Abstract
Reactive oxygen species (ROS) are a major cause of cellular injury in an increasing number of diseases, including cancer. Most ROS are created in the cell through normal cellular metabolism. They can be produced by environmental insults such as ultraviolet light and toxic chemicals, as well as by the inflammatory process. Interception of ROS or limiting their cellular effects is a major role of antioxidants. Due to their content of phenolic and flavonoid compounds, berries exhibit high antioxidant potential, exceeding that of many other foodstuffs. Through their ability to scavenge ROS and reduce oxidative DNA damage, stimulate antioxidant enzymes, inhibit carcinogen-induced DNA adduct formation and enhance DNA repair, berry compounds have been shown to inhibit mutagenesis and cancer initiation. Berry constituents also influence cellular processes associated with cancer progression including signaling pathways associated with cell proliferation, differentiation, apoptosis and angiogenesis. This review article summarizes laboratory and human studies, demonstrating the protective effects of berries and berry constituents on oxidative and other cellular processes leading to cancer development.
Introduction
Most chronic diseases, including cardiovascular disease, arthritis, diabetes and many types of cancer are caused, in part, by the conversion of cellular macromolecules to specific reactive oxygen species (ROS) during normal cellular metabolism (1). ROS produced by other means including the metabolism of environmental toxins and carcinogens, by ionizing radiation and by phagocytic cells involved in the inflammatory response also contribute to these diseases. There are five recognized types of ROS; i.e. singlet oxygen (′O2), superoxide anions (O2.−), hydrogen peroxide (H2O2), hydroxyl radical (OH.) and nitric oxide (NO−). Although some types are more injurious to cells than others, there is no question that, collectively, these ROS cause significant oxidative damage to cellular DNA, proteins and lipids leading to the development of chronic diseases including cancer (2).
Epidemiological studies indicate that persons who consume a diet rich in fruits and vegetables have a reduced risk of cancer at multiple sites (3–7), although the evidence may not be as strong as reported earlier (8). Since fruits and vegetables are major sources of antioxidants (among other factors), it is hypothesized that these antioxidants are largely responsible for their cancer-preventive effects. This hypothesis is supported by both epidemiological data, implicating micronutrient antioxidants in reduction of cancer risk and by experimental data in vitro and in vivo (2,9–11). The antioxidant effects of fruits and vegetables are due, in significant part, to their content of flavonoids and phenolic acids (12–14). Some of the extensively investigated polyphenolic compounds, with demonstrated cancer-preventative effects, such as, the catechins in green tea, theaflavins in black tea, curcumin in turmeric, resveratrol in red wine, quercitin in apples and the anthocyanins in berries, are antioxidants that exhibit significant radical scavenging activity (12,15–18).
Berries are among the most widely consumed fruits in the human diet. They are richly abundant in antioxidant flavonoids and phenolic acids, as well as antioxidant vitamins, carotenoids, tannins, lignans and stilbenes (19). Commercially the most important berries include members of the genus Vaccinium (blueberry, lingonberry, cranberry, bilberry), Rubus (blackberry, black raspberry, red raspberry, arctic raspberry/bramble, cloudberry), Fragaria (strawberry) and Sambucus (elderberry, red elderberry). Recently, the different varieties of berries, their use for nutritional and medicinal purposes and factors influencing their chemical composition have been summarized (20). The present review will discuss research progress in the identification of the active cancer-inhibitory constituents of berries and in determining their mechanisms of action. In addition, the available clinical evidence to suggest that berries might exhibit anticarcinogenic effects in humans will be summarized.
Bioactive phytochemicals in berries
Berries contain a wide range of bioactive phytochemicals, the most prominent of which are phenolic in nature; i.e. they have hydroxyl (−OH) groups on aromatic rings. Phytochemicals are non-nutritive constituents produced by secondary metabolism in plants. They are known to defend plants against predators, microbial infections and ultraviolet light, to regulate metabolic pathways and provide color and flavor to the plant (20). The bioactive phytochemicals in berries fall into several structural and chemical classes including phenolic acids (hydroxycinnamic and hydroxybenzoic acids), flavonoids (anthocyanins, flavanols, flavonols), condensed tannins (proanthocyanins), hydrolyzable tannins (ellagitannins and gallotannins), stilbenoids, lignans, triterpenes and sterols (19). The structures of these berry bioactives are shown in Figure 1, and their chemical features, biological activities and content in different berry types have been described in detail by Seeram (19). Among the most prevalent compounds in berries are the anthocyanins and ellagitannins that, collectively, are responsible for much of their antioxidant activity (21–24). In addition to the bioactives listed in Figure 1, berries contain many other constituents known to exhibit cancer-preventive effects including vitamins, such as A, C, E and folic acid, and minerals, such as calcium, selenium and zinc (25). The inhibitory effects of most of these constituents are due, at least in part, to their antioxidant capacity.
Fig. 1.
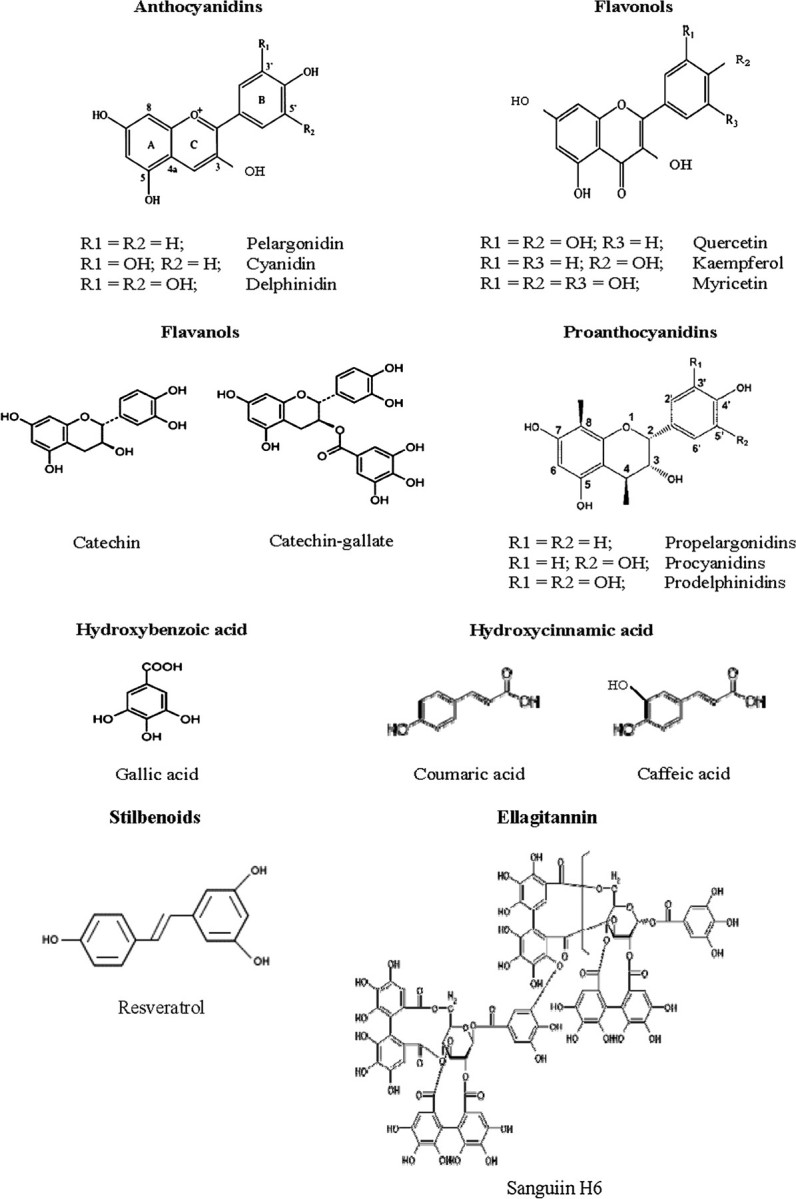
Structures of bioactives found in berries: anthocyanins, flavonol, flavanols, proanthocyanidins, hydroxycinnamic and hydroxybenzoic acids, stilbenoids and ellagitannins.
Laboratory studies of the anticancer effects of berry bioactives
Berry bioactives have many roles in cancer prevention. These include protection against oxidative DNA damage by the scavenging of ROS, inhibition of the formation of carcinogen-induced DNA adducts, enhancement of DNA repair, inhibition of carcinogen-induced tumorigenesis in animals and modulation of signaling pathways involved with cellular proliferation, apoptosis, inflammation, angiogenesis and cell cycle arrest. The following sections will summarize laboratory studies, both in vitro and in vivo, to determine the mechanisms by which berries and berry components exert anticarcinogenic effects.
Scavenging ROS and inhibition of oxidative DNA adduct formation
The ability of different berry juices (blackberry, strawberry, raspberry, cranberry and blueberry) to scavenge specific ROS including superoxide radicals (O.−), hydrogen peroxide (H2O2), hydroxyl radicals (OH.) and singlet oxygen (′O2) was investigated by Wang and Jiao (Table I) (15). They found that the scavenging activity of berries varied as a function of berry type and cultivar, as well as the types of ROS that were scavenged. In general, blackberries had the highest antioxidant capacity for inhibition of O2.−, H2O2 and OH.. Strawberries were second best for these same three radicals; however, they were superior to blackberries in the scavenging of ′O2. Cranberries had the lowest inhibition of H2O2 activity, whereas blueberries had the lowest antioxidant capacity against OH. and ′O2. Thus, there were significant differences among the different berry types in their abilities to scavenge different ROS.
Table I.
Comparison of the mean values of the scavenging capacity of juice from different berry species (blackberry, blueberry, cranberry, raspberry and strawberry) on active oxygen species (O2.−, H2O2, OH. and ′O2)
| % Inhibitiona |
||||
| Cultivar | O2− | H2O2 | OH. | ′O2 |
| Blackberryb | 64.3 | 66.3 | 72.0 | 12.4 |
| Blueberry | 60.1 | 61.2 | 58.7 | 7.71 |
| Cranberry | 59.0 | 59.8 | 64.2 | 8.64 |
| Raspberry | 57.3 | 60.9 | 66.9 | 8.88 |
| Strawberry | 64.2 | 65.3 | 68.6 | 15.41 |
| LSD 0.05 | 3.02 | 2.33 | 1.79 | 1.16 |
| Significant cultivarc | ** | ** | ** | ** |
LSD, least significant difference.
Data are taken from Wang and Jiao (15).
Data expressed as percent inhibition of radical (O2.−, H2O2, OH. and 'O2) production in the presence of 0.1 ml of fruit juice.
Data for the thornless blackberry were derived from the mean value calculated from six cultivars (Black Satin, Chester Thornless, Hull Thornless, Smoothstem, Thornfree, Triple Crown); blueberry data were derived from the mean value calculated from five cultivars (Bluecrop, Elliot, wild blueberry #1, wild blueberry #2, wild blueberry #3); cranberry data were derived from the mean value calculated from five cultivars (Ben Lear, Cropper, Early Black, Franklin, Howes); raspberry data were derived from the mean value calculated from five cultivars (Autumn Bliss, Jewel, Canby, Sentry, Summit); strawberry data were derived from the mean value calculated from six cultivars (Allstar, Delmarvel, Earliglow, Latestar, Lester, Red Chief).
**Nonsignificant or significant at P ≤ 0.05.
In another study, the antioxidant capacity of Oregon grown blackberries, boysenberries, marionberries, black raspberries and red raspberries was compared using the oxygen radical absorbance capacity (ORAC) assay (26). Antioxidant capacity, expressed as Trolox equivalents/gram of fresh berries, was greatest in black raspberries, followed by boysenberries, red raspberries, blackberries and marionberries. The antioxidant capacity of these berries was related to their content of total phenolics and anthocyanins. It should be mentioned, however, that caution should be used in drawing conclusions regarding the antioxidant capacity of different berry types since, in addition to type and cultivar, the antioxidant capacity of berries can vary as a function of the climate and soil conditions in which they are grown, degree of ripeness, storage temperature, methods used for industrial processing and the specific assay used to measure antioxidant capacity (27–29).
In addition to the direct scavenging of ROS, the antioxidant capacity of berries is also related to their content of antioxidant enzymes. For example, the juices of strawberries and blackberries have been shown to contain antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase and glutathione reductase, and the activities of these enzymes is positively correlated with antioxidant capacity of the berries (30). Thus, the ability of berry bioactives to scavenge ROS, coupled with the presence of antioxidant enzymes in berries, may result in attenuation of ROS concentration to maintain an intracellular oxidation and reduction (redox) balance in the berries themselves (19). It remains to be determined whether these berry enzymes exhibit antioxidant activity in mammals because it is not known whether they remain functional in the gastrointestinal tract.
The prevention of ROS-induced DNA damage is probably a first line of defense against the multistage process of carcinogenesis. Most studies with berry-based antioxidants have demonstrated their protective effects against oxidative DNA damage in human lymphocytes (31,32). For example, pretreatment of freshly isolated human lymphocytes from non-smokers with quercetin (1–100 μM) for 1 h was shown to protect against oxidative DNA damage induced by H2O2 as measured by the Comet assay (31). Similar findings were reported by Duthie et al. (32), who treated freshly isolated human lymphocytes with quercetin and myricetin. Inhibition of H2O2-induced DNA damage was prevented by quercetin at concentrations ranging from 10 to 50 μM and by myricetin at concentrations of ≥100 μM. In one animal study, the administration of 2.5, 5 and 10% lyophilized black raspberry (LBR) diets, which are abundant in anthocyanins and other antioxidants, given to rats treated with the colon carcinogen, azoxymethane (AOM), led to 73, 81 and 83% reductions, respectively, in the levels of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) (33). These results suggest that the berries markedly reduced oxidative DNA damage in AOM-exposed rats.
In contrast to these results, two human intervention studies failed to demonstrate a protective effect of berry bioactives against oxidative DNA damage. Moller et al. (34) placed three groups of healthy subjects on a low flavonoid diet for 3 weeks, supplemented with either a black current juice, a black current anthocyanin concentrate or a placebo. Vitamin C increased in plasma of the black current juice group, but the levels of endogenous oxidative DNA damage, as measured by the Comet assay, were unaffected by any of the treatments. In a study using cranberry juice (35), 20 healthy female subjects consumed 250 ml of cranberry juice 3×/day for 2 weeks. This treatment led to an increase in vitamin C in the plasma but not in plasma phenolics. There was no change in strand breakage or resistance/sensitivity to H2O2 in lymphocyte DNA. In addition, there was no treatment-related reduction in the level of 8-OHdG either in the lymphocyte DNA or in the urine. Thus, there are conflicting results of the potential inhibitory effects of berries and berry constituents on oxidative DNA damage in humans. This has been discussed in a recent article by Freese (36), who suggested that additional well-controlled studies using validated biomarkers are needed in groups with a high risk of DNA damage (e.g. smokers).
Inhibition of carcinogen-induced DNA adducts
Diets composed of berries and berry constituents that appear to produce reductions in the formation of oxidative DNA adducts have also been shown to inhibit the formation of DNA adducts from chemical carcinogens (Table II). Kresty et al. (25) reported that male rats fed diets containing either 5 or 10% LBRs or 0.04% ellagic acid for 14 days followed by a single injection of the esophageal carcinogen, N-nitrosomethylbenzylamine (NMBA), had reduced levels (73, 80 and 38%, respectively) of O6-methylguanine adducts in the DNA of their esophagus. In later experiments, the feeding of 5 and 10% LBRs to NMBA-treated rats of the same strain for 14–21 days was shown to result in enhanced mRNA expression levels of cytochromes P450 2a2 and 3a13 in the esophagus and of glutathione-S-transferase activity in the liver (37). It remains to be determined whether these effects are responsible for the reduced levels of O6-methylguanine adducts in the esophagus. Similar results were reported by Carlton et al. (38), who found that diets containing 5 or 10% lyophilized strawberries produced similar reductions in O6-methylguanine adducts in the DNA of esophagi from NMBA-treated rats. Casto et al. (39), in a study of oral cancer using Syrian golden hamsters, demonstrated a reduction in 7,12-dimethylbenz(a)anthracene (DMBA)-induced DNA adducts in the cheek pouch after feeding hamsters for 2 weeks with 5% LBRs. The adducts in berry-fed hamsters were reduced by 29 and 55% at 24 and 48 h, respectively, after topical application of DMBA to the surface of the cheek pouch.
Table II.
Effects of dietary lyophilized strawberries and black raspberries on NMBA-induced DNA adduct formation in the rat esophagus
| Treatment | pmol O6-meGua/mg DNA | % Inhibition |
| NMBA (0.25 mg/kg) | 4.4 ± 0.9 | — |
| NMBA + 5% STRWs | 1.4 ± 0.1a | 68 |
| NMBA + 10% STRWs | 1.9 ± 0.7a | 57 |
| NMBA + 5% BRs | 1.2 ± 0.3a | 73 |
| NMBA + 10% BRs | 0.9 ± 0.2a | 80 |
STRWs, strawberries; BRs, black raspberries; O6-meGua, O6-methylguanine. Rats were fed 5 or 10% lyophilized STRWs or BRs for 14 days and then given a single subcutaneous injection of NMBA at 0.25 mg/kg body wt. The esophageal DNA was isolated and O6-meGua measured. STRW data are taken from Carlton et al. (38) and BR data are from Kresty et al. (25).
Significantly different from NMBA controls (P < 0.05).
Wilms et al. (31) found that quercetin inhibits the formation of DNA adducts in human lymphocytes treated with benzo(a)pyrene [B(a)P]. Freshly isolated lymphocytes were treated with quercetin (0–100 μM), followed by treatment with 1 μM of B(a)P. Benzo(a)pyrene diol-epoxide (BPDE)–DNA adducts, evaluated by 32P-post-labeling, decreased in a dose-dependent manner and were undetectable in cells treated with 100 μM of quercetin. In contrast, when subjects were treated for 4 weeks with a quercetin-rich blueberry/apple juice, freshly isolated lymphocytes were not protected from BPDE-adduct formation following B(a)P treatment. Although the reasons for this discrepancy between in vitro and in vivo results are not known, the rather poor bioavailability of quercetin, and potentially other compounds in blueberry/apple juice, in vivo may be responsible for the differences.
Effects on DNA repair
A number of reports suggest that antioxidants in foods may have a dual action; i.e. reducing oxidative DNA damage by scavenging ROS and promoting DNA repair by enhancing the removal of oxidative adducts from DNA. Most research in support of the latter mechanism has revealed that cells or tissues treated with food constituents, after gamma irradiation, exhibit a faster rate of DNA repair than untreated cells or tissues. For example, Maurya et al. (40) examined the effect of ferulic acid (a phenolic acid in berries) on the induction of DNA strand breaks in peripheral blood leukocytes and bone marrow cells of mice exposed to whole-body γ-radiation. Intraperitoneal administration of ferulic acid (50–100 mg/kg body wt), 1 h prior to 4 Gy γ-radiation, resulted in a dose-dependent decrease in DNA strand breaks in both cell types. Administration of 50 mg/kg body wt of ferulic acid, after whole-body irradiation of mice, resulted in a disappearance of DNA strand breaks at a faster rate than was observed in irradiated controls, suggesting enhanced DNA repair in ferulic acid-treated animals. Similar results were obtained with troxerutin (a derivative of the flavonoid, rutin) by Maurya et al. (41). Thus, at least two compounds found in berries are effective in enhancing DNA repair, as evidenced by a more rapid rejoining of irradiation-induced DNA strand breaks in berry-treated cells. In contrast, another investigation found that resveratrol had only a modest effect. Ellagic acid had no effect, on stimulation of the DNA repair protein, O6-methylguanine-DNA-methyltransferase, in human lymphocytes and in glioblastoma and colon cancer cells (42). These results suggest that these berry compounds may have little or no effect on repair of O6-alkylguanine adducts in DNA.
Inhibition of carcinogen-induced tumors in animals
Berry bioactives have been shown to prevent chemically induced tumors in multiple animal model systems (43). Some of the earliest studies were done with ellagic acid [see summary by Stoner and Mukhtar (44)] that has been shown to inhibit carcinogen-induced cancer in the rodent lung, skin, esophagus, liver, colon and mammary gland. Mechanistic studies indicate that ellagic acid inhibits carcinogen-induced DNA adduct formation, scavenges the reactive metabolites of polycyclic hydrocarbons, stimulates the activity of various isoforms of glutathione-S-transferase and occupies sites in DNA that might otherwise react with carcinogens (45). Ellagic acid has also been shown to inhibit the activity of the enzyme, ornithine decarboxylase, resulting in reduced polyamine levels in mouse skin and an inhibition of skin tumorigenesis (46). Interest in ellagic acid as a chemopreventive agent was significantly reduced, however, by early studies that demonstrated its poor bioavailability in rodents (47,48).
While conducting studies with ellagic acid in the 1980s, our laboratory decided to identify foods in which it might be found (it was not known at that time to be widely present in berries). In examining a series of fruits, we found high concentrations (630–1500 μg/g dry wt) of ellagic acid in blackberries, red raspberries and strawberries and lesser amounts in cranberries (49). The ellagic acid was far more abundant in the pulp and seed of the berries; very little was found in the juice. Based upon these observations, we decided to take a ‘food-based’ approach to cancer prevention and determine if lyophilized berry powders would exhibit inhibitory effects on chemically induced cancer in animals. The rationale for this stems from the fact that berries are 85–90% water; thus, removal of water by the lyophilization process would increase the concentration of ellagic acid in berry powder at least 8- to 9-fold. At the time, we were unaware of the many other chemopreventive agents in berries. In initial tumor bioassays, lyophilized berry powders were evaluated for their ability to inhibit NMBA-induced esophageal carcinogenesis in rats when fed to animals either before, during and after NMBA treatment (complete prevention protocol) or only after treatment with NMBA (postinitiation protocol). The berry powders were fed at 5 and 10% of synthetic AIN-76A diet, which, in humans, would be equivalent to the consumption of 2–4 cups of fresh berries per day.
The chemopreventive effects of lyophilized strawberries, black raspberries and blackberries on NMBA-induced tumors (papillomas), in the rat esophagus, when administered in the complete prevention protocol are given in Table III. Administration of 5 and 10% LBRs and strawberries had no effect on tumor incidence but significantly reduced tumor multiplicity when compared with NMBA controls (P < 0.05) (25,38). Surprisingly, 5% dietary blackberries were effective in reducing tumor multiplicity, whereas 10% blackberries were ineffective (43). There were no significant differences in tumor size among any of the groups in these studies. One mechanism for the chemopreventive effects of strawberries and black raspberries in the complete prevention protocol was that of inhibiting the formation of mutagenic O6-methylguanine adducts in the DNA of esophageal epithelium (Table II).
Table III.
Effects of lyophilized strawberries, black raspberries and blackberries on NMBA-induced rat esophageal tumorigenesis when administered in the diet before, during and after NMBA treatment (complete prevention protocol)
| Treatment | Rats (n) | Tumor incidence (% inhibition) | Tumor multiplicity (% inhibition) |
| Vehicle control | 15 | 0 | 0.0 |
| 10% STRWs | 15 | 0 | 0.0 |
| NMBA control | 15 | 100 | 4.1 ± 0.2 |
| NMBA + 5% STRWs | 15 | 100 | 3.1 ± 1.0 (24)a |
| NMBA + 10% STRWs | 15 | 80 (20) | 1.8 ± 1.4 (56)a |
| Vehicle control | 15 | 0 | 0.0 |
| 10% BRs | 14 | 0 | 0.0 |
| NMBA control | 13 | 100 | 3.2 ± 0.3 |
| NMBA + 5% BRs | 14 | 79 (21) | 1.9 ± 0.4 (41)a |
| NMBA + 10% BRs | 13 | 92 (8) | 1.6 ± 0.3 (50)a |
| Vehicle control | 10 | 0 | 0.0 |
| 10% BBs | 10 | 0 | 0.0 |
| NMBA control | 17 | 82 | 2.8 ± 0.6 |
| NMBA + 5% BBs | 20 | 75 (8) | 1.5 ± 0.4 (46)a |
| NMBA + 10% BBs | 18 | 70 (15) | 2.3 ± 0.5 (18) |
The chemopreventive effects of lyophilized strawberries and LBRs when administered to rats after treatment with NMBA (postinitiation protocol) are shown in Table IV. When administered postinitiation, LBRs reduced tumor incidence whereas strawberries had no effect on tumor incidence (25,38). Both strawberries and LBRs produced significant reductions in tumor multiplicity; however, the 5% dietary concentrations were more effective than the 10%. The reason(s) for this result is unknown, but did not appear to be due to toxic effects of 10% berries for esophageal epithelium. In the postinitiation protocol, LBRs were found to inhibit the conversion of preneoplastic (dysplastic) lesions to papillomas and to reduce cellular proliferation, as determined by measuring the expression of proliferating cell nuclear antigen in esophageal epithelium, at different time points during the bioassay (25). In these studies, the inhibitory effects of all lyophilized berry types on tumor multiplicity were not dose related; i.e. 10% berry diets were not twice as effective in reducing the number of NMBA-induced tumors as 5% diets. Similarly, the inhibitory effects of ellagic acid on NMBA-induced tumors in the rat esophagus were not dose related (50). These results suggest that there may be a maximum threshold level in the uptake of dietary berry phenolics into the esophagus, beyond which additional amounts of dietary phenolics have no added benefit.
Table IV.
Effects of lyophilized strawberries and black raspberries on NMBA-induced rat esophageal tumorigenesis when administered in the diet after NMBA treatment (postinitiation protocol)
| Tumor multiplicity |
||||
| Treatment | Rat (n) | Tumor incidence (%) | Tumors/rat (mean ± standard error) | % Inhibition |
| Vehicle control | 10 | 0 | 0 | 0 |
| 10% STRWs | 10 | 0 | 0 | 0 |
| NMBA control | 14 | 100 | 4.8 ± 0.6 | 0 |
| NMBA + 5% STRWs | 15 | 100 | 3.0 ± 0.3a | 38 |
| NMBA + 10% STRWs | 15 | 100 | 3.3 ± 0.4a | 31 |
| Vehicle control | 10 | 0 | 0 | 0 |
| 10% BRs | 10 | 0 | 0 | 0 |
| NMBA control | 15 | 87 | 1.4 ± 0.3 | 0 |
| NMBA + 5% BRs | 15 | 40a | 0.5 ± 0.2a | 64 |
| NMBA + 10% BRs | 15 | 47a | 0.8 ± 0.3a | 43 |
LBRs were also found to inhibit AOM-induced colon carcinogenesis in rats, when given in a postinitiation protocol (Table V) (33). When fed at 2.5, 5.0 and 10% of the diet, LBRs did not reduce tumor incidence; however, they reduced the total tumor (adenoma + adenocarcinoma) multiplicity by 42, 45 and 71% (P < 0.05 in all groups), respectively, relative to AOM controls. Inhibition of oral cancer by the dietary administration of LBRs was demonstrated in the hamster cheek pouch by Casto et al. (39) (Table VI). Tumors were induced by painting both cheek pouches of hamsters with DMBA. Hamsters were fed either 5 or 10% LBRs in AIN-76A diet, prior to, during and after treatment with DMBA. Treatment with 5% LBRs caused a significant reduction in tumor multiplicity (P < 0.05), but the reduction with 10% berries was not significant. The reasons for the lack of inhibitory effect in animals administered 10% LBRs were not determined.
Table V.
Effects of lyophilized black raspberries on AOM-induced rat colon tumors when administered in the diet after AOM treatment (postinitiation protocol)
| Tumor multiplicity |
||||
| Treatment | Rat (n) | Tumor incidence (%) | Tumors/rat (mean ± standard error) | Inhibition (%) |
| Vehicle control | 18 | 0 | 0 | 0 |
| 5% BRs | 18 | 0 | 0 | 0 |
| AOM control | 18 | 67 | 2.33 ± 0.5 | 0 |
| AOM + 2.5% BRs | 18 | 78 | 1.35 ± 0.3a | 42 |
| AOM + 5% BRs | 18 | 89 | 1.27 ± 0.2a | 45 |
| AOM + 10% BRs | 18 | 50 | 0.67 ± 0.2a | 71 |
BRs, black raspberries. Data are taken from Harris et al. (33).
Statistically significant relative to AOM control (P < 0.05).
Table VI.
Effect of lyophilized black raspberries on DMBA-induced tumors in the hamster cheek pouch when administered in the diet before, during and after DMBA treatment (complete prevention protocol)
| Tumor multiplicity |
||||
| Treatment | Rat (n) | Tumor incidence (%) | Tumors/rat | % Inhibition |
| DMBA control | 15 | 100 | 3.20 | 0 |
| DMBA + 5% BRs | 14 | 93 | 1.93 | 40 |
| DMBA + 10% BRs | 15 | 100 | 2.60 | 19 |
BRs, black raspberries. Data are taken from Casto et al. (39).
Statistically significant relative to DMBA control (P < 0.05).
A recent study from our laboratory showed that an anthocyanin-rich fraction, derived from LBRs and an alcohol:water (80:20) extract of LBRs, were equally as effective as a 5% LBR diet in reducing NMBA-induced tumors in the rat esophagus (51). The content of anthocyanins in the anthocyanin-rich diet and in the alcohol:water extract diet was essentially identical to that in the 5% LBR diet (∼3.5 μmol/g diet). Since all three diets contained the same amounts of anthocyanins and were equally effective in preventing esophageal cancer, these data suggest the importance of the anthocyanins as chemopreventive agents in black raspberries. However, in this same study, another diet containing an alcohol-insoluble (residue) fraction of LBRs, that contained only trace amounts of anthocyanins, was nearly as effective as the anthocyanin-rich and alcohol:water extract diets in preventing NMBA-induced esophageal tumorigenesis. Recent results suggest that the residue fraction contains high levels of ellagitannins (S.S. Hecht, S. Carmella, L-S Wang and G.D. Stoner, unpublished data). Thus, both the anthocyanins and ellagitannins appear to be important for the chemopreventive effects of berries.
In contrast to the above observations, Carlton et al. (52), found that a diet containing 10% lyophilized strawberries was ineffective in reducing lung tumors in mice that were induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone or B(a)P. Mice were administered the strawberry diet beginning 1 week before initial carcinogen treatment and throughout the study. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and B(a)P were administered intraperitonially over a 2 week period beginning at week 2 of the bioassay. At 24 weeks, there were no differences in lung tumor incidence or multiplicity in any of the groups. The successes in reducing carcinogen-induced cancers in the oral cavity, esophagus and colon, where berry components come into direct contact with the tissues, and the failure to inhibit lung tumors in mice suggest that (i) either lung tumors fail to respond to berry components or (ii) the active components in berries fail to reach the lung in sufficient amounts to elicit protection. The latter conclusion may be a more logical explanation since studies on the uptake of anthocyanins and ellagitannins from berry juices in rodents have shown that these compounds are poorly absorbed and their plasma levels decline rapidly (53,54).
Modulation of signaling pathways associated with cell proliferation, apoptosis, inflammation, angiogenesis, cell cycle arrest and cell–cell communication
Numerous studies have investigated the effects of berry phytochemicals on signaling pathways associated with cancer development (19). These include in vitro studies of the ability of berry extracts and their purified derivatives to inhibit cell growth and to stimulate apoptosis and differentiation. Although many of these studies have focused on the effects of berry extracts, some of them have involved bioassay-directed fractionation of extracts to identify the active constituents in berries. Results from in vitro studies have also been confirmed in vivo in a limited number of investigations. This review will attempt to discuss only some of the in vitro and in vivo results on the effects of berry constituents on cellular functions and signaling pathways in the paragraphs that follow.
In vitro studies.
Most in vitro studies of the antiproliferative effects of berry extracts and components have been conducted with human tumor cell lines. For example, extracts from strawberry cultivars have been shown to reduce the proliferation of human HepG2 liver tumor cells and of human colon (HT29) and breast (MCF-7) cancer cells in a dose-dependent manner (55). However, there was no correlation between the antioxidant capacity of the cultivars or the levels of anthocyanins and flavonols in the extracts and their antiproliferative effects for the three cell lines. Jurinac et al. (56) compared the effects of a red raspberry extract with ellagic acid alone on the growth of human colon cancer LS174 cells and normal immune competent cells. Results from this comparison indicated that the extract produced a selective inhibitory effect on the growth of the colon cancer cells, which correlated positively with its content of ellagic acid.
Seeram et al. (57) evaluated the antiproliferative effects of methanol extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry and strawberry for human oral (KB and CAL27), breast (MCF-7), colon (HT29, HCT116) and prostate (LNCaP) cancer cell lines. The extent of inhibition of cell proliferation was found to vary markedly with both the type of berry extract and the specific cell line studied. The effects of the different berry extracts on cell apoptosis was assessed using HT29 cells that were treated with 200 μg/ml of each berry extract (a significantly higher level than can be achieved in vivo). Only black raspberry and strawberry extracts produced a significant induction of apoptosis. The authors concluded that there are several factors that must be considered when using cell culture studies to rank the chemopreventive activities of berry extracts. These include the cell line being used, the artificially high concentrations of extract, stability of extract components in different media, length of treatment time, differential uptake of phenolics and generation of artifacts such as H2O2 that is known to induce apoptosis.
Bioactivity-guided fractionation has been used to identify the active constituents in different berry types. Using bioactivity-guided fractionation, Murphy et al. (58) identified triterpenoid esters in cranberries as important for inhibition of the growth of human MCF-7 breast, ME180 cervical and PC3 prostate tumor cell lines. Similarly, Han et al. (59) used bioactivity-guided fractionation to identify chemopreventive phytochemicals in black raspberries. They compared the effects of several extract fractions of black raspberries with those of the berry constituents: ellagic acid, ferulic acid and β-sitosterol on the growth and cell cycle of normal, premalignant and malignant human oral cavity cells. An ethanol extract was found to inhibit growth of the premalignant and malignant but not the normal oral cell lines. Ellagic acid inhibited the growth of normal, premalignant and malignant cell lines, whereas ferulic acid and β-sitosterol exhibited selectivity for the premalignant and malignant lines. Thus, these workers concluded that ferulic acid and β-sitosterol were effective bioactives in black raspberries. Using western blot analysis, all treatments were found to affect different cyclins and cyclin-dependent kinases in the premalignant and malignant lines. This led to the conclusion that the growth-inhibitory effects of black raspberries on premalignant and malignant oral epithelial cells reside in the specific effects of different berry components on signaling pathways regulating cell cycle progression. Rodrigo et al. (60) also evaluated the preventative effects of an ethanol fraction of black raspberries on human oral cancer cell lines and found that the extract inhibited cell proliferation, vascular endothelial growth factor (VEGF) production and nitric oxide synthase activity and stimulated apoptosis and terminal cell differentiation. In an extension of these studies, another ethanol fraction of black raspberries was extensively subfractionated by high-performance liquid chromatography and the individual subfractions compared for their ability to downregulate the expression levels of nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1) in mouse epidermal JB-6 Cl 41 cells (61). Interestingly, the major constituents of the most active subfractions were the three most prevalent anthocyanins in black raspberries: cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside and cyanidin-3-O-(2G-xylosylrutinoside). These results further suggest that the cyanidin glucosides (anthocyanins) account for at least some of the chemopreventive activity of black raspberries.
The effects of berry extracts on signal transduction pathways have also been discussed (62). In initial studies with the JB-6 Cl 41 mouse epidermal cell model, Huang et al. (63) demonstrated the inhibitory effects of a methanol fraction of black raspberries and of other purified fractions on transactivation of AP-1 and NF-κB induced by BPDE in JB-6 Cl 41 cells. The inhibitory effects appeared to be mediated via inhibition of mitogen-activated protein kinase activation and inhibitory subunit κB phosphorylation, respectively. Pretreatment of cells with the methanol fraction did not result in an inhibition of BPDE binding to DNA; thus, this was not a mechanism of reduced AP-1 and NF-κB activation. None of the tested fractions affected p53-dependent transcription activity. The ability of black raspberries to inhibit tumor development may be mediated by impairing signal transduction pathways leading to activation of AP-1 and NF-κB. This concept is supported by the observation that the methanol fraction markedly inhibited activation of PI-3K, Akt and p70 S6 kinase, suggesting that another mechanism for the chemopreventive activity may be through inhibition of the PI-3K/Akt/AP-1/VEGF pathway (64).
The effects of individual berry bioactives on growth and apoptosis of human cancer cell lines have also been investigated. Quercetin, a flavonol found in several varieties of berries and black currents, at concentrations up to 70–80 mg/kg dry wt, was found to inhibit growth and stimulate apoptosis of lung cancer cells (65). Survival of a lung cancer cell line (NCI-H209) was decreased in a concentration-dependent manner after treatment with quercetin glucuronide. Importantly, quercetin was effective in vitro at doses (1–10 μM) that are pharmacologically relevant in vivo. Quercetin treatment resulted in an increase in cell cycle arrest with the cells accumulating in the S and G2/M phases of the cell cycle and in subG0 while undergoing apoptosis. Apoptosis was associated with decreases in mitochondrial membrane potential and in the antiapoptotic protein, Bcl-2, and increases in the proapoptotic proteins, p21CIP1/WAF1, Bak, Bax, cytochrome c and caspase 3. Nguyen et al. (66) treated A549 human lung epithelial cells with quercetin and found a similar dose-dependent reduction in cell viability and induction of apoptosis. Most of the reduction in cell viability at the two highest doses (43.5 and 58 μM) was attributed to apoptosis. In addition, quercetin treatment led to phosphorylation of the extracellular signal-related kinase, ERK 1/2, and ERK 1/2 activation was accompanied by phosphorylation of the mitogen-activated kinase, MEK.
Individual anthocyanins in berries have also been shown to exhibit chemopreventive effects in vitro. Ding et al. (67) investigated the effects of cyanidin-3-O-glucoside, isolated from blackberries, on gene expression in JB-6 cells. Cyanidin-3-O-glucoside pretreatment led to a dose-dependent decrease in the expression of cyclooxygenase-2 (COX-2) and activities of AP-1, NF-κB and tumor necrosis factor (TNF)α, when the cells were treated with 12-O-tetradecanolyphorbol-13-acetate or ultraviolet. Finally, an anthocyanin-rich preparation, from berries (wild blueberry, bilberry, cranberry, elderberry, raspberry and strawberry), was found to reduce VEGF expression in a spontaneously immortalized human keratinocyte cell line (HaCaT) and inhibit endothelial tube formation in a Matrigel assay (68).
It should be mentioned that there is growing concern that observations of the effects of phenolic antioxidants in vitro as a measure of their chemopreventive activity may be deceptive and should not be extrapolated to activity in vivo (69–72). Long et al. (70) suggested that the effects of several antioxidants in vitro are artifacts due to the production of hydrogen peroxide generated from these antioxidants in the culture medium. They pointed out that H2O2 can produce many of the effects observed in vitro with phenolics. These effects include elevation of intracellular Ca++ levels, activation of transcription factors, inhibition of cell proliferation, cytotoxicity, alteration of certain signal transduction pathways, stimulation of apoptosis, expression of adhesion molecules such as intercellular adhesion molecule 1, promotion of differentiation or cell senescence, downregulation of activation of AP-1 and suppression of protein kinase activation. These findings are supported by the work of other investigators (69,71,72), who also suggest caution in interpreting the results of studies of the effects of antioxidants on normal and tumor cells in vitro.
An additional concern of considerable importance in the extrapolation of in vitro data to the in vivo situation is that of dose extrapolation. Most, if not all, berry bioactives produce chemopreventive effects in cell cultures when added at micromolar concentrations (∼10 to 150 μM). In contrast, pharmacokinetic studies indicate that berry bioactives, such as the anthocyanins and ellagitannins, reach only nanomolar concentrations (∼1 to 20 nM) in blood and tissues when administered in the diet (13,77). Thus, caution must be used when extrapolating in vitro results to the in vivo situation; perhaps, the most appropriate use of the in vitro data is that of identifying possible mechanistic biomarkers for clinical investigations.
In vivo studies.
The effects of diets containing lyophilized berry powders on the expression of genes involved in signal transduction have been investigated. As indicated in Table IV, at dietary concentrations of 5 and 10%, lyophilized strawberry and black raspberry powders significantly reduced tumor multiplicity by 30–60%, when administered in a postinitiation scheme, indicating their ability to inhibit tumor progression in the rat esophagus (25,38). The molecular mechanisms of this inhibitory effect on tumor progression were investigated by Chen et al. (73), who showed that the 5% black raspberry diet downregulated the mRNA and protein expression levels of COX-2 and c-Jun (a component of AP-1), in preneoplastic tissues of NMBA-treated esophagus. This reduction in COX-2 expression correlated with lowered levels of prostaglandin E2 in esophageal epithelium that might be expected to reduce cell proliferation. The inhibition in c-Jun expression might also be expected to reduce the rate of cell proliferation since this gene is a component of the transcription activator, AP-1, which is induced by activated Ras oncogenic proteins (74). Nearly all NMBA-induced tumors in the rat esophagus have an activated H-ras oncogene (75). The reduction in inducible nitric oxide synthase (iNOS) expression correlated with lowered levels of nitrate/nitrite in the esophagus and, along with reduced COX-2, might be expected to inhibit inflammatory events in the tissue. The lowered levels of VEGF expression were accompanied by a significant reduction in microvessel density in the esophagus, indicating that the berries inhibited angiogenesis (76). Thus, these studies demonstrated that lyophilized berries reduce molecular markers of cell proliferation, inflammation and angiogenesis in carcinogen-treated rat esophagus. A summary of the effects of berry bioactives on cellular functions and signal transduction pathways is shown in Figure 2.
Fig. 2.
The effects of berry bioactives on cellular functions and signal transduction pathways. All genes shown are downregulated by berry bioactives except for intercellular adhesion molecule 1, p21CIP1/WAF1, Bak, Bax, cytochrome c and caspase 3 which are upregulated.
Clinical studies of the anticancer effects of berry bioactives
There are relatively few clinical studies of the anticancer effects of berries. Most of the available data on berry bioactives, in humans, derive from pharmacokinetic studies of the absorption, distribution, metabolism and excretion of berry compounds, obtained either from foodstuffs or as purified compounds. These studies have been summarized extensively by Seeram (19). Recently, our laboratory conducted a phase I trial in 11 subjects to determine the safety/tolerability of LBRs and to measure anthocyanins and ellagic acid in plasma and urine (77). Subjects were fed 45 g (equivalent to a 5% berry diet in animals) of black raspberry powder as a slurry in water daily for 7 days. Blood and urine samples were collected prior to and after berry treatment. The berries were found to be well tolerated; the only clinical observation was a low incidence of mild or moderate constipation in four of the 11 subjects. Maximum concentrations of anthocyanins and ellagic acid in plasma occurred at 1–2 h, and maximum quantities in urine appeared from 0 to 4 h. The uptake of the anthocyanins into plasma was reflective of their relative concentrations in black raspberry powder with cyanidin-3-rutinoside > cyanidin-3-xylosylrutinoside > cyanidin-3-glucoside = cyanidin-3-sambubioside. Overall, the uptake of ellagic acid and the anthocyanins was <1% of the administered dose. Interestingly, the major metabolites of the anthocyanins in urine were methylated derivatives and neither glucuronide nor sulfate conjugates.
Several investigations of the effects of berry powders on cancer development in the human esophagus, colon and oral cavity are either ongoing or have been completed. A brief discussion of studies from which data has been reported is as follows.
Barrett's esophagus
Barrett's esophagus (BE) affects an estimated 700 000 US adults and remains the only recognized precursor lesion to esophageal adenocarcinoma, a cancer that has increased dramatically in incidence throughout most of the western world over the last three decades (78). A study was undertaken to determine if the oral administration of black raspberry powder would influence parameters associated with the progression of Barrett's lesions (79). Twenty BE patients consumed 32 or 45 g (female and male, respectively) of black raspberry powder daily, as a slurry in water for 26 weeks. Biopsies of Barrett's lesions were taken before and after berry treatment for biomarker analysis. Based upon interim results from 10 of the 20 patients, berry treatment did not result in a reduction in segment length of Barrett's lesions at 26 weeks. Urine was collected from each subject at study baseline, at week 12 of study and at 26 weeks and evaluated for the oxidative damage biomarkers; 8-OHdG and 8-epi-prostaglandin F2α (8-Iso-PGF2). Levels of urinary 8-Iso-PGF2 were significantly reduced (Figure 3), but there was no significant change in mean levels of urinary 8-OHdG (although five of the 10 subjects had reduced levels). The authors concluded that the daily consumption of lyophilized black raspberries promotes reductions in the urinary excretion of two biomarkers of oxidative stress, 8-Iso-PGF2, and to a lesser more-variable extent, 8-OHdG, among patients with BE. One concern from this study is that the transit time of the black raspberry slurry across the esophagus, when consumed orally, is very rapid and may not permit sufficient localized absorption of berry compounds into Barrett's lesions to be effective.
Fig. 3.
Changes of mean urinary 8-epi-prostaglandin F2α levels in BE patients during 26 weeks of study.
Colon polyps and cancer
Based upon positive results of the inhibitory effects of LBRs on colon tumor development in rodents (33), we initiated a study in 50 subjects with colorectal cancer and/or polyps to determine if the oral administration of LBRs would modulate biomarkers of colon cancer development. Biopsies of normal and tumor/polyp tissues are collected at baseline. Subjects consume 20 g of freeze-dried LBR powder, as a slurry in water, three times per day (60 g total), until their scheduled surgery date, usually within 2–4 weeks. Posttreatment biopsy specimens are collected during the surgery. Pre- and posttreatment specimens from 23 patients have been analyzed to date for the effects of LBRs on cell proliferation using Ki67, apoptosis by TUNEL and angiogenesis by staining for CD105. Proliferation and angiogenesis biomarkers were reduced significantly by the berry treatment, whereas apoptosis was enhanced (80).
Oral cancer
Based upon animal data demonstrating a protective effect of LBRs on chemically induced cancer in the hamster cheek pouch (39), studies have been undertaken to determine if LBRs might exert a protective effect on the development of oral cancer in humans. A preliminary phase I study evaluated the utility of a mucoadhesive gel for the localized delivery of berry compounds to human oral mucosa (81). Black raspberry gels containing 5 and 10% berry powder were formulated to insure the stability of the anthocyanins. Maximum stability of these compounds in the gel was observed at pH 3.5 and at a temperature of 4°C. When the gel was applied topically to the oral mucosa of normal subjects, the anthocyanins were found to be readily absorbed into the tissue as evidenced by detectable blood levels within 5 min after gel application. In addition, all four anthocyanins in black raspberries were found to penetrate into explant tissues of oral mucosa, in vitro, with the greatest penetration being from the 10% berry gel. Based upon these results, the effects of topical application of the 10% black raspberry gel to oral intraepithelial neoplastic lesions in 17 patients and normal tissues in 10 patients, 4×/day for 6 weeks, were assessed (82). None of the 27 participants developed black raspberry gel-associated toxicities. Histologic regression of the intraepithelial neoplastic lesions was observed in a subset of patients as well as a statistically significant reduction in loss of heterozygosity at three tumor suppressor gene (INK4a/ARF, p53, FHIT) loci. Gene expression studies revealed that the berry gel reduced the expression of COX-2 and iNOS in dysplastic lesions and uniformally suppressed genes associated with RNA processing, growth factor recycling and inhibition of apoptosis (83). The authors concluded that further evaluation of berry gels for chemoprevention of oral intraepithelial neoplastic lesions is warranted.
Conclusions
Laboratory and clinical studies provide strong evidence of the cancer-preventive potential of berries. The ability of berries to intervene at all stages of the carcinogenesis process is undoubtedly related to the fact that they contain multiple known chemopreventive agents, including flavonoids, vitamins, minerals and phytosterols. Thus, berries and their components reduce oxidant and carcinogen-induced genetic damage and enhance DNA repair. They modulate events associated with tumor progression such as rates of cell proliferation and apoptosis and the processes of inflammation and angiogenesis. Although the antioxidant activity of berries is undoubtedly important for their preventative efficacy, it is unlikely that this is the sole mechanism for their inhibitory effects. In vitro studies have clearly demonstrated the ability of berry compounds to enter cells and to bind covalently to cellular proteins (84); this could be responsible for at least some of their effects on cell signaling pathways and other cellular functions.
Investigations in animals indicate that dietary berry bioactives are effective in preventing cancer in the oral cavity, esophagus and colon. Preliminary results suggest that they might also exhibit preventative effects in the human oral cavity, esophagus and colon. In these tissues, localized absorption of berry compounds appears to be important for their chemopreventive effects. Berries were not effective in preventing lung cancer in mice when administered in the diet (52), presumably because they do not reach the lung in sufficient concentrations for chemoprevention. This is consistent with pharmacokinetic studies in animals and humans in which it has been shown that the uptake of berry bioactives, such as the anthocyanins, ellagitannins and quercetin, into the bloodstream is low; i.e. in the nanomolar range. Thus, the future development of whole berry formulations that augment the absorption of berry bioactives is worthy of pursuit and could result in more effective prevention in the oral cavity, esophagus and colon, as well as in other organ sites in which berries have not been shown to exhibit chemopreventive efficacy.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- AP-1
activator protein-1
- B(a)P
benzo(a)pyrene
- BE
Barrett's esophagus
- BPDE
benzo(a)pyrene diol-epoxide
- COX-2
cyclooxygenase-2
- DMBA
7,12-dimethylbenz(a)anthracene
- LBR
lyophilized black raspberry
- NF-κB
nuclear factor kappa B
- NMBA
N-nitrosomethylbenzylamine
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
References
- 1.Weisburger JH. Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem. Toxicol. 1999;37:943–948. doi: 10.1016/s0278-6915(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 2.Ames BN, et al. Endogenous mutagens and the causes of aging and cancer. Mutat. Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz KA, et al. Vegetables, fruit and cancer. II mechanisms. Cancer Causes Control. 1991;2:427–442. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- 4.Block G, et al. Fruit, vegetables and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 5.Willett WC. Diet, nutrition, and avoidable cancer. Environ. Health Perspect. 1995;103:165–170. doi: 10.1289/ehp.95103s8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vainio H, et al. Fruit and Vegetables. Lyon, France: IARC Press; 2003. [Google Scholar]
- 7.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 8.Vainio H, et al. Fruit and vegetables in cancer prevention. Nutr. Cancer. 2006;54:111–142. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 9.Eberhardt MV, et al. Antioxidant activity of fresh apples. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, et al. Antioxidant and antiproliferative activities of fruits. J. Agric. Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 11.Chu Y-F, et al. Antioxidant and antiproliferative activities of vegetables. J. Agric. Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 12.Kahkonen MP, et al. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001;49:4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 13.Stoner GD, et al. Chemoprevention by fruit phenolic compounds. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Promising Cancer Chemoprevention Agents. New Jersey: Humana Press, Inc.; 2004. vol. 1, 419–435. [Google Scholar]
- 14.Stoner GD, et al. Foods and food components in the prevention of cancer. In: Neeser J-R, German JB, editors. Bioprocesses and Biotechnology for Functional Foods and Nutraceuticals. New York: Marcel Dekker, Inc.; 2003. pp. 331–373. Chapter 17. [Google Scholar]
- 15.Wang SY, et al. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000;48:5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 16.Leonard SS, et al. Antioxidant properties of fruit and vegetable juices: more to the story than ascorbic acid. Ann. Clin. Lab. Sci. 2002;32:193–200. [PubMed] [Google Scholar]
- 17.Leonard SS, et al. Resveratrol scavanges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Comm. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 18.Rietveld A, et al. Antioxidant effects of tea: evidence from human clinical trials. J. Nutr. 2003;133:3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- 19.Seeram NP. Berries. In: Heber D, Blackburn G, Go V, Milner J, editors. Nutritional Oncology. Amsterdam, the Netherlands: Elsevier, Inc.; 2006. pp. 615–628. Chapter 37. [Google Scholar]
- 20.deBoer JD. A Survey of Research into the Health Benefits of Berries. Victoria, Canada: DeBoer Consulting; 2005. Berries and their role in human health; pp. 1–103. [Google Scholar]
- 21.Connor AM, et al. Variation and heritability estimates for antioxidant activity, total phenolic content, and anthocyanin content in blueberry progenies. J. Am. Soc. Hort. 2002;127:82–88. [Google Scholar]
- 22.Wada L, et al. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002;50:3495–3500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 23.Cerda B, et al. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J. Agric. Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 24.Aaby K, et al. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa) J. Agric. Food Chem. 2005;53:4032–4040. doi: 10.1021/jf048001o. [DOI] [PubMed] [Google Scholar]
- 25.Kresty LA, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 26.Huang D, et al. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 27.Connor AM, et al. Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hort. Sci. 2002;127:89–97. [Google Scholar]
- 28.Connor AM, et al. Variability in antioxidant activity in blueberry and correlations among different antioxidant assays. J. Am. Soc. Hort. Sci. 2002;127:238–244. [Google Scholar]
- 29.Connor AM, et al. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 2002;50:893–898. doi: 10.1021/jf011212y. [DOI] [PubMed] [Google Scholar]
- 30.Jiao H, et al. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J. Agric. Food Chem. 2000;48:5672–5676. doi: 10.1021/jf000765q. [DOI] [PubMed] [Google Scholar]
- 31.Wilms LC, et al. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat. Res. 2005;582:155–162. doi: 10.1016/j.mrgentox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Duthie SJ, et al. Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutat. Res. 1997;393:223–231. doi: 10.1016/s1383-5718(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 33.Harris GK, et al. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer. 2002;40:125–133. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 34.Moller P, et al. Oxidative DNA damage in circulating mononuclear blood cells after ingestion of blackcurrant juice or anthocyanin-rich drink. Mutat. Res. 2004;551:19–26. doi: 10.1016/j.mrfmmm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Duthie SJ, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006;45:113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 36.Freese R. Markers of oxidative DNA damage in human interventions with fruit and berries. Nutr. Cancer. 2006;54:143–147. doi: 10.1207/s15327914nc5401_14. [DOI] [PubMed] [Google Scholar]
- 37.Reen RK, et al. Modulation of N-nitrosomethylbenzylamine metabolism by black raspberries in the esophagus and liver of Fischer 344 rats. Nutr. Cancer. 2006;54:47–57. doi: 10.1207/s15327914nc5401_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlton PS, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–446. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 39.Casto BC, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–4016. [PubMed] [Google Scholar]
- 40.Maurya DK, et al. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol. Cell Biochem. 2005;280:209–217. doi: 10.1007/s11010-005-0170-4. [DOI] [PubMed] [Google Scholar]
- 41.Maurya DK, et al. Protection of cellular DNA from gamma-radiation-induced damages and enhancement in DNA repair by troxerutin. Mol. Cell Biochem. 2005;280:57–68. doi: 10.1007/s11010-005-8052-3. [DOI] [PubMed] [Google Scholar]
- 42.Niture SK, et al. Increased expression of the MGMT repair protein mediated by cysteine prodrugs and chemopreventative natural products in human lymphocytes and tumor cell lines. Carcinogenesis. 2007;28:378–389. doi: 10.1093/carcin/bgl155. [DOI] [PubMed] [Google Scholar]
- 43.Stoner GD, et al. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr. Cancer. 2006;54:33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoner GD, et al. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 45.Maas J, et al. Ellagic acid, an anticarcinogen in fruits, especially in strawberries: a review. Hort. Science. 1991;26:10–14. [Google Scholar]
- 46.Perchellet JP, et al. Antitumor-promoting effects of gallotannins, ellagitannins, and flavonoids in mouse skin in vivo. In: Huang M-T, Osawa T, Ho C-T, Rosen RT, editors. Food Phytochemicals for Cancer Prevention—Fruits and Vegetables. Washington, DC: American Cancer society; 1994. pp. 303–327. [Google Scholar]
- 47.Smart RC, et al. Effect of ellagic acid and 3-O-decylellagic acid on the formation of benzo(a)pyrene-derived DNA adducts in vivo and on the tumorigenicity of 3-methylcholanthrene in mice. Carcinogenesis. 1986;7:1669–1675. doi: 10.1093/carcin/7.10.1669. [DOI] [PubMed] [Google Scholar]
- 48.Boukharta M, et al. Biodistribution of ellagic acid and dose-related inhibition of lung tumorigenesis in A/J mice. Nutr. Cancer. 1992;18:181–189. doi: 10.1080/01635589209514218. [DOI] [PubMed] [Google Scholar]
- 49.Daniel EM, et al. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Comp. Anal. 1989;2:338–349. [Google Scholar]
- 50.Mandal S, et al. Inhibition of N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 51.Stoner GD, et al. Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlton PS, et al. Failure of dietary lyophilized strawberries to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-and benzo[a]pyrene-induced lung tumorigenesis in strain A/J mice. Cancer Lett. 2000;159:113–117. doi: 10.1016/s0304-3835(00)00464-x. [DOI] [PubMed] [Google Scholar]
- 53.Felgines C, et al. Blackberry anthocyanins are slightly bioavailable in rats. J. Nutr. 2002;132:1249–1253. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- 54.Borges G, et al. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007;6:714–725. doi: 10.1002/mnfr.200700024. [DOI] [PubMed] [Google Scholar]
- 55.Olsson ME, et al. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006;54:1248–1255. doi: 10.1021/jf0524776. [DOI] [PubMed] [Google Scholar]
- 56.Jurinac Z, et al. Antiproliferative action of water extracts of seeds or pulp of five different raspberry cultivars. Food Chem. 2005;93:39–45. [Google Scholar]
- 57.Seeram NP, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry. and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 58.Murphy BT, et al. Identification of triterpene hydroxycinnamates with in vitro antitumor activity from whole cranberry fruit (Vaccinium macrocarpon) J. Agric. Food Chem. 2003;51:3541–3545. doi: 10.1021/jf034114g. [DOI] [PubMed] [Google Scholar]
- 59.Han C, et al. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr. Cancer. 2005;51:207–217. doi: 10.1207/s15327914nc5102_11. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigo K, et al. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr. Cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hecht SS, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo(a)pyrene-7,8-diol-9,10-epoxide induced NFκB and AP-1 activity. Carcinogenesis. 2006;27:1617–1626. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu H, et al. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr. Cancer. 2006;54:69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- 63.Huang C, et al. Inhibition of BPDE-induced transactivation of AP-1 and NFκB by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 64.Huang C, et al. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and vascular endothelial growth factor transcription by targeting the phosphoinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–588. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 65.Yang JH, et al. Inhibition of lung cancer cell growth by querceitn glucuronides via G2/M arrest and induction of apoptosis. Drug Metab. Dispos. 2006;34:296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen TT, et al. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–659. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- 67.Ding M, et al. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- 68.Bagchi D, et al. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 69.Yang GY, et al. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 70.Long LH, et al. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (-)-epigallocatechin, (-)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- 71.Chai PC, et al. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem. Biophys. Res. Commun. 2003;304:650–654. doi: 10.1016/s0006-291x(03)00655-7. [DOI] [PubMed] [Google Scholar]
- 72.Roques SC, et al. Hydrogen peroxide generation in caco-2 cell culture medium by addition of phenolic compounds: effect of ascorbic acid. Free Radical Res. 2002;36:593–599. doi: 10.1080/10715760290025979. [DOI] [PubMed] [Google Scholar]
- 73.Chen T, et al. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of COX-2, iNOS and c-Jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westwick JK, et al. Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc. Natl Acad. Sci. 1994;91:6030–6034. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, et al. Mutational activation of the cellular Harvey ras oncogene in rat esophageal papillomas induced by methylbenzylnitrosamine. Cancer Res. 1990;50:1591–1595. [PubMed] [Google Scholar]
- 76.Chen T, et al. Black raspberries inhibit N-Nitrosomethylbenzylamine-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006;27:2301–2307. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- 77.Stoner GD, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 78.Spechler S. Barrett's esophagus. N. Engl. J. Med. 2002;346:836–842. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 79.Kresty LA, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr. Cancer. 2006;54:148–156. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 80.Wang L-S, et al. Effect of Freeze-Dried Black Raspberries on Human Colorectal Cancer Lesions. 2007. American Association for Cancer Research Special Conference in Cancer Research. Advances in Colon Cancer Research, #B31. [Google Scholar]
- 81.Mallery SR, et al. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: implications for oral cancer chemoprevention. Pharmaceut. Res. 2007;24:728–737. doi: 10.1007/s11095-006-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shumway BS, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-4096. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mallery SR, et al. Application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whitley AC, et al. Intestinal epithelial cell accumulation of the cancer chemopreventive polyphenol ellagic acid—extensive covalent binding to protein and DNA. Biochem. Pharmacol. 2003;66:907–915. doi: 10.1016/s0006-2952(03)00413-1. [DOI] [PubMed] [Google Scholar]




