Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production, complement activation, and immune complex deposition, resulting in tissue and organ damage. An understanding of the mechanisms responsible for homeostatic control of inflammation, which involve both innate and adoptive immune responses, will enable the development of novel therapies for SLE. Regulatory T cells (Treg) play critical roles in the induction of peripheral tolerance to self- and foreign antigens. Naturally occurring CD4+CD25+ Treg, which characteristically express the transcription factor forkhead box protein P3 (Foxp3), have been intensively studied because their deficiency abrogates self-tolerance and causes autoimmune disease. Moreover, regulatory cytokines such as interleukin-10 (IL-10) also play a central role in controlling inflammatory processes. This paper focuses on Tregs and Treg-associated cytokines which might regulate the pathogenesis of SLE and, hence, have clinical applications.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production, immune complex deposition, and various clinical systemic manifestations that affect various organs. The pathogenesis of SLE involves complex interactions between genetic and environmental factors and the adaptive and innate immune systems. The breakdown of immunologic self-tolerance, that is, the control of self- and non-self-discrimination, results in the development of autoimmune diseases. Therefore, elucidating the mechanisms that regulate self-tolerance is important for protecting against self-directed immune responses and autoimmune diseases. On the other hand, proinflammatory cytokines are involved in the generalized immune dysregulation of SLE, as well as the local inflammatory response, which leads to tissue injury. The regulation of the proinflammatory activity of these cytokines is perceived to be mediated by anti-inflammatory and immunosuppressive cytokines such as interleukin- (IL-) 10, transforming growth factor- (TGF-) β, IL-27, or IL-35.
Treatment for SLE, as well as other autoimmune diseases, relies on the use of corticosteroids and immunosuppressive drugs, which are nonspecific and can cause adverse effects. Improved diagnosis and management of the disease have contributed to an improvement in its prognosis. However, patients with SLE still display increased mortality compared with the general population. Thus, there is a need for novel therapies that are specific and display improved efficacy and lower toxicity than the current therapies for SLE. Knowledge about Tregs and regulatory cytokines would not only provide new insights into the pathogenesis of SLE but could be also used to develop various clinical applications.
2. Role of Tregs in Autoimmune Diseases
The regulation of lymphocyte survival is required for the maintenance of lymphoid homeostasis, which prevents the development of autoimmune diseases. The existence of autoreactive T cells in healthy individuals suggests that peripheral tolerance mechanisms exist to control the responses of these cells. Accumulating evidence has indicated that clonal deletion and anergy, as well as T-cell-mediated control of self-reactive T cells contribute to the maintenance of self-tolerance. Tregs are now recognized as the mediators of peripheral tolerance and potent suppressors of excessive immune responses. Several Treg subtypes with distinct phenotypes, cytokine production profiles, and modes of action have been described. In the CD4+ regulatory T-cell compartment, CD4+CD25+ T cells (CD4+CD25+ Treg) and IL-10-producing type 1 T-regulatory cells (Tr1) have been described [1, 2]. Knowledge about the various developmental pathways and mechanisms of action of Treg-associated cytokines is required to develop novel specific therapies for SLE.
3. Role of CD4+CD25+ Treg in SLE
Extensive studies in mice and humans have indicated that CD4+CD25+ Treg belong to an important subset of Tregs. CD4+CD25+ Treg, which is naturally occurring and expresses forkhead-winged helix protein-3 (Foxp3), is a potent inhibitor of various immune responses [3]. Depletion or functional defect of CD4+CD25+ Treg leads to the development of autoimmune diseases in normal animals. CD4+CD25+ Treg are produced by the thymus as a distinct and mature subpopulation of T cells. Genetic alterations that affect the development or function of CD4+CD25+ Treg result in the development of autoimmune disease like IPEX syndrome and other inflammatory disorders in humans. In addition, decreased numbers of CD4+CD25+ Treg were found in some studies of SLE patients, especially during active disease. On the other hand, several investigators have reported that defective CD4+CD25+ Treg activity is correlated with the downregulated expression of Foxp3 [4–6]. Miyara et al. reported that CD4+CD25+ Treg isolated from SLE patients exhibited the same phenotypic and functional characteristics as corresponding cells in healthy controls [7]. However, lupus CD4+CD25+ Treg do not accumulate in either the lymph nodes or the inflamed kidneys and are more susceptible to Fas-induced apoptosis than those of healthy control. The accumulated data suggest that strategies for enhancing the function of CD4+CD25+ Treg might be beneficial for patients with SLE. The differences between the results of the various studies of CD4+CD25+ Treg in SLE patients might have been due to differences in the stage and activity of disease, disease manifestations, and the confounding influence of medical therapies. In addition, the use of different surface markers for defining CD4+CD25+ Treg might also have affected the results of these studies. Recently, Miyara et al. identified three subpopulations among human Foxp3 expressing cells, CD45RA+Foxp3low resting Treg, CD45RA−Foxp3high activated Treg, and CD45RA−Foxp3low cytokine-secreting cells. They reported that CD45RA−Foxp3low non-Treg fraction increased to form a distinct population in active SLE [8].
Lupus-prone mouse models, which are more homogeneous than SLE patients and can be left untreated, might give us more precise information about CD4+CD25+ Treg. (NZB × NZW) F1 (BWF1) and (SWR × NZB) F1 (SNF1) mice, which spontaneously develop lupus-like disease, had a lower percentage of CD4+CD25+ Treg than non-SLE-prone mice [9]. Reduced numbers of CD4+CD25+ Treg were also detected in mice that were congenic for the NZM2410 sle1 locus [10], and the reduced number of CD4+CD25+ Treg was associated with downregulated Foxp3 expression. Other lupus-prone MRL/lpr mice exhibited a normal percentage of CD4+CD25+ Treg, and their Foxp3 mRNA and protein expression levels were not altered. However, MRL/lpr CD4+CD25+ Treg exhibited a reduced capacity to suppress the proliferation and secretion of proinflammatory cytokines in effector cells [11]. In BWF1 mice, the number and frequency of CD4+CD25+ Treg were increased, and in vitro suppressive activity in lymphoid organs was intact [12]. However, the adoptive transfer of exogenously expanded CD4+CD25+ Treg to BWF1 mice reduced renal pathology and improved survival in BWF1 mice, supporting the protective role of these cells in lupus pathogenesis [13], and the induction of mucosal tolerance via the administration of the histone peptide H471 restored the lower numbers of CD4+CD25+ Treg in BWF1 mice to the levels of normal mice [9]. Kang et al. showed that administration of low doses of the nucleosomal histone peptide H471–94 for tolerance induction in SNF1 mice ameliorates the manifestations of the disease, prolongs survival, and increases the number of CD4+CD25+ Treg. Low-dose H471–94 therapy suppressed interferon- (IFN-) γ production by pathogenic T cells, autoantibody production, and lupus-associated responses upon their adoptive transfer in vivo [14]. High intravenous doses of a synthetic peptide (pConsensus [pCons]) based on a shared CDR1/framework 2 epitope encoded within the variable heavy chain regions of several murine anti-dsDNA immunoglobulins also exhibited therapeutic activity in BWF1 mice to prolong survival [15]. The administration of the tolerogenic peptide led to the expansion of peptide-reactive CD4+CD25+ Treg that inhibited the production of anti-dsDNA antibody-producing cells in vitro [16, 17].
Thus, Treg cell therapy could be a rational approach for the treatment of lupus, and investigators are currently attempting to expand its use to include the treatment of other autoimmune diseases. The specific activities of CD4+CD25+ Treg have been demonstrated in different animal models of autoimmune diseases (e.g., autoimmune diabetes, autoimmune thyroiditis, and experimental autoimmune encephalomyelitis (EAE)). For instance, CD4+CD25+ Treg expressing the T-cell receptor (TCR) specific to an islet antigen were reported to efficiently suppress and even reverse early onset of diabetes in nonobese diabetic (NOD) mice, whereas polyclonal CD4+CD25+ Treg were considerably less effective [18, 19]. This suggests that regulatory T-cell function depends on the antigen specificity. Therefore, expanding antigen-specific CD4+CD25+ Treg may be a promising approach in treating autoimmune diseases including SLE [20].
4. Role of IL-10 in SLE
IL-10 impedes the activation/expansion of autoreactive lymphocytes via various mechanisms. It is produced mainly by monocytes/macrophages and T-cell subsets including Tr1, CD4+CD25+ Treg, and T-helper (Th) 1 cells. IL-10 regulates immune cell function through a transmembrane receptor complex, composed of IL-10 receptor α (Rα) and IL-10Rβ. In monocytes/macrophages, IL-10 diminishes the production of inflammatory mediators, inhibits antigen presentation, and prevents specific and nonspecific immune reactions that cause tissue damage. At the same time, monocytes/macrophages increase antigen uptake and function as scavengers. IL-10 prevents the activation of antigen-presenting cells (APC) and downregulates the expression of costimulatory molecules. Recent studies have also revealed that IL-10 regulates autoreactive T cells in NOD mice [21, 22]. T cells that were generated in vitro and produced high levels of IL-10 inhibited the development of EAE [23]. The generation of antigen-specific T cells that produce IL-10 at sites of inflammation would be a promising strategy to the treatment of autoimmunity. Furthermore, IL-10 appears to play a role in Treg commitment and function [24] and so might be beneficial in SLE.
On the other hand, IL-10 boosts B-cell proliferation and immunoglobulin class switching, resulting in enhanced antibody production and increased inflammation. Several stimuli, including anti-dsDNA antibodies and immune complexes containing FcγRII, trigger IL-10 production [25, 26]. IL-10 has also immunostimulatory effects on CD8+ T cells and NK cells, especially in combination with other cytokines, such as IL-18 [27, 28]. In patients with SLE, an elevated IFN-γ/IL-10 ratio was observed in active class IV lupus nephritis and vice versa in class V nephritis [29]. IL-10 is overproduced by the B cells and monocytes of SLE patients [30–32], displays increased serum levels in SLE patients [32, 33], and is associated with disease activity [34]. Interestingly, continuous therapy from young age with IL-10 antibodies ameliorated autoimmunity in BWF1 mice [35]. In accordance with the therapeutic effect of anti-IL-10 antibodies, the continuous administration of recombinant IL-10 increased disease activity [35]. Interestingly, coadministration of blocking anti-tumor necrosis factor (TNF) antibodies cancelled the protective effect of anti-IL-10 antibodies [35], suggesting some unknown immunoregulatory balance between these two cytokines in BWF1 mice. Moreover, IL-10 blockade limited the renal damage in animal models of lupus nephritis [36]. The observed downregulation of T-cell activation in peripheral blood mononuclear cells from SLE patients was consistent with these effects [37]. In a small, uncontrolled, open-label study involving patients with mild disease, anti-IL-10 monoclonal antibody improved cutaneous lesions, joint symptoms, and the SLE disease activity index [38]. B-cell secretion of IL-10 might regulate dendritic cell (DC) and T-cell function, promoting Th2 deviation of the immune response [39]. In turn, IL-10 might contribute to a number of the earlier peripheral B-cell abnormalities observed in SLE, including plasma cell expansion [40]. These findings suggest that anti-IL-10 therapy with an agent that is suitable for use in humans might benefit some patients with SLE. However, increased numbers of IL-10-producing cells were also reported in first-degree relatives as well as healthy spouses [41, 42]. The contribution of IL-10 genotypes and IL-10 promoter polymorphisms to IL-10 overproduction has not been confirmed yet [42, 43]. In addition to environmental factors, both genetic and disease-induced events are required for the pathogenesis of lupus. Some of the proinflammatory effects of IL-10 might be displayed in the presence of other cytokines, such as IL-18 [28, 44], and the cytokines produced endogenously at inflammatory sites during various disease stages might modify the effect of IL-10. The modified development and characterization of IL-10 should be investigated further to aid the development of novel immunosuppressive therapies.
We recently reported on IL-10-secreting CD4+CD25−Foxp3− Treg that characteristically express both the lymphocyte activation gene-3 (LAG-3) and early growth response gene-2 (Egr-2), and the ectopic expression of Egr-2 conferred suppressive functions on naïve CD4+ cells [45]. The adoptive transfer of CD4+CD25−LAG3+ Treg from MRL/+ mice suppressed the progression of nephritis and autoantibody production in MRL/lpr mice (unpublished observation). In consistency with previous report [11], CD4+CD25+ Treg from MRL/+ mice revealed no significant therapeutic effect upon being transferred to MRL/lpr mice. These results indicate that IL-10-producing CD4+CD25−LAG3+ Treg play a critical role in preventing the development of autoimmune diseases characterized with autoantibody production.
Interestingly, Huber et al. reported that both Foxp3− and Foxp3+ regulatory CD4+ T cells control Th17 cells in an IL-10-dependent manner [46]. Th17 is a distinct helper T-cell subset that produces IL-17. Accumulated data suggest that IL-17 contributes to the pathogenesis of SLE. In lupus-prone BXD2 mice, IL-17 increases the number of IL-17-producing T cells, which help B cells, and accelerates germinal center formation in the spleen [47]. The frequency of IL-17-producing T cells also showed an increase in the peripheral blood of SLE patients. Patients with SLE display higher plasma levels of IL-17 and IL-23 than healthy controls, and their plasma IL-17 levels exhibit a positive correlation with disease activity [48]. CD3+CD4−CD8− double-negative (DN) T cells that produce IL-17 and IFN-γ expand in the peripheral blood of SLE patients, but not in healthy individuals [49]. In addition, DN T cells and IL-17-producing T cells are observed in the kidneys of patients with lupus nephritis. IL-17, IFN-γ, and IL-13 are the main cytokines produced by T cells that infiltrate in the kidneys of nephritic MRL/lpr mice [50]. IL-17-producing CD4+ T cells express IL-10Rα in vivo, and T cell-specific blockade of IL-10 signaling leads to a selective increase in the numbers of IL-17 and IFN-γ-producing CD4+ T cells during intestinal inflammation [46]. Both CD4+Foxp3− IL-10-producing cells and CD4+ Foxp3+ regulatory (Foxp3+ Treg) cells were found to control the numbers of Th17 cells and IL-17- and IFN-γ-producing cells in an IL-10-dependent manner in vivo. In addition, IL-10 treatment of mice with established colitis decreased the numbers of Th17 cells and IL-17 and IFN-γ-producing CD4+ T-cell frequencies through direct signaling in T cells. Consistent with our results, CD4+Foxp3− IL-10-producing cells expressed LAG-3, in contrast to Foxp3+ Treg. Therefore, investigating the functions of CD4+CD25−LAG3+ Treg might lead to the development of novel treatments for SLE.
5. Role of TGF-β in SLE
TGF-β functions to suppress inflammation and is important for the tolerance induced by oral antigen administration and for protecting against lymphopenia-induced colitis and thyroiditis mediated by the transfer of CD4+ T cells [51]. TGF-β can regulate autoaggressive T-cell responses by prevention of the APC maturation and can inhibit the differentiation of naïve CD4+ T cells into Th1 or Th2 effector cells by inhibiting T-bet, a Th1-specific transcription factor, and GATA-3, a transcription factor for Th2 differentiation [52–54]. In contrast to the development of naturally occurring CD4+CD25+ Treg [55], the peripheral induction of CD4+CD25+ Treg [56] depends on the effect of TGF-β. TGF-β signaling via TGF-β receptor is also required for the de novo expression of Foxp3 [57]. TGF-β signaling is required for the suppressive ability and the in vivo expansion of Tregs [58]. The TGF-β-induced transcription factor mother against decapentaplegic homologue 3 (SMAD3) have been shown to control the activity of a Foxp3 intronic enhancer element in cooperation with NFAT [59]. Recently, it has been shown that TGF-β increases the amount of acetylated Foxp3 protein bound to active chromatin sites, suggesting that TGF-β prolongs the half-life of Foxp3 RNA species and/or phosphorylates the chromatin-bound Foxp3, which might enable other transcription factors to undergo cellular compartment transitions for other transcription factors [60]. The suppressive effects of TGF-β can be transmitted to effector T cells through the soluble forms of this cytokine, or direct contact with Tregs, which display TGF-β on their surface [61]. When cell-to-cell contact takes place, TGF-β molecules on the surfaces of Tregs are triggered to aggregate by signals originating from CTLA-4 upon cell-to-cell contact [61]. T cells that cannot respond to TGF-β, thus, escape control by Tregs [62] to result in generalized autoimmunity in vivo [63].
Interestingly, the serum concentration of TGF-β1 is decreased in patients with active SLE, and urinary TGF-β1 levels are increased in patients with lupus nephritis [64, 65]. Patients with decreased numbers of CD4+CD25+ Treg tend to display lower serum TGF-β1 levels and higher urinary TGF-β1 levels. TGF-β1 might even play dual roles in murine lupus, immune regulation, and promotion of chronic end organ damage [66]. BWF1 mice have reduced expression of TGF-β1 in the spleen, and TGF-β1 or TGF-β1-producing T cells suppress autoantibody production. In contrast, the expression of TGF-β1 protein and TGF-β-signaling proteins increased in the kidneys. The levels of TGF-β1 in the kidneys and urine correlate with the extent of chronic lesions that represent local fibrosis. TGF-β1 blockade by treatment of these mice with an anti-TGF-β antibody in vivo selectively inhibits chronic fibrotic lesions without affecting autoantibody production and tissue inflammation. The use of TGF-β1 in association with CD4+CD25+ Treg might have potential as a novel therapeutic approach for autoimmune disease. However, we should be aware of chronic organ damage by TGF-β1.
6. Roles of IL-27 in SLE
IL-27 is a member of the IL-12 family cytokines composed of Epstein-Barr virus-induced gene 3 (EBI-3) and p28 subunits [67, 68]. IL-27 is mainly produced by activated monocytes/macrophages and DCs but is also expressed by other cell types including the parenchymal cells [69–71]. The murine p28 subunit can be secreted by itself, but the human p28 subunit has to be bound to EBI-3 for secretion. Therefore, the expression of both the EBI-3 subunit and p28 subunit within the same cell appears to be necessary for IL-27 production in humans [72]. While the expression of each subunit is differentially regulated, toll-like receptor (TLR) signaling is an important trigger of the expression of the EBI-3 and p28 subunits [72]. It was reported that TLR4 mediates the upregulation of IL-27 expression via MyD88-dependent and independent mechanisms. In addition, NF-κB signaling has also contributed to the induction of IL-27 expression [73, 74]. The administration of IL-27 suppressed the cytokine production of activated T cells in vitro [75]. IL-27 and its receptor, IL-27R, play an immunosuppressive role and suppress the production of proinflammatory cytokines. IL-27R consists of WSX-1 and gp130. WSX-1 is expressed in T cells, B cells, NK cells, monocytes, mast cells, dendritic cells (DCs), and endothelial cells [76]. Similar to other cytokine receptors, IL-27R signaling activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways in a cell-type-dependent manner [77–79]. Collectively, these findings revealed a novel role for IL-27/WSX-1 as an attenuator of proinflammatory cytokine production, which is important for preventing excessive inflammation.
IL-27 is a pleiotropic cytokine that has both offensive and defensive properties. IL-27 is unique in that although it suppresses immune responses, it also plays a proinflammatory role by inducing Th1 differentiation [80]. The role of WSX-1 in Th1 differentiation has also been examined in WSX-1−/− mice. WSX-1−/− mice exhibited impaired IFN-γ production compared with wild-type mice [81, 82]. IL-27/WSX-1 signaling induces STAT1 or STAT3 activation, followed by the induction and activation of T-bet. Though naive CD4+ T cells do not produce IFN-γ in response to IL-27 stimulation, additional stimulation with IL-12 induces IFN-γ production by naive CD4+ T cells. IL-27/WSX-1 suppresses the expression of GATA-3 in a STAT1-dependent or independent manner, thereby, contributing to Th1 differentiation [83].
Experimental inflammatory responses were also enhanced in WSX-1−/− mice [84]. Recent studies have demonstrated that IL-27 promotes IL-10 production by CD4+ T cells [85, 86] and inhibits Th17 cells in mice and humans [87, 88]. These results suggest a dual regulatory mechanism of IL-27 for controlling autoimmunity and tissue inflammation. The IL-10-producing CD4+ T cells elicited by IL-27 are T-bet+FoxP3−IFN-γ+ [85, 86, 89–91], and STAT1 and STAT3 activities are involved in the induction of IL-10 by IL-27 [85]. The induction of c-Maf, IL-21, and ICOS expression seems to be required to IL-27-mediated, Tr1-like cell differentiation [92]. Whereas IL-10 induction is critical for IL-27-mediated immune suppression, IL-10-independent anti-inflammatory mechanisms have been indicated [85, 89]. For instance, IL-27 demonstrated suppressive effects in an IL-10-deficient milieu, and it even suppressed IL-10 production in some conditions [75]. In addition, IL-27 also suppresses inflammation by inhibiting Th17 cell differentiation in vitro [71]. IL-27 was reported to suppress the expression of the Th17-specific transcription factor RORγt [88].
Recently, the effects of IL-27 signaling on autoimmune responses in MRL/lpr mice were investigated. Deficiency of the WSX-1 gene resulted in the development of a disease resembling human membranous glomerulonephritis (WHO class V), and sera levels of IgG1 and IgE were increased. WSX-1 −/− MRL/lpr T cells exhibited significantly decreased IFN-γ production along with elevated IL-4 expression [93], and EBI-3 deficiency in MRL/lpr mice resulted in a disease resembling human membranous glomerulonephritis and sialadenitis [94]. On the other hand, transgenic overexpression of the WSX-1 gene in the T cells of MRL/lpr mice strongly suppressed the development of glomerulonephritis and improved survival, suggesting protective role of high-dose IL-27 in lupus [95]. Microarray analysis of glomerular gene expression in murine lupus nephritis revealed increased Ebi3 expression [96]. In addition, decreased serum IL-27 levels were observed in patients with SLE, especially those with nephritis. These findings suggest a protective role for IL-27 in SLE [97]. However, both the regulatory and proinflammatory functions of IL-27 should be investigated further, especially in humans. Recently, it was reported that the WSX-1 is required to support IL-21 production and follicular helper T-cell survival in a T-cell intrinsic manner [98]. Recombinant IL-27 molecules that have a long-lasting effect in vivo, small chemical compounds that promote IL-27/WSX-1 signaling, and antagonistic or agonistic anti-IL-27R antibodies would be useful as novel therapies for SLE.
7. Role of IL-35 in SLE
IL-35 is a newly identified inhibitory cytokine that belongs to the IL-12 cytokine family [99, 100]. It is composed of the IL-12α (p35) and EBI-3, which is considered to be a downstream target of Foxp3. IL-35 is preferentially expressed in CD4+CD25+ Treg, and ectopic expression of IL-35 confers regulatory activity on naïve T cells, whereas recombinant IL-35 suppresses T-cell proliferation [101]. IL-35 is required for maximal regulatory function in vivo as CD4+CD25+ Treg deficient in either subunit fail to control homeostatic T-cell expansion or inflammatory bowel disease [101]. Given that IL-35 was discovered relatively recently, our understanding of its biological activity is still limited. Compared with IL-12 and IL-27, generation and purification of recombinant IL-35 are challenging due to its instability. It is tempting to speculate that the apparent poor stability of IL-35 might underlie its important physiological roles and limit potency over short range. On the other hand, DC that secretes IL-12 or IL-27 might be precluded from generating IL-35, because of preferential pairing of IL-12 and IL-27.
IL-35 fusion protein was suggested to inhibit the differentiation of Th17 cells in vitro and to ameliorate collagen-induced arthritis (CIA) and suppress IL-17 secretion in vivo [102]. To precisely characterize the function of IL-35, the determination of the IL-35 receptor and its expression pattern is required. Its receptor will also be composed of a new combination of known family receptor chains [101], or the receptor might be composed of novel subunits. Lastly, it is unknown whether IL-35 can induce the formation of Tregs. This is a shared feature of the other two inhibitory cytokines IL-10 and TGF-β, and; thus, it remains plausible that IL-35 has a similar ability. Since TGF-β-induced Treg (Th3) and IL-10-induced Tr1 cells have very distinct transcriptional and functional profiles [103, 104], IL-35-induced regulatory populations may also exhibit quite distinct phenotype. The linkage between the regulatory role of IL-35 and lupus pathogenesis remains to be investigated.
8. Conclusions
Accumulating data have revealed that the numbers and the function of CD4+CD25+ Treg are decreased in SLE. Other than CD4+CD25+ Treg, there are several Treg populations including IL-10-producing Tr1-like cells. Cooperation between these subsets of Tregs might be required for optimal immunoregulatory function (Figure 1). Investigations of Treg-associated cytokines, such as IL-10, TGF-β, IL-27, and IL-35, might aid the development of novel therapies for SLE. Indeed, the generation of T cells producing high levels of immunosuppressive cytokines in response to antigen specific stimulation successfully prevented autoimmune disease in animal models. Therapeutic approaches that induce functional Tregs with relevant antigen-specificities would restore immune homeostasis in patients and protect them from further autoimmune response. Further investigations in animal models and humans will hopefully allow lupus to be treated with Tregs and Treg-associated cytokines.
Figure 1.
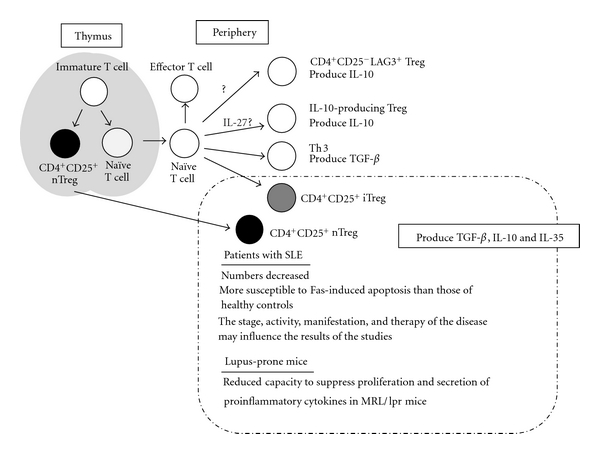
The subsets of regulatory T cells. These Treg cells are candidates which prevent breakdown of self-tolerance and autoimmune diseases.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annual Review of Immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Sharabi A, Mozes E. Harnessing regulatory T cells for the therapy of lupus and other autoimmune diseases. Immunotherapy. 2009;1(3):385–401. doi: 10.2217/imt.09.2. [DOI] [PubMed] [Google Scholar]
- 4.Crispin JC, Martínez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. Journal of Autoimmunity. 2003;21(3):273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 5.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. Journal of Immunology. 2007;178(4):2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 6.Hahn BH, Anderson M, Le E, la Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis and Rheumatism. 2008;58(8):2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 7.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. Journal of Immunology. 2005;175(12):8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 8.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Wu HY, Staines NA. A deficiency of CD4+CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004;13(3):192–200. doi: 10.1191/0961203303lu1002oa. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Cuda C, Morel L. Genetic determination of T cell help in loss of tolerance to nuclear antigens. Journal of Immunology. 2005;174(12):7692–7702. doi: 10.4049/jimmunol.174.12.7692. [DOI] [PubMed] [Google Scholar]
- 11.Parietti V, Monneaux F, Décossas M, Muller S. Function of CD4+,CD25+ Treg cells in MRL/lpr mice is compromised by intrinsic defects in antigen-presenting cells and effector T cells. Arthritis and Rheumatism. 2008;58(6):1751–1761. doi: 10.1002/art.23464. [DOI] [PubMed] [Google Scholar]
- 12.Abe J, Ueha S, Suzuki J, Tokano Y, Matsushima K, Ishikawa S. Increased Foxp3+ CD4+ regulatory T cells with intact suppressive activity but altered cellular localization in murine lupus. American Journal of Pathology. 2008;173(6):1682–1692. doi: 10.2353/ajpath.2008.080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand black/New Zealand white lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. Journal of Immunology. 2006;177(3):1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 14.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. Journal of Immunology. 2005;174(6):3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 15.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-β. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8810–8815. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.la Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand black × New Zealand white)F1 mice suppress in vitro production of antibodies to DNA. Journal of Immunology. 2004;173(5):3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki Y, Amakawa R, Ito T, et al. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis and Rheumatism. 2001;44(2):432–441. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. Journal of Experimental Medicine. 2007;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. Journal of Immunology. 2005;175(5):3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 20.Mendlovic S, Brocke S, Shoenfeld Y, et al. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(7):2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. Journal of Immunology. 2001;167(11):6087–6091. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+T cells in autoimmune diabetes. Nature Immunology. 2001;2(12):1117–1125. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- 23.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. Journal of Experimental Medicine. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nature Reviews Immunology. 2002;2(6):389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 25.Rönnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcγRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Annals of the Rheumatic Diseases. 2003;62(1):37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun KH, Yu CL, Tang SJ, Sun GH. Monoclonal anti-double-stranded DNA autoantibody stimulates the expression and release of IL-1β, IL-6, IL-8, IL-10 and TNF-α from normal human mononuclear cells involving in the lupus pathogenesis. Immunology. 2000;99(3):352–360. doi: 10.1046/j.1365-2567.2000.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KW, de Waal-Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 28.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. European Journal of Immunology. 1999;29(9):2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Uhm WS, Na K, Song GW, et al. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology. 2003;42(8):935–938. doi: 10.1093/rheumatology/keg255. [DOI] [PubMed] [Google Scholar]
- 30.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. Journal of Experimental Medicine. 1999;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 32.Hondowicz BD, Alexander ST, Quinn WJ, 3rd, et al. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. International Immunology. 2007;19(4):465–475. doi: 10.1093/intimm/dxm011. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JS, Schneider P, Kalled SL, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. Journal of Experimental Medicine. 2000;192(1):129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houssiau FA, Lefebvre C, Berghe MV, Lambert M, Devogelaer JP, Renauld JC. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4(5):393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 35.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. Journal of Experimental Medicine. 1994;179(1):305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravirajan CT, Wang Y, Matis LA, et al. Effect of neutralizing antibodies to IL-10 and C5 on the renal damage caused by a pathogenic human anti-dsDNA antibody. Rheumatology. 2004;43(4):442–447. doi: 10.1093/rheumatology/keh083. [DOI] [PubMed] [Google Scholar]
- 37.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Interleukin-10 blockade corrects impaired in vitro cellular immune responses of systemic lupus erythematosus patients. Arthritis and Rheumatism. 2000;43(9):1976–1981. doi: 10.1002/1529-0131(200009)43:9<1976::AID-ANR8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Llorente L, Richaud-Patin Y, Garcia-Padilla C, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic Lupus erythematosus. Arthritis and Rheumatism. 2000;43(8):1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. Journal of Experimental Medicine. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobi AM, Odendahl M, Reiter K, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2003;48(5):1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 41.Gröndal G, Kristjansdottir H, Gunnlaugsdottir B, et al. Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in icelandic multicase families. Arthritis and Rheumatism. 1999;42(8):1649–1654. doi: 10.1002/1529-0131(199908)42:8<1649::AID-ANR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 42.van der Linden MW, Westendorp RG, Sturk A, Bergman W, Huizinga TWJ. High interleukin-10 production in first-degree relatives of patients with generalized but not cutaneous lupus erythematosus. Journal of Investigative Medicine. 2000;48(5):327–334. [PubMed] [Google Scholar]
- 43.Alarcon-Riquelme ME, Lindqvist AKB, Jonasson I, et al. Genetic analysis of the contribution of IL10 to systemic lupus erythematosus. Journal of Rheumatology. 1999;26(10):2148–2152. [PubMed] [Google Scholar]
- 44.Zhang X, Giangreco L, Broome HE, Dargan CM, Swain SL. Control of CD4 effector fate: transforming growth factor β1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. Journal of Experimental Medicine. 1995;182(3):699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamura T, Fujio K, Shibuya M, et al. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huber S, Gagliani N, Esplugues E, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu HC, Yang PA, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunology. 2008;9(2):166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 48.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clinical Immunology. 2008;127(3):385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Crispin JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. Journal of Immunology. 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Ito S, Chino Y, et al. Use of laser microdissection in the analysis of renal-infiltrating T cells in MRL/lpr mice. Modern Rheumatology. 2008;18(4):385–393. doi: 10.1007/s10165-008-0074-8. [DOI] [PubMed] [Google Scholar]
- 51.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nature Immunology. 2001;2(9):816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 52.Gorelik L, Flavell RA. Transforming growth factor-β in T-cell biology. Nature Reviews Immunology. 2002;2(1):46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 53.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. Journal of Experimental Medicine. 2002;195(11):1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. European Journal of Immunology. 2000;30(9):2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Piccirillo CA, Letterio JJ, Thornton AM, et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. Journal of Experimental Medicine. 2002;196(2):237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. Journal of Immunology. 2001;166(12):7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 57.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunology. 2005;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 58.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. Journal of Immunology. 2004;173(11):6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 59.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunology. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 60.Samanta A, Li B, Song X, et al. TGF-β and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14023–14027. doi: 10.1073/pnas.0806726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-β-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. Journal of Immunology. 2006;177(4):2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 62.Fahlén L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-β escape control by CD4+ CD25+ regulatory T cells. Journal of Experimental Medicine. 2005;201(5):737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nature Immunology. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 64.Xing Q, Su H, Cui J, Wang B. Role of Treg cells and TGF-β1 in patients with systemic lupus erythematosus: a possible relation with lupus nephritis. Immunological Investigations. 2011 doi: 10.3109/08820139.2011.578189. [DOI] [PubMed] [Google Scholar]
- 65.Hammad AM, Youssef HM, El-Arman MM. Transforming growth factor β 1 in children with systemic lupus erythematosus: a possible relation with clinical presentation of lupus nephritis. Lupus. 2006;15(9):608–612. doi: 10.1177/0961203306071873. [DOI] [PubMed] [Google Scholar]
- 66.Saxena V, Lienesch DW, Zhou M, et al. Dual roles of immunoregulatory cytokine TGF-β in the pathogenesis of autoimmunity-mediated organ damage. Journal of Immunology. 2008;180(3):1903–1912. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 68.Pan HF, Tao JH, Ye DQ. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opinion on Therapeutic Targets. 2010;14(5):479–484. doi: 10.1517/14728221003769911. [DOI] [PubMed] [Google Scholar]
- 69.Smits HH, van Beelen AJ, Hessle C, et al. Commensal gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. European Journal of Immunology. 2004;34(5):1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 70.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Research. 2005;1040(1-2):202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 71.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunology. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 72.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunology Letters. 2008;117(2):123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-γ-mediated pathways. Journal of Experimental Medicine. 2007;204(1):141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wirtz S, Becker C, Fantini MC, et al. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-κB activation. Journal of Immunology. 2005;174(5):2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 75.Yoshimura T, Takeda A, Hamano S, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. Journal of Immunology. 2006;177(8):5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 76.Hibbert L, Pflanz S, de Waal-Malefyt R, Kastelein RA. IL-27 and IFN-α signal via STAT1 and STAT3 and induce T-Bet and IL-12Rβ2 in naive T cells. Journal of Interferon and Cytokine Research. 2003;23(9):513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 77.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. Journal of Immunology. 2003;170(10):4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 78.Artis D, Villarino A, Silverman M, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. Journal of Immunology. 2004;173(9):5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 79.Ouaked N, Mantel PY, Bassin C, et al. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. Journal of Immunology. 2009;182(2):1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. Journal of Leukocyte Biology. 2009;86(6):1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida H, Hamano S, Senaldi G, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 82.Chen Q, Ghilardi N, Wang H, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 83.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naïve CD4+ T cells through STAT1-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. Journal of Immunology. 2007;179(10):6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 85.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunology. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 86.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature Immunology. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 87.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. Journal of Immunology. 2009;183(4):2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. Journal of Immunology. 2009;182(9):5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature Immunology. 2007;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 90.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. Journal of Immunology. 2008;180(5):2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 91.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological Reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 92.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. Journal of Immunology. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimizu S, Sugiyama N, Masutani K, et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) Journal of Immunology. 2005;175(11):7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 94.Igawa T, Nakashima H, Sadanaga A, et al. Deficiency in EBV-induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Modern Rheumatology. 2009;19(1):33–41. doi: 10.1007/s10165-008-0117-1. [DOI] [PubMed] [Google Scholar]
- 95.Sugiyama N, Nakashima H, Yoshimura T, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor α(WSX-1) Annals of the Rheumatic Diseases. 2008;67(10):1461–1467. doi: 10.1136/ard.2007.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teramoto K, Negoro N, Kitamoto K, et al. Microarray analysis of glomerular gene expression in murine lupus nephritis. Journal of Pharmacological Sciences. 2008;106(1):56–67. doi: 10.1254/jphs.fp0071337. [DOI] [PubMed] [Google Scholar]
- 97.Li TT, Zhang T, Chen GM, et al. Low level of serum interleukin 27 in patients with systemic lupus erythematosus. Journal of Investigative Medicine. 2010;58(5):737–739. doi: 10.231/JIM.0b013e3181d88f7b. [DOI] [PubMed] [Google Scholar]
- 98.Batten M, Ramamoorthi N, Kljavin NM, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. Journal of Experimental Medicine. 2010;207(13):2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collison LW, Vignali DAA. Interleukin-35: odd one out or part of the family? Immunological Reviews. 2008;226(1):248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Current Opinion in Immunology. 2009;21(6):612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 102.Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. European Journal of Immunology. 2007;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 103.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature Immunology. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]


