Abstract
Primary angiosarcoma of the breast is a rare malignant tumor. We report a case of breast primary cutaneous angiosarcoma in a patient with a strong family history of malignancy. For definitive diagnosis, a tissue biopsy is needed, with immunostaining for the presence of blood vessel endothelial markers CD31 and CD34. Total mastectomy is the preferred method of surgical treatment. Chemotherapy has not been shown to increase overall survival, but in some instances it may improve local control and disease-free survival. Surgery combined with radiation may increase local control, but patients at high risk of recurrence may benefit from adjuvant treatment as well. We discuss the potential benefits from various treatments for primary cutaneous breast angiosarcoma.
Angiosarcoma of the breast is a very rare malignant tumor, with few patients surviving long term (1). It may occur as a primary tumor, as a complication of radiation therapy after breast conservation, or during pregnancy (2–4). True primary angiosarcomas account for <0.04% of malignant breast neoplasms (5), and primary angiosarcomas of the breast have a reported incidence of 17 new cases per million women (6). We describe a case of primary cutaneous angiosarcoma of the breast in a patient with a family history of malignancy.
CASE REPORT
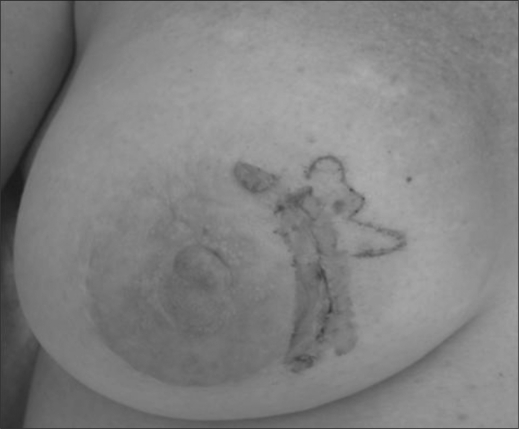
A 54-year-old Caucasian woman visited her dermatologist with complaints of a “rash” on her breast present for approximately 3 months. She was referred for biopsy, which revealed cutaneous angiosarcoma. She presented to our office with complaints of thickened, purple discoloration located around the 4:00 position on the right breast (Figure 1). The patient's history was unremarkable except for a history of breast implants along with a record of several breast surgeries, most recently in 2006, for correction of fibrotic capsulation around the implant. She also had trauma to her right breast following a skiing accident about 4 to 5 months before presentation. A punch biopsy showed findings suggestive of chronic inflammation and malignancy. Magnetic resonance imaging (MRI) of the breast revealed hematoma associated with skin thickening. A second punch biopsy of the lesion revealed an atypical intradermal vascular proliferation suspicious for malignancy.
Figure 1.
Angiosarcoma of the breast.
A full-thickness skin biopsy was then performed. Histologically, the tumor consisted of nodules of vascular proliferation with atypical spindle cells in a cuff-like appearance (Figure 2a). The neoplastic cells showed moderate to focally marked pleomorphic nuclei, a high mitotic index, and an overall infiltrative pattern. Immunostaining indicated that these cells strongly expressed CD34 (Figure 2b) and CD31 (not shown) but did not express HHV8. These findings led to a diagnosis of angiosarcoma.
Figure 2.
Histology of the grade II tumor. (a) Angiosarcoma stained with hematoxylin and eosin (original magnification 400×). (b) Angiosarcoma stained with antibody to CD34 (original magnification 400×).
The patient had a negative metastatic workup (complete blood count, complete metabolic profile and computed tomography scan). She then received a right mastectomy. The pathology of the tumor revealed a grade 2 (7) tumor 4.5 cm in diameter. The patient is currently undergoing adjuvant chemotherapy with single-agent paclitaxel every 3 weeks.
She has a strong family history of malignancy. Her father died at the age of 64 of chronic leukemia, reportedly diagnosed at 62 years of age. Her brother died as a result of glioblastoma diagnosed at age 56, and her sister died at age 43 of non-Hodgkin's lymphoma, initially diagnosed at the age of 43. In addition, her paternal aunt was diagnosed with breast cancer in her 60s.
DISCUSSION
Angiosarcoma of the breast
The first documented case of breast angiosarcoma was presented by Borrman in 1907 (8). Different from breast carcinomas, primary angiosarcoma of the breast occurs sporadically in young women, usually during the third and fourth decades of life. The probability of developing angiosarcoma of the breast has been attributed to multiple risk factors, including trauma, radiation, lymphedema, and breast implants. In a retrospective study of almost 200,000 women with breast cancer, those who received adjuvant radiotherapy were at a 16-fold increased risk for development of angiosarcoma (9). Lymphedema as a result of axillary lymph node dissection is considered a risk for developing angiosarcoma; however, there are no definitive data to support this claim. The same is true for trauma and breast implants. Both have been observed to occur in cases of breast angiosarcoma, but there are no definitive data to support these claims. On the other hand, there are reports of cases of angiosarcoma of the breast and chest wall associated with implants and fibrocystic breast disease (10, 11). Whether the combination of trauma and reaction to breast implants was the cause of cutaneous angiosarcoma in our patient is an intriguing idea, but one that needs evidence to support it. Due to the rarity of angiosarcoma, especially a primary cutaneous angiosarcoma of the breast, it is difficult to make any conclusions as to causality.
Patients with primary breast angiosarcoma normally present with a palpable mass. Bluish skin discoloration occurs in up to a third of patients and is thought to be attributable to the vascular nature of the tumor (5). In a study of a series of 24 breast angiosarcoma cases, the mean tumor size of the mass at presentation was 5.9 cm (5). Mammographic findings tend to be nonspecific for angiosarcoma, while with ultrasound, angiosarcoma typically presents as a heterogeneous, hyperechoic, hypervascular mass (5). MRI is more likely to image an angiosarcoma; however, this was not true in our case. An MRI of angiosarcoma shows a heterogeneous mass with low signal intensity on T1-weighted images, but signal intensity is high in images that are heavily T2-weighted (5, 12). Although not definitive, MRI is useful in ascertaining the extent of tumor and in planning surgery. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) may be used for staging of angiosarcoma (13). One case report showed focal, intense accumulation of FDG in angiosarcomas of the heart, with a standard uptake value of 7.5 (14).
A definitive preoperative diagnosis may be achieved with fine-needle aspiration cytology or a core needle biopsy (15). Immunohistochemical stains for epithelial markers (pancytokeratin), endothelial markers (CD34 and CD31), and other sarcoma markers are helpful in making the correct diagnosis (16). Rosen's method for grading breast angiosarcoma correlates well with the clinical outcome, as a low grade is associated with a better outcome (7). His system is very similar to the French soft tissue sarcoma grading system, where various histologic aspects of the tumor are scored (tumor differentiation, mitotic count, tumor necrosis) and added together to give a final histologic grade (17). Rosen's study gave estimated probabilities of disease-free survival of 5 years following initial treatment: stage I, 76%; stage II, 70%; and stage III, 15%. Our patient had stage II disease (7). Thus, prompt localization and identification of angiosarcoma is vital in the treatment of this disease.
Total mastectomy alone is the preferred method of surgical treatment (18). Sarcomas are less likely to spread to the lymph nodes, as Sher et al demonstrated (3). Sarcomas most commonly spread to the lung. In 31 cases of breast angiosarcoma, only two had lymph node invasion (3). Studies examining the efficacy of adjuvant chemotherapy are lacking, due in part to the low incidence of breast angiosarcomas. One retrospective study revealed that 36% of patients with primary angiosarcoma received chemotherapy in an adjuvant or neoadjuvant setting (7). Sher et al reported that adjuvant chemotherapy using an anthracycline and ifosfamide or gemcitabine and a taxane did not significantly improve recurrence-free survival compared with patients who did not receive chemotherapy (38 vs. 31 patients; hazard ratio, 0.47; P = 0.11). However, administration of chemotherapy at the time of recurrence resulted in a 48% response rate (3). In the case of secondary angiosarcomas induced by radiation treatment, docetaxel showed promise for treating secondary breast angiosarcomas that were refractory to anthracycline-based chemotherapy (19). Bevacizumab, the anti–vascular endothelial growth factor antibody, has been used as treatment for angiosarcomas to block blood vessel growth, but the results have been variable. Currently two phase II clinical trials are investigating the use of bevacizumab in cases of sarcoma, including angiosarcoma. The goal of the first trial, which has completed accrual, is to determine the effect of treatment with bevacizumab alone and to measure disease-free survival in patients with angiosarcoma (20). The second trial, which is still accruing patients, involves treatment with bevacizumab in combination with gemcitabine and docetaxel in patients with various sarcomas, including angiosarcoma (21). Once results of these studies are available, we may better know the effect of this adjuvant therapy in cases of primary breast angiosarcomas.
For patients with sarcomas of the breast, it has been suggested that radiation therapy after surgical resection may have a beneficial effect on outcome, especially for patients with microscopically positive margins (18). There was no statistical correlation of adjuvant radiation therapy with survival in this study, due to the small number of patients and the retrospective nature of the study. But, patients at high risk of recurrence (with large, high-grade tumors) may benefit from adjuvant treatment with improved local control and disease-free survival (18). Adjuvant radiation therapy should be administered especially when the margins of resection are microscopically involved after definitive surgical treatment, such as in this case.
Li-Fraumeni syndrome
Li-Fraumeni syndrome (LFS) is a cancer predisposition syndrome associated with soft tissue sarcoma, osteosarcoma, premenopausal breast cancer, brain tumors, adrenocortical carcinoma, and a variety of other neoplasms (22). More than 70% of individuals diagnosed clinically have an identified disease-causing germline mutation in TP53, the only gene known to be associated with LFS (23).
Since our patient had a personal history of sarcoma, as well as a family history of brain tumors, leukemia, and lymphoma, she met with a genetic counselor to assess the possibility of LFS. Using sequencing and deletion/duplication studies, no mutation was found in her p53 gene. Based on the patient's family history of malignancy, there is likely a genetic predisposition to cancer. At this time, it is not known what gene(s) are contributing to this familial cancer risk.
Conclusion
Primary cutaneous angiosarcoma of the breast is a very rare disease. PET–computed tomography is useful for staging workup. Definitive diagnosis is based on pathology results. Total mastectomy is the preferred treatment. Although no clinical trial proves the benefit of adjuvant chemotherapy or radiation therapy, both therapies should be considered in patients at high risk of recurrence.
References
- 1.Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer. 1980;46(2):368–371. doi: 10.1002/1097-0142(19800715)46:2<368::aid-cncr2820460226>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Yap J, Chuba PJ, Thomas R, Aref A, Lucas D, Severson RK, Hamre M. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52(5):1231–1237. doi: 10.1016/s0360-3016(01)02799-7. [DOI] [PubMed] [Google Scholar]
- 3.Sher T, Hennessy BT, Valero V, Broglio K, Woodward WA, Trent J, Hunt KK, Hortobagyi GN, Gonzalez-Angulo AM. Primary angiosarcomas of the breast. Cancer. 2007;110(1):173–178. doi: 10.1002/cncr.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingaszner LC, Enzinger FM, Taylor HB. Hemangiosarcoma of the breast. Cancer. 1965;18:352–361. [Google Scholar]
- 5.Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007;242(3):725–734. doi: 10.1148/radiol.2423060163. [DOI] [PubMed] [Google Scholar]
- 6.Desbiens C, Hogue JC, Levesque Y. Primary breast angiosarcoma: avoiding a common trap. Case Reports in Oncological Medicine. 2011;2011(Article ID 517047):5. doi: 10.1155/2011/517047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer. 1988;62(10):2145–2151. doi: 10.1002/1097-0142(19881115)62:10<2145::aid-cncr2820621014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Borrman R. Metastasenbildung bei histologish gutartigen geschwulsten: Fall von Metastasierendem Angiom. Beitr Pathol Anat. 1907;40:372–393. [Google Scholar]
- 9.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92(1):172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.Cuesta-Mejías T, de León-Bojorge B, Abel de la Peña J, Valenzuela-Tamariz J. Breast angiosarcoma in a patient with multiple surgical procedures and breast implant. Report of a case [article in Spanish] Ginecol Obstet Mex. 2002;70:76–81. [PubMed] [Google Scholar]
- 11.Saunders ND, Marshall JS, Anderson RC. A case of chest wall angiosarcoma associated with breast implants. J Thorac Cardiovasc Surg. 2007;134(4):1076–1077. doi: 10.1016/j.jtcvs.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 12.Liberman L, Dershaw DD, Kaufman RJ, Rosen PP. Angiosarcoma of the breast. Radiology. 1992;183(3):649–654. doi: 10.1148/radiology.183.3.1584913. [DOI] [PubMed] [Google Scholar]
- 13.Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190(2):533–538. doi: 10.2214/AJR.07.2909. [DOI] [PubMed] [Google Scholar]
- 14.Freudenberg LS, Rosenbaum SJ, Schulte-Herbrüggen J, Eising EG, Lauenstein T, Wolff A, Bockisch A. Diagnosis of a cardiac angiosarcoma by fluorine-18 fluordeoxyglucose positron emission tomography. Eur Radiol. 2002;12(Suppl 3):S158–S161. doi: 10.1007/s00330-002-1478-z. [DOI] [PubMed] [Google Scholar]
- 15.Gherardi G, Rossi S, Perrone S, Scanni A. Angiosarcoma after breast-conserving therapy: fine-needle aspiration biopsy, immunocytochemistry, and clinicopathologic correlates. Cancer. 2005;105(3):145–151. doi: 10.1002/cncr.21035. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol. 2009;13(3):147–150. doi: 10.1016/j.anndiagpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Coindre JM. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 18.Kaklamanos IG, Birbas K, Syrigos KN, Vlachodimitropoulos D, Goutas N, Bonatsos G. Breast angiosarcoma that is not related to radiation exposure: a comprehensive review of the literature. Surg Today. 2011;41(2):163–168. doi: 10.1007/s00595-010-4341-x. [DOI] [PubMed] [Google Scholar]
- 19.Mano MS, Fraser G, Kerr J, Gray M, Evans V, Kazmi A, Canney P. Radiation-induced angiosarcoma of the breast shows major response to docetaxel after failure of anthracycline-based chemotherapy. Breast. 2006;15(1):117–118. doi: 10.1016/j.breast.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Agulnik M. Bevacizumab in treating patients with angiosarcoma. ClinicalTrials.gov. Available at http://clinicaltrials.gov/ct2/show/NCT00288015?term=NCT00288015&rank=1 2010; accessed November 28, 2011.
- 21.D'Adamo D. Gemcitabine and docetaxel with bevacizumab in selected sarcoma subtypes. ClinicalTrials.gov. Available at http://clinicaltrials.gov/ct2/show/NCT00887809?term=NCT00887809&rank=1 2011; accessed November 28, 2011.
- 22.Birch JM. The Li-Fraumeni cancer family syndrome. J Pathol. 1990;161(1):1–2. doi: 10.1002/path.1711610102. [DOI] [PubMed] [Google Scholar]
- 23.Schneider K, Garber J. Li-Fraumeni syndrome. 1999 Jan 19 [updated 2010 Feb 09]. In Pagon RA, Bird TD, Dolan CR, Stephens K, eds. GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle, 1993. Available at http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=li-fraumeni; accessed November 8, 2011.