Abstract
The new ternary phase pentaterbium lithium tristannide, Tb5LiSn3, crystallizes in the hexagonal Hf5CuSn3 structure type, which is a ‘filled’ version of the binary RE
5Sn3 phases (Mn5Si3-type) (RE is rare earth). The asymmetric unit contains two Tb sites (site symmetries 3.2 and m2m), one Li site (site symmetry  .m) and one Sn site (site symmetry m2m). The 14-vertex Frank–Kasper polyhedra are typical for Li and Tb atoms. The environment of the Sn atom is a pseudo-Frank–Kasper polyhedron with a coordination number of 13 for the tin atom. One of the Tb atoms is enclosed in a 17-vertex polyhedron. The metallic type of bonding was indicated by an analysis of the interatomic distances.
.m) and one Sn site (site symmetry m2m). The 14-vertex Frank–Kasper polyhedra are typical for Li and Tb atoms. The environment of the Sn atom is a pseudo-Frank–Kasper polyhedron with a coordination number of 13 for the tin atom. One of the Tb atoms is enclosed in a 17-vertex polyhedron. The metallic type of bonding was indicated by an analysis of the interatomic distances.
Related literature
For the Hf5CuSn3 structure type, see: Rieger & Parthé (1965 ▶). For related structures, see: Pavlyuk & Bodak (1992a ▶,b ▶); Pavlyuk et al. (1989 ▶, 1991 ▶, 1993 ▶). For the magnetic properties of related compounds, see: Tran et al. (2008 ▶).
Experimental
Crystal data
Tb5LiSn3
M r = 1157.72
Hexagonal,
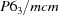
a = 9.0122 (14) Å
c = 6.5744 (13) Å
V = 462.4 (2) Å3
Z = 2
Mo Kα radiation
μ = 45.56 mm−1
T = 293 K
0.07 × 0.05 × 0.03 mm
Data collection
Oxford Diffraction Xcalibur3 CCD diffractometer
Absorption correction: analytical (CrysAlis RED; Oxford Diffraction, 2008 ▶) T min = 0.322, T max = 0.657
1907 measured reflections
216 independent reflections
207 reflections with I > 2σ(I)
R int = 0.021
Refinement
R[F 2 > 2σ(F 2)] = 0.021
wR(F 2) = 0.066
S = 1.33
216 reflections
14 parameters
Δρmax = 1.08 e Å−3
Δρmin = −1.47 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2008 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2008 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041328/ff2031sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041328/ff2031Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
Financial support from the Ministry of Education and Science, Youth and Sport of Ukraine (N 0111U001089) is gratefully acknowledged.
supplementary crystallographic information
Comment
The RE5TM3 (RE - rare earth, T - Cu, Ag and M - Sn, Pb) ternary stannides crystallize in a hexagonal Hf5CuSn3 (superstructure to Ti5Ga4-type) with space group P63/mcm (Rieger and Parthé, 1965). These intermetallic compounds are characterized by two different sites for the RE atoms located at 4 d and 6 g, respectively. The Sn or Pb atoms are located at the next 6 g site and the transitions atoms occupy 2 b site.The RE5TM3 intermetallics are 'filled' version of the binary RE5M3 phases which crystallize in Mn5Si3 structure type. It is also possible, that the transition metals fill the octahedral voids.
For the Ce based compounds, Ce5TM3, investigated by (Tran et al., 2008) are found multiple magnetic phase transitions at low temperatures and discussed the role of f-spd hybridization on the evolution of heavy-fermion behaviour.
We detected the new ternary compound during the systematic study of ternary alloys of Tb—Li—Sn system from the concentration region with low content of lithium. The powder diffraction pattern of this compound is similar to the powder pattern of the RE5Sn3 (RE - rare-earth metals) binary phases, but has some differences. So we decided to further study this phase using single-crystal method. Obtained single-crystal data show that the title compound crystallizes with the hexagonal space group P63/mcm as a Hf5CuSn3 type. The projection of the unit cell and coordination polyhedra of the atoms are shown in Fig. 1. The distribution of tin and lithium atoms in three-dimensional-nets consisted of Tb atoms are shown in Fig. 2.
The number of neighbouring atoms correlates well with the dimensions of the central atoms. The Tb atoms are enclosed in 14- and 17-vertex polyhedra. The coordination polyhedron of the Sn atom is pseudo Frank-Kasper polyhedron with CN=13. Lithium atom is surrounded by 14 neighbours atoms in the form of 14-vertex Frank-Kasper polyhedron. The shortest interatomic distances in the title compound are in the typical for intermetallic compounds ranges and indicate metallic type of bonding.
In the title compound lithium atoms occupy the same crystallographic position that the atoms of transition metal in the original structure type. The same was observed previously when we studied RELiSn2 compounds with the CeNiSi2 structure type (Pavlyuk et al., 1989), RELiGe with the ZrNiAl type (Pavlyuk et al., 1991 and Pavlyuk & Bodak, 1992a), RE3Li2Ge3 with Hf3Ni2Si3 type (Pavlyuk & Bodak, 1992b), solid solutions RLixCu2 -xSi2 and RLixCu2 -xGe2 (Pavlyuk et al., 1993).
Experimental
Terbium, lithium and tin, all with a nominal purity more than 99.9 wt. %, were used as starting elements. First, the pieces of the pure metals with a stoichiometry Tb55Li10Sn35 were pressed into pellet, enclosed in tantalum crucible and placed in a resistance furnace with a thermocouple controller. Heating rate from room temperature to 670 K was equal 5 K per minute. At this temperature the alloy was held over 2 d and then the temperature was increased from 670 to 1070 K over 1 h. Then the alloy was annealed at this temperature for 8 h and slowly cooled down to room temperature. After the melting and annealing procedures, the total weight loss was less than 2%. Small good quality single-crystal of the title compound was isolated from alloy.
Figures
Fig. 1.
The projection of the unit cell and coordination polyhedra of the atoms.
Fig. 2.
The distribution of tin and lithium atoms in three-dimensional-nets consisted of Tb atoms.
Crystal data
| Tb5LiSn3 | Dx = 8.315 Mg m−3 |
| Mr = 1157.72 | Mo Kα radiation, λ = 0.71073 Å |
| Hexagonal, P63/mcm | Cell parameters from 1907 reflections |
| Hall symbol: -P 6c 2 | θ = 2.6–27.4° |
| a = 9.0122 (14) Å | µ = 45.56 mm−1 |
| c = 6.5744 (13) Å | T = 293 K |
| V = 462.4 (2) Å3 | Prism, metallic dark grey |
| Z = 2 | 0.07 × 0.05 × 0.03 mm |
| F(000) = 956 |
Data collection
| Oxford Diffraction Xcalibur3 CCD diffractometer | 216 independent reflections |
| Radiation source: fine-focus sealed tube | 207 reflections with I > 2σ(I) |
| graphite | Rint = 0.021 |
| Detector resolution: 0 pixels mm-1 | θmax = 27.4°, θmin = 2.6° |
| ω scans | h = −11→11 |
| Absorption correction: analytical (CrysAlis RED; Oxford Diffraction, 2008) | k = −11→11 |
| Tmin = 0.322, Tmax = 0.657 | l = 0→8 |
| 1907 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Primary atom site location: structure-invariant direct methods |
| R[F2 > 2σ(F2)] = 0.021 | Secondary atom site location: difference Fourier map |
| wR(F2) = 0.066 | w = 1/[σ2(Fo2) + (0.0268P)2 + 3.977P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.33 | (Δ/σ)max < 0.001 |
| 216 reflections | Δρmax = 1.08 e Å−3 |
| 14 parameters | Δρmin = −1.47 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Tb1 | 0.25088 (10) | 0.0000 | 0.2500 | 0.0479 (3) | |
| Tb2 | 0.3333 | 0.6667 | 0.0000 | 0.0535 (3) | |
| Sn3 | 0.60694 (14) | 0.0000 | 0.2500 | 0.0493 (4) | |
| Li4 | 0.0000 | 0.0000 | 0.0000 | 0.055 (13) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Tb1 | 0.0478 (4) | 0.0481 (5) | 0.0479 (5) | 0.0241 (3) | 0.000 | 0.000 |
| Tb2 | 0.0536 (4) | 0.0536 (4) | 0.0532 (6) | 0.0268 (2) | 0.000 | 0.000 |
| Sn3 | 0.0491 (5) | 0.0493 (7) | 0.0496 (6) | 0.0247 (4) | 0.000 | 0.000 |
| Li4 | 0.07 (2) | 0.07 (2) | 0.03 (2) | 0.033 (11) | 0.000 | 0.000 |
Geometric parameters (Å, °)
| Tb1—Li4 | 2.7952 (8) | Tb2—Tb1xii | 3.8093 (7) |
| Tb1—Li4i | 2.7952 (8) | Tb2—Tb1xi | 3.8093 (7) |
| Tb1—Sn3ii | 3.1066 (11) | Tb2—Tb1xiii | 3.8093 (8) |
| Tb1—Sn3iii | 3.1066 (11) | Tb2—Tb1ix | 3.8093 (7) |
| Tb1—Sn3 | 3.2090 (16) | Sn3—Tb1xvii | 3.1066 (11) |
| Tb1—Sn3iv | 3.5281 (8) | Sn3—Tb1xviii | 3.1066 (11) |
| Tb1—Sn3v | 3.5281 (8) | Sn3—Tb2vii | 3.2247 (6) |
| Tb1—Tb2vi | 3.8093 (7) | Sn3—Tb2vi | 3.2247 (6) |
| Tb1—Tb2vii | 3.8093 (7) | Sn3—Tb2ix | 3.2247 (6) |
| Tb1—Tb2viii | 3.8093 (7) | Sn3—Tb2viii | 3.2247 (6) |
| Tb1—Tb2ix | 3.8093 (7) | Sn3—Tb1iv | 3.5281 (8) |
| Tb1—Tb1x | 3.9161 (17) | Sn3—Tb1v | 3.5281 (8) |
| Tb2—Sn3xi | 3.2247 (6) | Li4—Tb1xix | 2.7952 (8) |
| Tb2—Sn3xii | 3.2247 (6) | Li4—Tb1xx | 2.7952 (8) |
| Tb2—Sn3xiii | 3.2247 (6) | Li4—Tb1xiii | 2.7952 (8) |
| Tb2—Sn3ix | 3.2247 (6) | Li4—Tb1x | 2.7952 (8) |
| Tb2—Sn3xiv | 3.2247 (6) | Li4—Tb1xiv | 2.7952 (8) |
| Tb2—Sn3iii | 3.2247 (6) | Li4—Li4i | 3.2872 (6) |
| Tb2—Tb2xv | 3.2872 (6) | Li4—Li4xxi | 3.2872 (6) |
| Tb2—Tb2xvi | 3.2872 (6) | ||
| Li4—Tb1—Li4i | 72.03 (3) | Sn3xiv—Tb2—Tb1xii | 144.94 (2) |
| Li4—Tb1—Sn3ii | 82.67 (2) | Sn3iii—Tb2—Tb1xii | 123.785 (13) |
| Li4i—Tb1—Sn3ii | 82.67 (2) | Tb2xv—Tb2—Tb1xii | 64.439 (7) |
| Li4—Tb1—Sn3iii | 82.67 (2) | Tb2xvi—Tb2—Tb1xii | 115.561 (7) |
| Li4i—Tb1—Sn3iii | 82.67 (2) | Sn3xi—Tb2—Tb1xi | 53.50 (2) |
| Sn3ii—Tb1—Sn3iii | 161.86 (5) | Sn3xii—Tb2—Tb1xi | 110.39 (2) |
| Li4—Tb1—Sn3 | 143.985 (13) | Sn3xiii—Tb2—Tb1xi | 59.520 (16) |
| Li4i—Tb1—Sn3 | 143.985 (13) | Sn3ix—Tb2—Tb1xi | 51.60 (2) |
| Sn3ii—Tb1—Sn3 | 99.07 (2) | Sn3xiv—Tb2—Tb1xi | 123.785 (13) |
| Sn3iii—Tb1—Sn3 | 99.07 (2) | Sn3iii—Tb2—Tb1xi | 144.94 (2) |
| Li4—Tb1—Sn3iv | 147.31 (3) | Tb2xv—Tb2—Tb1xi | 115.561 (7) |
| Li4i—Tb1—Sn3iv | 75.28 (2) | Tb2xvi—Tb2—Tb1xi | 64.439 (7) |
| Sn3ii—Tb1—Sn3iv | 93.283 (5) | Tb1xii—Tb2—Tb1xi | 63.16 (2) |
| Sn3iii—Tb1—Sn3iv | 93.283 (5) | Sn3xi—Tb2—Tb1xiii | 59.520 (16) |
| Sn3—Tb1—Sn3iv | 68.70 (3) | Sn3xii—Tb2—Tb1xiii | 123.785 (13) |
| Li4—Tb1—Sn3v | 75.28 (2) | Sn3xiii—Tb2—Tb1xiii | 53.50 (2) |
| Li4i—Tb1—Sn3v | 147.31 (3) | Sn3ix—Tb2—Tb1xiii | 144.94 (2) |
| Sn3ii—Tb1—Sn3v | 93.283 (5) | Sn3xiv—Tb2—Tb1xiii | 110.39 (2) |
| Sn3iii—Tb1—Sn3v | 93.283 (5) | Sn3iii—Tb2—Tb1xiii | 51.60 (2) |
| Sn3—Tb1—Sn3v | 68.70 (3) | Tb2xv—Tb2—Tb1xiii | 64.439 (7) |
| Sn3iv—Tb1—Sn3v | 137.41 (5) | Tb2xvi—Tb2—Tb1xiii | 115.561 (7) |
| Li4—Tb1—Tb2vi | 102.887 (9) | Tb1xii—Tb2—Tb1xiii | 102.753 (8) |
| Li4i—Tb1—Tb2vi | 136.924 (8) | Tb1xi—Tb2—Tb1xiii | 93.848 (16) |
| Sn3ii—Tb1—Tb2vi | 54.444 (14) | Sn3xi—Tb2—Tb1ix | 123.785 (13) |
| Sn3iii—Tb1—Tb2vi | 140.12 (2) | Sn3xii—Tb2—Tb1ix | 59.520 (16) |
| Sn3—Tb1—Tb2vi | 53.886 (12) | Sn3xiii—Tb2—Tb1ix | 144.94 (2) |
| Sn3iv—Tb1—Tb2vi | 100.83 (2) | Sn3ix—Tb2—Tb1ix | 53.50 (2) |
| Sn3v—Tb1—Tb2vi | 51.970 (13) | Sn3xiv—Tb2—Tb1ix | 51.60 (2) |
| Li4—Tb1—Tb2vii | 136.924 (8) | Sn3iii—Tb2—Tb1ix | 110.39 (2) |
| Li4i—Tb1—Tb2vii | 102.887 (9) | Tb2xv—Tb2—Tb1ix | 115.561 (7) |
| Sn3ii—Tb1—Tb2vii | 140.12 (2) | Tb2xvi—Tb2—Tb1ix | 64.439 (7) |
| Sn3iii—Tb1—Tb2vii | 54.444 (14) | Tb1xii—Tb2—Tb1ix | 93.848 (16) |
| Sn3—Tb1—Tb2vii | 53.886 (12) | Tb1xi—Tb2—Tb1ix | 102.753 (8) |
| Sn3iv—Tb1—Tb2vii | 51.970 (13) | Tb1xiii—Tb2—Tb1ix | 160.55 (2) |
| Sn3v—Tb1—Tb2vii | 100.83 (2) | Tb1xvii—Sn3—Tb1xviii | 78.14 (5) |
| Tb2vi—Tb1—Tb2vii | 107.77 (2) | Tb1xvii—Sn3—Tb1 | 140.93 (2) |
| Li4—Tb1—Tb2viii | 136.924 (8) | Tb1xviii—Sn3—Tb1 | 140.93 (2) |
| Li4i—Tb1—Tb2viii | 102.887 (9) | Tb1xvii—Sn3—Tb2vii | 73.951 (16) |
| Sn3ii—Tb1—Tb2viii | 54.444 (14) | Tb1xviii—Sn3—Tb2vii | 137.78 (3) |
| Sn3iii—Tb1—Tb2viii | 140.12 (2) | Tb1—Sn3—Tb2vii | 72.61 (2) |
| Sn3—Tb1—Tb2viii | 53.886 (12) | Tb1xvii—Sn3—Tb2vi | 137.78 (3) |
| Sn3iv—Tb1—Tb2viii | 51.970 (13) | Tb1xviii—Sn3—Tb2vi | 73.951 (16) |
| Sn3v—Tb1—Tb2viii | 100.83 (2) | Tb1—Sn3—Tb2vi | 72.61 (2) |
| Tb2vi—Tb1—Tb2viii | 51.122 (14) | Tb2vii—Sn3—Tb2vi | 145.22 (4) |
| Tb2vii—Tb1—Tb2viii | 86.152 (16) | Tb1xvii—Sn3—Tb2ix | 73.951 (16) |
| Li4—Tb1—Tb2ix | 102.887 (9) | Tb1xviii—Sn3—Tb2ix | 137.78 (3) |
| Li4i—Tb1—Tb2ix | 136.924 (8) | Tb1—Sn3—Tb2ix | 72.61 (2) |
| Sn3ii—Tb1—Tb2ix | 140.12 (2) | Tb2vii—Sn3—Tb2ix | 61.287 (15) |
| Sn3iii—Tb1—Tb2ix | 54.444 (14) | Tb2vi—Sn3—Tb2ix | 107.56 (2) |
| Sn3—Tb1—Tb2ix | 53.886 (12) | Tb1xvii—Sn3—Tb2viii | 137.78 (3) |
| Sn3iv—Tb1—Tb2ix | 100.83 (2) | Tb1xviii—Sn3—Tb2viii | 73.951 (16) |
| Sn3v—Tb1—Tb2ix | 51.970 (13) | Tb1—Sn3—Tb2viii | 72.61 (2) |
| Tb2vi—Tb1—Tb2ix | 86.152 (16) | Tb2vii—Sn3—Tb2viii | 107.56 (2) |
| Tb2vii—Tb1—Tb2ix | 51.122 (14) | Tb2vi—Sn3—Tb2viii | 61.287 (15) |
| Tb2viii—Tb1—Tb2ix | 107.77 (2) | Tb2ix—Sn3—Tb2viii | 145.22 (4) |
| Li4—Tb1—Tb1x | 45.533 (9) | Tb1xvii—Sn3—Tb1iv | 73.62 (2) |
| Li4i—Tb1—Tb1x | 45.533 (9) | Tb1xviii—Sn3—Tb1iv | 73.62 (2) |
| Sn3ii—Tb1—Tb1x | 50.93 (2) | Tb1—Sn3—Tb1iv | 111.30 (3) |
| Sn3iii—Tb1—Tb1x | 110.93 (2) | Tb2vii—Sn3—Tb1iv | 68.510 (11) |
| Sn3—Tb1—Tb1x | 150.0 | Tb2vi—Sn3—Tb1iv | 125.693 (6) |
| Sn3iv—Tb1—Tb1x | 108.33 (2) | Tb2ix—Sn3—Tb1iv | 125.693 (6) |
| Sn3v—Tb1—Tb1x | 108.33 (2) | Tb2viii—Sn3—Tb1iv | 68.510 (11) |
| Tb2vi—Tb1—Tb1x | 99.726 (11) | Tb1xvii—Sn3—Tb1v | 73.62 (2) |
| Tb2vii—Tb1—Tb1x | 148.420 (11) | Tb1xviii—Sn3—Tb1v | 73.62 (2) |
| Tb2viii—Tb1—Tb1x | 99.726 (11) | Tb1—Sn3—Tb1v | 111.30 (3) |
| Tb2ix—Tb1—Tb1x | 148.420 (11) | Tb2vii—Sn3—Tb1v | 125.693 (6) |
| Sn3xi—Tb2—Sn3xii | 163.38 (4) | Tb2vi—Sn3—Tb1v | 68.510 (11) |
| Sn3xi—Tb2—Sn3xiii | 72.44 (2) | Tb2ix—Sn3—Tb1v | 68.510 (11) |
| Sn3xii—Tb2—Sn3xiii | 96.334 (10) | Tb2viii—Sn3—Tb1v | 125.693 (6) |
| Sn3xi—Tb2—Sn3ix | 96.334 (10) | Tb1iv—Sn3—Tb1v | 137.41 (5) |
| Sn3xii—Tb2—Sn3ix | 72.44 (2) | Tb1xix—Li4—Tb1xx | 88.933 (19) |
| Sn3xiii—Tb2—Sn3ix | 97.06 (4) | Tb1xix—Li4—Tb1 | 91.067 (19) |
| Sn3xi—Tb2—Sn3xiv | 96.334 (10) | Tb1xx—Li4—Tb1 | 180.0 |
| Sn3xii—Tb2—Sn3xiv | 97.06 (4) | Tb1xix—Li4—Tb1xiii | 180.0 |
| Sn3xiii—Tb2—Sn3xiv | 163.38 (4) | Tb1xx—Li4—Tb1xiii | 91.067 (19) |
| Sn3ix—Tb2—Sn3xiv | 96.334 (10) | Tb1—Li4—Tb1xiii | 88.933 (19) |
| Sn3xi—Tb2—Sn3iii | 97.06 (4) | Tb1xix—Li4—Tb1x | 91.067 (19) |
| Sn3xii—Tb2—Sn3iii | 96.334 (10) | Tb1xx—Li4—Tb1x | 91.067 (19) |
| Sn3xiii—Tb2—Sn3iii | 96.334 (10) | Tb1—Li4—Tb1x | 88.933 (19) |
| Sn3ix—Tb2—Sn3iii | 163.38 (4) | Tb1xiii—Li4—Tb1x | 88.933 (19) |
| Sn3xiv—Tb2—Sn3iii | 72.44 (2) | Tb1xix—Li4—Tb1xiv | 88.933 (19) |
| Sn3xi—Tb2—Tb2xv | 120.643 (7) | Tb1xx—Li4—Tb1xiv | 88.933 (19) |
| Sn3xii—Tb2—Tb2xv | 59.357 (8) | Tb1—Li4—Tb1xiv | 91.067 (19) |
| Sn3xiii—Tb2—Tb2xv | 59.357 (7) | Tb1xiii—Li4—Tb1xiv | 91.067 (19) |
| Sn3ix—Tb2—Tb2xv | 120.643 (8) | Tb1x—Li4—Tb1xiv | 180.00 (7) |
| Sn3xiv—Tb2—Tb2xv | 120.643 (7) | Tb1xix—Li4—Li4i | 126.015 (13) |
| Sn3iii—Tb2—Tb2xv | 59.357 (7) | Tb1xx—Li4—Li4i | 126.015 (13) |
| Sn3xi—Tb2—Tb2xvi | 59.357 (7) | Tb1—Li4—Li4i | 53.985 (13) |
| Sn3xii—Tb2—Tb2xvi | 120.643 (7) | Tb1xiii—Li4—Li4i | 53.985 (13) |
| Sn3xiii—Tb2—Tb2xvi | 120.643 (8) | Tb1x—Li4—Li4i | 53.985 (13) |
| Sn3ix—Tb2—Tb2xvi | 59.357 (8) | Tb1xiv—Li4—Li4i | 126.015 (13) |
| Sn3xiv—Tb2—Tb2xvi | 59.357 (7) | Tb1xix—Li4—Li4xxi | 53.985 (13) |
| Sn3iii—Tb2—Tb2xvi | 120.643 (7) | Tb1xx—Li4—Li4xxi | 53.985 (13) |
| Tb2xv—Tb2—Tb2xvi | 180.0 | Tb1—Li4—Li4xxi | 126.015 (13) |
| Sn3xi—Tb2—Tb1xii | 110.39 (2) | Tb1xiii—Li4—Li4xxi | 126.015 (13) |
| Sn3xii—Tb2—Tb1xii | 53.50 (2) | Tb1x—Li4—Li4xxi | 126.015 (13) |
| Sn3xiii—Tb2—Tb1xii | 51.60 (2) | Tb1xiv—Li4—Li4xxi | 53.985 (13) |
| Sn3ix—Tb2—Tb1xii | 59.520 (16) | Li4i—Li4—Li4xxi | 180.0 |
Symmetry codes: (i) −x, −y, z+1/2; (ii) −y, x−y−1, z; (iii) −x+y+1, −x+1, z; (iv) −x+1, −y, −z+1; (v) −x+1, −y, −z; (vi) x, y−1, z; (vii) −x+1, −y+1, z+1/2; (viii) x, y−1, −z+1/2; (ix) −x+1, −y+1, −z; (x) −x+y, −x, z; (xi) y, −x+y+1, −z; (xii) x, y+1, z; (xiii) −y, x−y, z; (xiv) x−y, x, −z; (xv) x, y, −z+1/2; (xvi) x, y, −z−1/2; (xvii) −y+1, x−y, z; (xviii) −x+y+1, −x, z; (xix) y, −x+y, −z; (xx) −x, −y, −z; (xxi) −x, −y, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FF2031).
References
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Oxford Diffraction (2008). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Pavlyuk, V. & Bodak, O. (1992a). Akad. Nauk SSSR Izv. Met. 6, 207–210.
- Pavlyuk, V. & Bodak, O. (1992b). Inorg. Mater. 28, 877–879.
- Pavlyuk, V., Bodak, O. & Bruskov, V. (1991). Dop. Akad. Nauk Ukraine, 1, 112–114.
- Pavlyuk, V., Bodak, O. & Kevorkov, D. (1993). Dop. Akad. Nauk Ukraine, 9, 84–87.
- Pavlyuk, V., Bodak, O., Pecharskii, V., Skolozdra, R. & Gladyshevskii, E. (1989). Inorg. Mater. 25, 962–965.
- Rieger, W. & Parthé, E. (1965). Monatsh. Chem. 96, 232–241.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tran, V. H., Gamza, M., Slebarski, A. & Miiller, W. (2008). J. Alloys Compd, 451, 457–460.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041328/ff2031sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041328/ff2031Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




