PLK1-mediated phosphorylation of pericentrin induces proper organization of the spindle pole–specific pericentriolar matrix and subsequent centrosome maturation.
Abstract
The microtubule-organizing activity of the centrosome oscillates during the cell cycle, reaching its highest level at mitosis. At the onset of mitosis, the centrosome undergoes maturation, which is characterized by a drastic expansion of the pericentriolar matrix (PCM) and a robust increase in microtubule-organizing activity. It is known that PLK1 is critical for the initiation of centrosome maturation. In this paper, we report that pericentrin (PCNT), a PCM protein, was specifically phosphorylated by PLK1 during mitosis. Phosphoresistant point mutants of PCNT did not recruit centrosomal proteins, such as CEP192, GCP-WD (γ-complex protein with WD repeats), γ-tubulin, Aurora A, and PLK1, into the centrosome during mitosis. However, centrosomal recruitment of CEP215 depended on PCNT irrespective of its phosphorylation status. Furthermore, ectopic expression of PLK1-PCNT fusion proteins induced the centrosomal accumulation of CEP192, GCP-WD, and γ-tubulin even in interphase cells, mimicking centrosome maturation. Based on these results, we propose that PLK1-mediated phosphorylation of PCNT initiates centrosome maturation by organizing the spindle pole–specific PCM lattice.
Introduction
Centrosome consists of a pair of centrioles surrounded by a protein meshwork named pericentriolar matrix (PCM). Centrosomes are the primary microtubule-organizing center in which microtubules are nucleated and anchored (Job et al., 2003). The microtubule-organizing activity of the centrosome oscillates during the cell cycle, reaching its peak during mitosis. At the onset of mitosis, PCM should be expanded to organize a large amount of short-lived spindle microtubules. This expansion of PCM is called centrosome maturation. If centrosome maturation is inhibited, monopolar spindles are formed as a result of reduction of microtubules emanated from the spindle poles.
PLK1 is a mitotic kinase that phosphorylates multiple substrates for execution of diverse mitotic events in a coordinated manner (Petronczki et al., 2008). PLK1 is also critical for centrosome maturation because inhibition of PLK1 activity results in a monopolar spindle with reduced microtubule-organizing activity (Lane and Nigg, 1996; Sumara et al., 2004; Lénárt et al., 2007; Santamaria et al., 2007). Pericentrin (PCNT), CEP192, and CEP215, which are required for centrosome maturation, were suggested as the substrates of PLK1 (Santamaria et al., 2011). However, it remains to be investigated how PLK1 executes centrosome maturation.
PCNT is a large coiled-coil protein that serves as a scaffold for anchoring many PCM proteins (Zimmerman et al., 2004; Haren et al., 2009; Buchman et al., 2010). Mutations in the PCNT gene are associated with several human disorders, including primordial dwarfism (Griffith et al., 2008; Rauch et al., 2008; Anitha et al., 2009; Numata et al., 2009; Delaval and Doxsey, 2010). PCNT is required for centrosome maturation because its centrosomal level augments at the onset of mitosis, and its depletion results in monopolar spindles (Zimmerman et al., 2004). In addition to PCNT, CEP215 and CEP192 are also involved in recruitment of γ-tubulin into the spindle poles (Gomez-Ferreria et al., 2007; Fong et al., 2008; Zhu et al., 2008; Haren et al., 2009; Lee and Rhee, 2010). However, it remains to be investigated how these PCM proteins are coordinated to execute centrosome maturation.
In this study, we show that PCNT is phosphorylated by PLK1 in mitosis. Furthermore, we revealed that the PLK1-mediated phosphorylation of PCNT is essential for the initiation step of centrosome maturation.
Results and discussion
PCNTB is necessary for spindle formation and centrosome maturation
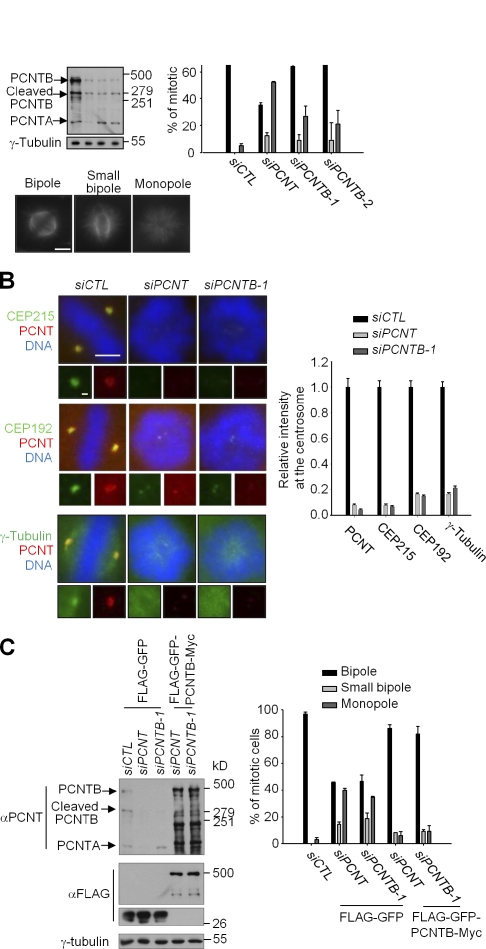
There are two isoforms of PCNT: PCNTB is a larger clone that is 340 kD in size and that shares its N-terminal end with PCNTA (220 kD; Fig. S1 A; Doxsey et al., 1994; Flory et al., 2000; Li et al., 2001; Flory and Davis, 2003). We detected the PCNTA- and PCNTB-specific bands at the expected positions (Fig. 1 A). We also detected an additional band (275 kD; Fig. 1 A). This band may be a proteolytic fragment of PCNTB because it was depleted along with PCNTB and was detected in PCNTB-stable cell lines (Fig. 1 A and not depicted).
Figure 1.
PCNTB is necessary for centrosome maturation and spindle formation. (A) HeLa cells were transfected with siCTL, siPCNT, siPCNTB-1, and siPCNTB-2. 48 h later, the lysates were subjected to immunoblotting and immunostaining analyses. The phenotypes were classified as bipole, small bipole, and monopole. n ≥ 200 per group in two independent experiments. Error bars represent SD. Bar, 5 µm. (B) HeLa cells were transfected with siCTL, siPCNT, and siPCNTB-1. 48 h later, the cells were coimmunostained with the indicated antibodies. Bar, 5 µm. (insets) Magnified views of the centrosomes. Bar, 1 µm. n ≥ 40 per group in two independent experiments. Error bars represent SEM. (C) The PCNT-depleted HeLa cells were rescued with Flag-GFP-PCNTB-Myc. Immunoblot analysis confirmed knockdown and overexpression. 40 h later, the cells were treated with monastrol for 6 h to synchronize the cell in mitosis and subsequently with MG132 for 1.5 h to block exiting of the mitosis. n ≥ 100 per group in two independent experiments. Error bars represent SD.
PCNT was depleted with PCNTB-specific siRNAs (siPCNTB-1 and siPCNTB-2; Zimmerman et al., 2004) and with pan-PCNT siRNA (siPCNT; Fig. 1 A; Srsen et al., 2006). As previously reported, PCNT depletion resulted in defects in spindle formation, such as a monopole and a small bipole in which the distance between the two poles was significantly reduced (Fig. 1 A; Zimmerman et al., 2004). When the B isoform of PCNT was selectively depleted, defects in spindle formation were still observed (Fig. 1 A). These results reveal that PCNTB is necessary for bipolar spindle formation.
On the contrary to the aforementioned result, a previous study reported that PCNTB is not important for spindle formation and the recruitment of γ-tubulin ring complex (Zimmerman et al., 2004). To resolve this issue, we determined the centrosomal levels of PCNT, γ-tubulin, and the PCM proteins for γ-tubulin recruitment in PCNT-depleted cells. The centrosomal PCNT disappeared in cells transfected with both PCNTB and pan-PCNT siRNAs, suggesting that the centrosomal PCNT in mitotic cells is largely of the B isoform (Fig. 1 B). Centrosomal levels of CEP215, CEP192, and γ-tubulin were significantly reduced in the spindle poles of both pan-PCNT– and PCNTB-depleted cells (Fig. 1 B; Gomez-Ferreria et al., 2007; Zhu et al., 2008; Haren et al., 2009). We also performed rescue experiments with the ectopic PCNTB protein. We treated the cells with monastrol and released and treated with MG132 to make the cells enter metaphase synchronously and be blocked from exiting the mitosis. The results showed that ectopic PCNTB rescued the defects of spindle formation in both pan-PCNT– and PCNTB-depleted cells (Fig. 1 C). These results indicate that the B isoform, rather than the A isoform, is the major PCNT species critical for mitotic spindle formation. Therefore, we decided to focus on the PCNTB for further investigation.
PLK1 phosphorylation at S1235 and S1241 of PCNT is necessary for microtubule nucleation and spindle formation in mitosis
PLK1 phosphorylation of PCNT has been previously suggested (Soung et al., 2009; Santamaria et al., 2011). Indeed, we observed that PCNTB of the mitotic cells migrated more slowly in SDS-PAGE than that of the S phase–arrested cells did (Fig. S1 B). The slow-migrating band of PCNTB disappeared with a phosphatase treatment, suggesting that PCNTB is phosphorylated specifically during mitosis (Fig. S1 B). Furthermore, when mitotic cells were treated with BI2536, which is a PLK1 inhibitor, the slow-migrating band of PCNTB disappeared, suggesting that PLK1 is responsible for mitotic phosphorylation of PCNTB (Fig. S1 C).
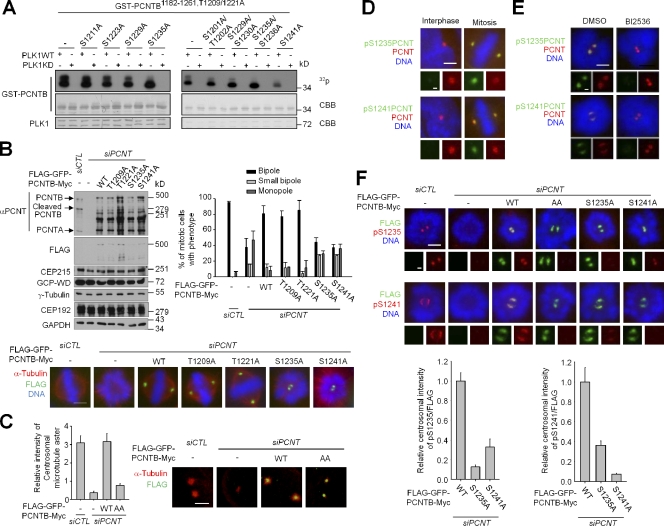
In vitro kinase assays were performed to identify the PLK1 phosphorylation sites within PCNTB. PLK1 phosphorylated PCNTB1182–1261 the most strongly out of all of the truncated GST-PCNTB mutants (Fig. S1, D–F). The specific phosphorylation sites were pinpointed to T1209, T1221, S1235, and S1241 by using the phosphoresistant point mutants as substrates (Figs. 2 A and S2 G).
Figure 2.
The PLK1 phosphorylations on S1235 and S1241 of PCNT are important for spindle formation and centrosomal microtubule nucleation in mitosis. (A) In vitro kinase assays were performed with the indicated point mutants of GST-PCNTB1182–1261. The immunoprecipitates of constitutively active (Myc-GFP-PLK1CA) and kinase-dead (Myc-GFP-PLK1KD) PLK1 were used as enzymes. CBB, Coomassie Brilliant blue; WT, wild type. (B) PCNT-depleted HeLa cells were rescued with the phosphoresistant point mutants of Flag-GFP-PCNTB-Myc at S1209, S1221, S1235, and S1241. 40 h later, the cells were treated with monastrol for 6 h to synchronize the cell in mitosis and subsequently with MG132 for 1.5 h to block exiting of the mitosis. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Bar, 5 µm. n ≥ 100 per group in two independent experiments. Error bars represent SD. (C) The microtubule regrowth assay was performed with the PCNT-depleted HeLa cells rescued with Flag-GFP-PCNTB-Myc-WT and -AA (S1235A and S1241A). Bar, 5 µm. n ≥ 20 per group in two independent experiments. Error bars represent SEM. (D) HeLa cells were coimmunostained with the antibodies specific for PCNT and phospho-PCNT (pS1235PCNT or pS1241PCNT). Bar, 5 µm. (insets) Magnified views of the centrosomes. Bar, 1 µm. (E) HeLa cells were incubated with monastrol for 6 h and subsequently treated with BI2536 for 3 h. The cells were coimmunostained with the antibodies specific for PCNT and phospho-PCNT. Bar, 5 µm. (insets) Magnified views of the centrosomes. Bar, 1 µm. (F) The PCNT-depleted HeLa cells were rescued with the PCNTB-WT, -AA, S1235A, or S1241A. 40 h later, the cells were treated with monastrol for 6 h and immunostained with antibodies specific for FLAG and phospho-PCNT. Bar, 5 µm. (insets) Magnified views of the centrosomes. Bar, 1 µm. n ≥ 20 per group in two independent experiments. Error bars represent SEM.
We performed the rescue experiments with the phosphoresistant PCNTB mutants to determine their functional importance in bipolar spindle formation. All forms of phosphoresistant PCNTB were normally targeted to the centrosome, indicating that these PLK1 phosphorylations are dispensable for centrosomal localization of PCNTB (Fig. 2 B). T1209A and T1221A mutants of PCNTB rescued the spindle defect phenotypes of PCNT-depleted cells, whereas S1235A and S1241A mutants did not (Fig. 2 B). We also detected that the microtubule nucleation activity was significantly reduced in the phosphoresistant PCNTB-expressing cells (Fig. 2 C). The observations that the cells with phosphoresistant PCNT had defects in spindle formation with a reduced microtubule nucleation activity led us to conclude that the phosphorylation of PCNT is important for centrosome maturation.
S1235 and S1241 of PCNT are conserved among vertebrates but do not fit with the proposed PLK1 consensus sequence (Fig. S1 G; Nakajima et al., 2003). There is no sequence homology between human PCNT and Drosophila melanogaster pericentrin-like protein except the pericentrin-AKAP450 centrosomal targeting domain at the C-terminal end. Therefore, it is possible that the biological activities of PCNT and Drosophila pericentrin-like protein might not be controlled by PLK1 in an identical manner. Rather, it is known that Cnn, the Drosophila homologue of CEP215, has a major role in PCM maintenance in fly (Martinez-Campos et al., 2004; Conduit et al., 2010).
To investigate the phosphorylation of PCNT in vivo, we raised phosphoantibodies specific for pS1235 and pS1241 of PCNT. The phosphoantibodies detected their own antigen peptides specifically by immunoblot analysis (Fig. S1 H). They also detected the GST-PCNTB fusion protein only when the specific sites were phosphorylated by PLK1 in vitro (Fig. S1 I). The phosphoantibodies detected the centrosomes of mitotic cells but not those of interphase cells (Fig. 2 D). Furthermore, the phospho-PCNT levels were significantly reduced with the BI2536 treatment, indicating that S1235 and S1241 of PCNT are phosphorylated by PLK1 during mitosis (Fig. 2 E). However, we do not rule out a possibility that these sites are also phosphorylated by the other kinases.
Specificity of the phospho-PCNT antibodies was also confirmed by the rescue experiments (Fig. 2 F). Interestingly, the pS1235 antibody immunostained the PCNTBS1241A-expressing cells but to a lesser extent (Fig. 2 F). A similar immunostaining pattern was also observed with the pS1241PCNT antibody (Fig. 2 F). These results suggest that phosphorylation at S1235 and S1241 is partially interdependent on each other.
PLK1 phosphorylation of PCNT is essential for recruitment of the PCM proteins for centrosome maturation
It is known that centrosomal PCNTs are significantly reduced in the BI2536-treated cells (Fig. S2 A; Haren et al., 2009). However, BI2536 has a little effect on the centrosomal PCNT levels once the cells have already reached M phase (Fig. S2 A). We determined the centrosomal levels of the PCM proteins essential for centrosome maturation in the BI2536-treated cells after their cell cycles were arrested at M phase with monastrol. The centrosomal levels of CEP192, GCP-WD (γ-complex protein with WD repeats), γ-tubulin, and Aurora A were significantly reduced in the BI2536-treated cells (Fig. S2 B); this finding suggests that PLK1 is essential for the recruitment of these PCM proteins during M phase. However, the centrosomal CEP215 levels were not affected by the PLK1 inhibitor, suggesting that the centrosomal recruitment of CEP215 is independent of the phosphorylation of PCNT (Fig. S2 B).
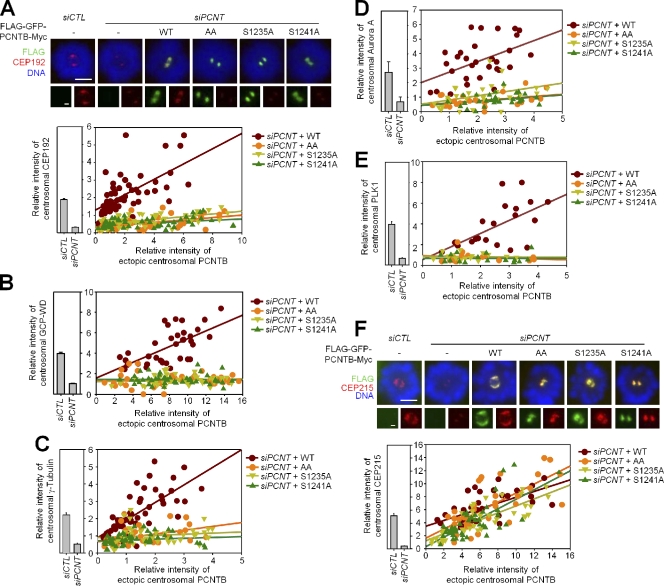
We performed rescue experiments with the phosphoresistant PCNT mutants to examine whether the phosphorylation of PCNT is required for centrosomal recruitment of the PCM proteins during mitosis. Mitotic cells were treated with monastrol to avoid any complications caused by the spindle pole shapes of the cells. The centrosomal levels of endogenous CEP192 were positively correlated with those of wild-type PCNTB (Fig. 3 A). However, the centrosomal CEP192 levels remained minimal with the phosphoresistant PCNTB mutants S1235 and/or S1241 (Fig. 3 A). The centrosomal levels of GCP-WD and γ-tubulin and Aurora A were also correlated with wild-type PCNTB but not with the phosphoresistant PCNTB mutants (Fig. 3, B–D). The centrosomal localization of PLK1 is dependent on the phosphorylation of PCNT, suggesting a positive feedback mechanism in centrosomal recruitment of PLK1 (Fig. 3 E). The interdependency of the phosphorylation of S1235 and S1241 supports this suggestion (Fig. 2 F). However, the centrosomal levels of CEP215 were correlated with the centrosomal levels of PCNTB irrespective of phosphorylation status at S1235 and S1241 (Fig. 3 F). These results indicate that phosphorylation of PCNT is critical for centrosomal recruitment of selected PCM proteins, such as CEP192, GCP-WD, γ-tubulin, Aurora A, and PLK1 in mitotic cells. In contrast, centrosomal recruitment of CEP215 is independent of the specific phosphorylation of PCNT.
Figure 3.
PLK1 phosphorylation at S1235 and S1241 of PCNT is essential for recruitment of the PCM proteins for centrosome maturation. (A–F) The PCNT-depleted HeLa cells were rescued with Flag-GFP-PCNTB-Myc (PCNTBWT, PCNTBS1235A, PCNTBS1241A, and PCNTBAA). 40 h later, the cells were treated with monastrol for 6 h and coimmunostained with the FLAG antibody along with the antibodies specific to the indicated PCM proteins. Bars, 5 µm. (insets) Magnified views of the centrosomes. Bars, 1 µm. Centrosomal intensities of ectopic PCNTB and the PCM proteins were measured and plotted in the linear regression graphs. Error bars represent SEM. n > 20 per experimental group.
We performed the rescue experiments with phosphoresistant PCNTB mutants in interphase cells. First, the centrosomal levels of CEP192, GCP-WD, and γ-tubulin were much smaller than those of PCNTB in interphase cells (Fig. S2 C). The depletion and rescue of PCNT did not affect the centrosomal levels of these PCM proteins in interphase cells (Fig. S2 C). These results suggest that PCNT has a little role in centrosomal recruitment of these PCM proteins in interphase cells. In contrast, the centrosomal levels of CEP215 were comparable with those of PCNTB in interphase cells (Fig. S2 C). The centrosomal CEP215 levels were reduced in PCNT-depleted cells and restored with ectopic PCNTB irrespective of the presence of the phosphoresistant mutations (Fig. S2 C). These results indicate that PCNT is critical for the centrosomal recruitment of CEP215 in both interphase and mitotic cells. However, the centrosomal recruitment of CEP215 is independent of the PLK1-mediated phosphorylation of PCNT.
It was previously reported that CEP215 directly interacts with γ-tubulin and recruits it to the centrosome (Fong et al., 2008). However, we revealed that γ-tubulin does not follow CEP215 until PCNT is specifically phosphorylated by PLK1 (Fig. 3 C). This suggests that CEP215 should cooperate with phospho-PCNT to recruit γ-tubulin during centrosome maturation. Consistent with this hypothesis, it was shown that γ-tubulin is normally localized to the centrosome in chick cells in which endogenous CEP215 is replaced with a CNN1 deletion mutant (Barr et al., 2010).
Centrosomal accumulation of the PCM proteins during centrosome maturation
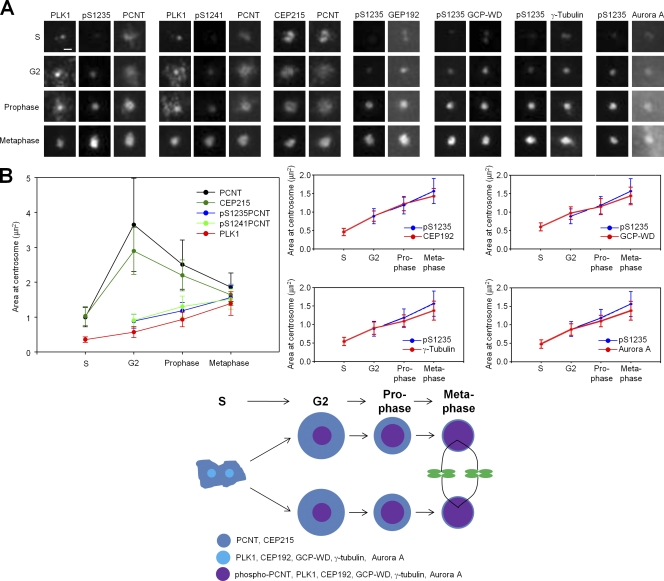
We examined the centrosomal localization of the selected PCM proteins during the cell cycle. The immunostaining patterns of CEP192, GCP-WD, and γ-tubulin appear as two separate spots in interphase cells (Figs. 4 A and S2 D; Haren et al., 2006; Lüders et al., 2006; Gomez-Ferreria et al., 2007; Zhu et al., 2008). PCNT and CEP215 are also stained as two dots until early S phase and become scattered thereafter (Figs. 4 A and S2 D; Dictenberg et al., 1998).
Figure 4.
Centrosomal accumulation of the PCM proteins during centrosome maturation. (A) HeLa cells were coimmunostained with the indicated antibodies. Bar, 1 µm. (B) The centrosomal area of the indicated proteins was analyzed statistically. n ≥ 40 per group in two independent experiments. Error bars represent SD.
PLK1 appears as two small dots in S phase but forms a single large dot when the cell progresses to M phase (Fig. 4 A). The phospho-PCNT appears at G2 phase and gradually becomes prominent like PLK1 (Fig. 4 A). The centrosomal staining patterns of CEP192, GCP-WD, γ-tubulin, and Aurora A are similar to those of phospho-PCNT, which forms a large, discrete dot at the M phase centrosome (Fig. 4 A). However, the centrosomal localization of CEP215 and PCNT had a different pattern than phospho-PCNT. The CEP215 and PCNT centrosomal areas reached a maximum in G2 phase and then slightly reduced in prophase and metaphase (Fig. 4 A). The centrosomal areas of the PCM proteins and phospho-PCNT were quantified and statistically analyzed (Fig. 4 B).
Ectopic expression of the PLK1-PCNT fusion protein induces centrosomal recruitment of the PCM proteins even in interphase cells
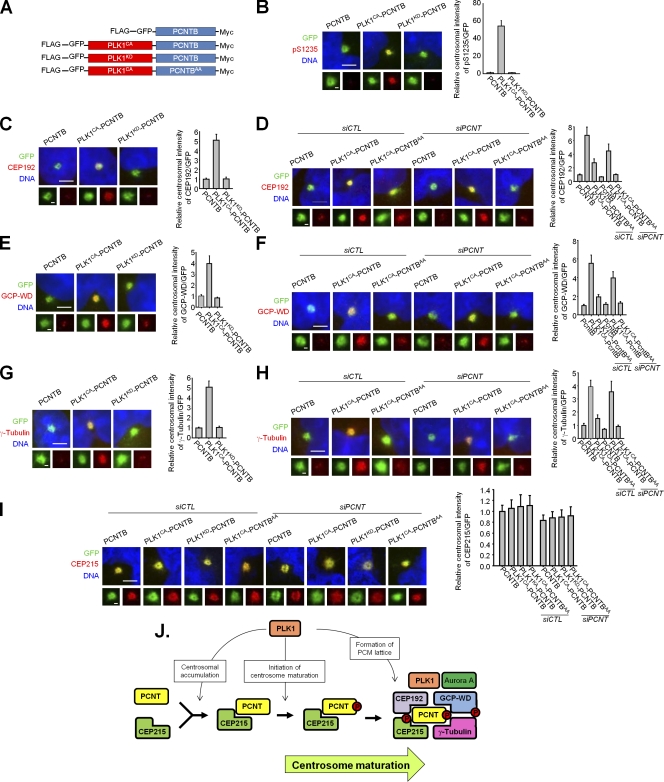
The requirement of PLK1-mediated phosphorylation of PCNT for centrosomal recruitment of the PCM proteins was examined with ectopic expression of PLK1-PCNTB fusion proteins in interphase cells (Fig. 5 A). As expected, ectopic PCNT protein was phosphorylated by linked active PLK1 kinase domain even in interphase cells (Fig. 5 B). CEP192, GCP-WD, and γ-tubulin were recruited to the centrosomes in the constitutively active PLK1 (PLK1CA)–PCNTB-expressing cells but not in the kinase-dead PLK1 (PLK1KD)–PCNTB-expressing cells (Fig. 5, C, E, and G). Their centrosomal recruitment depends on the PLK1 phosphorylation of PCNT because the recruitments were significantly reduced in the PLK1CA-PCNTBS1235A and S1241A (PCNTBAA)–expressing cells (Fig. 5, D, F, and H). These results support the hypothesis that PCNT phosphorylation regulates centrosomal recruitment of the PCM proteins essential for centrosome maturation. However, CEP215 was recruited to the centrosome in all of the PLK1-PCNTB–expressing cells irrespective of the phosphorylation status of PCNTB (Fig. 5 I).
Figure 5.
Ectopic expression of the PLK1-PCNTB fusion proteins induces centrosomal recruitment of the PCM proteins even in interphase cells. (A) Constructs of the PLK1-PCNTB fusion proteins in which the constitutively active (PLK1CA) or kinase-dead (PLK1KD) catalytic domain of PLK1 is linked to PCNT–wild type or PCNT-AA. (B–I) The PLK1-PCNTB–expressing HeLa cells were coimmunostained with the GFP antibody along with the indicated antibodies (red). Endogenous PCNT proteins were depleted in some experimental groups. Bars, 5 µm. (insets) Magnified views of the centrosomes. Bars, 1 µm. n ≥ 40 per group in two independent experiments. Error bars represent SEM. (J) A working model. P, phosphorylated.
These results indicate that activation of centrosomal PLK1 is essential for the initiation of centrosome maturation. However, it remains to be determined whether PLK1-mediated phosphorylation of PCNT at S1235 and S1241 is sufficient for execution of centrosome maturation. In fact, PLK1 is known to phosphorylate the additional PCM proteins, including GCP-WD (Haren et al., 2009; Zhang et al., 2009; Santamaria et al., 2011). It is unfortunate that phosphomimetic PCNT mutants are not available because the phosphorylation of S1241 can’t be mimicked with aspartate and glutamate (Fig. S3). This issue should be examined in an alternative way.
Working model
Based on the present data, we propose a model for the process of centrosome maturation (Fig. 5 J). Before entering mitosis, both PCNT and CEP215 are accumulated into the centrosome in a PLK1 activity–dependent manner that has not yet been elucidated. At the onset of mitosis, PLK1 phosphorylates S1235 and S1241 of PCNT, and this phosphorylation induces the recruitment of a group of PCM proteins essential for assembly of a γ-tubulin ring complex–rich lattice. The PCM lattice gradually becomes enlarged during centrosome maturation and eventually organizes a spindle pole for mitosis. Other centrosomal proteins might be phosphorylated by PLK1 to complete the centrosome maturation.
Expansion of the PCM lattice was also observed in the centrosomes of Drosophila early embryos (Conduit et al., 2010). Conduit et al. (2010) proposed that Drosophila Cnn, a homologue of human CEP215, is incorporated into the region of the PCM immediately surrounding the centriole. Asl and Dspd-2, homologues of human CEP152 and CEP192, are known to be essential for the incorporation of Cnn (Conduit et al., 2010). Once the cell cycle progresses to M phase, these protein complexes spread outward throughout the rest of the PCM and form a dynamic lattice that allows other centrosomal components to be stably retained in the PCM (Conduit et al., 2010). It remains to be determined whether PCM expansion in the Drosophila early embryos is identical to centrosome maturation in mammalian cells or not.
Materials and methods
Cell culture
HeLa and 293T cells were cultured in DME supplemented with 10% FBS and antibiotics.
siRNA, DNA constructs, and transfection
siRNAs used in this study were siPCNT (5′-GCAGCUGAGCUGAAGGAGATT-3′; Srsen et al., 2006), siPCNTB-1 (5′-UGGACGUCAUCCAAUGAGATT-3′; Zimmerman et al., 2004), siPCNTB-2 (5′-GCUCUGAUUUAUCAAAAGATT-3′), and scrambled siCTL (5′-GCAAUCGAAGCUCGGCUACTT-3′). The full-length PCNTB cDNA was a gift from M. Takahashi (Biosignal Research Center, Kobe University, Kobe, Japan; Takahashi et al., 2002). FLAG-GFP-PCNTB-Myc was prepared by subcloning PCNTB into p3xFLAG-CMV10 with EGFP and the Myc tag. For kinase assays, the truncated mutants of PCNTB were subcloned into pGEX-4T2 (GE Healthcare). Transfection was performed with Fugene HD (DNA; Roche) or RNAiMAX (siRNA; Invitrogen) according to the manufacturers’ manuals.
Antibodies
The anti-PCNT and -CEP215 antibodies were used as previously described (Lee and Rhee, 2010; Kim and Rhee, 2011). The anti-CEP192 antibody was a gift from L. Pelletier (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada; Zhu et al., 2008). Antibodies specific for PLK1 (sc-17783; Santa Cruz Biotechnology, Inc.), α-tubulin (ab18251; Abcam), β-tubulin (T0198; Sigma-Aldrich), γ-tubulin (sc-7396; Santa Cruz Biotechnology, Inc.), GFP (sc-9996 and sc-8334; Santa Cruz Biotechnology, Inc.), FLAG (F3165; Sigma-Aldrich), CEP192 (A302-324A; Bethyl Laboratories, Inc.), GCP-WD (ab57336; Abcam), and Aurora A (3092; Cell Signaling Technology) were purchased. Alexa Fluor 488, 555, and 647 dyes (Invitrogen) were used for the labeling of rabbit polyclonal antibodies. Anti–mouse IgG-HRP (A9044; Sigma-Aldrich), protein A–HRP (539253; EMD), and Alexa 488, 555, and 647 (Invitrogen) were used as secondary antibodies for immunoblotting and immunostaining analyses.
Immunofluorescence, microscopy, and statistical analyses
Immunofluorescence, microscopy, and statistical analyses were performed as previously described (Kim and Rhee, 2011). In brief, HeLa cells were cultured on a 12-mm coverslip and fixed with cold methanol for 10 min or 3.7% PFA for 15 min. Fixed cells were permeabilized and blocked with 3% BSA in 0.5% PBS with Triton X-100 for 20 min. Antibodies were diluted in 0.1% PBS with Triton X-100 with 3% BSA. The incubation time was 1 h for primary antibodies and 30 min for fluorescence-conjugated secondary antibodies. DAPI solution was used at the final step for DNA staining. The cells were mounted onto a slide glass and imaged using a 60×/1.25 oil Iris objective lens (UFlanFl; Olympus) observed with a fluorescence microscope (IX51; Olympus) equipped with a charge-coupled device camera (QICAM Fast 1394; QImaging). The images were obtained from the best-fitted single focal plan to the centrosome and analyzed using ImagePro (version 5.0; Media Cybernetics) and ImageJ (National Institutes of Health) software and statistically analyzed with SigmaPlot (Systat Software). The region of interest was defined by drawing a circle including the centrosome. Background value is measured from the same-sized circle as a circle including the centrosome in an adjacent region. In the case of monopolar cells, the same method was applied, and two centrosomes were measured together. In the regrowth assay experiment (Fig. 2 C), the images were obtained by z projection (0.45-µm interval) with a confocal microscope (LSM700; Carl Zeiss) and analyzed using ImageJ and statistically analyzed with SigmaPlot.
In vitro kinase assay
The kinase-active Myc-GFP-PLK1 and kinase-dead Myc-GFP-PLK1K82R proteins were immunoprecipitated from 293T cells. GST-PLK11–356, GST-PLK11–356, K82R, and truncated GST-PCNTB mutants were purified from bacteria. PLK1 and PCNTB were mixed in a kinase assay buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT, and 5 µM ATP) with 0.25 µCi γ-[32P]ATP for 30 min at 30°C.
Rescue experiments
HeLa cells were simultaneously plated and transfected with siRNA by using RNAiMAX. 3 h after siRNA transfection, the plasmids encoding siRNA-resistant PCNTB were transfected with Fugene HD. 40 h after DNA transfection, the cells were treated with 100 µM monastrol for 6 h to synchronize the cells in mitosis, washed three times with PBS, and incubated with 20 µM MG132 for 1.5 h to block the exiting of mitosis.
Microtubule regrowth assay
Microtubule regrowth assay was performed as previously described (Kim and Rhee, 2011). The cells were incubated with 1 µg/ml nocodazole for 3 h and placed in ice for 1 h to depolymerize microtubules. Microtubule regrowth was triggered by transfer to drug-free medium at 37°C.
Online supplemental material
Figs. S1–S3 include the data about the phosphorylation of PCNT and its function. Fig. S1 shows that PCNTB is a substrate of PLK1. Fig. S2 shows PLK1 phosphorylation of PCNTB and the centrosomal localization pattern of the PCM proteins during the cell cycle. Fig. S3 shows that the aspartate or glutamate substitution at S1241 of PCNT cannot rescue the knockdown phenotype of PCNT. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201106093/DC1.
Acknowledgments
We thank Drs. L. Pelletier and M. Takahashi, who kindly provided us with the CEP192 antibody and the PCNTB clone, respectively.
This study was supported by grants from the Bio Imaging Research Center at the Gwangju Institute of Science and Technology, the Basic Science Research Program (20110025944), and the Science Research Center Program (R11-2005-009-03005-0). K. Lee was supported by the second stage of the Brain Korea 21 Project in 2007.
Footnotes
Abbreviations used in this paper:
- PCM
- pericentriolar matrix
- PCNT
- pericentrin
References
- Anitha A., Nakamura K., Yamada K., Iwayama Y., Toyota T., Takei N., Iwata Y., Suzuki K., Sekine Y., Matsuzaki H., et al. 2009. Association studies and gene expression analyses of the DISC1-interacting molecules, pericentrin 2 (PCNT2) and DISC1-binding zinc finger protein (DBZ), with schizophrenia and with bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B:967–976 10.1002/ajmg.b.30926 [DOI] [PubMed] [Google Scholar]
- Barr A.R., Kilmartin J.V., Gergely F. 2010. CDK5RAP2 functions in centrosome to spindle pole attachment and DNA damage response. J. Cell Biol. 189:23–39 10.1083/jcb.200912163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman J.J., Tseng H.C., Zhou Y., Frank C.L., Xie Z., Tsai L.H. 2010. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 66:386–402 10.1016/j.neuron.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Conduit P.T., Brunk K., Dobbelaere J., Dix C.I., Lucas E.P., Raff J.W. 2010. Centrioles regulate centrosome size by controlling the rate of Cnn incorporation into the PCM. Curr. Biol. 20:2178–2186 10.1016/j.cub.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Delaval B., Doxsey S.J. 2010. Pericentrin in cellular function and disease. J. Cell Biol. 188:181–190 10.1083/jcb.200908114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg J.B., Zimmerman W., Sparks C.A., Young A., Vidair C., Zheng Y., Carrington W., Fay F.S., Doxsey S.J. 1998. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141:163–174 10.1083/jcb.141.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J., Stein P., Evans L., Calarco P.D., Kirschner M. 1994. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 76:639–650 10.1016/0092-8674(94)90504-5 [DOI] [PubMed] [Google Scholar]
- Flory M.R., Davis T.N. 2003. The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics. 82:401–405 10.1016/S0888-7543(03)00119-8 [DOI] [PubMed] [Google Scholar]
- Flory M.R., Moser M.J., Monnat R.J., Jr, Davis T.N. 2000. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA. 97:5919–5923 10.1073/pnas.97.11.5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K.W., Choi Y.K., Rattner J.B., Qi R.Z. 2008. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the γ-tubulin ring complex. Mol. Biol. Cell. 19:115–125 10.1091/mbc.E07-04-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ferreria M.A., Rath U., Buster D.W., Chanda S.K., Caldwell J.S., Rines D.R., Sharp D.J. 2007. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 17:1960–1966 10.1016/j.cub.2007.10.019 [DOI] [PubMed] [Google Scholar]
- Griffith E., Walker S., Martin C.A., Vagnarelli P., Stiff T., Vernay B., Al Sanna N., Saggar A., Hamel B., Earnshaw W.C., et al. 2008. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 40:232–236 10.1038/ng.2007.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren L., Remy M.H., Bazin I., Callebaut I., Wright M., Merdes A. 2006. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172:505–515 10.1083/jcb.200510028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren L., Stearns T., Lüders J. 2009. Plk1-dependent recruitment of γ-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE. 4:e5976 10.1371/journal.pone.0005976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Valiron O., Oakley B. 2003. Microtubule nucleation. Curr. Opin. Cell Biol. 15:111–117 10.1016/S0955-0674(02)00003-0 [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee K. 2011. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J. Cell Sci. 124:338–347 10.1242/jcs.078329 [DOI] [PubMed] [Google Scholar]
- Lane H.A., Nigg E.A. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701–1713 10.1083/jcb.135.6.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Rhee K. 2010. CEP215 is involved in the dynein-dependent accumulation of pericentriolar matrix proteins for spindle pole formation. Cell Cycle. 9:774–783 [PubMed] [Google Scholar]
- Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J.J., Hoffmann M., Rettig W.J., Kraut N., Peters J.M. 2007. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17:304–315 10.1016/j.cub.2006.12.046 [DOI] [PubMed] [Google Scholar]
- Li Q., Hansen D., Killilea A., Joshi H.C., Palazzo R.E., Balczon R. 2001. Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1. J. Cell Sci. 114:797–809 [DOI] [PubMed] [Google Scholar]
- Lüders J., Patel U.K., Stearns T. 2006. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8:137–147 10.1038/ncb1349 [DOI] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J.W. 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165:673–683 10.1083/jcb.200402130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Toyoshima-Morimoto F., Taniguchi E., Nishida E. 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278:25277–25280 10.1074/jbc.C300126200 [DOI] [PubMed] [Google Scholar]
- Numata S., Iga J., Nakataki M., Tayoshi S., Tanahashi T., Itakura M., Ueno S., Ohmori T. 2009. Positive association of the pericentrin (PCNT) gene with major depressive disorder in the Japanese population. J. Psychiatry Neurosci. 34:195–198 [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Lénárt P., Peters J.M. 2008. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev. Cell. 14:646–659 10.1016/j.devcel.2008.04.014 [DOI] [PubMed] [Google Scholar]
- Rauch A., Thiel C.T., Schindler D., Wick U., Crow Y.J., Ekici A.B., van Essen A.J., Goecke T.O., Al-Gazali L., Chrzanowska K.H., et al. 2008. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 319:816–819 10.1126/science.1151174 [DOI] [PubMed] [Google Scholar]
- Santamaria A., Neef R., Eberspächer U., Eis K., Husemann M., Mumberg D., Prechtl S., Schulze V., Siemeister G., Wortmann L., et al. 2007. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol. Biol. Cell. 18:4024–4036 10.1091/mbc.E07-05-0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A., Wang B., Elowe S., Malik R., Zhang F., Bauer M., Schmidt A., Silljé H.H., Körner R., Nigg E.A. 2011. The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol. Cell. Proteomics. 10:M110.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung N.K., Park J.E., Yu L.R., Lee K.H., Lee J.M., Bang J.K., Veenstra T.D., Rhee K., Lee K.S. 2009. Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Dev. Cell. 16:539–550 10.1016/j.devcel.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srsen V., Gnadt N., Dammermann A., Merdes A. 2006. Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J. Cell Biol. 174:625–630 10.1083/jcb.200606051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I., Giménez-Abián J.F., Gerlich D., Hirota T., Kraft C., de la Torre C., Ellenberg J., Peters J.M. 2004. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr. Biol. 14:1712–1722 10.1016/j.cub.2004.09.049 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamagiwa A., Nishimura T., Mukai H., Ono Y. 2002. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring γ-tubulin ring complex. Mol. Biol. Cell. 13:3235–3245 10.1091/mbc.E02-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen Q., Feng J., Hou J., Yang F., Liu J., Jiang Q., Zhang C. 2009. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome. J. Cell Sci. 122:2240–2251 10.1242/jcs.042747 [DOI] [PubMed] [Google Scholar]
- Zhu F., Lawo S., Bird A., Pinchev D., Ralph A., Richter C., Müller-Reichert T., Kittler R., Hyman A.A., Pelletier L. 2008. The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr. Biol. 18:136–141 10.1016/j.cub.2007.12.055 [DOI] [PubMed] [Google Scholar]
- Zimmerman W.C., Sillibourne J., Rosa J., Doxsey S.J. 2004. Mitosis-specific anchoring of γ tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 15:3642–3657 10.1091/mbc.E03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]