Abstract
Molecular typing was used to examine surveillance definitions for recurrent Clostridium difficile–associated diarrhea. Among 102 patients, 85 had a second episode within 8 weeks, 88% of which were relapses. Of 49 second episodes occurring after > 8 weeks, 65% were relapses. Categorization of a recurrent episode occurring after >8 weeks as a new infection may misrepresent the majority of episodes for surveillance.
In recent years, Clostridium difficile infection (CDI) has become more frequent, severe, and difficult to treat [1–3]. It is now recommended that healthcare facilities perform active surveillance for CDI, and many states in the United States have mandated facility-specific public reporting of healthcare-associated CDI rates [4]. To establish a uniform approach to surveillance, the Centers for Disease Control and Prevention constituted an Ad Hoc C. difficile Surveillance Working Group to develop interim surveillance definitions for new, recurrent, and healthcare facility–associated CDI. A particular challenge is classification of persons who have >1 episode of CDI. For these patients, the Ad Hoc Working Group determined that a second episode of CDI should be categorized as either a relapse or a second new infection (reinfection), based on the interval between positive test results. Specifically, they recommended that a relapse be defined as a second episode occurring within 2–8 weeks of the index case; a second new episode was defined as occurring ≥8 weeks after the index case [4]
Polymerase chain reaction (PCR) ribotyping, based on polymorphisms in the 16S-23S ribosomal RNA interspacer region, is a rapid method for typing of C. difficile isolates with good discriminatory power [5]. It is easy to perform and highly reproducible and is widely used in Europe for hospital-based surveillance of CDI [6]. In this study, we applied PCR ribotyping to specimens from a series of patients with ≥2 episodes of CDI to determine whether the second episode was due to the same strain as the first or a different strain. We then consider these results in the context of the Ad Hoc Working Group’s current recommendations [4]. The accuracy of surveillance definitions is increasingly important given the public scrutiny of healthcare-associated conditions and the possibility that reimbursement eventually may be decreased when patients develop a healthcare-associated infection such as CDI.
METHODS
Memorial Sloan-Kettering Cancer Center is 470-bed tertiary care cancer center in New York City. Between January 2008 and June 2010, patients with 2 episodes of CDI occurring ≥2 weeks apart were identified by means of microbiology records, and the intervals between episodes were determined. Patients with >1 recurrence (≥3 episodes) were examined by intervals between first and second then second and third episodes; the time from the first to the third episode was not analyzed. Patients who had had episodes before the start of the study period were excluded. The institutional review board reviewed the study and granted a Health Insurance Portability and Accountability Act waiver of authorization.
Diagnosis of Clostridium difficile Infection
Before September 2009, all stool samples submitted to the Microbiology Laboratory were evaluated by using the C. difficile cytotoxin neutralization assay. After September 2009, a 2-step algorithm was implemented, using an enzyme immunoassay for the detection of C. difficile common antigen glutamate dehydrogenase as a screening assay, followed by testing of all glutamate dehydrogenase–positive stools with the cytotoxin neutralization assay. Isolates were studied by PCR ribotyping, as described elsewhere by Bidet et al [5]. The American Type Culture Collection strain BAA-1805 (C. difficile, North American pulse-field type 1 [NAP1]; toxinotype III, binary toxin positive) was included as a reference strain in all gels.
Statistical Analysis
Statistical analysis was performed using Fisher's exact and χ2 tests and GraphPad Quick Calcs software. Microsoft Excel graphing (linear regression) was used to calculate the slope, y-intercept, and correlation coefficient for data shown in the Figure 1.
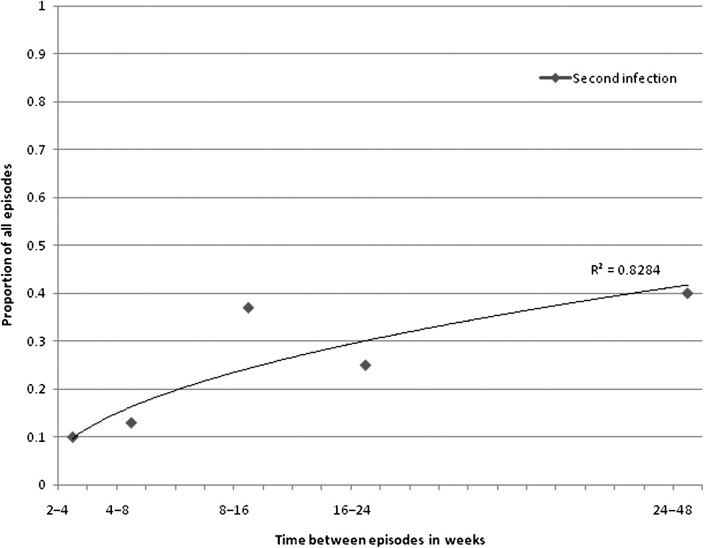
Figure 1.
Relapse and second infection as determined by molecular typing, depicted by time from the initial episode.
RESULTS
The incidence of CDI during the 30-month study period was 21 cases/10 000 patient days, and the CDI rate of hospital-acquired disease was 9.8 cases/10 000 patient days. A total of 134 paired isolates of C. difficile from 102 patients were analyzed by PCR ribotyping. Among the 134 pairs, 85 were collected 2–8 weeks apart, and 49 were collected >8 weeks apart. Twenty-four patients (24%) had ≥3 episodes of CDI. No outbreaks were detected by molecular typing during the study period.
Episodes of Clostridium difficile Infection >2 and <8 Weeks Apart
Eighty-five paired isolates of C. difficile from 70 patients were analyzed by PCR ribotyping. The mean interval between these episodes was 28 days, and the median was 25 days (range, 14–55 days). For 75 (88%) of 85 episodes, the second strain was identical to the original infecting strain, reflecting relapse. The likelihood of having a second infection did not vary for pairs collected <4 or >4 weeks apart (90% vs 86.5%) (Table 1). Limiting the analysis to comparison only between the index case and the second episode (ie, excluding the third and fourth episodes) did not change the results (Table 1).
Table 1.
Relapse Versus Second Infection as Determined by Polymerase Chain Reaction Ribotyping and Sorted by Interval Between Episodes
| All recurrent episodes, no. (%) (n = 134) |
Index and second episode only from patients with >1 recurrence, no. (%) (n = 102) |
|||||
| Interval between episodes, weeks | Total | Relapse | Second infection | Total | Relapse | Second infection |
| 2–4 | 48 | 43 (90) | 5 (10) | 37 | 32 (86.5) | 5 (13.5) |
| 4–8 | 37 | 32 (86.5) | 5 (13.5) | 28 | 24 (86) | 4 (14) |
| >8 | 49 | 32 (65) | 17 (35) | 37 | 24 (65) | 13 (35) |
Table 2.
Relapse Stratified by Strain Type of Original Infecting Clostridium difficile Isolates (North American Pulse-Field Type 1 [NAP1] Versus Non-NAP1)
| Same strain at relapse/Total infections, no. (%) |
|||
| Interval between episodes, weeks | NAP1 | Non-NAP1 | P value |
| <8 | 25/25 (100) | 52/60 (87) | .05 |
| >8 | 8/10 (80) | 24/39 (62) | .23 |
Episodes of Clostridium difficile Infection ≥8 Weeks Apart
Forty-nine paired isolates of C. difficile from 48 patients were analyzed. The mean interval between episodes was 125 days, and the median 98 days (range, 56–337 days). None of the patients had symptomatic C. difficile infection between the episodes examined. For 32 (65%) of 49 cases, PCR ribotyping showed identical strains for both episodes. As with episodes recurring within 8 weeks, exclusion of the third and fourth episodes did not change the results (Table 1; Figure 1).
Impact of North American Pulse-Field Type 1 Strain
The proportion of cases due to the NAP1 strain varied widely during the study period. A 3-month prevalence survey done from June to August 2008 showed a 40% prevalence of NAP1. More recently, a 10-week study from March to May 2010 showed that the prevalence of NAP1 had decreased to 16%. Exclusion of NAP1 isolates from the study did not change the findings (Table 2).
Multiple Episodes of Clostridium difficile Infection
Among the 24 patients with ≥3 episodes of CDI, 17 had ≥4 episodes. The mean duration between the first and second episodes was 7.3 weeks, and the mean duration between the second and third episodes was 9.8 weeks. Eleven of 17 patients (65%) had the same strain isolated for all episodes. Exclusion of the third and fourth episodes from the analysis did not change the results.
DISCUSSION
We examined C. difficile isolates from 102 patients with repeated episodes of CDI. Our findings suggest that for those with a second episode within 8 weeks of the index case, almost all second episodes are due to the same strain. However, even for episodes occurring >8 weeks after the initial episode, the majority (65%) are also due to the original infecting strain and thus represent relapse and not a second new infection (Figure 1). Our study also suggests that patients with initial infection due to NAP1 usually have relapses from this strain rather than second infections from another strain.
Molecular typing has been used in 6 previously published studies to distinguish relapse from second new infection. The proportion of patients with true relapse, as characterized by DNA fingerprinting, has varied from 25% to 67% in these reports [7–12]. Most of the studies were performed before the emergence of the NAP1 strain and are limited by relatively small sample sizes. In addition, only a small fraction of multiple isolates analyzed represent episodes that occurred >8 weeks apart.
Distinguishing relapses from second new infections by means of PCR ribotyping may have certain limits. Recently, Sethi et al used culture and typing to prospectively follow up 52 patients treated for CDI for up to 1–4 weeks after treatment [13]. They observed that more than half of these patients became asymptomatic carriers of C. difficile, and the strain type isolated from a patient’s environment and/or skin usually matched that causing CDI. They posited that asymptomatic shedding of C. difficile could contribute to horizontal transmission. This pathophysiologic explanation would represent a true “new infection” that, on ribotyping with our methods, would appear to be a relapse rather than a new infection and fundamentally change the interpretation of our findings. However, the contribution of this possible route of disease development is not yet settled.
Our study has several limitations. First, we did not use the most discriminatory typing methods to determine genetic relatedness between strains. We did not assess for clinical resolution of symptoms between episodes. However, we adopted this study methodology to follow the laboratory-based reporting system proposed by the Surveillance Working Group and chose PCR ribotyping because of its ease of performance and potential for widespread use as an epidemiologic tool.
Finally, the contribution of NAP1 cannot be fully determined; this strain has a higher rate of therapeutic failure, so relapse may appear more frequent. Our findings, unlike those of the previous 6 reported studies, may have influenced by the presence of the NAP1 strain. Most hospitals now have endemic NAP1 disease; our hospital and findings may therefore be more representative of the actual dynamics of CDI in the 21st century. Exclusion of NAP1 strains from the results, however, did not change our conclusions.
According to the proposed definitions by the Ad Hoc C. difficile Surveillance Working Group, second CDI episodes that occur >8 weeks after the index case should be classified as second infections from a new strain. However, we found that the majority of these cases are in fact due to relapse from the same strain causing the index episode. Such a distinction is important: in the era of public reporting, hospital rates of CDI are being monitored closely. Misclassification of a case as a “new case” will artificially inflate hospital rates of disease, resulting in inaccurate data and, possibly, deleterious economic effects for institutions. It is crucial therefore to base surveillance definitions on evidence, including molecular typing results from patients with multiple episodes of CDI.
Notes
Acknowledgments.
We thank Teresa Childers for statistical analysis.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant K23-AI0838880 to M. K.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–72. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 3.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–42. doi: 10.1503/cmaj.050978. . PMCID: 1266326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–5. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 5.Bidet P, Barbut F, Lalande V, Burghoffer B, Petit JC. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol Lett. 1999;175:261–6. doi: 10.1111/j.1574-6968.1999.tb13629.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuijper EJ, Barbut F, Brazier JS, et al. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;13:18942. [PubMed] [Google Scholar]
- 7.Alonso R, Gros S, Pelaez T, Garcia-de-Viedma D, Rodriguez-Creixems M, Bouza E. Molecular analysis of relapse vs re-infection in HIV-positive patients suffering from recurrent Clostridium difficile associated diarrhoea. J Hosp Infect. 2001;48:86–92. doi: 10.1053/jhin.2001.0943. [DOI] [PubMed] [Google Scholar]
- 8.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. . PMCID: 86814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;159:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill GL, Beaman MH, Riley TV. Relapse versus reinfection with Clostridium difficile. Epidemiol Infect. 1991;107:627–35. doi: 10.1017/s0950268800049323. . PMCID: 2272080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang-Feldman Y, Mayo S, Silva Jr J Jr, Cohen SH. Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent clostridium difficile-associated diarrhea. J Clin Microbiol. 2003;41:3413–4. doi: 10.1128/JCM.41.7.3413-3414.2003. . PMCID: 165369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox MH, Fawley WN, Settle CD, Davidson A. Recurrence of symptoms in Clostridium difficile infection–relapse or reinfection? J Hosp Infect. 1998;38:93–100. doi: 10.1016/s0195-6701(98)90062-7. [DOI] [PubMed] [Google Scholar]
- 13.Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31:21–7. doi: 10.1086/649016. [DOI] [PubMed] [Google Scholar]