone and 15d-PGJ2 have also been shown to inhibit growth factor (e.g., PDGF, CTGF and angiotensin II)-induced VSMC migration [54,71,191]. Besides, PPARγ ligands can induce apoptosis in VSMCs. TGF-β, phosphor-SMAD2, IRF-1 and GADD45 are some the key factors implicated in PPARγ-induced apoptosis of VSMCs [54,71,191,192]. Finally, there is evidence that inflammatory cytokine (TNF-α)-mediated gene and protein expression of VCAM-1, MCP-1 and fractalkine (CX3CL1) is downregulated by PPARγ activation in cultured VSMCs through the inhibition of NF-κB and C/EBP transcription factors [54,71,191,192].
PPARγ is expressed in murine-derived macrophages, neointimal lesions, macrophage-derived foam cells, atherosclerotic lesions and differentiated human monocyte-derived macrophages [54,71,97,98]. PPARγ expression is also detected in other inflammatory cells such as human plasma peripheral blood T cells, human CD4+ T cells, and mature dendritic cells from the spleen. PPARγ is also expressed in mouse T-helper cells and macrophage cell lines such as THP-1 and RAW 264.7 cells. PPARγ activation has been shown to attenuate expression of inflammatory cytokines (TNF-α, CCR-2, IL-6 and IL-1), inducible NO synthase, MMP-9, scavenger receptor A and oxidative burst. PPARγ agonists can stimulate IL-10 production. PPARγ ligands also inhibit expression of adhesion molecules, repress chemotaxis and promote apoptosis by interfering with NF-κB signaling [54,71]. Besides, PPARγ exerts a significant effect on macrophage lipid (cholesterol) metabolism. Activation of PPARγ is shown to upregulate the expression of fatty-acid transporter CD36, a member of the class B scavenger receptors [54,71,158]. This receptor/transporter facilitates uptake and internalization of oxidized LDL into the macrophages. Activated PPARγ promotes lipid (cholesterol) efflux from macrophages and in doing so interferes with their transformation into lipid-laden foam cells. This antiatherogenic action of PPARγ appears to be mediated by ABCA1 and ABCG1 cholesterol transporters [54,71,193]. In support of this, it is shown that macrophages deficient in PPARγ express relatively lower levels of ABCA1 and ABCG1 [193].
Recently accumulated evidence suggests that PPARγ is a key regulator of M1/M2 polarization and that the anti-inflammatory role of PPARγ in atherosclerosis is mediated by alternatively activated M2 phenotype macrophages [186,194,195]. The M2 macrophage differs from the classically activated M1 macrophage, by predominantly exhibiting an anti-inflammatory phenotype, instead of a proinflammatory phenotype. PPARγ agonists have been shown to inhibit macrophage activation and suppress M1 phenotypes including expression of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 [185]. PPARγ agonists also promote differentiation of macrophages into a M2 phenotype which, in turn, results in increased functional expression of PPARγ and M2 markers such as arginase-1, TARC, mannose receptor and CD36 [185,194–197]. Furthermore, under in vitro culture conditions, exposure of microphages to Th1 cytokine IFNg and Th2 cytokine IL-4, result in the generation of M1 and M2 phenotypes, respectively [194,195,198]. Interestingly, macrophage activation, which yields M1 and M2 types of macrophages, can be reversed by exposing cells to the opposite class of cytokine [195]. Finally, increasing evidence now indicates that PPARγ in its capacity as an atheroprotective agent also serves as a key determinant of distinct monocyte-derived subpopulations that play an anti-inflammatory, homeostatic role in atherogenesis [195].
PPARγ activation has been shown to influence the events connected with the development and progression of atherosclerotic lesions [54,71,156,199]. A majority of studies indicate that PPARγ and its ligands exert direct antiatherosclerotic actions. For example, treatment with the TZD class of PPARγ agonists reduces the size of atherosclerotic lesions in two mouse models of atherosclerosis which were fed a different diet: the LDL receptor-null mice (LDL-R−/−) and mice gene ablated for apolipoprotein E (apoE−/−) [54,71,154,156,168,200]. In keeping with the anti-inflammatory properties of PPARγ and TZDs, the aortas from these animals show decreased accumulation of macrophages in lesions and attenuated expression of proatherogenic agents. Interestingly, these changes occur independently of improvements in dyslipidemia, glycemic control and hypertension [150,200], which support direct vascular effects. In addition, it is shown that LDL-R-null mice transplanted with PPARγ-deficient bone marrow developed more severe atherosclerosis as compared with mice transplanted with bone marrow from control animals [146], further confirming the antiatherosclerotic actions of PPARγ.
PPAR ligands/agonists & their relevance to MetS, T2DM & CVD
As previously discussed, PPARs (PPARα, -β/δ and -γ) control the expression of a wide range of target genes required for the normal functioning of metabolic pathways involved in the glucose, lipid and cholesterol metabolism, and any dysregulation of these metabolic pathways can lead to the development of obesity, MetS, T2DM and CVD [73,106,158,201–203]. All three isoforms are known to be activated by a wide range of structurally diverse endogenous (Table 9) and synthetic (Tables 6, 9, 10 & 11) ligands, which add further diversity to the already expanded scope of their metabolic actions. Because of this, PPARs are considered attractive drug targets, and over the years many pharmaceutical companies around the world have committed an enormous amount of their research and development efforts into developing new PPAR-modulating therapeutic agents for the treatment of obesity, components of MetS and CVD. So far, these efforts have resulted in the development of two classes of drugs, fibrates and TZD, which activate PPARα and PPARγ, respectively, and which since have been marketed and are being used clinically. More new and safer drugs including a PPARβ/δ-specific compound are under various stages of development. No PPARβ/δ-specific drugs, however, are currently marketed or in routine clinical use.
Table 9.
Partial list of endogenous peroxisome proliferator-activated receptor ligands (activators).
| PPARα | PPARγ/β | PPARγ |
|---|---|---|
| Fatty acids | ||
|
| ||
| Docahexanoic acid | Docahexanoic acid | Docahexanoic acid |
| Arachidonic acid | Arachidonic acid | Arachidonic acid |
| Linoleic acid | Linoleic acid | |
| Saturated fatty acids (C6–C18) (weak) | Saturated fatty acids (C6–C18) (very weak) | |
| ETYA | ||
|
| ||
| Eicosanoids | ||
|
| ||
| 15d-PGJ2 | 15d-PGJ2 | 15d-PGJ2 |
| PGJ2 | PGJ2 | PGJ2 |
| Prostacyclin (PGI2) | Prostacyclin (PGI2) | PGA1/2 |
| PGA1/2 | PGA1/2 | PGB2 |
| PGB2 | PGB2 | 8-(R)HETE |
| 8-HEPE | 8-(S)HETE | |
| 8-(R)HETE | 15-HETE | |
| 8-(S)HETE | 9-(R/S)HODE | |
| 12-HETE | 13-(R/S)HODE | |
| LTB4 | 13-(S)HpODE | |
| 9-(R/S)HODE | 9-oxoODE | |
| 13-(R/S)HODE | 13-oxoODE | |
| 20,8,9-HEET | ||
| 20,11,12-HEET | ||
| 20,14,15-HEET | ||
|
| ||
| Lipoproteins | ||
|
| ||
| None yet identified | Components of VLDL | Components of oxidized LDL |
ETYA: Eicosatetraynoic acid; HEET: Hydroxyepoxyeicosatrienoic acid; HEPE: Hydroxyeicosapentaenoic acid; HETE: Hydroxyepoxyeicosatetraenoic acid; HODE: Hydroxyoctadecadienoic acid; HpODE: Hydroperoxyoctadecadienoic acid; LTB4: Leukotriene B4; VLDL: Very-low-density lipoprotein
Table 10.
Dual/pan peroxisome proliterator-activated receptor agonists in development.
| Agonist | Company | Developmental phase |
|---|---|---|
| PPARα/γ | ||
|
| ||
| Muraglitazar | Bristol-Myers Squibb | Discontinued |
| Naveglitazar | Eli Lilly and Ligand Pharmaceuticals | Phase II; discontinued |
| Ragaglitazar | Dr Reddy's laboratories/Novo Nordisk | Phase II; discontinued |
| Tesaglitazar (Galida) | AstraZeneca | Phase III; discontinued |
| MK-0767/KRP-297 | Kyorin Pharmaceutical Co. Ltd/Banyu | Discontinued |
| Farglitazar | Pharmaceutical Co. Ltd/Merck & Co. | Discontinued |
| Aleglitazar | GlaxoSmithKline | Phase II |
| Chiglitazar (CS038) | Hoffmann-La Roche | Phase II |
| AVE0847 | Sherizhen Chipscreen Biosciences | Phase II |
| Imiglitazar | Sanofi-Aventis | Discontinued |
| AZD 6610 | Takeda Pharmaceutical/AstraZeneca | Discontinued |
|
| ||
| PPARα/δ(β) | ||
|
| ||
| T913659 | Tularik (now Amgen) | Investigational agonist |
| GW2433 | GlaxoSmithKline | Investigational agonist |
|
| ||
| PPARγ/δ(β) | ||
|
| ||
| Compound 23 | GlaxoSmithKline | Preclinical |
| Compound 20 | Eli Lilly Pharmaceutical | Preclinical |
| Propionic acid derivative | Eli Lilly Pharmaceutical | Preclinical |
|
| ||
| PPARα/δ(β)/γ pan | ||
|
| ||
| Sipoglitazar | Takeda Pharmaceutical | Phase III; discontinued |
| Netoglitazone | Perlegen and Mitsubishi Tanabe Pharma | Phase II |
| Sodelglitazar | GlaxoSmithKline | Discontinued |
| GW-625019 | GlaxoSmithKline | Discontinued |
| Indeglitazar | Plexxikon and Wyeth | Phase II; discontinued |
| LY465608 | Eli Lilly Pharmaceutical | Preclinical |
| DRL 11605 | Perlecan Pharma | Preclinical |
Table 11.
Selective PPARγ modulators in development.
| SPPARγM | Company/reference | Developmental phase |
|---|---|---|
| GW0072 | GlaxoSmithKline | Preclinical: full antagonist of rosiglitazone and insulin sensitizer in vitro; lowers plasma and triglyceride levels in mice |
| FMOC-l-leucine | Multiple | Preclinical: weak PPARγ agonist; attenuates hyperglycemia in db/db mice; exhibit insulin sensitizing properties in normal mice |
| nTZDpa | Merck | Preclinical: ameliorates hyperinsulinemia and hyperglycemia in high-fat fed mice |
| Compound (14) | Merck | Preclinical: not fully characterized |
| Compound (15) | Merck | Preclinical: not fully characterized |
| Compound (16) | Merck | Preclinical: not fully characterized |
| MK-0533 | Merck | Phase II; discontinued |
| Metaglidasen (MBX-10) | Metabolex Inc./Johnson & Johnson | Phase II/III: improves insulin sensitivity, hyperinsulinemia, hyperglycemia, and dyslipidemia in several rodent models of insulin resistance and Type 2 diabetes |
| MBX-2044 | Metabolex Inc | Phase II: more potent than MBX-102 in terms of its metabolic function |
| AMG-131(T-131)/INT-131 | Amgen (Previously Tularik)/InteKrin Therapeutics Inc. | Phase IIa completed: more efficacious than rosiglitazone in reducing plasma glucose, insulin, triglycerides, free fatty acids and glucose-induced insulin secretion in Zucker fa/fa rats |
| PAT-5A | Dr Reddy's laboratory | Preclinical: selective PPARγ partial agonist with some modulator-like activity; lowers plasma glucose levels and improves insulin sensitivity |
| Balaglitazone (DRF-2593) | Dr Reddy's laboratory/Rheosciences (Denmark)/Novo Nordisk | Phase III; discontinued |
| FK-614 | Astellas | Discontinued |
| PA-082 | Roche | Preclinical: a modestly potent, selective partial PPARγ agonist with properties to release corepressor N-CoR and recruit coactivator proteins SRC-1, SRC-3 and TIF2; it is also capable of enhancing insulin-stimulated glucose transport in adipocytes |
| NHRI-compound | NHRI, Taipei, Taiwan | Preclinical: novel partial PPARγ agonist |
| KR-62980 | Korean Research Institute Chemical Technology | Preclinical: potent, selective partial agonist |
| S26948 | [237] | Preclinical: potent SPPARγM with a unique in vitro and in vivo profile |
NHRI: National Health Research Institutes; SPPARγM: Selective peroxisome proliferator-activated receptor modulator; SRC: Saccharomyces cerevisiae; TIF: Transcription intermediacy factor.
Fibrates & other PPARα agonists
The fibrate class of drugs, including clofibrate, ciprofibrate, benzafibrate and gemfibrozil, are low-affinity PPARα agonists, which are used primarily to lower circulating TGs and FFA levels [204], they raise plasma HDL-C levels and show a modest effect on lowering LDL-C. In addition to lipid-lowering effects, several large clinical trials using fibrate drugs (weak PPARα agonists), gemfibrazil, fenofibrate and benzafibrate have been carried out to test their clinical efficacy in reducing cardiovascular risk [72,166,193,204,205]. The key findings of these trials are summarized in Table 12. Based on these various clinical trials, it is quite apparent that direct antiatherosclerotic actions of PPARα agonists beyond lipid control remains to be fully substantiated. Because fenofibrate, gemfibrozil and benzafibrate are relatively weak PPARα agonists and raise HDL-C levels only modestly in certain patient populations, it is possible that their direct antiatherosclerotic effects are not strong enough to register a significant impact on the pathogenesis of CVD. Given this, there is a greater need for the design and synthesis of more potent and more specific PPARα agonists, which will not only improve atherogenic dyslipidemia but also effectively ameliorate atherosclerosis and offer other therapeutic benefits over that of existing fibrate drugs. Several of such potent PPARα agonists including LY518674 [206], AVE8134 [207], GW590735 [208] and DRF10945 (Table 6) are already under various stages of development.
Table 12.
Clinical trials to test the efficacy of PPAR agonists in the prevention of cardiovascular disease.
| Clinical trials | Key findings | Ref. |
|---|---|---|
| WHO clofibrate study | Clofibrate treatment caused approximately 20% reduction in relative risk of nonfatal MI No changes were noted in overall CHD-related deaths Significant increases in the incidence of non-CHD-related deaths were reported in clofibrate-treatment group |
[58,204] |
|
| ||
| The Helsinki Heart study (HHS) | A 34% reduction in primary end point of CHD risk was reported in genfibrozil-treated group Significant increases in HDL levels along with decreases in LDL-C and TG levels in genfibrozil-treated group |
[58,71] |
|
| ||
| The Diabetes Atherosclerosis Intervention Study (DAIS) | An angiographic trial focusing exclusively on Type 2 diabetic patients to determine the impact of fenofibrate treatment on selected angiographic markers of atherosclerosis and relative risk reduction The fenofibrate group exhibited a significant reduction in the levels of three angiographic markers and a 23% RRR of combined cardiac end points |
[58] |
|
| ||
| Veterans Affairs High-Density Intervention trial (VA-HIT) | Gemfibrozil reduced the risk major cardiovascular events (i.e., CAD death, nonfatal MI or stroke) independent of LDL-C levels in patients with a prior history of CAD and low plasma HDL-C | [58,71,204,205] |
|
| ||
| Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) | No significant treatment effect of fenofibrate was found on the incidence of the cardiovascular events or the overall mortality | [260] |
|
| ||
| Benzafibrate Infarction Prevention (BIP) trial | No significant reduction in cardiovascular events was reported despite significant changes in LDL-C and HDL-C | [58,206,261] |
|
| ||
| Lower Extremity Arterial Disease Event Reduction (LEADER) trial | Contrary to the significant changes in plasma lipid profile, benzafibrate treatment did not reduce the extent of combined cardiovascular end point in older men with a history of lower extremity vascular disease | [204] |
|
| ||
| The Prospective PioglitAzone Clinical Trial in Macrovascular Events (PROACTIVE) | Pioglitazone can reduce the risk of secondary macrovascular events in a high-risk patient population with Type 2 diabetes | [261,262] |
|
| ||
| A Diabetes Outcomes and Progression Trial (ADOPT) | Although not particularly tailored to evaluate effects on cardiovascular events, the trial demonstrated no beneficial actions of rosiglitazone on CAD but showed a trend towards an increased risk of MI | [263] |
|
| ||
| Diabetes Reduction | These are ongoing clinical trials | [264] |
| Assessment with Ramipril and Rosiglitazone Medication (DREAM) Action to Control Cardiovascular Risk in Diabetes (ACCORD) |
Current data from DREAM and ADOPT trials reported no statistically significant effect of rosiglitazone on cardiovascular events, changes in the rates of myocardial ischemia or cardiovascular deaths | [60,264,265] |
| Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) | Results from RECORD trial confirmed that addition of rosiglitazone to glucose lowering therapy in people with Type 2 diabetes is associated with increased risk of heart failure and of some fractures mainly in women | [266] |
| Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation (PERISCOPE) | A recently published report from the PERISCOPE Trial indicated a significant effect of pioglitazone on the atheroma volume | [266,267,304] |
| Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone (CHICAGO study) | The CHICAGO study demonstrated reduced carotic intima-media thickness progression in type diabetic patients treated with pioglitazone | [268,269] |
| Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) | [270] | |
CHD: Coronary heart disease; HDL-C: HDL-cholesterol; LDL-C: LDL-cholesterol; MI: Myocardial infarction; TG: Triglyceride.
Thiazolidinediones & other PPARγ agonists
The TZDs or glitazone PPARγ agonists represent a class of synthetic insulin-sensitizing agents that are widely prescribed for the treatment of hyperglycemia in patients with T2DM (Table 6) [64,174,182,207,208]. The first such TZD, troglitazone (Rezulin) was approved for clinical use in 1997 but was voluntarily withdrawn from the market due to the development of severe idiosyncratic hepatotoxicity in a small percentage of diabetic patients taking this drug. Its two successors, rosiglitazone (Avandia, GlaxoSmithKline) and pioglitazone (Actos, Pharmaceuticals) are currently marketed for the treatment of T2DM and represent important agents in the treatment of this disease both as a monotherapy and in combination with sulfonylurea insulin secretagogue, insulin or another insulin-sensitizing agent, metformin [61,200,209,210]. In animals and humans, both pioglitazone and rosiglitazone lower blood glucose, decrease hemoglobin A1c and improve insulin sensitivity in skeletal muscle, liver and adipose tissue [61,166,167,210,211]. These two PPARγ agonists also show modest effects on plasma lipoprotein lipids in humans, although pioglitazone has been shown to exert a somewhat superior effect in improving the plasma lipoprotein profile in patients with T2DM and dyslipidemia [169,170].
While pioglitazone and rosiglitazon have many beneficial effects in T2DM, they also have some adverse events associated with long-term use, including weight gain, edema, headache, congestive heart failure, hypoglycemia, myalgia, increased fracture risk and bone-marrow abnormalities [205,210,212–216]. Because of these adverse effects, efforts are underway to generate new PPARγ agonists that are not only highly efficient and specific but also exhibit minimum side effects. Two of such new-generation PDZ-based PPARγ agonists, balaglitazone and rivoglitazone (CS-011), are undergoing clinical trials. Several non-TZD PPARγ agonists have also been described which are currently at the preclinical stage (Table 6) [210,217,218].
As noted earlier, PDZ activation of PPARγ has also been shown to influence the development and progression of atherosclerotic lesions in experimental animal models, for example, treatment of various animal models of atherosclerotic disease with TDZs is associated with a significant reduction in the extent of plaque formation [54,71,150,158,218,201,219]. In humans, some studies support potential vascular benefits of TZDs including decreased carotid artery intimal medial thickness [220], improved endothelial reactivity [221], and levels of inflammatory markers and mediators in response to TDZs in both diabetic patients [190] and human subjects [222]. However, whether or not currently used TZDs, pioglitazone and rosiglitazone, are protective of CVD in humans is questionable [223–225]. Until now only a few large clinical trials have reported the effects of TZDs on cardiovascular events and the findings differ considerably as summarized in Table 12 [156,158,189,193,200].
Dual/pan PPAR agonists
The idea of developing highly efficacious dual- and pan-PPAR agonists stems from the beneficial effects seen in human clinical trials with the PPARα and PPARγ agonists and the promising, mostly preclinical, efficacy seen with certain PPARβ/δ-specific ligands. It is based on the concept that combining the therapeutic benefits of PPARα, -β/δ and -γ in various combinations, would result in the development of super dual or pan agonists that effectively improve multiple metabolic abnormalities associated with MetS and T2DM, including obesity, insulin resistance and hyperinsulinemia, hyperglycemia, atherogenic dyslipidemia (low HDL, high TE) and inflammation. The medical need for such designer dual and pan agonists is also indicated by minimizing the cardiovascular risk especially, in T2DM patients, who are considered at much greater risk of developing CVD [224]. The currently marketed TDZs, and for that matter, any of the currently available antidiabetic drugs, do not appear to improve cardiovascular outcomes [226]. Only benzafibrate, which is a weak activator of PPARα, -β/δ and -γ, is shown to improve many metabolic abnormalities commonly associated with MetS and T2DM except cardiovascular events. These wide-range beneficial effects of benzafibrate provide additional support to the concept of dual/pan PPARs and overall rationale to develop new pharmacological agents that target more than one PPAR isoform [227].
Unfortunately, so far the efforts to develop new dual/pan PPARs have not met with much success. While a large number of structurally diverse dual/pan agonists have been introduced and/or patented by various pharmaceutical companies, further clinical development of the majority of these agonists including MK-0767, muraglitazar, tesaglitazar, rasaglitazar, farglitazar, imiglitazar, ragaglitazar and naveglitazar has been discontinued for a variety of safety reasons (Table 10) [199,228–233]. Similarly, over the years efforts are underway to develop PPAR pan (α/γ/δ[β]) agonists in the clinical management of T2DM and core components of MetS [226,231]. Unfortunately, until now these efforts have also not been very successful. A number of PPAR pan agonists, such as sipoglitazar, sodelglitazar (GW-677954), indeglitazar (DPM-204 and PLX-204) and GW-625019 have been terminated due to serious safety concerns [226,231]. Only one compound, netoglitazone (PGX-510, formerly MCC-555) developed by Perlegan and Mitsubishi is currently undergoing Phase II clinical trials. Another PPAR pan compound, DRL 11605, designed by Perlecan Pharma is in its initial stages of development. This is the only known compound targeted towards obesity [210]. Several additional PPAR pan agonists undergoing preclinical testing have been described [210,229].
Selective PPAR modulators
The current disappointing clinical outcomes with the development of the PPAR dual and pan agonists have spurred the search for additional strategies to create novel PPAR-targeted drugs that can deliver superior therapeutic indexes. One such strategy that has gained considerable popularity in recent years is based on the selective PPAR modulator (SPPARM) concept. The molecular basis of SPPARM activity involves binding of SPPARM ligands in distinct manners to the ligand-binding domain of PPARs causing confirmation changes in the receptor which facilitate differential interactions with coactivators or corepressor proteins, and subsequently initiate or suppress transcription of specific target genes, ultimately resulting in differential biological responses [210,229,234–237]. This concept in principle is borrowed from the strategies that led to the successful development of selective estrogen receptor modulator drugs, tamoxifen and raloxifene, which act in a tissue-specific manner and provide much improved therapeutical benefits compared with the natural estrogen receptor ligand [210,229,234–237]. PPARγ has attracted most interest in the identification and clinical characterization of SPPARMs owing to two main reasons: first to minimize the impact of adverse effects that accompany full activation of the PPARγ agonist; and second, the genetic evidence both in humans and mice indicates that suboptimal activation of PPARγ results in more beneficial metabolic effects [210,229,234]. These latter observations led to a general understanding that a modest activation instead of full activation of PPARγ may be a preferred therapeutic strategy to develop PPARγ-specific antidiabetic SPPARγMs drugs to increase insulin sensitivity and lower plasma glucose levels but at the same prevent weight gain through adipogenesis.
In recent years, a number of SPPARγMs with differential potency and selectivity have been described (Table 11). Most of these compounds, however, are at the preclinical phase of their development; only three compounds (MBX-102, MBX-2044 and INT-131) have progressed to PhaseII/III clinical trials. Further clinical development of three additional compounds, MK-0533, balaglitazone and FK-614, has been halted due to serious safety concerns.
Conclusion & expert commentary
MetS represents a clustering of cardiometabolic abnormalities that increases an individual's risk of developing T2DM and CVD. The current epidemic of obesity, a direct consequence of over-nutrition and sedentary lifestyles, is credited with the rising prevalence of both MetS and T2DM. The MetS-linked cardiometabolic abnormalities and T2DM also impact heavily on the incidence of CVD, the leading cause of morbidity and mortality around the world. Because the MetS is a cluster of different clinical conditions, and not a single disease, it presents a formidable therapeutic challenge. Currently, the therapeutic management of MetS is being achieved through the use of combination therapy, but even so, core risk factors, including atherogenic dyslipidemia, insulin resistance, inflammation and hypertension are often poorly controlled. These issues highlight the urgent need for the development of new, safer and effective drugs that can be used as valuable clinical tools in the management of individual components of MetS as well as T2DM and associated cardiovascular complications.
PPARs are the members of a superfamily of nuclear hormone receptors comprising of three isoforms, PPARα, -β/δ and -γ, which act as ligand activated transcription factors. PPARs are considered master regulators of adipogenesis, lipid metabolism, energy balance, inflammation, blood pressure and atherosclerosis. These characteristics, coupled with their involvement in metabolic diseases, make PPARs an ideal target for the development of new pharmacological tools to treat individual risk factors. Indeed, two classes of synthetic PPAR agonists, the antidiabetic insulin-sensitizing TZDs (PPARγ agonists) and lipid-lowering fibrate derivatives (weak PPARα agonists) are widely prescribed for the treatment of hyperglycemia in patients with T2DM and for improving hyperlipidemia by the lowering of circulating TG and FFA levels, respectively. However, despite their therapeutic importance, these two drugs affect only a single component of MetS (i.e., fibrates ameliorate hyperlipidemia without impacting hyperglycemia while TZDs improve insulin sensitivity and hyperglycemia with minimal or no effect on hyperlipidemia), which limits their utility as a monotherapy and, more importantly, their use is often associated with adverse side effects, particularly treatment with TZDs. In addition, a number of clinical trials using various fibrate drugs, including gemfibrazil, fenofibrate and benzafibrate largely, but not uniformly, support the notion that these drugs may also have a direct and significant protective effect against CVD. Similarly, based on the outcomes from a number of clinical trials, it appears that the currently used TZD class of PPARγ agonists (rosiglitazone and pioglitazone) provides very little protection against overall cardiac mortality.
Because of these various reasons, and to improve treatment strategies in the management of MetS and associated diabetes and cardiovascular complications, extensive efforts are underway to develop safer and more effective single PPAR agonists, together with dual, pan and partial agonists and peroxisome proliferator-activated receptor modulator (SPARM) compounds for treating these problems. Several compounds that selectively and potently activate PPARα (LY518674, AVE8134, GW590735 and DRF-10945), PPARδ/β (GW501516) and PPARγ (balaglitazone and rivoglitazone) in cell and animal models are undergoing Phase II/III clinical trials. These trials appear to be progressing satisfactorily, but a lot more work still needs to be done to establish whether any of these experimental potent agonists show corresponding improvement in their efficacy, clinical therapeutic index and safety profile. To develop new potent agonists that target more than one PPAR isoform and provide superior therapeutic indexes and/or additional benefits, the concept of dual (PPARα/γ, α/δ[β] and γ/δ[β]) and pan (PPARα/δ[β]/γ) PPAR agonists has been put forward and a considerable amount of effort has been invested in exploring the clinical utility of these two new classes of compounds. Unfortunately, development of most of the novel and selective dual PPARα/γ or pan PPARα/δ(β)/γ agonists has been terminated due to various safety reasons. Currently, only three PPARα/γ dual agonists (aleglitazar, chiglitazar and AVE0847) and one pan agonist (netoglitazone) is the subject of Phase II clinical trials, and although based on past outcomes, their further clinical development does not appear to be very promising. At present there are no experimental PPARα/δ(β) dual agonists under investigation and only three PPARγ/δ(β) dual agonists are being evaluated at the preclinical stage.
To date the search for novel SPPARMs has primarily focused on PPARγ receptor (selective SPPARγM) compounds as previously discussed. However, given that ligand binding pockets of the receptors show considerable sequence homology, this concept can be extended to the other two PPAR subtypes as well. Table 11 lists the potential SPPARγM compounds currently under investigation. While most of these compounds are at the preclinical phase of their development, three compounds (MBX-102, MBX-2044 and INT-131) have progressed to Phase II/III clinical trials. Further clinical development of three additional compounds, MK-0533, balaglitazone and FK-614, have been halted due to serious safety concerns. Based on such a high failure rate, it is difficult to predict the toxicity, efficacy and clinical outcomes of the remaining SPPARγM. However, recent clinical trials with metaglidasen showing clinical efficacy with little adverse effects have given confidence that the SPPARγM could eventually become the next generation of insulin sensitizers.
Despite numerous setbacks in the clinical development of novel and potent single, dual, pan and partial agonists, the search for new agents, in addition to SPPARγMs, targeting three PPAR isotypes either separately or in combination, is still the most attractive, viable and important approach. In this context, continuing efforts to further delineate PPAR physiology, pharmacology and molecular functions may identify additional novel targets that can be exploited in the development of superior, efficacious and tissue-/PPAR isotype-specific agonists for the treatment of MetS. Moreover, development of more specific and reliable translational models and biomarkers to better understand the safety and efficacy in the clinical development of potential PPAR agonists should help greatly. Finally, a detailed mechanistic understanding of the role of PPARs in MetS and major metabolic organs in response to diabetes and cardiovascular systems is necessary in order to design PPAR-specific therapeutic agents that specifically target the individual components of MetS, the diabetic tissues or the cellular events connected with CVD.
Future perspective
Metabolic syndrome represents a clustering of cardiometabolic risk factors that is considered a direct consequence of overnutrition, sedentary lifestyles and resultant obesity. MetS is associated with an approximate doubling of CVD risk, and a fivefold increased risk for T2DM. Its prevalence is increasing rapidly in the USA and worldwide and, as a result, will probably have a major impact on the global incidence of T2DM and CVD. Because MetS is a cluster of different clinical conditions and not a single disease, and there are currently no specific therapies to treat all of these conditions, there is an urgent need for development of new safer and effective drugs that can be used as valuable clinical tools in the management of individual components of MetS and MetS-associated diabetic and cardiovascular complications. The ability of PPAR transcription factors to serve as master regulators of many metabolic processes, including lipid, glucose and energy homeostasis, inflammation and cardiovascular events, has made them an ideal target for the development of new pharmacological tools to treat individual risk factors. Indeed, whereas the TZD class of insulin-sensitizing PPARγ agonists are the preferred drugs in the management of insulin resistance and hyperglycemia, the fibrate class of weak PPARα agonists with hypolipidemic properties is commonly used to treat hyperlipidemia. However, because TZDs and fibrate only impact individual components of MetS (i.e., insulin resistance/hyperglycemia and hyperlipidemia, respectively), exhibit significant undesirable side effects especially with the use of TZDs, and are not particularly effective against CVD. In recent years considerable efforts have been invested in developing more effective single PPAR agonists, and also dual, pan and partial agonists as well as SPARM compounds with a goal to cover the entire spectrum of MetS. Unfortunately, until now such efforts have not resulted in the development of highly efficacious, selective and safer PPAR agonists, particularly, dual (PPARα/γ) or pan (PPARα/δ[β]/γ) agonists. Despite these setbacks, specific targeting still remains the most attractive, viable and important approach in the search for new single, dual, pan and partial PPAR agents in addition to SPPARγMs. Further refinement of experimental strategies, group-specific chemical modification of potential compounds, and development of specific and reliable translational models and biomarkers to better understand safety and efficacy, all should greatly aid in the future clinical development of novel types of PPAR agonists. Moreover, future efforts to further delineate PPAR physiology, pharmacology and molecular functions may identify additional novel targets that can also be exploited in the development of superior, efficacious and tissue-/PPAR isotype-specific agonists for the treatment of MetS.
Executive summary
-
■
Metabolic syndrome (MetS) represents a clustering of cardiometabolic risk factors that is associated with an approximate doubling of cardiovascular disease (CVD) risk and fivefold increased risk for Type 2 diabetes mellitus (T2DM).
-
■
Its prevalence is increasing rapidly in the USA and worldwide and will have a major impact on the global incidence of T2DM and CVD.
-
■
Because MetS is a cluster of different clinical conditions, and not a single disease, the clinical management of MetS is currently being achieved through the use of combination therapy and lifestyle changes but, even so, core risk factors, particularly dyslipidemia and insulin resistance, are often poorly controlled.
-
■
Given this, there is an immediate need for the clinical development of new, safer and effective drugs that can be used as valuable clinical tools in the management of individual components of this syndrome.
-
■
Because peroxisome proliferators-activated receptor (PPARs) are involved in the regulation of several metabolic processes, including lipid and carbohydrate metabolism, and are involved in the pathophysiology of metabolic diseases, this makes them an ideal target for the development of new pharmacotherapies to treat individual risk factors.
-
■
Indeed, two classes of synthetic PPAR agonists, the antidiabetic insulin-sensitizing thiazolidinediones (PPARγ agonists) and lipid-lowering fibrate derivatives (weak PPARα agonists) are widely prescribed for the treatment of hyperglycemia in patients with T2DM and for improving hyperlipidemia by lowering circulating thiazolidinediones and free fatty acid levels, respectively.
-
■
Despite lipid-lowering activity fibrates, a number of clinical trials using various fibrate drugs, including gemfibrazil, fenofibrate and benzafibrate, largely, but not uniformly, support the direct protective effect of these drugs against CVD. Similarly, based on outcomes from a number of clinical trials, it appears that the currently used tryglicerides class of PPARγ agonists (rosiglitazone and pioglitazone) also provides very little protection against overall cardiac mortality.
-
■
To improve treatment strategies in the management of MetS and associated diabetes and cardiovascular complications, extensive efforts are underway to develop safer and more effective single PPAR agonists, along with dual, pan and partial agonists and SPARM compounds for treating these problems.
-
■
Unfortunately, until now such efforts have not resulted in the development of highly efficacious, selective and safer PPAR agonists, particularly, dual, pan or partial PPAR agonists.
-
■
Further refinement of experimental strategies, group specific chemical modification of potential compounds, development of specific and reliable translational models and biomarkers and a greater understanding of the physiology, pharmacology and molecular functions of PPARs should greatly aid in the future development of superior, efficacious and safer PPAR agonists for the treatment of MetS.
Acknowledgments
Financial & competing interests disclosure This work was supported by the Office of Research and Development, Medical Service, Department of Veterans Affairs, and grants from the National Institutes of Health (HL033881 and HL092473).
Footnotes
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Plump AS, Lum PY. Genomics and cardiovascular drug development. J. Am. Coll. Cardiol. 2009;53:1089–1100. doi: 10.1016/j.jacc.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Weiner SD, Rabbani LE. Secondary prevention strategies for coronary heart disease. J. Throm. Thrombolysis. 2010;29(1):8–24. doi: 10.1007/s11239-009-0381-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics: 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 5.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular diseases: pathophysiology, evaluation and effect of weight loss: an update of the 1997 American Heart Association Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 6.Aronne LJ, Isoldi KK. Overweight and obesity: key components of cradiometabolic risk. Clin. Cornerstone. 2007;8:29–37. doi: 10.1016/s1098-3597(07)80026-3. [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and Type 2 diabetes mellitus pandemic: Part I: increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and Type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009;4:113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calkin AC, Allen TJ. Diabetes mellitus-associated atherosclerosis: mechanisms involved and potential for pharmacological invention. Am. J. Cardiovasc Drugs. 2006;61:15–40. doi: 10.2165/00129784-200606010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Smith SC. Multiple risk factors for cardiovascular disease and diabetes mellitus. Am. J. Med. 2007;120:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande AD, Harris-Hays M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haslam DW, James WP. Obesity. Lancet. 2005;366:107–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am. J. Med. Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan A. Obesity, metabolic syndrome, and Type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr. Rev. 2007;65:S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 15.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in Type 2 diabetes. Nat. Rev. Mol. Cell. Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 16.Levesque J, Lamarche B. The metabolic syndrome: definitions, prevalence and mangement. J. Nutrigenet. Nutrigenomics. 2008;1:100–108. doi: 10.1159/000112457. [DOI] [PubMed] [Google Scholar]
- 17.Lien LF, Guyton JR. Metabolic syndrome. Dermatol. Ther. 2008;21:362–375. doi: 10.1111/j.1529-8019.2008.00218.x. [DOI] [PubMed] [Google Scholar]
- 18.Azhar S, Kelley G. PPARα: its role in the human metabolic syndrome. Future Lipidol. 2007;2:31–53. [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM. Metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 22.Miranda PJ, DeFronzo RA, Califf RM. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am. Heart J. 2005;149:33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–210. [PubMed] [Google Scholar]
- 24.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce KD, Byrne CD. The metabolic syndrome: common origins of a multifactorial disorder. Postgrad. Med. J. 2009;85:614–621. doi: 10.1136/pgmj.2008.078014. [DOI] [PubMed] [Google Scholar]
- 26.Potenza MV, Mechanick JI. The metabolic syndrome: definition, global impact, and pathophysiology. Nutr. Clin. Pract. 2009;24:560–577. doi: 10.1177/0884533609342436. [DOI] [PubMed] [Google Scholar]
- 27.Khashab MA, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr. Gastroenterol. Rep. 2008;10:73–80. doi: 10.1007/s11894-008-0012-0. [DOI] [PubMed] [Google Scholar]
- 28.Apridonidze T, Essah PA, Luorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 29.Jerico C, Knobel H, Montero M, et al. Metabolic syndrome among HIV-infected patients: prevalence, characteristics, and related factors. Diabetes Care. 2005;28:132–137. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 30.Poyhiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metab. Syndr. Relat. Disord. 2009;7:279–288. doi: 10.1089/met.2008.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: part 1. Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Metab. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation: European Group for the Study of insulin Resistance (EGIR) Diabet. Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 33.Executive summary of the third report of the Nationnal Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Grudy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 36.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 37.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 38.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 39.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin. Pharmacol. Ther. 2007;82:509–524. doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 40.Spinler SA. Challenges associated with metabolic syndrome. Pharmacotherapy. 2006;26:209S–217S. doi: 10.1592/phco.26.12part2.209S. [DOI] [PubMed] [Google Scholar]
- 41.Epidemiology of paediatric metabolic syndrome and Type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2007;4:285–296. doi: 10.3132/dvdr.2007.055. [DOI] [PubMed] [Google Scholar]
- 42.Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr. Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Li C, Zhao G, Pearson WS, Mokad AH. Prevalence of the metabolic syndrome among US adolescents using the definition from the Internatioanl Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 44.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. J. Clin. Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semple RK, Chatterjee VK, O'Rahilly S. PPARγ and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiege JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006;45:120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Duval C, Müller M, Kersten S. PPARα and dyslipidemia. Biochim. Biophys. Acta. 2007;1771:961–972. doi: 10.1016/j.bbalip.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 50.Wagner KD, Wagner N. Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010;125:423–435. doi: 10.1016/j.pharmthera.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in Type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2006;26:28–40. doi: 10.1161/01.ATV.0000191663.12164.77. [DOI] [PubMed] [Google Scholar]
- 52.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 53.Reilly SM, Lee C-H. PPARδ as a therapeutic target in metabolic disease. FEBS Lett. 2008;582:26–31. doi: 10.1016/j.febslet.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson E, Grieve DJ. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol. Ther. 2009;122:246–263. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol.Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 57.Hansen MK, Connolly TM. Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Curr. Opin. Investig. Drug. 2008;9:247–255. [PubMed] [Google Scholar]
- 58.Barter PJ, Rye K-A. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb. Vasc. Biol. 2008;28:39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 59.Remick J, Weintraub H, Setton R, et al. Fibrate therapy: an update. Cardiol. Rev. 2008;16:129–141. doi: 10.1097/CRD.0b013e31816b43d3. [DOI] [PubMed] [Google Scholar]
- 60.Yki-Järvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;35:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 61.Barnett AH. Redefining the role of thiazolidinediones in the management of Type 2 diabetes. Vasc. Health Risk Manag. 2009;5:141–151. doi: 10.2147/vhrm.s4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprecher DL, Massien C, Pearce G, et al. Triglyceride: high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist. Arterioscler. Thromb. Vasc. Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 63.Risérus U, Sprecher D, Johnson T, et al. Activation of peroxisome proliferator-activated receptor (PPAR) δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 64.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michalik L, Auwerx J, Berger JP, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 66.Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor γ as a drug target in the pathogenesis of insulin resistance. Pharmacol. Ther. 2006;111:145–173. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Gurevich I, Flores AM, Aneskievich BJ. Corepressors of agonist bound nuclear receptors. Toxicol. Appl. Pharmacol. 2007;223:288–298. doi: 10.1016/j.taap.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu S, Reddy JK. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim. Biophys. Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Gozalez FJ, Shah YM. PPARα: Mechansim of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2008;246:2–8. doi: 10.1016/j.tox.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recep. Signal. 2010;8:2002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamblin M, Chang L, Fan Y, Zhang J, Chen YE. PPARs and the cardiovascular system. Antioxid. Redox Signal. 2009;11:1–38. doi: 10.1089/ars.2008.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dayspring T, Pokrywka G. Fibrate therapy in patients with metabolic syndrome and diabetes mellitus. Curr. Atheroscler. Rep. 2006;8:356–364. doi: 10.1007/s11883-006-0032-x. [DOI] [PubMed] [Google Scholar]
- 73.Fruchart J-C. Peroxisome proliferator-activated receptor-α (PPARα): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu. Rev. Biocem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 75.Naito H, Yamanoshita O, Kamijima M, et al. Association of V227A PPARα polymorphism with altered serum biochemistry and alcohol drinking in Japanese men. Pharmacogenet. Genomics. 2006;16:569–577. doi: 10.1097/01.fpc.0000220565.90466.79. [DOI] [PubMed] [Google Scholar]
- 76.Yong EL, Li J, Liu MH. Single gene contributions: genetic variants of proliferator-activated receptor (isoforms α, β/δ and γ) and mechanisms of dyslipidemias. Curr. Opin. Lipidol. 2008;19:106–112. doi: 10.1097/MOL.0b013e3282f64542. [DOI] [PubMed] [Google Scholar]
- 77.Liu MH, Li J, Shen P, Husna B, Tai ES, Yong EL. A natural polymorphism in the peroxisome prolifertaor-activated receptor-α hinge region attenuates transcription due to defective release of nuclear receptor coreprssor from chromatin. Mol. Endocrinol. 2008;22:1078–1092. doi: 10.1210/me.2007-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan E, Tan CS, Deurenberg-Yap M, Chia KS, Chew SK, Tai ES. The V227A polymorphism at the PPARA locus is associated with serum lipid concentrations and modulates the association between dietary polyunsaturated fatty acid intake and serum high density lipoprotein concentrations in Chinese women. Atherosclerosis. 2006;187:309–315. doi: 10.1016/j.atherosclerosis.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Sparsø T, Hussain MS, Andersen G, et al. Relationships between the functional PPARa Leu162Val polymorphism and obesity, Type 2 diabetes, dyslipidemia, and related quantitative traits in studies of 5799 middle-aged white people. Mol. Genet. Metab. 2007;90:205–209. doi: 10.1016/j.ymgme.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka T, Ordovas JM, Delgado-Lista J, et al. Peroxisome proliferator-activated receptor a polymorphisms and postprandial lipemia in healthy men. J. Lipid Res. 2007;48:1402–1408. doi: 10.1194/jlr.M700066-JLR200. [DOI] [PubMed] [Google Scholar]
- 81.Uthurralt J, Gordish-Dressman H, Bradbury M, et al. PPARa L162V underlies variation in serum triglycerides and subcutaneous fat volume in young males. BMC Med. Genet. 2007;8:55. doi: 10.1186/1471-2350-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudkowska I, Verrault M, Barbier O, Vohl M-C. Differences in transcription activation by the two allelic (L162V polymorphic) variants of PPARα after omega-3 fatty acid treatment. PPAR Res. 2009;369602 doi: 10.1155/2009/369602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silbernagel G, Stefan N, Hoffmann MM, et al. The L162V polymorphism of the peroxisome proliferator activated receptor α gene (PPAR) is not associated with Type 2 diabetes, BMI or body fat composition. Exp. Clin. Endocrinol. Diabetes. 2009;117:113–118. doi: 10.1055/s-0028-1082069. [DOI] [PubMed] [Google Scholar]
- 84.Doney AS, Fischer B, Lee SP, Morris AD, Leese G, Palmer CN. Association of common variation in the variation in the PPARα gene withincident myocardial infarction in individuals with Type 2 diabetes: a Go-DARTS study. Nucl Recept. 2005;3:4. doi: 10.1186/1478-1336-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc. Res. 2007;73:269–277. doi: 10.1016/j.cardiores.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 86.Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J. Mol Med. 2007;85:697–706. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- 87.Madarazo JA, Kelley DP. The PPAR trio: regulators of mitochondrial energy metabolism in health and disease. J. Mol. Cell. Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Barak Y, Kim S. Genetic manipulations of PPARs: effects on obesity and metabolic disease. PPAR Res. 2007;12781 doi: 10.1155/2007/12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park SY, Cho YR, Finck BM, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-α causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 90.Sambandhan N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD. Chronic activation of PPARα is detrimental to cardiac recovery after ischemia. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 91.Duncan JG, Fong JL, Mederiros DM, Finck BN, Kelley DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-α/PGC-1α gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev Diabt Stud. 2005;3:108–117. doi: 10.1900/RDS.2006.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panagia M, Gibbons GF, Radda GK, Clarke K. PPAR-α activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2677–H2683. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- 94.Loichot C, Jesel L, Tesse A, et al. Deletion of peroxisome proliferator-activated receptor-α induces an alteration of cardiac functions. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H161–H166. doi: 10.1152/ajpheart.01065.2004. [DOI] [PubMed] [Google Scholar]
- 95.Gélinas R, Labarthe F, Bouchard B, et al. Alterations in carbohydrate metabolism and its regulation in PPARα null mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1571–H1580. doi: 10.1152/ajpheart.01340.2007. [DOI] [PubMed] [Google Scholar]
- 96.Sweets PJH, Teunissen BEJ, Willemsen PHM, et al. Cardiac hypertrophy is enhanced in PPARα−/− mice in response to chronic pressure overload. Cardiovas. Res. 2008;78:79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- 97.Schiffrin EL. Peroxisome proliferator-activated receptors and cardiovascular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1037–H1043. doi: 10.1152/ajpheart.00677.2004. [DOI] [PubMed] [Google Scholar]
- 98.Moraes L, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Zandbergen F, Plutzky J. PPARα in atherosclerosis and inflammation. Biochim. Biophys. Acta. 2007;1771:972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcription nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 101.Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ. Res. 2004;94:1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 102.Chinetti-Gbaguidi G, Rigamonti E, Helin L, et al. Peroxisome proliferator-activated α controls cellular cholesterol trafficking in macrophages. J. Lipid Res. 2005;46:2717–2725. doi: 10.1194/jlr.M500326-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Hennuyer N, Tailleux A, Torpier G, et al. PPARα, but not PPARγ, activators decrease macrophage-lawn atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2005;25:1897–1902. doi: 10.1161/01.ATV.0000175756.56818.ee. [DOI] [PubMed] [Google Scholar]
- 104.Li AC, Binder CJ, Gutierrez A, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Babaev VR, Ishiguro H, Ding L, et al. Macrophage expression of peroxisome proliferator-activated receptor-α reduces atherosclerosis in low-density lipoprotein-deficient mice. Circulation. 2007;116:1404–1412. doi: 10.1161/CIRCULATIONAHA.106.684704. [DOI] [PubMed] [Google Scholar]
- 106.Barish GD, Nakar V, Evans RM. PPARδ: a dagger in the heart of metabolic syndrome. J. Clin. Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilgore KS, Billin A. PPARβ/δ ligands as modulators of the inflammatory response. Curr. Opin. Investig. Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- 108.Bishop-baily D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-β/δ in inflammation. Pharmacol. Ther. 2009;124:141–150. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 109.Oliver WR, Shenk JL, Snaith MR, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc. Natl Acad. Sci. USA. 98:5305–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wallace JM, Schwarz M, Coward P, et al. Effects of peroxisome proliferator-activated receptor α/δ agonists on HDL-cholesterol in vervet monkeys. J. Lipid Res. 2005;46:1009–1016. doi: 10.1194/jlr.M500002-JLR200. [DOI] [PubMed] [Google Scholar]
- 111.Barish GD, Atkins AR, Downes M, et al. PPARδ regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl Acad. Sci. USA. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tokata Y, Liu J, Fen Y, et al. PPARδ-mediated anti-inflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc. Natl Acad, Sci. USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi S, Tanaka T, Sakai J. New therapeutic target for metabolic syndrome. Endocr. J. 2007;54:347–357. doi: 10.1507/endocrj.kr-99. [DOI] [PubMed] [Google Scholar]
- 114.Kang K, Hatano B, Lee C-H. PPARδ agonists and metabolic diseases. Curr. Atheroscler. Rep. 2007;9:72–77. doi: 10.1007/BF02693931. [DOI] [PubMed] [Google Scholar]
- 115.Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor δ. Pharmacol.Rev. 2009;61:373–393. doi: 10.1124/pr.109.001560. [DOI] [PubMed] [Google Scholar]
- 116.van der Veen JN, Kruit JK, Havinga R, et al. Reduced cholesterol absorption upon PPARδ activation coincides with decreased intestinal expression of NPC1L1. J. Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- 117.Akiyama TE, Lambert G, Nicol CJ, et al. Peroxisome proliferator β/δ regulates very low density lipoprotein production and catabolism in mice on a western diet. J. Biol. Chem. 2004;279:20874–20881. doi: 10.1074/jbc.M312802200. [DOI] [PubMed] [Google Scholar]
- 118.Nadra K, Anghel SI, Joye E, et al. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor β(δ) Mol. Cell. Biol. 2006;26:3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee CH, Olson P, Hevener A, et al. PPARδ regulates glucose metabolism and insulin sensitivity. Proc. Natl Acad. Sci. USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived TH2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Odegaard JI, Richardo-Gonzalez RR, Eagle AR, et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Desvergne B. PPARδ/β: the lobbyist switching macrophage allegiance in favor of metabolism. Cell Metab. 2008;7:467–469. doi: 10.1016/j.cmet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 123.Cheng L, Ding G, Qin Q, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-δ deletion pertubs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 124.Cheng LY, Qin Q, Liu J, Liu J, Lo WK, Brako LA, Yang Q. High-fat feeding in cardiomyocyte-restricted PPARδ knockout mice leads to cardiac overexpression of lipid metabolic genes but fails to rescue cardiac phenotypes. J. Mol. Cell. Cardiol. 2009;47:536–543. doi: 10.1016/j.yjmcc.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burkat EM, Sambandam N, Han X, et al. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. J. Clin. Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:1532–1539. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sprecher DL. Lipids, lipoproteins, and peroxisome proliferator activated receptor δ. Am. J. Cardiol. 2007;100(11A):N20–N24. doi: 10.1016/j.amjcard.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Reilly SM, Lee C-H. PPARδ as a therapeutic target in metabolic disease. FEBS. Lett. 2008;582:26–31. doi: 10.1016/j.febslet.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aberle J, Hopfer I, Beil FU, Seedorf U. Association of the T+294C polymorphism in PPAR δ with low HDL cholesterol and coronary heart disease risk in women. Int. J. Med. Sci. 2006;3:108–111. doi: 10.7150/ijms.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grarup N, Albrechtsen A, Ek J, et al. Variation in the peroxisome proliferator-activated receptor δ gene in relation to common metabolic traits in 7,495 middle-aged white people. Diabetologia. 2007;50:1201–1208. doi: 10.1007/s00125-007-0668-2. [DOI] [PubMed] [Google Scholar]
- 131.Hautala AJ, Leon AS, Skinner JS, Rao DC, Bouchard C, Rankinen T. Peroxisome proliferator-activated receptor-δ polymorphisms are associated with physical performance and plasma lipids: the HERITAGE Family Study. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2498–H2505. doi: 10.1152/ajpheart.01092.2006. [DOI] [PubMed] [Google Scholar]
- 132.Robitaille J, Gaudet D, Pérusse L, Vohl MC. Features of the metabolic syndrome are modulated by an interaction between the peroxisome proliferator-activated receptor δ −87T>C polymorphism and dietary fat in French-Canadians. Int. Obes (Lond) 2007;31:411–417. doi: 10.1038/sj.ijo.0803450. [DOI] [PubMed] [Google Scholar]
- 133.Thamer C, Machann J, Stefan N, et al. Variations in PPARδ determine the change in body composition during lifestyle intervention: a whole-body magnetic resonance study. J. Clin. Endocrinol. Metab. 2008;93:1497–1500. doi: 10.1210/jc.2007-1209. [DOI] [PubMed] [Google Scholar]
- 134.Burch LR, Donnelly LA, Doney ASF, et al. Peroxisome proliferator-activated receptor-δ genotype influences metabolic phenotype and may influence lipid response to statin therapy in humans: a genetic of diabetes audit and research tayside study. J. Clin. Endocrinol. Metab. 2010;95:1830–1837. doi: 10.1210/jc.2009-1201. [DOI] [PubMed] [Google Scholar]
- 135.Stefan N, Thamer C, Staiger H, et al. Genetic variations in PPARδ and and PPARGC1A determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J. Clin Endocrinol. Metab. 2007;92:1827–1833. doi: 10.1210/jc.2006-1785. [DOI] [PubMed] [Google Scholar]
- 136.Eynon N, Meckel Y, Alves AJ, et al. Is there an interaction between PPARδ T294C and PPARGC1A Gly482Ser polymorphisms and human endurance performance? Exp. Physiol. 2009;94:1147–1152. doi: 10.1113/expphysiol.2009.049668. [DOI] [PubMed] [Google Scholar]
- 137.Aberle J, Hopfer I, Beil FU, Seedorf U. Assocaition of peroxisome proliferator-activated receptor δ +294T/C with body mass index and interaction with peroxisome proliferator-activated receptor α 162V. Int. J. Obes. (Lond.) 2006;30:1709–1713. doi: 10.1038/sj.ijo.0803345. [DOI] [PubMed] [Google Scholar]
- 138.Vänttinen M, Nuutila P, Kuulasmaa T, Pihlajamäki J. Single nucleotide polymorphisms in the peroxisome prolifertaor-activated receptor δ gene are associated with skeletal muscle glucose uptake. Diabetes. 2005;54:3587–3591. doi: 10.2337/diabetes.54.12.3587. [DOI] [PubMed] [Google Scholar]
- 139.Shin HD, Park BL, Kim LH, et al. Genetic polymorphisms in peroxisome proliferator-activated receptor δ associated with obesity. Diabetes. 2004;53:847–851. doi: 10.2337/diabetes.53.3.847. [DOI] [PubMed] [Google Scholar]
- 140.Hu C, Jia W, Fan Q, Zhang R, Wang C, Lu J, Xiang K. Peroxisome proliferator-activated receptor (PPAR) δ genetic polymorphism and its association with insulin resistance index and fasting plasma gluose concentrations in Chinese subjects. Diabet. Med. 2006;23:1307–1312. doi: 10.1111/j.1464-5491.2006.02001.x. [DOI] [PubMed] [Google Scholar]
- 141.Holzapfel J, Heun R, Lütjohann D, Jessen F, Maier W, Kölsch H. PPARδ haplotype influences cholesterol metabolism but is no risk factor for Alzheimer's disease. Neurosci. Lett. 2006;408:57–61. doi: 10.1016/j.neulet.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 142.Castriloo A, Tontonoz P. Nuclear receptors in Macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu. Rev. Cell. Dev. Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 143.Kilgore KS, Billin AN. PPARb/d ligands as modulators of the inflammatory response. Curr. Opin. Investig. Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- 144.Fan Y, Wang Z, Tang Z, et al. Suppression of proinflammatory adhesion molecules by PPAR-d in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 145.Piqueras L, Sanz MJ, Perretti M, et al. Activation of PPARβ/δ inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J. Leukoc. Biol. 2009;86:115–122. doi: 10.1189/jlb.0508284. [DOI] [PubMed] [Google Scholar]
- 146.Liang YJ, Liu YC, Chen CY, et al. Comparison of PPARδ and PPARγ in inhibiting the proinflammatory effects of C-reactive protein in endothelial cells. Int. J. Cardiol. 2009 doi: 10.1016/j.ijcard.2009.03.100. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 147.Fang Y, Wang Y, Tang Z, et al. Suppression adhesion molecules by PPAR-δ in vascular human and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 148.Lim HJ, Lee S, Park JH, et al. PPAR δ agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009;202:446–454. doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 149.Kim HJ, Ham SA, Kim SU, et al. Transforming growth factor-β1 is a molecular target for the peroxisome proliferator-activated receptor δ. Circ. Res. 2008;102:193–200. doi: 10.1161/CIRCRESAHA.107.158477. [DOI] [PubMed] [Google Scholar]
- 150.Barish GD, Evans RM. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocriol. Metab. 2004;15:158–1165. doi: 10.1016/j.tem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Wang N. PPAR-δ in vascular pathophysiology. PPAR Res. 2008;164163:1–10. doi: 10.1155/2008/164163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARδ agonist GW0742X reduces atherosclerosis in LDLR (−/−) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 153.Zhang H, Pi R, Li R, et al. PPARβ/δ activation inhibits angiotensin II-induced collagen type I expression in rat cardiac fibroblasts. Arch. Biochem. Biophys. 2007;460:25–32. doi: 10.1016/j.abb.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 154.Brunelli L, Cieslik A, Alcorn JL, et al. Peroxisome proliferator activated receptor-δ upregulates 14–3–3 epsilon in human endothelial cells via CCAAT/enhancer binding protein-β. Circ. Res. 2007;100:e59–e71. doi: 10.1161/01.RES.0000260805.99076.22. [DOI] [PubMed] [Google Scholar]
- 155.Sprecher DL, Massien C, Pearce G, et al. Triglyceride: high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist. Arterioscler. Thromb. Vasc. Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 156.Jandeleit-Dahm KAM, Calkin A, Tikellis C, Thomas M. Direct antiatherosclerotic effects of PPAR agonists. Curr. Opin. Lipidol. 2009;20:24–29. doi: 10.1097/mol.0b013e32831f1b18. [DOI] [PubMed] [Google Scholar]
- 157.Ahmed W, Ziouzenkova O, Brown J, et al. PPARs and their metabolic modulation: new mechanisms for transcription regulation? J. Intern. Med. 2007;262:184–198. doi: 10.1111/j.1365-2796.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- 158.Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor γ as a drug target in the pathogenesis of insulin resistance. Pharmacol. Ther. 2006;111:145–173. doi: 10.1016/j.pharmthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 159.Medina-Gomez G, Gray S, Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor γ (PPARγ) and PPARγcoactivator (PGC1) Public Health Nutr. 2007;10:1132–1137. doi: 10.1017/S1368980007000614. [DOI] [PubMed] [Google Scholar]
- 160.Medina-Gomez G, Virtue S, Lelliott C, et al. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-γ2 isoform. Diabetes. 2005;54:1706–1716. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Medina-Gomez G, Gray SL, Yetukuri L, et al. PPARγ2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2009;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Christodoulides C, Vidal-Puig A. PPARs and adipocyte function. Mol. Cell. Endocrinol. 2010;318:1–2. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 163.Chen Y, Jimenez AR, Medh JD. Identification and regulation of novel PPAR-γ splice variants in human THP-1 macrophages. Biochim. Biophys. Acta. 2006;1759:32–43. doi: 10.1016/j.bbaexp.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou J, Wilson KM, Medh JD. Genetic analysis of four novel peroxisome proliferator activated receptor-γ splice variants in monkey macrophages. Biochem. Biophys. Res. Commun. 2002;293:274–283. doi: 10.1016/S0006-291X(02)00138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez AZ. Peroxisome proliferator-activated receptors in the modulation of the immune/inflammatory response in atherosclerosis. PPAR Res. 2008;2008:285842. doi: 10.1155/2008/285842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic target for metabolic disease. Trends Pharmacol. Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 167.Bhatia V, Viswanathan P. Insulin resistance and PPAR insulin sensitizers. Curr. Opin. Investig. Drugs. 2006;7:89–897. [PubMed] [Google Scholar]
- 168.Hansen MK, Connolly TM. Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Curr. Opin. Investig. Drugs. 2008;9:247–255. [PubMed] [Google Scholar]
- 169.Goldberg RB, Kendell DM, Deeg MA, et al. A comparison of lipid and glycemic effects on serum lipoprotein particle concentrations and sizes in patients with Type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 170.Deeg MA, Buse JB, Goldberg RB, et al. Pioglitazone and rosiglitazone have different effects on serum lipoproparticle concentrations and sizes in patients with Type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 171.Larsen TM, Toubro S, Astrup A. PPARγ agonists in the treatment of Type II diabetes: is increased fatness commensurate with long-term efficacy? Int. J. Obes. Relat. Metab. Disord. 2003;27:147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 172.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am. J. Med. 2007;120:S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 173.Argmann CA, Cock TA, Auwerx J. Peroxisome proliferator-activated receptor γ: the more the merrier? Eur: J. Clin. Invest. 2005;35:82–92. doi: 10.1111/j.1365-2362.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 174.Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM, Sigmund CD. Does peroxisome proliferator-activated receptor-γ (PPARγ) protect from hypertension directly through effects in the vasculature? J. Biol. Chem. 2010;285:9311–9316. doi: 10.1074/jbc.R109.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Duan SZ, Ivashchenko CY, Whitesall SE, et al. Hupotension, lipodystrophy, and insulin resistance in generalized PPARγ-deficient mice rescued from embryonic lethality. J. Clin. Invest. 2007;117:812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Medina-Gomez G, Gray SL, Yetukuri L, et al. PPARγ2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genetics. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jeninga EH, Gurnell M, Kalkhoven E. Functional implications of genetic variation in human PPARγ. Trends Endocrinol. Metab. 2009;20:380–387. doi: 10.1016/j.tem.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 178.Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, Higgins JPT. The association between the peroxisome proliferator-activated receptor-γ2 (PPATG2) Pro12Ala gene variant and Type 2 diabetes mellitus: a HuGE review and meta analysis. Am. J. Epidemiol. 2010;171:645–655. doi: 10.1093/aje/kwp450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Heikkinen S, Argmann C, Fiege JN, et al. The pro12Ala PPARγ2 variant determines meatbolism at the gene-enviroment interface. Cell Metab. 2009;9:88–98. doi: 10.1016/j.cmet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 180.Tsai YS, Kim HJ, Takahashi N, et al. Hypertension, and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J. Clin. Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gray SL, Dalla Nora E, Backlund EC, et al. Decreased brown adipocyte recruitment and thermogenic capacity in mice with impaired peroxisome prolifertaor-activated receptor (P465L PPARγ) function. Endocrinology. 2006;147:5708–5714. doi: 10.1210/en.2006-0684. [DOI] [PubMed] [Google Scholar]
- 182.Gray SL, Dalla Nora E, Grosse J, et al. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor γ function (P465L PPARγ) in mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 183.Beyer AM, Baumbach GL, Halabi CM, et al. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Verrier E, Wang L, Wadham C, et al. PPARγ agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ. Res. 2004;94:1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 185.Duan SH, Usher M, Mortensen RM. PPARs: the vasculature, inflammation and hypertension. Curr. Opin. Nephrol. Hyper. 2009;18:128–133. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- 186.Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor-γ ligands regulate endothelial membrane superoxide production. Am. J. Physiol. Cell Physiol. 2005;288:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 187.Kronke G, Kadl A, Ikonomu E, et al. Expression of heme oxygenase-1 in human vascular cells is activated by peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 188.Bruemmer D, Blaschke F, Law RE. New targets for PPARγ in the vessel wall: implications for restenosis. Int. J. Obes. 2005;29(Suppl. 1):S26–S30. doi: 10.1038/sj.ijo.0802910. [DOI] [PubMed] [Google Scholar]
- 189.Kuusisto J, Andrulionyte L, Laakksko M. Atherosclerosis and cardiovascular risk reduction with PPAR agonists. Curr. Atheroscler. Rep. 2007;9:274–280. doi: 10.1007/s11883-007-0033-4. [DOI] [PubMed] [Google Scholar]
- 190.Ptasinska A, Wang S, Zhang J, Wesley RA, Danner RL. Nitric oxide activation of peroxisome proliferator-activated receptor γ through a p38 MAPK signaling pathway. FASEB J. 2007;21:950–961. doi: 10.1096/fj.06-6822com. [DOI] [PubMed] [Google Scholar]
- 191.Gizard F, Bruemmer D. Transcriptional control of vascular smooth muscle cell proliferation by peroxisome proliferator-activated receptor-γ: therapeutic implications for cardiovascular diseases. PPAR Res. 2008;429123 doi: 10.1155/2008/429123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-agonists. Am. J. Cardiol. 2007;99(Suppl. 99):27B–40B. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 193.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 194.Bouhlel MA, Derudas B, Rigamonti E, et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 195.Gerry JM, Pascual G. Narrowing in on cardiovascular disease: the atheroprotective role of peroxisome proliferator-activated receptor γ. Trends Cardiovasc. Med. 2008;18:39–44. doi: 10.1016/j.tcm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 196.Berry A, Balard P, Coste A, et al. IL-3 induces expression of CD36 in human monocytes through PPARγ activation. Eur. J. Immunol. 2007;37:1642–1652. doi: 10.1002/eji.200636625. [DOI] [PubMed] [Google Scholar]
- 197.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Porcheray F, Viaud S, Rimaniol AC, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kendall DM, Rubin CJ, Mohideen P, et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (α/γ peroxisome proliferators activated receptor activator, in patients with Type 2 diabetes inadequately controlled with metformin monotherapy: a double-blinded, randomized, pioglitazone-comoartaive study. Diabetes Care. 2005;29:1016–1023. doi: 10.2337/diacare.2951016. [DOI] [PubMed] [Google Scholar]
- 200.Rangwala SM, Lazar MA. Peroxisome proliferator activated receptor γ in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 201.Balint BL, Nagy L. Selective modulators of PPAR activity as new therapeutic tools in metabolic diseases. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:33–43. doi: 10.2174/187153006776056620. [DOI] [PubMed] [Google Scholar]
- 202.Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and Type 2 diabetes mellitus. Curr. Diabetes Rev. 2007;3:33–39. doi: 10.2174/157339907779802067. [DOI] [PubMed] [Google Scholar]
- 203.Rosenson RS. Effects of peroxisome proliferator-activated receptors on lipoprotein metabolism and glucose control in Type 2 diabetes mellitus. Am. J. Cardiol. 2007;99:96B–104B. doi: 10.1016/j.amjcard.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 204.Remick J, Weintraub H, Setton R, Offenbacher J, Fisher E, Schwartzbard A. Fibrate therapy. Cardiol. Rev. 2008;16:129–141. doi: 10.1097/CRD.0b013e31816b43d3. [DOI] [PubMed] [Google Scholar]
- 205.Robinson J. Should we use PPAR agonists to reduce cardiovascular risk? PPAR Res. 2008;891425 doi: 10.1155/2008/891425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Singh JP, Kauffman R, Bensch W, et al. Identification of a novel selective peroxisome proliferators-activated receptor a agonist, 2-methyl-2-(4-{3-{1-(4-methylbenzyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl]propyl} phenoxy)proppanoic acid (LY618674), that produces marked changes in serum lipids and apolipoprotein A-1 expression. Mol. Pharmacol. 2005;68:763–768. doi: 10.1124/mol.105.010991. [DOI] [PubMed] [Google Scholar]
- 207.Linz W, Wohlfart P, Baader M, et al. The peroxisome proliferator-activated receptor-α (PPAR-α) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacol. Sin. 2009;30:935–946. doi: 10.1038/aps.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sierra ML, Benton V, Boullay A-B, et al. Substituted 2-[(4-aminomethyl)phenoxy]-2-methylpropionic acid PPARα agonists. 1. Discovey of a novel series potent HDLC raising agents. J. Med. Chem. 2007;50:685–695. doi: 10.1021/jm058056x. [DOI] [PubMed] [Google Scholar]
- 209.Akiyama TE, Meinke PT, Berger JP. PPAR ligands: potential therapies for metabolic syndrome. Curr. Diab. Rep. 2005;5:45–52. doi: 10.1007/s11892-005-0067-3. [DOI] [PubMed] [Google Scholar]
- 210.Cho N, Momose Y. Peroxisome proliferators-activated receptor γ agonists as insulin sensitizers: from the discovery to recent progress. Curr. Top. Med. Chem. 2008;8:1483–1507. doi: 10.2174/156802608786413474. [DOI] [PubMed] [Google Scholar]
- 211.Waugh J, Keating GM, Plosker GL, et al. Pioglitazone: a review of of its use in Type 2 diabetes mellitus. Drugs. 2006;66:410–418. doi: 10.2165/00003495-200666010-00005. [DOI] [PubMed] [Google Scholar]
- 212.Chang F, Jaber LA, Berlie HD, O'Connell MB. Evolution of paeroxisome proliferator-activated receptor agonists. Ann. Pharmacother. 2007;41:973–983. doi: 10.1345/aph.1K013. [DOI] [PubMed] [Google Scholar]
- 213.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 214.Cheng AYY, Fantus IG. Thiazolidinedione-induced congestive heart failure. Ann. Pharmacother. 2004;38:817–820. doi: 10.1345/aph.1D400. [DOI] [PubMed] [Google Scholar]
- 215.Digman C, Klein AK, Pittas AG. Leukopenia and thrombocytopenia caused by thiazolidinediones. Ann. Intern. Med. 2005;143:465–466. doi: 10.7326/0003-4819-143-6-200509200-00016. [DOI] [PubMed] [Google Scholar]
- 216.Hampton T. Diabetes drugs tied to fractures in women. JAMA. 2007;297:1645. doi: 10.1001/jama.297.15.1645. [DOI] [PubMed] [Google Scholar]
- 217.Chen X, Osborne MC, Rybczynski PJ, et al. Pharmacological profile of a novel, non-TZD PPARγ agonist. Diabetes Obes. Metab. 2005;7:536–546. doi: 10.1111/j.1463-1326.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 218.Carmona MC, Louche K, Lefebvre B, et al. On behalf of the consortium of the French Ministry of Research and Technology: S 26948: a new specific peroxisome proliferators-activated receptor γ modulator with potent antidiabetes and antiatherogenic effects. Diabetes. 2007;56:2797–2808. doi: 10.2337/db06-1734. [DOI] [PubMed] [Google Scholar]
- 219.Calkin AC, Forbes JM, Smith CM, et al. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler. Thromb. Vasc. Biol. 2005;25:1903–1909. doi: 10.1161/01.ATV.0000177813.99577.6b. [DOI] [PubMed] [Google Scholar]
- 220.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients witout diabetes. Arterioscler. Thromb. Vasc. Biol. 2004;24:930–934. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- 221.Campia U, Matuskey LA, Panza JA. Peroxisome proliferators-activated receptor-γ activation with pioglitazone improves endothelium dependent dialation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113:867–875. doi: 10.1161/CIRCULATIONAHA.105.549618. [DOI] [PubMed] [Google Scholar]
- 222.Meisner F, Walcher D, Gizard F, et al. Effect of rosiglitazone treatment on plaque inflammation and collagen content in nondiabetic patients: data from a randomized placebo-controlled trial. Arterioscler. Thromb. Vasc. Biol. 2006;26:845–850. doi: 10.1161/01.ATV.0000203511.66681.7f. [DOI] [PubMed] [Google Scholar]
- 223.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infraction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 224.Rosen CJ. The rosiglitazone story-lessons from an FDA Adviosry Committee Meeting. N. Engl. J. Med. 2007;357:844–846. doi: 10.1056/NEJMp078167. [DOI] [PubMed] [Google Scholar]
- 225.Dogrell SA. Does rosiglitazone increase cardiovascular outcomes? Expert Opin. Pharmacother. 2007;8:2665–2669. doi: 10.1517/14656566.8.15.2665. [DOI] [PubMed] [Google Scholar]
- 226.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and Type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and Type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009;4:113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tenenbaum A, Motro M, Fisman EZ. Dual and pan-peroxisome proliferators-activated receptors (PPAR) co-agonism: the benzafibrate lessons. Cardiovasc. Diabetol. 2005;14(4):14. doi: 10.1186/1475-2840-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rubenstrunk A, Hanf R, Hun DW, Fruchart JC, Stales B. Safety issues and prospects for future generations of PPAR modulators. Biochim. Biophys. Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 229.Sharer BG, Billin AN. The next generation of PPAR drugs: do we have the tools to find them? Biochim. Biophys. Acta. 2007;1771:1082–1093. doi: 10.1016/j.bbalip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 230.Nissen SE, Wolski K, Topol EJ. Effect of muraglitizar on death and major adverse cardiovascular events in patients with Type 2 diabetes mellitus. JAMA. 2005;294:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 231.Bay H, McElhattan J, Bryzinski BS, On behalf of the Gallant 6 Study Group A double-blind, randomized trial of tesaglitazar versus pioglitazone in patients with Type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2007;4:181–193. doi: 10.3132/dvdr.2007.039. [DOI] [PubMed] [Google Scholar]
- 232.Fagerberg B, Edwards S, Halmos T, et al. Tesaglitazar, a novel dual peroxisome proliferator-activated receptor α/γ agonist, dose-dependently improves the metabolic abnormalities associated with insulin resistance in a non-diabetic population. Diabetologia. 2005;48:1716–1725. doi: 10.1007/s00125-005-1846-8. [DOI] [PubMed] [Google Scholar]
- 233.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am. J. Cardiol. 2007;99(Suppl. 1):S3–S18. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 234.Feldman PL, Lambert MH, Henke BR. PPAR modulators and PPAR pan agonists for metabolic diseases: the next generation of drugs targeting peroxisome proliferator-activated receptors? Curr. Top. Med. Chem. 2008;8:728–749. doi: 10.2174/156802608784535084. [DOI] [PubMed] [Google Scholar]
- 235.Balint BL, Nagy L. Selective modulators of PPAR activity as new therapeutic tools in metabolic diseases. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:33–43. doi: 10.2174/187153006776056620. [DOI] [PubMed] [Google Scholar]
- 236.Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet. Gynecol. Surv. 2008;63:163–181. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 237.Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatment. Anticancer Agents Med. Chem. 2009;9:481–499. doi: 10.2174/187152009788451833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheung C, Akiyama TE, Ward JM, et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor α. Cancer Res. 2004;64:3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- 239.Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. The PPARα-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPARα. Toxicol. Sci. 2008;101:132–139. doi: 10.1093/toxsci/kfm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Imai T, Takakuwa R, Marchand S, et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl Acad. Sci. USA. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kintscher U, Law RE. PPARγ-mediated insulin sensitization: the importance of fat versus muscle. Am. J. Physiol. Endocrinol. Metab. 2005;288:E287–E291. doi: 10.1152/ajpendo.00440.2004. [DOI] [PubMed] [Google Scholar]
- 242.He W, Barak Y, Hevener, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resisatnce in fat and liver but not in muscle. Proc. Natl Acad. Sci. USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jones JR, Barrick C, Kim K-A, et al. Deletion of PPARγ in a adipose tissues of mice protects against high fat diet-induced obesity and insulin resisatnce. Proc. Natl Acad. Sci. USA. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 245.Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clinical Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hevener AL, He W, Barak Y, et al. Muscle-specific PPARγ deletion causes insulin resistance. Nature Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 248.Zhong S, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 249.Chang L, Villacorta L, Zhang J, et al. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-γ deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang N, Symons JD, Zhang H, et al. Distinct functions of vascular endothelial and smooth muscle PPARγ in regulation of blood oressure and vascular tone. Toxicol. Pathol. 2009;37:21–27. doi: 10.1177/0192623308328545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Halabi CM, Beyer AM, de Lange W, et al. Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kleinhenz JM, Kleinhenz DJ, You S, et al. Disruption of endothelial peroxisome proliferator-activated receptor-γ reduces vascular nitric oxide production. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1647–H1654. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARγ in endothelial cells influences high fat diet-induced hypertension. Am. J. Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 254.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improve insulin resistance. Nature. 2007;447:1116–1121. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Son NH, Park TS, Yamashita H, et al. Cradiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J. Clin. Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Amin RH, Mathews ST, Camp HS, Ding L, Left T. Selective activation of PPARγ in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009;298:E28–E37. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 257.Zhang J, Fu M, Cui T, et al. Selective disruption of PPARγ2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl Acad. Sci. USA. 2004;101:10703–10708. doi: 10.1073/pnas.0403652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Medina-Gomez G, Virtue S, Lelliott C, et al. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-γ2 isoform. Diabetes. 2005;54:1706–1718. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Koutnikova H, Cock TA, Watanabe M, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPARγ hypomorphic mice. Proc. Natl Acad. Sci. USA. 2003;100:14457–114462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with Type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet. 2005;26:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 261.Betteridge DJ, DeFronzo RA, Chilton RJ. PROactive: time for critical appraisal. Eur. Heart J. 2008;29:969–983. doi: 10.1093/eurheartj/ehn114. [DOI] [PubMed] [Google Scholar]
- 262.Erdman E, Dormandy J, Wilcox R, Massi-Benedetti M, Charbonnel B. PROactive: pioglitazone in the treatment of Type 1 diabetes: results of the PROactive study. Vasc. Health Risk Manag. 2007;3:355–370. [PMC free article] [PubMed] [Google Scholar]
- 263.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. N. Engl. J. Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 264.Gerstein HC, Yusuf J, Bosch J, et al. DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) trial investigators: effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 265.Buse JB. Atrion to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am. J. Cradiol. 2007;99(Suppl. 1):S21–S33. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 266.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type diabetes (RECORD): a multicenter, randomized, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 267.Nissen SE, Nicholls SJ, Wolski K, et al. Compariosn of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with Type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 268.Betterge DJ. CHICAGO, PERISCOPE and PROactive: CV risk modification in diabetes with pioglitazone. Fundam. Clin. Pharmacol. 2009;23:675–679. doi: 10.1111/j.1472-8206.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 269.Polonsky T, Mazzone T, Dvaidson M. The clinical implications of the CHICAGO study for the management of cardiovascular risk in patients with Type 2 diabetes. Trends Cardiovasc. Med. 2009;19:94–99. doi: 10.1016/j.tcm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 270.Magee ME, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 diabetes (BARI 2D) trial. Am. J. Cardiol. 2006;97(Suppl. 1):20–30. doi: 10.1016/j.amjcard.2006.02.024. [DOI] [PubMed] [Google Scholar]
Websites
- 301.International cardiovascular disease statistics: statistical fact sheet: populations (2008 update) American Heart Association www.americanheart.org/downloadable/heart/1201543457735FS06INT08.pdf.
- 302.International cardiovascular disease statistics: heart disease and stroke statistics (2008 update at-a-glance) American Heart Association www.americanheart.org/downloadable/heart/200078608862HS _Stats%202008.final.pdf.
- 303.American Diabetes Association: national fact sheet, 2005. www.diabetesorg/diabetes-statisticsjsp.
- 304.Pioglitazone effect on regression of intravascular sonographic coronary obstruction perspective evaluation (PERISCOPE): current controlled trials website. www.controlled-trials.com/mect/PERISSCOPE/1059/122553.html. [PubMed]