Abstract
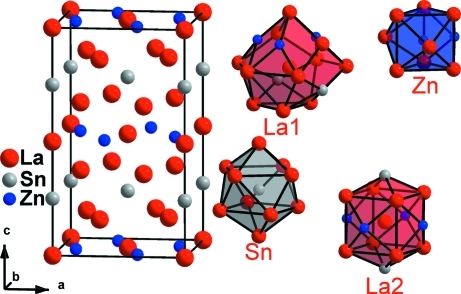
A single crystal of pentalanthanum dizinc stannide, La5Zn2Sn, was obtained from the elements in a resistance furnace. It belongs to the Mo5SiB2 structure type, which is a ternary ordered variant of the Cr5B3 structure type. The space is filled by bicapped tetragonal antiprisms from lanthanum atoms around tin atoms sharing their vertices. Zinc atoms fill voids between these bicapped tetragonal antiprisms. All four atoms in the asymmetric unit reside on special positions with the following site symmetries: La1 (..m); La2 (4/m..); Zn (m.2m); Sn (422).
Related literature
For general background to {Tb,La}–Zn–{Sn,Pb} ternary systems, see: Manfrinetti & Pani, (2005 ▶); Oshchapovsky et al. (2010 ▶, 2011 ▶); Pavlyuk et al. (2009 ▶). For related structures, see: Bertaut (1953 ▶). For isotypic structures, see: Aronsson (1958 ▶).
Experimental
Crystal data
La5Zn2Sn
M r = 944.04
Tetragonal,
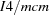
a = 8.3277 (12) Å
c = 14.334 (3) Å
V = 994.1 (3) Å3
Z = 4
Mo Kα radiation
μ = 28.10 mm−1
T = 293 K
0.04 × 0.04 × 0.01 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2004 ▶) T min = 0.340, T max = 0.765
9291 measured reflections
419 independent reflections
346 reflections with I > 2σ(I)
R int = 0.091
Refinement
R[F 2 > 2σ(F 2)] = 0.027
wR(F 2) = 0.056
S = 1.14
419 reflections
14 parameters
Δρmax = 1.60 e Å−3
Δρmin = −1.59 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶) and JANA2006 (Petricek et al., 2006 ▶); molecular graphics: DIAMOND (Brandenburg, 2006 ▶) and VESTA (Momma & Izumi, 2008 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811042413/ru2016sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811042413/ru2016Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
Financial support from the Ministry of Education and Science, Youth and Sport of Ukraine (N 0111U001089) is gratefully acknowledged.
supplementary crystallographic information
Comment
Ternary compounds formed by rare-earth, transition metal and d-metal often have interesting physical and chemical properties e. g. strong ferromagnetism, hydrogen storage capabilities and so on. The systematic investigation of the components interaction in the {Tb, La}-Zn-{Sn,Pb} ternary systems can lead to development of functional materials (for crystal structures of ternary compounds see: TbZnSn –Manfrinetti & Pani, (2005), Pavlyuk et al., (2009), TbZnSn2 - Pavlyuk et al., (2009), Tb13ZnSn13-Oshchapovsky et al., (2010) and LaZn12.37-Oshchapovsky et al., (2011)).
The title compound crystallizes in Mo5SiB2 (Aronsson, 1958) structure type which is an ordered superstructure of Cr5B3 type (Bertaut, 1953). Unit cell projection together with coordination polyhedra are given in Fig.1. Coordination polyhedra of the La1 atoms are 16- vertex polyhedra. Sn atoms are surrounded by ten neighbours forming bicapped tetragonal antiprism. La2 atoms are enclosed into trigon - tetrahexahedron with CN=14. And coordination polyhedra of the Zn atoms are bicapped trigonal prisms with CN=9. Coordination polyhedra of Sn atoms share their vertices forming three dimensional framework. The voids in this framework are filled by zinc atoms. (See graphical abstract).
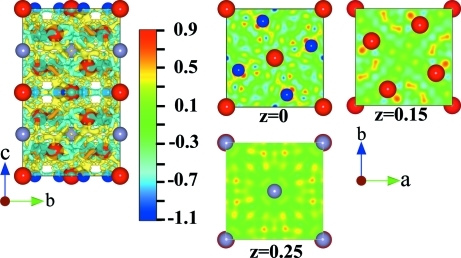
The way of bond formation in this compound was assumed using only X-ray diffraction data. Further structure refinement was carried out by means of Jana2006 software package using anharmonic ADP for La1 and Zn atoms. Anharmonic displacement parameters for other atoms were not refined because in case of their refinement their standard deviations were larger than obtained values. As the result we gained lower absolute values of peak and hole in the difference Fourier map (1.02 and -1.17 e Å-3 respectively). The resulting isosurface drawn at the level 0.308 e/Å3 and sections of difference Fourier map are given in Fig. 2. These maps and sections are noisy but some trends in location of positive and negative regions can be noticed. Positive residual electron density is mostly situated around zinc atoms and near layers made of tin atoms. Negative residual density is mostly located between lanthanum atoms which means that lanthanum atoms donate their electrons to zinc and tin atoms. Similar behaviour of lanthanum atoms can be observed in the LaZn12.37 compound using electronic structure calculations (See Oshchapovsky et al., (2011)). As a conclusion this compound besides dominate metallic bonding has a weak ionic interaction between lanthanum and zinc and tin atoms.
Experimental
Small good quality single-crystal of title compound was isolated from alloy with composition La7ZnSn2 during systematic investigation of lanthanum-rich region of La—Zn—Sn ternary system. The samples with high lanthanum contents were prepared by melting of pieces of pure metals in evacuated quartz ampoule with subsequent annealing at 600 0C for 30 days. Further phase analysis showed the existence of title compound in sample with composition La7ZnSn2 as well as in the other lanthanum-rich ternary alloys. However they were non equilibrium.
Figures
Fig. 1.
Unit cell projection and coordination polyhedra in the La5Zn2Sn compound
Fig. 2.
Isosurface drawn at 0.308 e/Å3 and sections of difference Fourier map after the refinement of crystal structure of the La5Zn2Sn compound
Crystal data
| La5Zn2Sn | Dx = 6.308 Mg m−3 |
| Mr = 944.04 | Mo Kα radiation, λ = 0.71073 Å |
| Tetragonal, I4/mcm | Cell parameters from 1223 reflections |
| Hall symbol: -I 4 2c | θ = 5.7–26.1° |
| a = 8.3277 (12) Å | µ = 28.10 mm−1 |
| c = 14.334 (3) Å | T = 293 K |
| V = 994.1 (3) Å3 | Plate, grey |
| Z = 4 | 0.04 × 0.04 × 0.01 mm |
| F(000) = 1580.0 |
Data collection
| Bruker APEXII CCD diffractometer | 419 independent reflections |
| Radiation source: sealed tube | 346 reflections with I > 2σ(I) |
| graphite | Rint = 0.091 |
| Detector resolution: 8.366 pixels mm-1 | θmax = 30.1°, θmin = 2.8° |
| φ and ω scans | h = −11→11 |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | k = −11→11 |
| Tmin = 0.340, Tmax = 0.765 | l = −20→19 |
| 9291 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Primary atom site location: structure-invariant direct methods |
| R[F2 > 2σ(F2)] = 0.027 | Secondary atom site location: difference Fourier map |
| wR(F2) = 0.056 | w = 1/[σ2(Fo2) + (0.0144P)2 + 16.4322P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.14 | (Δ/σ)max = 0.004 |
| 419 reflections | Δρmax = 1.60 e Å−3 |
| 14 parameters | Δρmin = −1.59 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| La1 | 0.66685 (5) | 0.16685 (5) | 0.14853 (4) | 0.02054 (16) | |
| La2 | 0.0000 | 0.0000 | 0.0000 | 0.0357 (3) | |
| Zn | 0.12383 (12) | 0.62383 (12) | 0.0000 | 0.0139 (3) | |
| Sn | 0.0000 | 0.0000 | 0.2500 | 0.0145 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| La1 | 0.01937 (19) | 0.01937 (19) | 0.0229 (3) | 0.00430 (19) | 0.000 | 0.000 |
| La2 | 0.0291 (4) | 0.0291 (4) | 0.0490 (8) | 0.000 | 0.000 | 0.000 |
| Zn | 0.0123 (4) | 0.0123 (4) | 0.0171 (7) | 0.0013 (5) | 0.000 | 0.000 |
| Sn | 0.0129 (3) | 0.0129 (3) | 0.0177 (5) | 0.000 | 0.000 | 0.000 |
Geometric parameters (Å, °)
| La1—Zni | 3.2436 (9) | La2—La1xvii | 3.7630 (6) |
| La1—Znii | 3.2436 (9) | Zn—Znx | 2.917 (3) |
| La1—Zniii | 3.2573 (12) | Zn—La1xviii | 3.2436 (9) |
| La1—Sniv | 3.4268 (5) | Zn—La1xvi | 3.2436 (9) |
| La1—Snv | 3.4268 (5) | Zn—La1xix | 3.2436 (9) |
| La1—La1vi | 3.5068 (12) | Zn—La1xiv | 3.2436 (9) |
| La1—La2iv | 3.7630 (6) | Zn—La1xx | 3.2573 (12) |
| La1—La2vii | 3.7630 (6) | Zn—La1iii | 3.2573 (12) |
| La1—La1viii | 3.9301 (12) | Zn—La2xxi | 3.2980 (8) |
| La2—Znix | 3.2980 (8) | Zn—La2vii | 3.2980 (8) |
| La2—Znii | 3.2980 (8) | Sn—La1xxii | 3.4268 (5) |
| La2—Znx | 3.2980 (8) | Sn—La1xvii | 3.4268 (5) |
| La2—Znxi | 3.2980 (8) | Sn—La1xiv | 3.4268 (5) |
| La2—Snxii | 3.5835 (7) | Sn—La1v | 3.4268 (5) |
| La2—Sn | 3.5835 (7) | Sn—La1xxiii | 3.4268 (5) |
| La2—La1xiii | 3.7630 (6) | Sn—La1xxiv | 3.4268 (5) |
| La2—La1xiv | 3.7630 (6) | Sn—La1viii | 3.4268 (5) |
| La2—La1xv | 3.7630 (6) | Sn—La1xxv | 3.4268 (5) |
| La2—La1viii | 3.7630 (6) | Sn—La2xxvi | 3.5835 (7) |
| La2—La1xvi | 3.7630 (6) | ||
| Zni—La1—Znii | 53.44 (5) | Znii—La2—La1xvi | 54.461 (17) |
| Zni—La1—Zniii | 91.69 (2) | Znx—La2—La1xvi | 54.208 (18) |
| Znii—La1—Zniii | 91.69 (2) | Znxi—La2—La1xvi | 125.792 (18) |
| Zni—La1—Sniv | 93.75 (2) | Snxii—La2—La1xvi | 55.545 (10) |
| Znii—La1—Sniv | 146.93 (3) | Sn—La2—La1xvi | 124.455 (10) |
| Zniii—La1—Sniv | 93.506 (15) | La1xiii—La2—La1xvi | 111.09 (2) |
| Zni—La1—Snv | 146.93 (3) | La1xiv—La2—La1xvi | 68.91 (2) |
| Znii—La1—Snv | 93.75 (2) | La1xv—La2—La1xvi | 71.332 (10) |
| Zniii—La1—Snv | 93.506 (15) | La1viii—La2—La1xvi | 108.668 (10) |
| Sniv—La1—Snv | 118.450 (18) | Znix—La2—La1xvii | 54.461 (17) |
| Zni—La1—La1vi | 151.98 (2) | Znii—La2—La1xvii | 125.539 (17) |
| Znii—La1—La1vi | 151.98 (2) | Znx—La2—La1xvii | 125.792 (18) |
| Zniii—La1—La1vi | 96.86 (3) | Znxi—La2—La1xvii | 54.208 (18) |
| Sniv—La1—La1vi | 59.225 (9) | Snxii—La2—La1xvii | 124.455 (10) |
| Snv—La1—La1vi | 59.225 (9) | Sn—La2—La1xvii | 55.545 (10) |
| Zni—La1—La2iv | 55.564 (18) | La1xiii—La2—La1xvii | 68.91 (2) |
| Znii—La1—La2iv | 97.94 (3) | La1xiv—La2—La1xvii | 111.09 (2) |
| Zniii—La1—La2iv | 55.477 (10) | La1xv—La2—La1xvii | 108.668 (10) |
| Sniv—La1—La2iv | 59.571 (13) | La1viii—La2—La1xvii | 71.332 (10) |
| Snv—La1—La2iv | 146.931 (17) | La1xvi—La2—La1xvii | 180.000 (16) |
| La1vi—La1—La2iv | 108.903 (17) | Znx—Zn—La1xviii | 63.28 (2) |
| Zni—La1—La2vii | 97.94 (3) | Znx—Zn—La1xvi | 63.28 (2) |
| Znii—La1—La2vii | 55.564 (18) | La1xviii—Zn—La1xvi | 74.58 (3) |
| Zniii—La1—La2vii | 55.477 (10) | Znx—Zn—La1xix | 63.28 (2) |
| Sniv—La1—La2vii | 146.931 (17) | La1xviii—Zn—La1xix | 82.05 (3) |
| Snv—La1—La2vii | 59.571 (13) | La1xvi—Zn—La1xix | 126.56 (5) |
| La1vi—La1—La2vii | 108.903 (17) | Znx—Zn—La1xiv | 63.28 (2) |
| La2iv—La1—La2vii | 102.966 (18) | La1xviii—Zn—La1xiv | 126.56 (5) |
| Zni—La1—La1viii | 52.712 (14) | La1xvi—Zn—La1xiv | 82.05 (3) |
| Znii—La1—La1viii | 52.712 (14) | La1xix—Zn—La1xiv | 74.58 (3) |
| Zniii—La1—La1viii | 139.19 (2) | Znx—Zn—La1xx | 139.19 (2) |
| Sniv—La1—La1viii | 106.604 (9) | La1xviii—Zn—La1xx | 140.29 (2) |
| Snv—La1—La1viii | 106.604 (9) | La1xvi—Zn—La1xx | 140.29 (2) |
| La1vi—La1—La1viii | 123.951 (19) | La1xix—Zn—La1xx | 84.909 (18) |
| La2iv—La1—La1viii | 105.084 (8) | La1xiv—Zn—La1xx | 84.909 (18) |
| La2vii—La1—La1viii | 105.084 (8) | Znx—Zn—La1iii | 139.19 (2) |
| Zni—La1—La1xxvii | 87.66 (2) | La1xviii—Zn—La1iii | 84.909 (18) |
| Znii—La1—La1xxvii | 113.473 (17) | La1xvi—Zn—La1iii | 84.909 (18) |
| Zniii—La1—La1xxvii | 147.382 (8) | La1xix—Zn—La1iii | 140.29 (2) |
| Sniv—La1—La1xxvii | 54.057 (10) | La1xiv—Zn—La1iii | 140.29 (2) |
| Snv—La1—La1xxvii | 104.62 (2) | La1xx—Zn—La1iii | 81.63 (4) |
| La1vi—La1—La1xxvii | 70.91 (2) | Znx—Zn—La2xxi | 116.78 (2) |
| La2iv—La1—La1xxvii | 98.861 (14) | La1xviii—Zn—La2xxi | 70.228 (8) |
| La2vii—La1—La1xxvii | 156.689 (9) | La1xvi—Zn—La2xxi | 138.024 (12) |
| La1viii—La1—La1xxvii | 60.762 (9) | La1xix—Zn—La2xxi | 70.228 (8) |
| Zni—La1—La1xxii | 113.473 (17) | La1xiv—Zn—La2xxi | 138.024 (12) |
| Znii—La1—La1xxii | 87.66 (2) | La1xx—Zn—La2xxi | 70.06 (2) |
| Zniii—La1—La1xxii | 147.382 (8) | La1iii—Zn—La2xxi | 70.06 (2) |
| Sniv—La1—La1xxii | 104.62 (2) | Znx—Zn—La2vii | 116.78 (2) |
| Snv—La1—La1xxii | 54.057 (10) | La1xviii—Zn—La2vii | 138.024 (12) |
| La1vi—La1—La1xxii | 70.91 (2) | La1xvi—Zn—La2vii | 70.228 (8) |
| La2iv—La1—La1xxii | 156.689 (9) | La1xix—Zn—La2vii | 138.024 (12) |
| La2vii—La1—La1xxii | 98.861 (14) | La1xiv—Zn—La2vii | 70.228 (8) |
| La1viii—La1—La1xxii | 60.762 (9) | La1xx—Zn—La2vii | 70.06 (2) |
| La1xxvii—La1—La1xxii | 58.477 (18) | La1iii—Zn—La2vii | 70.06 (2) |
| Znix—La2—Znii | 180.0 | La2xxi—Zn—La2vii | 126.44 (4) |
| Znix—La2—Znx | 90.0 | La1xxii—Sn—La1xvii | 146.791 (18) |
| Znii—La2—Znx | 90.0 | La1xxii—Sn—La1xiv | 61.550 (18) |
| Znix—La2—Znxi | 90.0 | La1xvii—Sn—La1xiv | 129.77 (2) |
| Znii—La2—Znxi | 90.0 | La1xxii—Sn—La1v | 79.622 (8) |
| Znx—La2—Znxi | 180.0 | La1xvii—Sn—La1v | 132.159 (17) |
| Znix—La2—Snxii | 90.0 | La1xiv—Sn—La1v | 71.89 (2) |
| Znii—La2—Snxii | 90.0 | La1xxii—Sn—La1xxiii | 129.77 (2) |
| Znx—La2—Snxii | 90.0 | La1xvii—Sn—La1xxiii | 61.550 (18) |
| Znxi—La2—Snxii | 90.0 | La1xiv—Sn—La1xxiii | 146.791 (18) |
| Znix—La2—Sn | 90.0 | La1v—Sn—La1xxiii | 79.622 (8) |
| Znii—La2—Sn | 90.0 | La1xxii—Sn—La1xxiv | 132.159 (17) |
| Znx—La2—Sn | 90.0 | La1xvii—Sn—La1xxiv | 79.622 (8) |
| Znxi—La2—Sn | 90.0 | La1xiv—Sn—La1xxiv | 79.622 (8) |
| Snxii—La2—Sn | 180.0 | La1v—Sn—La1xxiv | 61.550 (18) |
| Znix—La2—La1xiii | 54.461 (17) | La1xxiii—Sn—La1xxiv | 71.89 (2) |
| Znii—La2—La1xiii | 125.539 (17) | La1xxii—Sn—La1viii | 71.89 (2) |
| Znx—La2—La1xiii | 125.792 (18) | La1xvii—Sn—La1viii | 79.622 (8) |
| Znxi—La2—La1xiii | 54.208 (18) | La1xiv—Sn—La1viii | 79.622 (8) |
| Snxii—La2—La1xiii | 55.544 (10) | La1v—Sn—La1viii | 146.791 (18) |
| Sn—La2—La1xiii | 124.456 (10) | La1xxiii—Sn—La1viii | 132.159 (17) |
| Znix—La2—La1xiv | 125.539 (17) | La1xxiv—Sn—La1viii | 129.77 (2) |
| Znii—La2—La1xiv | 54.461 (17) | La1xxii—Sn—La1xxv | 79.622 (8) |
| Znx—La2—La1xiv | 54.208 (18) | La1xvii—Sn—La1xxv | 71.89 (2) |
| Znxi—La2—La1xiv | 125.792 (18) | La1xiv—Sn—La1xxv | 132.159 (17) |
| Snxii—La2—La1xiv | 124.456 (10) | La1v—Sn—La1xxv | 129.77 (2) |
| Sn—La2—La1xiv | 55.544 (10) | La1xxiii—Sn—La1xxv | 79.622 (8) |
| La1xiii—La2—La1xiv | 180.000 (16) | La1xxiv—Sn—La1xxv | 146.791 (18) |
| Znix—La2—La1xv | 54.208 (18) | La1viii—Sn—La1xxv | 61.550 (18) |
| Znii—La2—La1xv | 125.792 (18) | La1xxii—Sn—La2xxvi | 64.885 (10) |
| Znx—La2—La1xv | 54.461 (17) | La1xvii—Sn—La2xxvi | 115.115 (10) |
| Znxi—La2—La1xv | 125.539 (17) | La1xiv—Sn—La2xxvi | 115.115 (10) |
| Snxii—La2—La1xv | 55.544 (10) | La1v—Sn—La2xxvi | 64.885 (10) |
| Sn—La2—La1xv | 124.456 (10) | La1xxiii—Sn—La2xxvi | 64.885 (10) |
| La1xiii—La2—La1xv | 71.332 (10) | La1xxiv—Sn—La2xxvi | 115.115 (10) |
| La1xiv—La2—La1xv | 108.668 (10) | La1viii—Sn—La2xxvi | 115.115 (10) |
| Znix—La2—La1viii | 125.792 (18) | La1xxv—Sn—La2xxvi | 64.885 (10) |
| Znii—La2—La1viii | 54.208 (18) | La1xxii—Sn—La2 | 115.115 (10) |
| Znx—La2—La1viii | 125.539 (17) | La1xvii—Sn—La2 | 64.885 (10) |
| Znxi—La2—La1viii | 54.461 (17) | La1xiv—Sn—La2 | 64.885 (10) |
| Snxii—La2—La1viii | 124.456 (10) | La1v—Sn—La2 | 115.115 (10) |
| Sn—La2—La1viii | 55.544 (10) | La1xxiii—Sn—La2 | 115.115 (10) |
| La1xiii—La2—La1viii | 108.668 (10) | La1xxiv—Sn—La2 | 64.885 (10) |
| La1xiv—La2—La1viii | 71.332 (10) | La1viii—Sn—La2 | 64.885 (10) |
| La1xv—La2—La1viii | 180.000 (18) | La1xxv—Sn—La2 | 115.115 (10) |
| Znix—La2—La1xvi | 125.539 (17) | La2xxvi—Sn—La2 | 180.0 |
Symmetry codes: (i) y, −x, −z; (ii) −y+1, x, z; (iii) −x+1, −y+1, −z; (iv) x+1, y, z; (v) −x+1/2, −y+1/2, −z+1/2; (vi) −x+3/2, −y+1/2, −z+1/2; (vii) −x+1/2, y+1/2, −z; (viii) −x+1, −y, z; (ix) y−1, −x, −z; (x) −x, −y+1, −z; (xi) x, y−1, z; (xii) −x, −y, −z; (xiii) −y, x−1, −z; (xiv) y, −x+1, z; (xv) x−1, y, −z; (xvi) y, −x+1, −z; (xvii) −y, x−1, z; (xviii) −y, x, −z; (xix) −y, x, z; (xx) −x+1, −y+1, z; (xxi) x, y+1, z; (xxii) −y+1/2, x−1/2, −z+1/2; (xxiii) y−1/2, −x+1/2, −z+1/2; (xxiv) x−1, y, z; (xxv) x−1/2, y−1/2, −z+1/2; (xxvi) −x, y, −z+1/2; (xxvii) y+1/2, −x+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RU2016).
References
- Aronsson, B. (1958). Acta Chem. Scand. 12, 31–37.
- Bertaut, F. (1953). C. R. Hebd. Seances Acad. Sci. 236, 1055–1056.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2004). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Manfrinetti, P. & Pani, M. (2005). J. Alloys Compd, 393, 180–184.
- Momma, K. & Izumi, F. (2008). J. Appl. Cryst. 41, 653–658.
- Oshchapovsky, I., Pavlyuk, V., Dmytriv, G. & White, F. (2011). Acta Cryst. E67, i43. [DOI] [PMC free article] [PubMed]
- Oshchapovsky, I., Pavlyuk, V., Fässler, T. F. & Hlukhyy, V. (2010). Chem. Met. Alloys, 3, 177–183.
- Pavlyuk, V., Oshchapovsky, I. & Marciniak, B. (2009). J. Alloys Compd, 477, 145–148.
- Petricek, V., Dusek, M. & Palatinus, L. (2006). JANA2006. Institute of Physics, Praha, Czech Republic.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811042413/ru2016sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811042413/ru2016Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report