Undetected contamination of food and water by oocysts causes human infections in North America. Risks often are unrecognized. Education alone cannot prevent suffering and economic consequences associated with congenital toxoplasmosis. Prenatal screening can facilitate prevention and treatment of congenital toxoplasmosis.
Abstract
(See the Editorial Commentary by Linn, on pages 1090–1.)
Background. Congenital toxoplasmosis presents as severe, life-altering disease in North America. If mothers of infants with congenital toxoplasmosis could be identified by risks, it would provide strong support for educating pregnant women about risks, to eliminate this disease. Conversely, if not all risks are identifiable, undetectable risks are suggested. A new test detecting antibodies to sporozoites demonstrated that oocysts were the predominant source of Toxoplasma gondii infection in 4 North American epidemics and in mothers of children in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS). This novel test offered the opportunity to determine whether risk factors or demographic characteristics could identify mothers infected with oocysts.
Methods. Acutely infected mothers and their congenitally infected infants were evaluated, including in-person interviews concerning risks and evaluation of perinatal maternal serum samples.
Results. Fifty-nine (78%) of 76 mothers of congenitally infected infants in NCCCTS had primary infection with oocysts. Only 49% of these mothers identified significant risk factors for sporozoite acquisition. Socioeconomic status, hometown size, maternal clinical presentations, and ethnicity were not reliable predictors.
Conclusions. Undetected contamination of food and water by oocysts frequently causes human infections in North America. Risks are often unrecognized by those infected. Demographic characteristics did not identify oocyst infections. Thus, although education programs describing hygienic measures may be beneficial, they will not suffice to prevent the suffering and economic consequences associated with congenital toxoplasmosis. Only a vaccine or implementation of systematic serologic testing of pregnant women and newborns, followed by treatment, will prevent most congenital toxoplasmosis in North America.
If mothers of infants with congenital toxoplasmosis could reliably identify exposure to Toxoplasma gondii, it would provide strong support for eliminating this disease by educating pregnant women about risk factors. Conversely, if not all risk factors are recognized, an undetectable environmental risk for pregnant women that cannot be eliminated by education alone may be present.
One author (K. B.) conducted in-person interviews of a cohort of North American mothers of infants with congenital toxoplasmosis regarding recognized risk factors [1]. Serum samples from 76 of these mothers were obtained in the perinatal period when their congenitally infected infants were born and have been stored by the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS). Recently, these serum samples were tested with a new assay to determine whether antibodies to the sporozoite form of T. gondii were present [2]. Antibodies to sporozoites are present only when humans or other animals have been infected with T. gondii oocysts, formed in cats. Antibodies to sporozoites are not present when the host is infected with bradyzoites in tissue cysts [2]. Tissues are infectious when consumed in undercooked meat [3]. We recently found, by testing serum samples for this antibody to a 11-kDa sporozoite protein, that oocyst infection also occurred in persons in 4 North American epidemics (ie, at a riding stable in Atlanta [4], at a research laboratory, in an Amish family [2] in the United States, and in Victoria, Canada [5]) and in mothers of infants in the NCCCTS [2]. Here, we correlate presence of this serum antibody to sporozoites with a mother’s recognition of risk factors for acquisition of T. gondii, either from materials contaminated with oocysts or bradyzoites during the months before delivery, demographic features, or presence and manifestations of clinical illness in mother and child after delivery.
METHODS
National Collaborative Chicago-based Congenital Toxoplasmosis Study Mothers
Seventy-six acutely infected mothers in the NCCCTS who transmitted T. gondii to their fetuses in utero provided serum samples for testing near the time when their infected child was born between 1981 and 1999. These mothers have been described in other publications of the NCCCTS, including the Amish and the Victoria, Canada, mothers [1, 2, 3, 6–27]. Our study was conducted according to the ethical standards for human experimentation established in the Declaration of Helsinki, with the prior approval of the Institutional Review Board of the University of Chicago and in accordance with Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all adult participants and from parents or legal guardians of minors.
Questions Asked Concerning Risk Factors
In addition to providing serum samples for serologic testing, the mothers were questioned about their possible exposure to common vehicles of T. gondii transmission, specifically cats, oocyst-contaminated soil, and meat not cooked to well-done. Each mother was asked whether she owned a cat, had cleaned a cat’s litter box, had gardened, had come into contact with a sandbox, or had very close, sustained, hours-long contact with a new kitten. For example, 1 mother kept her new kitten in bed with her at night and was scratched by this kitten while playing with it. The interviewer classified the type of exposure to cats for each mother. Cleaning a litter box, gardening, coming into contact with a sandbox, or very close, sustained contact with a kitten were defined as significant risk factors. Owning an indoor cat fed dry or canned food is considered a cat-associated risk factor, but not necessarily one that has substantial risk for exposure to oocysts for the cat owner. The mothers were also asked whether they had prepared or consumed raw or undercooked meat or other foods that may have harbored the parasite, such as raw eggs or unpasteurized dairy products. Details about the type and frequency of the exposure and when it occurred during the pregnancy were noted. Any symptoms of illness during pregnancy that could indicate infection, such as influenza-like symptoms, fever, night sweats, headache, or lymphadenopathy, were also recorded. Demographic information including maternal age, place of residence, race/ethnicity, method of payment for care, and the variables needed to calculate the family’s Hollingshead index (a measure of socioeconomic status) were obtained.
Evaluation of Mother and Child
Socioeconomic limitations were circumvented by providing complimentary travel and accommodations for all patients participating in the study. Each patient’s medical history and neuroradiological scans were reviewed. For the infants, evaluations were conducted by adult and pediatric specialists in infectious diseases, neurology, developmental psychology, developmental pediatrics, pediatric ophthalmology, audiology, and neuroradiology. Hematologic laboratory tests, including complete blood cell count, differential white blood cell count, and platelet counts were also performed. The evaluations were conducted at predetermined ages: birth and 1, 3.5, 5, 7.5, 10, 15, and 20 years [6–27].
Toxoplasma gondii Serologic Testing of Mother and Child
Toxoplasma gondii Immunoglobulin G Assays
The Biomérieux direct agglutination assay [21] was used to measure immunoglobulin G (IgG) in serum samples from the pregnant Amish women. The Sabin-Feldman dye test was used to measure IgG in all other samples tested [28]. The dye test is based on the observation that living parasites incubated with normal serum become swollen and stain deeply blue when methylene blue is added. Exposure to antibody-containing serum results in thin, distorted unstained parasites when the dye is added because of lysis of the organisms secondary to activation of complement.
Toxoplasma gondii–Specific Immunoglobulin M Assays
The immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) [21] was used to test serum samples from the mothers in the NCCCTS. The IgM ELISA [21] was used and, beginning on 1 April 1991, IgM Immunosorbent Agglutination Assay (ISAGA) [21] was used to test newborns’ serum samples for IgM-specific antibodies to T. gondii in the infants in our patient cohort [21]. The IgM ISAGA is more sensitive than the IgM ELISA for detection of IgM antibody specific to T. gondii in the infant [21]. Serologic testing for the persons in the Atlanta epidemic was performed as described elsewhere [4].
Other T. gondii–Specific Serologic Testing
During the later years of the study, immunoglobulin A (IgA) ELISAs were performed for NCCCTS mothers and their children [21]. In addition, differential agglutination tests [21] were performed for pregnant women in the NCCCTS, and avidity assays [21] were also performed.
Determination of Presence of Antibody to Sporozoites
This assay was performed as described elsewhere [2].
Statistical Methods
Correlations between the presence of serum antibody to the 11-kDa sporozoite protein and demographic characteristics, perceived risk factors, and clinical findings were examined using Fisher exact test for categorical variables and the Wilcoxon rank-sum test [29] for continuous or ordinal variables. The sensitivity, specificity, and positive and negative predictive values of the perceived risk factors were calculated by treating the presence of serum antibody as the gold standard, or true indicator of exposure to oocysts. Exact confidence intervals for proportions were based on the binomial distribution. All statistical analyses were performed using Stata software, version 10 (StataCorp) [30].
RESULTS
High Rate of Exposure to Oocysts in National Collaborative Chicago-based Congenital Toxoplasmosis Study Mothers
Seventy-eight percent of NCCCTS mothers tested, including 3 mothers who were part of an epidemic in Victoria, Canada, and the mother in an Amish family, were infected by sporozoites.
Lack of Correlation of Birth Year or Time of Year With Oocyst Exposure
Neither year (Figure 1) nor month of the year (Figure 2) when the infant was born was clearly associated with exposure to oocysts.
Figure 1.
Distribution of mothers with and without antibody to sporozoites in each year, 1981–1999.
Figure 2.
Lack of correlation between presence of antibody to sporozoites in maternal serum samples and month of birth of child.
Demographic Characteristics and Exposure to Oocysts
Socioeconomic status, age, race (white vs other), and hometown size classified as rural, urban, or suburban did not differentiate those who did or did not have antibodies to sporozoites (Table 1). Women exposed to sporozoites were from throughout the United States (Figure 3).
Table 1.
Correlation of Antibody to Sporozoites in Maternal Serum Samples and Maternal Demographic Characteristics
| Demographic factor | Overall (N = 76) | Positive (n = 59) | Negative (n = 17) | P value |
| SES scorea | 3.0 (1.5); 3 (n = 75) | 2.9 (1.5); 3 (n = 58) | 3.1 (1.4); 3 | .60 |
| Agea | 27.5 (6.3); 27 | 27.8 (6.2); 27 | 26.5 (6.4); 27 | .64 |
| Hometown sizeb | .43 | |||
| Rural | 28 | 27 | 29 | |
| Suburban | 25 | 22 | 35 | |
| Urban | 47 | 51 | 35 | |
| Caucasianb | 71 | 73 | 65 | .55 |
Abbreviation: SES, socioeconomic status.
Numbers are mean (standard deviation); median.
Numbers are percentages.
Figure 3.
Lack of correlation of antibody to sporozoites in maternal serum samples with hometown location and hometown size. Mothers with serum antibody to sporozoites and cysts are distributed across rural, urban, and suburban regions in the United States.
Sensitivity and Specificity of Risk Factors in Identifying Mothers Infected by Oocysts
Although some persons could be identified who had substantial exposure to a cat and likely oocyst exposure (eg, a mother who had moved into a house where dozens of cats had used the basement to defecate, and the mother had cleaned this basement while pregnant), the correlation between cat exposure and having antibody to T. gondii sporozoites was not robust (Table 2, Table 3). In addition, none of the mothers who were exposed during the water-borne Victoria epidemic [5] recognized cat exposure. Two of these mothers reported exposure to meat not cooked to well-done. It is of interest that the serum samples from 4 mothers of NCCCTS children who were in this epidemic had antibody to sporozoites, although several could only identify a rare meat exposure. There are no published data documenting oocyst exposure for other persons in the epidemic. At present, serum samples from other persons in this epidemic have not been tested using our sporozoite assay.
Table 2.
Correlation of Antibody to Sporozoites in Maternal Serum Samples and Recognition of Exposure to Uncooked Meat Versus Material Contaminated by Oocysts From Cats
| Overall (N = 76) | Positive (n = 59) | Negative (n = 17) | P value | |
| At least 1 significant cat risk factora,b | 45 | 49 | 29 | .18 |
| Any risk factora | 84 | 83 | 88 | 1.00 |
Numbers are percentages.
Significant cat exposure is defined in Methods as cleaning a cat’s litter box, gardening, coming into contact with a sandbox, or very close, sustained contact with a kitten. Owning an indoor cat fed dry or canned food is considered a risk factor associated with cats, but not necessarily one that has substantial risk for exposure to oocysts for the cat owner.
Table 3.
Specificities, Sensitivities, and Negative and Positive Predictive Values of Attributing Risk of Exposure Based on 76 Acutely Infected Mothers of Children Enrolled in National Collaborative Chicago-based Congenital Toxoplasmosis Study
| Specificitya | 95% CI | Sensitivityb | 95% CI | NPVc | 95% CI | PPVd | 95% CI | |
| At least 1 cat risk factor | 24 | 7–50 | 69 | 56–81 | 18 | 5–40 | 76 | 62–87 |
| (4/17) | (41/59) | (4/22) | (41/54) | |||||
| At least 1 significant cat risk factore | 71 | 44–90 | 49 | 36–63 | 29 | 16–45 | 85 | 69–95 |
| (12/17) | (29/59) | (12/42) | (29/34) |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Specificity indicates the percentage of individuals negative for sporozoite antibody who did not identify any cat risk factors.
Sensitivity is the percentage of persons positive for antibody who identified at least 1 cat risk factor.
NPV is the percentage of persons not identifying any risk factors who were negative for antibody to the 11-kDa sporozoite protein.
PPV is the percentage of persons identifying a risk factor who were positive for antibody to the sporozoite protein.
Significant cat exposure is defined in Methods as cleaning a cat's litter box, gardening, coming into contact with a sandbox, or very close, sustained contact with a kitten. Owning an indoor cat fed dry or canned food is considered a risk factor associated with cats, but not necessarily one that has substantial risk for exposure to oocysts for the cat owner.
Maternal Illness and Oocyst Exposure
Presence of specific symptoms or total number of symptoms did not correlate significantly with acquisition of oocysts by mothers (Figure 4).
Figure 4.
Lack of correlation of antibody to sporozoites in maternal serum samples with presence of maternal illness. Mothers’ symptoms include lymphadenopathy, fever, night sweats, headache, and influenza-like illness.
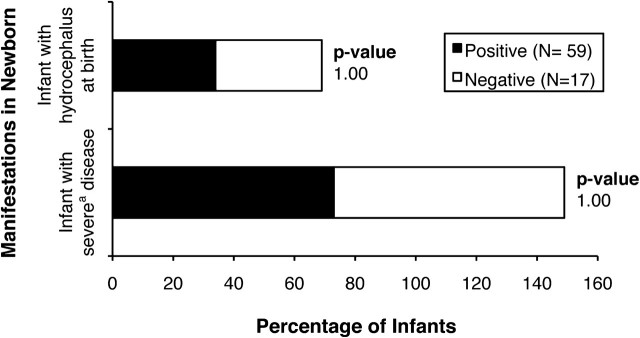
Severity of Illness in the Infant and Oocyst Exposure
Neither the severity of illness in the infant nor the presence of hydrocephalus correlated significantly with presence of maternal serum antibody to sporozoites (Figure 5).
Figure 5.
Lack of correlation of antibody to sporozoites in maternal serum samples and manifestations of infection in the newborn infant. aSevere disease is defined in references [14].
Maternal and Infant Serologic Tests
The magnitude of serologic tests for T. gondii–specific IgG, IgM, and IgA in mothers and infants did not correlate significantly with oocyst exposure. The acetone-fixed parasite titer in the differential agglutination test was higher in the serum samples from mothers with antibody to the 11-kDa sporozoite protein (P = .04; Table 4).
Table 4.
Correlation of Presence of Antibody to Sporozoites in Maternal Serum Samples and Pattern of Antibody Response to Toxoplasma gondii in Mother and/or Child at Birth of Child
| Antibody Assay | Overall (N = 76) | Positive (n = 59) | Negative (n = 17) | P value |
| IgA ELISAa | ||||
| Mother (n = 59) | 7.9 (7.6); 5.3 | 8.2 (7.8); 5.3 | 7.0 (7.0); 5.3 | .55 |
| Child (n = 58) | 9.8 (10.4); 3.5 | 10.3 (10.4); 4.7 | 7.8 (10.5); 2.9 | .53 |
| IgM ELISAa | ||||
| Mother (n = 68) | 5.0 (3.1); 4.5 | 5.2 (2.9); 4.6 | 4.2 (3.4); 2.8 | .15 |
| Child (n = 41) | 3.2 (3.6); 1.9 | 3.0 (3.4); 1.8 | 4.2 (4.2); 3.6 | .28 |
| IgG DTa | ||||
| Mother (n = 74) | 6545 (7512); 4096 | 6522 (8123); 4096 | 6622 (5150); 4096 | .30 |
| Child (n = 75) | 6059 (9542); 4096 | 6421 (10506); 4096 | 4823 (5085); 2048 | .75 |
| IgM ISAGAa | ||||
| Child (n = 47) | 6.7 (5.1); 9 | 6.8 (5.1); 9 | 6.3 (5.4); 8 | .67 |
| ACa (n = 60) | 1228 (575); 1600 | 1305 (528); 1600 | 946 (669); 800 | .04 |
| HSa (n = 60) | 2647 (1015); 3200 | 2494 (1100); 3200 | 3200 (0); 3200 | .02 |
Abbreviations: AC, results with acetone-fixed antigen; DT, Sabin-Feldman dye test; ELISA, enzyme-linked immnosorbent assay; HS, results with formalin-fixed antigen; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; ISAGA, immunoglobulin-M immunosorbent agglutination assay.
Numbers are mean (SD); median.
DISCUSSION
A new method to determine whether infection with T. gondii was acquired from oocysts in the preceding 8 months was reported recently [2]. This assay determined that oocyst infection induces an antibody specific to an 11-kDa sporozoite protein [2]. This antibody response differentiates between infections acquired by ingesting oocysts and infection caused by tissue cysts in pigs, mice, and humans [2]. Analysis of serum samples for presence of antibody to an 11-kDa sporozoite protein can determine whether T. gondii infection was caused by the acquisition of oocysts for 6–8 months after acquisition of the infection [2]. All persons received a diagnosis on the basis of serologic testing from the US reference laboratory. For adults (ie, for acutely infected mothers), these tests are highly reliable, essentially 100% sensitive, and specific in this reference laboratory. In an analysis of serum samples obtained in the United States and Canada, oocysts were found to be the predominant source of T. gondii infection in 78% of the mothers in our NCCCTS cohort and in 4 North American epidemics, including an outbreak in Victoria (3 of 3 mothers), 6 of 8 persons in an Amish community, in all 6 persons in a laboratory accident, and in a stable in Atlanta (15 [88%] of 17 persons) [2]. These findings indicate that oocysts are a major and often unrecognized source of infection in mothers of children with congenital toxoplasmosis and in epidemics in the United States.
It is important to both inform susceptible individuals and the general populations that meat should be cooked thoroughly, fruits and vegetables should be washed before consumption, and contact with oocysts should be avoided, particularly when changing cat litter. Nonetheless, the data reveal that ownership of a cat is not necessary to acquire T. gondii, because it has been shown that oocyst exposure is not always associated with cat ownership or with recognition of risk factors. In the United States, there are an estimated 73 million feral cats and 78 million domestic cats [31], which allows for environmental contamination by oocysts to be the source of infection even for those who do not own cats. Hill et al recently determined that there was a low prevalence of tissue cysts currently in retail meats in the United States, including beef, chicken, and pork [32]. The low frequency of infected meat suggests that cats potentially were a cause for much T. gondii infection in the United States.
Analysis of the correlation between the antibody specific to the sporozoite protein in serum samples from infected mothers and recognized risk factors indicates that exposure to oocysts was not identified in 31% of those who actually acquired the infection by this route. Although it is beneficial for physicians to advise pregnant women to avoid exposure to cat feces, not all exposure is from recognized sources; most appeared not to be. Thus, although education may help to prevent some cases of toxoplasmosis, it will not be sufficient to prevent a substantial proportion of these infections, because acquisition can be undetected and recognized risk factors are frequently absent.
Because T. gondii infection in the United States is diagnosed without a systematic, gestational serologic screening program, it largely presents as severe disease in the infant. There are substantial clinical manifestations detected in the newborn period [6–27] that lead to diagnosis. The route of acquisition in the mother did not correlate with severity of symptoms in the infant. This has varied only slightly during the past 3 decades (R. McLeod, K. Boyer, E. Mui, K. Wroblewski, T. Karrison, M. Sautter, G. Noble, S. Withers, C. Swisher, P. Heydemann, D. Lee, D. Burrowes, E. Holfels, M. Mets, P. Latkany, W. Mieler, D. Patel, P. Meier, unpublished data). Acquisition from oocysts, as shown by presence of antibodies to sporozoites, also has not changed over time.
There may be differences in other countries or regional differences in the United States that are not reflected in our cohort. Incidence in different populations may vary significantly, as shown by the National Health and Nutrition Examination Survey data showing 10%–14% chronic infection rates among persons in various regions of the United States, with more infection in the southern states and an association with poverty. Another example is that this assay determined that approximately 45% of pregnant women with acute acquired toxoplasmosis during gestation in Chile had antibody to sporozoites [2], in contrast to 78% of mothers in the present series.
Another example of differing incidence in different geographic regions or populations in the United States is evident in an Amish community in Lancaster, Pennsylvania. Serum sample from an Amish child from the NCCCTS whose mother had antibody to oocysts was tested. In addition, serum samples from the child’s mother and 7 siblings were tested. All but the congenitally infected infant and her 2-year-old sibling were acutely infected and had antibody to oocysts.
Because of concern for other pregnant women and their children in the Lancaster Amish community, 114 pregnant women from this community provided serum samples for T. gondii IgG testing. Fifty-nine of these women were seropositive (52%), and 55 women (48%) were T. gondii IgG seronegative. One of the IgG-seropositive women had IgM antibody at low titer. The 114 serum samples from women in the Amish community were tested later for antibody to sporozoites. The 55 serum samples from those who had no antibodies to T. gondii were seronegative controls. All 59 women who were T. gondii IgG seropositive and IgM seronegative were considered chronically infected persons for development of the assay [2]. None of these women had antibody to the sporozoite protein, and only 1 had T. gondii–specific IgM antibodies, indicating that their infection was remote in time [2]. Other seronegative and chronically infected seropositive controls also were described in Hill et al [2].
Severe damage may occur in immunocompromised patients or in fetuses infected during early pregnancy, underscoring the key role of host factors in the pathogenicity of T. gondii. However, data suggest that other factors also play a role in the disease. For example, the higher incidence and severity of ocular toxoplasomsis in tropical areas (South America and Africa), compared with nontropical areas (Europe and North America), may be associated with acquisition of infection from oocysts rather than tissue cysts, exposure to a higher parasite load because of diet or living conditions, and genetic differences in strain virulence or host susceptibility to toxoplasmosis. The new test detecting antibodies to sporozoites contained in oocysts described here will be important in the field of toxoplasmosis because it offers the opportunity to better know the epidemiology of toxoplasmosis by identifying the source of infection (cysts vs oocysts). It can also pave the way for a better understanding of the role of the life-cycle stage in the pathogenicity of T. gondii.
The predominance of maternal acquisition of oocysts and the lack of recognition of oocyst exposure indicates that environmental contamination by oocysts contributes substantially to the acquisition of T. gondii and consequent disease in North America. Not only are risk factors commonly associated with acquisition of T. gondii often unrecognized by those infected, but our analysis indicates that socioeconomic status, maternal clinical presentations, and ethnicity were not reliable predictors of acquisition of T. gondii oocysts. Thus, only systematic screening of pregnant women and/or a vaccine have the potential to prevent the fetal disease caused by acquisition of T. gondii during gestation by women in North America.
Notes
Acknowledgments.
We thank the families who generously worked with us in this study; Joseph McCammon for his assistance with this manuscript; J. S. Remington and the Palo Alto Research Institute and Stanford University in Palo Alto, California, for performing the serologic testing used to diagnose NCCCTS patients and Amish mothers; Michael Kirisits and Douglas Mack for processing samples; Frank Jagdis, Andrew Burnett, Patrick McLeod, Marianna Wilson, Holmes Morton, and Donna Robinson for patient referral, identification, and evaluation; and C. Loss and C. Munoz-Zanzi for development of the sporozoite assay.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases (R01AI027530 to R. M.); the Research to Prevent Blindness Foundation, the Stanley Foundation and Medical Research Institute (07R-1890 to R. M.), the United States Department of Agriculture, ARS (to D. H.); and gifts from the Blackmon, Brennan, Cornwell, Cussen, Dougiello, Jackson, Kapnick, Kiewiet, Koshland, Langel, Lipskar, Mann, Morel, Rooney-Alden, Rosenstein, Samuel, and Taub families. The funding source and study sponsors had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Potential conflicts of interest.
All authors:No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Other members of the Toxoplasmosis Study Group include Dianna Bardo, Delilah Burrowes, Audrey Cameron, Ellen Holfels, Paul Latkany, Douglas Mack, John Marcinak, James McAuley, Marilyn Mets, Sanford Meyers, William Mieler, Dushyant Patel, Jeanne Perkins, Peter Rabiah, James Rago, Nancy Roizen, Lazlo Stein, Andrew Suth, Marie Weissbourd, Teri Hull, Kathy Zebracki, and Caitlin Roache,
References
- 1.Boyer K, Holfels E, Roizen N, et al. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obstet Gynecol. 2005;192:564–71. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Hill D, Coss C, Dubey JP, et al. Identification of a sporozoite-specific antigen fromToxoplasma gondii. J Parasitol. 2011;97:328–37. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelong M, Bernard J, Desmonts G, Couvreur J. Acquired toxoplasmosis. Arch Fr Pediatr. 1960;17:281–331. [PubMed] [Google Scholar]
- 4.Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300:695–9. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AJ, Shortt SG, Isaac-Renton J, King A, Werker D, Bowie WR. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology. 1998;105:1032–7. doi: 10.1016/S0161-6420(98)96004-3. [DOI] [PubMed] [Google Scholar]
- 6.McLeod R, Mack D, Foss R, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Antimicrob Agents Chemother. 1992;36:1040–8. doi: 10.1128/aac.36.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee T, Wolters C, Stein L, et al. Absence of sensorineural hearing loss in treated infants and children with congenital toxoplasmosis. Otolaryngol Head Neck Surg. 1992;106:75. doi: 10.1177/019459989210600131. [DOI] [PubMed] [Google Scholar]
- 8.Swisher C, Boyer K, McLeod R. Neurologic findings in congenital toxoplasmosis. Ann Neurol. 1992;32:448. [Google Scholar]
- 9.McAuley J, Boyer K, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Roizen N, Swisher C, Stein M, et al. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics. 1995;95:11–20. [PubMed] [Google Scholar]
- 11.Patel DV, Holfels EM, Vogel NP, et al. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996;199:433–40. doi: 10.1148/radiology.199.2.8668790. [DOI] [PubMed] [Google Scholar]
- 12.Mets M, Holfels E, Boyer K, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–24. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 13.Roizen N, Kasza K, Karrison T, et al. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: implications for compensatory intervention strategies. Pediatrics. 2006;118:e379–90. doi: 10.1542/peds.2005-1530. [DOI] [PubMed] [Google Scholar]
- 14.Mack D, Johnson J, Roberts F, et al. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–8. doi: 10.1016/s0020-7519(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 15.Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brézin A. Clinical manifestations of ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:91–102. doi: 10.3109/09273948.2011.564068. [DOI] [PubMed] [Google Scholar]
- 16.McLeod R, Boyer K, Karrison T, et al. Outcomes of treatment of congenital toxoplasmosis, 1981–2004, the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 17.Arun V, Noble AG, McLeod R. Cataracts in congenital toxoplasmosis. J AAPOS. 2007;11:551–4. doi: 10.1016/j.jaapos.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson SE, de Roubaix LA, Cortina-Borja M, et al. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benevento J, Jager R, Noble AG, et al. Toxoplasmosis associated neovascular lesions treated successfully with ranibizumab and antiparasitic therapy. Arch Ophthalmol. 2008;126:1152–5. doi: 10.1001/archopht.126.8.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008;146:375–84. doi: 10.1016/j.ajo.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. 6th ed. Philadelphia, PA: WB Saunders; 2006. pp. 947–1091. [Google Scholar]
- 22.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology. 2008;115:553–9.e8. doi: 10.1016/j.ophtha.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 23.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–44. doi: 10.1590/s0074-02762009000200029. ISSN 0074–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamieson SE, Cordell H, Petersen E, McLeod R, Gilbert RE, Blackwell JM. Host genetic and epigenetic factors in toxoplasmosis. Mem Inst Oswaldo Cruz. 2009;104:162–9. doi: 10.1590/s0074-02762009000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witola WH, Mui E, Hargrave A, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–66. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cong H, Mui E, Witola WH, et al. Human immunome, bioinformatic analyses using HLA supermotifs and the parasite genome, binding assays, studies of human T cell responses, and immunization of HLA-A*1101 transgenic mice including novel adjuvants provide a foundation for HLA-A03 restricted CD8+T cell epitope based, adjuvanted vaccine protective against Toxoplasma gondii. Immunome Res. 2010;6:12. doi: 10.1186/1745-7580-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees MP, Fuller SJ, McLeod R, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J. Immunol. 2010;184:7040–6. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frenkel JK, Jacobs L. Ocular toxoplasmosis; pathogenesis, diagnosis and treatment. AMA Arch Ophthalmol. 1958;59:260–79. [PubMed] [Google Scholar]
- 29.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–3. [Google Scholar]
- 30.StataCorp. Stata statistical software: release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 31.Levy JK, Crawford PC. Humane strategies for controlling feral cat populations. J Am Vet Med Assoc. 2004;255:1354–60. doi: 10.2460/javma.2004.225.1354. [DOI] [PubMed] [Google Scholar]
- 32.Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]