Abstract
Background
Cigarette smoking is associated with increased cardiovascular morbidity and mortality in the general population, but the effect of smoking on these outcomes in the dialysis population is less well studied.
Study Design
Systematic review and meta-analysis of cohort studies.
Setting & Population
Adults treated with long-term hemodialysis or peritoneal dialysis.
Selection Criteria for Included Studies
Cohort studies of unselected dialysis patients reporting the association between smoking status and cardiovascular morbidity and/or mortality.
Predictor
Smoking status (determined by patient report).
Outcomes
1) All-cause or cardiovascular mortality; 2) Incident cardiovascular events
Results
We identified 34 studies which fulfilled all inclusion criteria. Of these, 26 studies provide data on smoking and mortality and 10 (n = 6538) were included in a meta-analysis. The pooled hazard ratio for all-cause mortality in smokers compared to non-smokers was 1.65 (95% CI , 1.26–2.14; p<0.001) Eleven studies provided data on smoking and incident cardiovascular events, 5 (pooled n = 845) were included in a meta-analysis. The pooled hazard ratio for composite cardiovascular events in smokers compared to non-smokers was 1.01 (95% CI, 0.98–1.05, p 0.4)‥
Limitations
Data for these meta-analyses were heterogeneous. Few individual studies assessed smoking as the primary variable of interest.
Conclusions
Active smoking is associated with a significant increase in all-cause mortality in dialysis patients, although there was not a corresponding increased risk of cardiovascular events.
In the general population the adverse health effects of cigarette smoking are well known. Smoking is the leading cause of preventable mortality in the US and accounts for approximately 443,000 deaths annually with 28% due to ischemic heart disease. 1 Internationally, smoking accounts for 1.6 million cardiovascular deaths each year with 54% due to ischemic heart disease and 25% due to cerebrovascular disease. 2 Despite the negative consequences of smoking, tobacco use remains common in the United States. The Center for Disease Control estimates that in 2008, approximately one out of five American adults were active smokers 3. The prevalence of smoking in dialysis patients is less clear. The most recent USRDS estimate, based on data from the End Stage Renal Disease Medical Evidence Report (Form 2728) as completed by dialysis providers, is that 6.2% of incident dialysis patients are smokers 4. However, the provider-generated data in the 2728 form has been shown to significantly underestimate true smoking prevalence 5. Dialysis patient questionnaires estimate the true prevalence of cigarette smoking to be between 14 and 15%.5
Amongst disease-specific populations, it would seem that individuals with end-stage renal disease (ESRD) would be especially vulnerable to the adverse consequences of smoking. The annual mortality in the dialysis population is very high with only 34% five-year survival 4, 6. Cardiovascular disease accounts for 40 % of all deaths in this population 4, 6.
Given that patients with ESRD have a high rate of cardiovascular disease, as do smokers with normal kidney function, we hypothesized that ESRD patients who smoke would be at extraordinarily high risk of cardiovascular disease and subsequent mortality. The answer to this question may seem obvious; however, several co-morbidities associated with increased mortality in the general population such as hypercholesterolemia 7, 8 hypertension 9 and obesity 10, 11 have not been shown to increase mortality in the ESRD population 12–14. In this study we systematically reviewed all publications that compared ESRD smokers to non-smokers with regards to mortality and/or cardiovascular morbidity.
Methods
Study Selection
A Medline search from 1970-present was conducted on January 17, 2011 with no language restrictions using the following criteria: “smoking AND (dialysis OR hemodialysis OR end stage renal disease OR chronic kidney disease OR chronic renal failure)” (Item S1, available as online supplementary material). Animal or pediatric studies (subjects under 19 years of age) were excluded without further review. Abstracts of the remaining studies were reviewed by two independent reviewers (S.E.L. and S.P.L), with any discrepancies adjudicated by an independent third party (D.A.B.). The reference lists for each of these studies and all of the references in the 2005 KDOQI guidelines on smoking in dialysis patients were also searched for relevant studies 15. In addition, all abstracts submitted to the American Society of Nephrology’s Renal Week annual meeting for the past three years were searched for potentially relevant data sets, and authors were contacted to provide data. Full text review of these studies was performed to assess fulfillment of the following inclusion criteria: cohort study design (either prospective or retrospective); unselected population comprised entirely of ESRD patients (treated either with hemodialysis or peritoneal dialysis); and compared between smokers and non-smokers one or more of the outcomes of interest. Outcomes of interest were either mortality (all-cause or cardiovascular) or cardiovascular morbidity (comprising incident cardiac events, incident peripheral vascular events, or incident cerebrovascular events).
Quantitative Data Synthesis
Given that only the estimates, not the individual data, from each study were available, we used a random effects meta-analysis model 16 to estimate the pooled hazard ratio with 95% confidence intervals (CIs). The random effects analysis calculates a weighted average of the estimates, and includes the original variance plus the between-studies variance. Thus the smaller the variances, the more weight an estimate will have in the pooled estimate. In all studies included, the adjusted hazard ratio was used for analysis. The presence of heterogeneity across studies was evaluated using Q-statistic. The analyses were carried out using “meta” package in software R version 2.12.1 on a WINDOWS XP platform.
Results
Study Identification
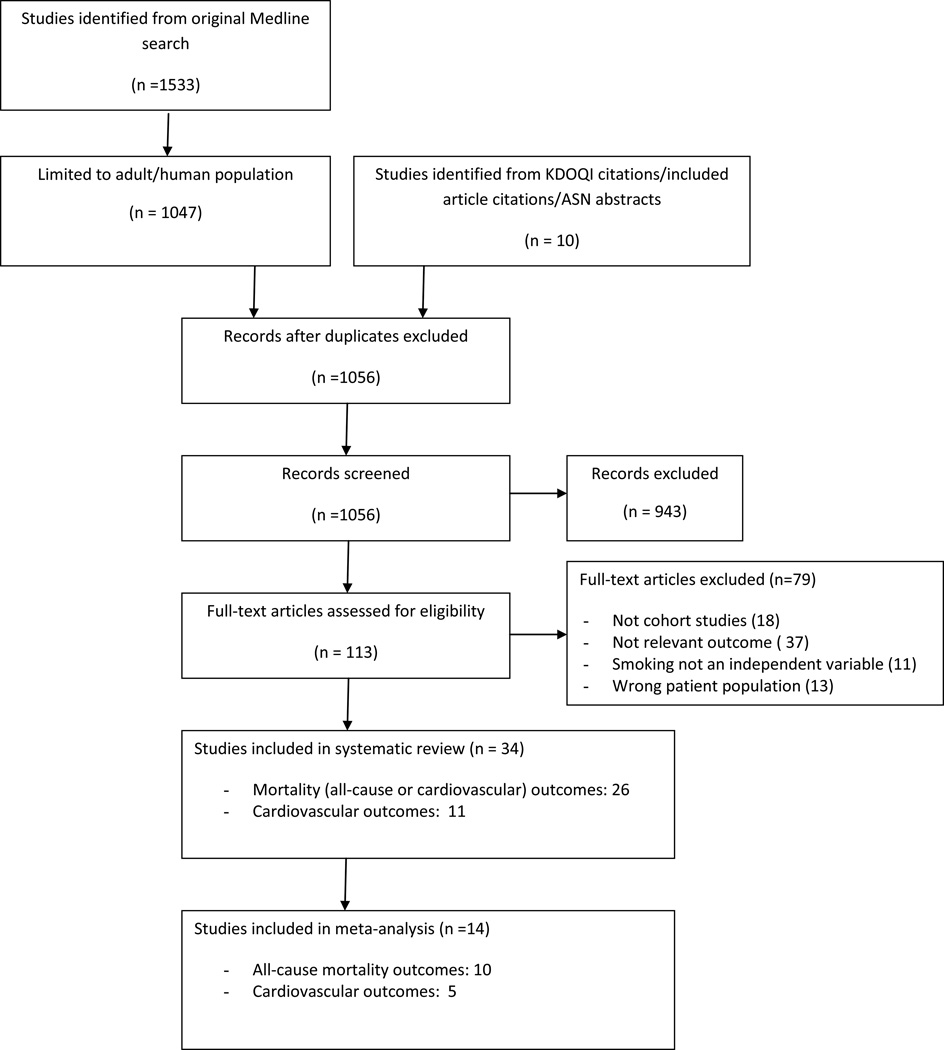
A summary of the study identification process is shown in Figure 1. The original Medline search indentified 1533 articles, this number decreased to 1047 with exclusion of animal or pediatric studies. Reference list review, ASN abstract search, and review of KDOQI citations identified an additional 10 articles. Of this total, 943 were excluded based on abstract review and 34 of the remaining studies fulfilled our inclusion criteria after full-text review. We divided studies by two outcomes - mortality or incident cardiovascular events. Several studies provided data on multiple outcomes of interest, and therefore there are a greater number of total outcomes analyzed than included studies. One article which fulfilled our inclusion criteria17 was not included, as another study 18 analyzed the same dataset using smoking as the primary variable of interest, not simply as a covariate.
Figure 1.
Flow diagram for study selection
Risk of Bias
The majority of studies included in this review continued patient follow-up for several years time, a duration which should allow for a valid estimation of the true frequency of the outcomes assessed. Studies included in this review were comprised of representative samples of ESRD patients, although many cohorts were single-center and ethnically homogeneous. In addition, most included studies required that patients be stable on dialysis for 3 months and free of acute medical conditions (often active infection or heart failure) for enrollment, which likely excluded a high-risk population from analysis. Publication bias is unlikely in this review given that nearly all included studies reported data on the effect of smoking only as a covariate, and not as the main rationale for publication. For those studies included in meta-analysis, the relationship between smoking and the main outcome of interest was reported via a multivariate-adjusted Cox proportional hazard model, which allowed for the control of other factors (age, gender, and other co-morbidities) which could obscure this relationship.
Mortality
We identified 26 cohort studies examining the relationship between cigarette smoking and mortality in dialysis patients (Table 1). There are numerous differences between these studies including cohort size, dialysis modality, and the definition of smoking (i.e. both former and current cigarette users considered as “smokers” versus considering current smokers only, with some not explicitly defining smoking status). Most of these studies were single-center cohorts, had a small number of patients, and were not specifically designed to address the relationship between smoking and mortality. In most cases smoking status was analyzed only as a covariate.
Table 1.
Studies reporting mortality rates.
| Reference | Included in meta- analysis? |
Dialysis Modality (n) |
Smoking definition | Mortality outcome |
Association | Magnitude** | Notes |
|---|---|---|---|---|---|---|---|
| Terrier et al, 2008 19 | N | HD (187) | Not specified | All-cause; CV | All-cause, no; CV, yes | All-cause: NA; CV: RR, 2.52 | |
| Plantinga et al, 2007 20 | N | HD (767); PD (274) | Current or former | All-cause | Partial (yes at 2 y, no at 4 y) | ||
| Braatvedt et al, 2006 21 | N | PD (1293) | Current or former | All-cause | Yes | RR, 1.22 (1.02 – 1.46) for current or former | Smoking is primary variable of interest |
| Wagner et al, 2006 22 | Y | HD (154) | Not specified | All-cause | Yes | HR, 3.03 (1.48 – 6.19) | |
| Kitahara et al, 2005 23 | Y | HD (785) | Current or former | All-cause | No | ||
| Cooper et al, 2004 24 | Y | HD (57); PD (52) | Current or former | All-cause | No | ||
| Koch et al, 2004 25 | Y | HD (322) | Not specified | All-cause | Yes | HR, 2.29 | |
| Foley et al, 2003 18 | Y | HD (2018); PD (1923) | Never, former (quit >1 y), former (quit < 1 y), or current | All-cause | Yes (current smoking only) | HR, 1.37 (1.15 – 1.64) for current smokers | Smoking is primary variable of interest |
| Zoccali et al, 2003 26 | Y | HD (227) | Current | All-cause; CV | All-cause: no; CV: no | ||
| Mallamaci et al, 2002 27 | N | HD (175) | Current | CV | No | ||
| Tepel et al, 2002 28 | Y | HD (188) | Not specified | All-cause | Yes | HR, 2.32 (1.30–4.12) | |
| Benedetto et al, 200129 | N | HD (91); PD (47) | Current | CV | No | ||
| Fleischmann et al, 2001 30 | N | HD (453) | Current, former, or lifetime nonsmoker | All-cause | No | ||
| Haubitz et al, 2001 31 | N | PD (34) | Not specified | All-cause | No | ||
| Shemin et al, 2001 32 | Y | HD (114) | Current | All-cause | Yes | HR, 3.24 (1.7–6.17) | |
| Shoji et al, 2001 33 | Y | HD (265) | Not specified | All-cause; CV | All-cause: no; CV: no | ||
| Blacher et al, 1999 34 | N | HD (241) | Current or former | All-cause; CV | All-cause: no; CV: no | ||
| Mazzuchi et al, 1999 35 | N | HD (531) | Current or quit ≤ 5 y | All-cause | Unclear* | ||
| Zimmerman et al, 1999 36 | N | HD (280) | Not specified | All-cause; CV | All-cause: no; CV: no | ||
| Blacher et al, 1998 37 | N | HD (79) | Current or former | All-cause; CV | All-cause: no; CV: no | ||
| Foley et al, 1997 38 | Y | 433 total – modality not specified | Current | All-cause (diabetic subgroup only) | Yes | HR, 2.3 (1.0–5.2) | Diabetic subgroup only |
| Bloembergen et al, 1996 39 | N | HD (2479) | Current or former | All-cause | No | ||
| Fishbane et al, 1996 40 | N | HD (132) | Not specified | All-cause; CV | All-cause: no; CV: no | ||
| Soucie et al, 1996 41 | N | HD (12,240); PD (3005) | Not specified | All-cause (≤90 d of initiation) | Yes | OR, 1.3 (1.0 – 1.7) | |
| Brown et al, 1994 42 | N | HD (84); PD (165); both (16) | > 3 cigarettes/d for > 3 y | CV | Yes | 38% vs 26% | |
| Postorino et al, 2008 43 | N | HD (537) | Current or former | All-cause | No | Abstract |
Abbreviations: HD = hemodialysis, PD = peritoneal dialysis; CV, cardiovascular; NA, not applicable; HR, hazard ratio; RR, risk ratio;
No reference group identified, so it is unclear whether this is a mortality harm or benefit with smoking.
where applicable, 95% confidence interval is shown in parentheses
Of the 26 total studies, two were specifically designed to investigate the relationship between smoking and mortality in ESRD patients and deserve additional attention. Foley et al. examined a large cohort (n=3941) of ESRD patients at the initiation of either peritoneal dialysis (PD) or hemodialysis (HD) 18. Patients were stratified into one of five groups based on their response to a questionnaire: lifelong non-smokers (56 %), current smokers (14 %), individuals who quit smoking more than 1 year prior (20 %), those who quit within one year (6 %), or status unknown (4 %). Compared to lifelong non-smoking, current smoking was associated with an increased risk of mortality (RR, 1.37) after adjustment for other demographic variables. Former smokers (even those who had quit within one year) were not at an increased mortality risk. Braatvedt et al. studied the relationship between smoking and mortality in a large cohort (n = 1293) of patients initiating PD 21. Patients were categorized as smokers, including both current (17%) or former smokers (45%), or lifetime non-smokers (38%) based on an interview. After follow-up ranging from 20–140 months, mortality was significantly higher for smokers (current or former) compared to lifetime non-smokers (RR, 1.22). There was no significant difference in survival between former or current smokers.
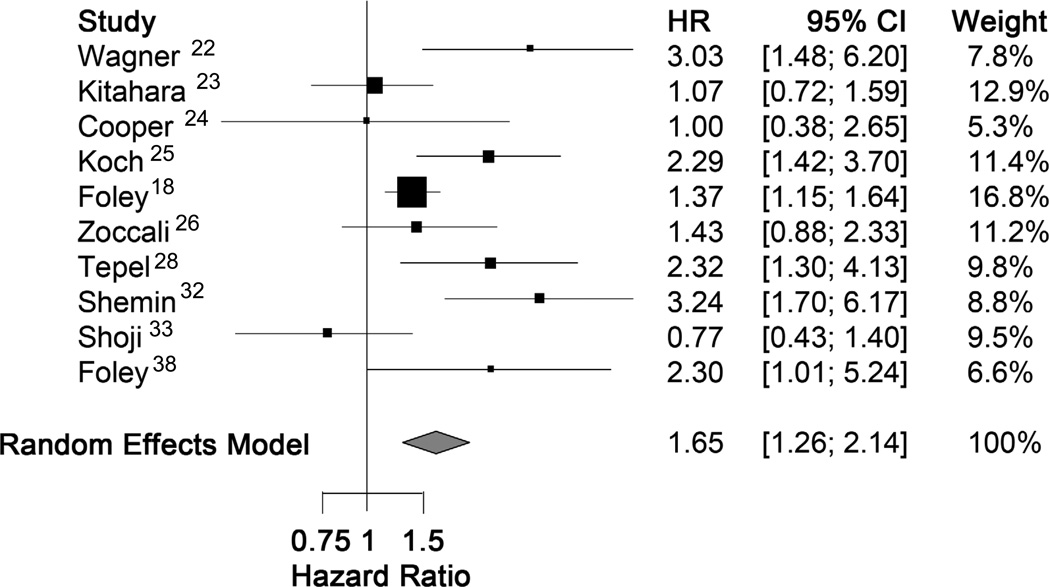
Ten studies (pooled n = 6538) provided estimates of the hazard ratio for all-cause mortality in smokers versus non-smokers, and could be included in a meta-analysis. The pooled hazard ratio for mortality comparing smokers to non-smokers was 1.65 (95%CI, 1.26–2.14; p<0.001) based on a random effects analysis. (Figure 2). There was significant heterogeneity amongst studies, with a Q-statistic p value of 0.003.
Figure 2.
Forest plot for all-cause mortality
Cardiovascular Morbidity
Eleven studies provided data on the relationship between cigarette smoking and a variety of cardiovascular events in the ESRD population (Table 2). The majority provided data for a composite cardiovascular outcome and were not specifically designed with smoking as the primary variable of interest. One study provided data for a variety of individual cardiovascular outcomes and was designed with smoking as the primary variable 18. As described earlier, patients in this large cohort were classified as non-smokers, current smokers, or former smokers. Using inpatient Medicare claim records, the rate of incident cardiovascular outcomes was assessed prospectively in this group. Over a mean follow-up of 2.2 years, current smokers were more likely to develop heart failure (RR, 1.59) and peripheral vascular disease (RR, 1.68) compared to lifelong non-smokers. Current smoking did not have a statistically significant impact on the rates of incident ischemic heart disease or cerebrovascular disease, and former smoking conferred no excess risk compared to lifelong non-smoking on any cardiovascular outcome.
Table 2.
Studies reporting incident cardiovascular events.
| Reference | Included in meta- analysis? |
Dialysis Modality (n) |
Smoking Definition | Outcome Assessed |
Association | Magnitude** |
|---|---|---|---|---|---|---|
| Sanchez-Perales et al, 2010 44 | N | 375 (HD); 84 (PD) | Not defined | CVA | No | |
| Combe et al, 2009 45 | N | 29,838 (HD) | Current/recent (quit < 1 y) or former (quit > 1 y) | PVD (peripheral amputation) | Yes for current/recent, no for former | HR, 1.33 (1.08 – 1.64) |
| Shah et al, 2008 46 | N | 193 (HD); 81 (PD) | Current or quit ≤ 1 y | Composite CV events | Yes | OR, 2.14 (1.02 – 4,42) |
| Schwaiger et al, 2006 47 | Y | 154 (HD) | Current or former | Composite CV events | Yes | HR, 1.90 (1.05 – 3.43) |
| Huybrechts et al, 2005 48 | Y | 179 (HD) | Current or former | Composite CV events | Yes | HR, 0.67 (non-smokers vs smokers) |
| Cooper et al, 2004 24 | Y | 109 (HD & PD) | Current or former | Composite CV events | No | |
| Foley et al, 2003 18 | N | 3941 (HD & PD) | Current, former (quit < 1 y), former (quit >1 y), or never | CHF; CHD; CVA; PVD | CHF: yes for current; CHD: no; CVA: no; PVD: yes for current | CHF: HR, 1.59 (1.16 – 2.17); CHD & CVA: NA; PVD: HR, 1.68 (1.27 – 2.22) |
| Mallamaci et al, 2002 27 | Y | 175 (HD) | Current | Composite CV events | No | |
| Zoccali et al, 2002 49 | Y | 228 (HD) | Not specified | Composite CV events | No | |
| Cressman et al, 1992 50 | N | 129 (HD) | Not specified | Composite CV events | No |
Abbreviations: HD = hemodialysis, PD = peritoneal dialysis, CV = cardiovascular, CVA = stroke, CHF = congestive heart failure, PVD = peripheral vascular disease, CHD = coronary heart disease; HR, hazard ratio; NA, not applicable; OR, odds ratio
where applicable, 95% confidence interval is shown in parentheses
In the majority of the remaining studies, data were provided for a composite cardiovascular outcome – different in each study but generally a combination of ischemic heart events (myocardial infarction or need for percutaneous coronary intervention or coronary bypass surgery), cerebrovascular events (transient ischemic attack or stroke), or peripheral vascular events (arterial thrombosis or need for revascularization procedure or amputation). Some also included cardiovascular death, congestive heart failure, and deep venous thrombosis as part of the composite outcome.
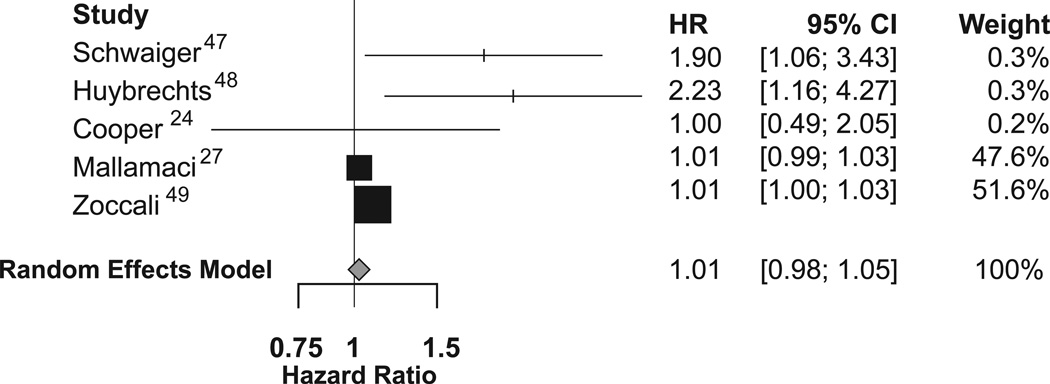
Five studies (pooled n = 845) provided an estimate of the hazard ratio for composite cardiovascular outcomes in smokers versus non-smokers, and could be included in a meta-analysis. The pooled hazard ratio for incident cardiovascular events in smokers compared to non-smokers was 1.01 (95%CI, 0.98–1.05; p=0.4) based on a random effects analysis. (Figure 3).
Figure 3.
Forest plot for incident cardiovascular events
Discussion
This review and meta-analysis summarizes the currently available data on the relationship between cigarette smoking and clinical outcomes in the ESRD population. With respect to mortality the data were heterogeneous, but the meta-analysis demonstrates a significantly higher risk of mortality in dialysis patients who smoke compared to those who do not. In support of this finding, both studies specifically designed to evaluate the mortality risk of smoking showed significantly increased mortality in active smokers compared to non-smokers 18, 21. The estimated magnitude of this effect found on meta-analysis (a 49 % risk increase) is clinically significant, and should prompt the clinician to address smoking cessation in addition to the myriad of other factors known to impact mortality in the dialysis population.
The data were also heterogeneous for incident cardiovascular events, but showed no significant increased risk in smokers compared to non-smokers. Given the conclusive mortality increase in smokers, and the presumption that this is due to accelerated vascular disease, how can these data be reconciled? It may be that the composite cardiovascular outcome used in our meta-analysis included several manifestations of vascular disease which were not influenced by smoking status, with the overall result that a composite outcome showed a non-significant result. To support this, the two largest studies examining cardiovascular outcomes in an ESRD population 18, 45 demonstrated a higher incidence of peripheral vascular events and heart failure in smokers, but there was no increased incidence of cerebrovascular or coronary vascular events. Alternatively, smoking may increase mortality through non-cardiovascular mechanisms.
One strength of this review is that we focused our analysis on the most rigorous data available. Given that a randomized controlled trial of smoking in the ESRD population is not feasible, we focused on cohort studies in an attempt to isolate a direct temporal relationship between cigarette smoking and the outcomes of interest. We eliminated studies with unclear design as well as those which included only a specific ESRD subpopulation. Further, we limited our review to the clinical outcomes most pertinent to the care of ESRD patients, rather than focusing on biochemical markers or radiographic/angiographic evidence of vascular disease.
Limitations of this review are inherent in the design of the included studies. In all included studies, smoking status was defined at study outset only, and no data were provided regarding changes in smoking habits during follow up. Further, smoking status was determined by self report, which may lead to misclassification 51, 52. Finally, the majority of studies reported data on cigarette smoking only as a covariate along with another major variable of interest. As such, sample sizes may not have been adequate to detect outcome differences in relation to smoking status. This may have been a major factor in the overall heterogeneity of our meta-analyses.
There are many opportunities for further work in this area. Studies using objective biomarkers of nicotine exposure (such as cotinine 53) may more accurately establish the relationship between cigarette smoking and adverse events. This would eliminate the potential misclassification bias introduced by self report. Also, the impact of successful smoking cessation on clinical outcomes in the ESRD population has not been evaluated. While significant, data from cohort studies can only demonstrate association, not causality. A demonstrated decrease in adverse events after smoking cessation would further compel providers to counsel against tobacco use. Studies examining the most successful means to achieve smoking cessation are also lacking in the ESRD population. Given the mortality benefit for non-smoking ESRD patients demonstrated in this review, a clinical trial to determine the most effective intervention (counseling versus the various forms of pharmacologic therapy) would be a logical next step.
The adverse effects of smoking in the ESRD population are less well-established than in the general population. Our meta-analysis shows that there is a significantly higher risk of mortality in dialysis patients who smoke compared to those who do not. Based on current data, however, there does not appear to be a higher incidence of cardiovascular events in smokers versus non-smokers with ESRD. Given our findings, we support the 2005 KDOQI guidelines advocating regular counseling and encouragement to stop smoking in all ESRD patients 15.
Supplementary Material
Acknowledgements
The authors wish to express their appreciation to Arthur Watts for his assistance in preparing the figures for this manuscript.
Support: This publication was made possible in part by Grant Number 1 UL1 RR024160 – 01 from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH), and the NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Contributor Information
Scott E. Liebman, University of Rochester Medical Center.
Steven P. Lamontagne, University of Rochester Medical Center.
Li-Shan Huang, Institute of Statistics, National Tsing Hua University, Hsin-Chu city, TAIWAN R.O.C.
Susan Messing, Department of Biostatistics and Computational Biology, University of Rochester Medical Center.
David A. Bushinsky, University of Rochester Medical Center.
REFERENCES
- 1.Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses --- United States, 2000--2004. MMWR weekly. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of Smoking in Global and Regional Cardiovascular Mortality. Circulation. 2005;112(4):489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- 4.Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 5.Longenecker JC, Coresh J, Klag MJ, et al. Validation of Comorbid Conditions on the End-Stage Renal Disease Medical Evidence Report: The CHOICE Study. J Am Soc Nephrol. 2000;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 6.Foley R, Parfrey P, Sarnak M. Clinical epidemiology of cardiovascular disease in chronic renal disease. American Journal of Kidney Disease. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd J, Cobbe SM, Ford I, et al. Prevention of Coronary Heart Disease with Pravastatin in Men with Hypercholesterolemia. N Engl J Med. 1995;333(20):1301–1308. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Pfeffer MA, Moye LA, et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Willett WC, Stampfer MJ, et al. Body Weight and Mortality among Women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 11.Gu D, He J, Duan X, et al. Body Weight and Mortality Among Men and Women in China. JAMA. 2006;295(7):776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55(4):1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Krane V, Marz W, et al. Atorvastatin in Patients with Type 2 Diabetes Mellitus Undergoing Hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse Epidemiology of Hypertension and Cardiovascular Death in the Hemodialysis Population: The 58th Annual Fall Conference and Scientific Sessions. Hypertension. 2005;45(4):811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. American Journal of Kidney Disease. 2005;45:16–153. [PubMed] [Google Scholar]
- 16.Whitehead A. Meta-Analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons, Ltd.; 2002. [Google Scholar]
- 17.Kestenbaum B, Gillen DL, Sherrard DJ, Seliger S, Ball A, Stehman-Breen C. Calcium channel blocker use and mortality among patients with end-stage renal disease. Kidney Int. 2002;61(6):2157–2164. doi: 10.1046/j.1523-1755.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 18.Foley RN, Herzog CA, Collins AJ. Smoking and cardiovascular outcomes in dialysis patients: The United States Renal Data System Wave 2 Study 1,2. Kidney Int. 2003;63(4):1462–1467. doi: 10.1046/j.1523-1755.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 19.Terrier N, Jaussent I, Dupuy A-M, et al. Creatinine index and transthyretin as additive predictors of mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2008;23(1):345–353. doi: 10.1093/ndt/gfm573. [DOI] [PubMed] [Google Scholar]
- 20.Plantinga LC, Fink NE, Levin NW, et al. Early, Intermediate, and Long-Term Risk Factors for Mortality in Incident Dialysis Patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. American Journal of Kidney Disease. 2007;49(6):831–840. doi: 10.1053/j.ajkd.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Braatvedt G, Rosie B, Bagg W, Collins J. Current and former smoking increases mortality in patients on peritoneal dialysis. N Z Med J. 2006;119(1234):1977–1987. [PubMed] [Google Scholar]
- 22.Wagner Z, Molnar M, Molnar G, et al. Serum Carboxymethyllysine Predicts Mortality in Hemodialysis Patients. American Journal of Kidney Disease. 2006;47(2):294–300. doi: 10.1053/j.ajkd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Kitahara T, Ono K, Tsuchida A, et al. Impact of Brachial-Ankle Pulse Wave Velocity and Ankle-Brachial Blood Pressure Index on Mortality in Hemodialysis Patients. American Journal of Kidney Disease. 2005;46(4):688–696. doi: 10.1053/j.ajkd.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Cooper BA, Penne EL, Bartlett LH, Pollock CA. Protein malnutrition and hypoalbuminemia as predictors of vascular events and mortality in ESRD. American Journal of Kidney Disease. 2004;43(1):61–66. doi: 10.1053/j.ajkd.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Koch M, Trapp R, Kulas W, Grabensee B. Critical limb ischaemia as a main cause of death in patients with end-stage renal disease: a single-centre study. Nephrol. Dial. Transplant. 2004;19(10):2547–2552. doi: 10.1093/ndt/gfh404. [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C, Mallamaci F, Tripepi G, et al. Chlamydia pneumoniae, overall and cardiovascular mortality in end-stage renal disease (ESRD) Kidney Int. 2003;64(2):579–584. doi: 10.1046/j.1523-1755.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 27.Mallamaci F, Zoccali C, Tripepi G, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002;61(2):609–614. doi: 10.1046/j.1523-1755.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 28.Tepel M, Giet MVd, Park A, Zidek W. Association of calcium channel blockers and mortality in haemodialysis patients. Clin. Sci. 2002;103(5):511–515. doi: 10.1042/cs1030511. [DOI] [PubMed] [Google Scholar]
- 29.Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic Value of Ultrasonographic Measurement of Carotid Intima Media Thickness in Dialysis Patients. J Am Soc Nephrol. 2001;12(11):2458–2464. doi: 10.1681/ASN.V12112458. [DOI] [PubMed] [Google Scholar]
- 30.Fleischmann E, Bower J, Salahudeen A. Are conventional cardiovascular risk factors predictive of two-year mortality in hemodialysis patients? Clin Nephrol. 2001;56(3):221–230. [PubMed] [Google Scholar]
- 31.Haubitz M, Brunkhorst R. C-reactive protein and chronic Chlamydia pneumoniae infection--long-term predictors for cardiovascular disease and survival in patients on peritoneal dialysis. Nephrology Dialysis Transplantation. 2001;16(4):809–815. doi: 10.1093/ndt/16.4.809. [DOI] [PubMed] [Google Scholar]
- 32.Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. American Journal of Kidney Disease. 2001;38(1):85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 33.Shoji T, Emoto M, Shinohara K, et al. Diabetes Mellitus, Aortic Stiffness, and Cardiovascular Mortality in End-Stage Renal Disease. J Am Soc Nephrol. 2001;12(10):2117–2124. doi: 10.1681/ASN.V12102117. [DOI] [PubMed] [Google Scholar]
- 34.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of Aortic Stiffness on Survival in End-Stage Renal Disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 35.Mazzuchi N, Carbonell E, Fernandez-Cean J. ESRD patients without co-morbid risk factors at the start of haemodialysis are ideal as survival comparison population. Nephrol. Dial. Transplant. 1999;14(5):1091–1096. doi: 10.1093/ndt/14.5.1091. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid Arterial Stiffness as a Predictor of Cardiovascular and All-Cause Mortality in End-Stage Renal Disease. Hypertension. 1998;32(3):570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Foley R, Culleton B, Parfrey P, et al. Cardiac disease in diabetic end-stage renal disease. Diabetologia. 1997;40(11):1307–1312. doi: 10.1007/s001250050825. [DOI] [PubMed] [Google Scholar]
- 39.Bloembergen WE, Stannard DC, Port FK, et al. Relationship of dose of hemodialysis and cause-specific mortality. Kidney Int. 1996;50(2):557–565. doi: 10.1038/ki.1996.349. [DOI] [PubMed] [Google Scholar]
- 40.Fishbane S, Youn S, Flaster E, Adam G, Maesaka J. K Ankle-arm blood pressure index as a predictor of mortality in hemodialysis patients. American Journal of Kidney Disease. 1996;27(5):668–672. doi: 10.1016/s0272-6386(96)90101-8. [DOI] [PubMed] [Google Scholar]
- 41.Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. Journal of the American Society of Nephrology. 1996;7(10):2169–2175. doi: 10.1681/ASN.V7102169. [DOI] [PubMed] [Google Scholar]
- 42.Brown JH, Hunt LP, Vites NP, Short CD, Gokal R, Maflick NP. Comparative mortality from cardiovascular disease in patients with chrome renal failure. Nephrol. Dial. Transplant. 1994;9(8):1136–1142. doi: 10.1093/ndt/9.8.1136. [DOI] [PubMed] [Google Scholar]
- 43.Postorino M, Marino C, Tripepi G, Zoccali C. Diverging relationships of waist-hip ratio and BMI with mortality risk in patients with end stage renal disease (ESRD): The role of inflammation. [ASN abstract SA-PO 2731] J Am Soc Nephrol. 2008;19:727A. [Google Scholar]
- 44.Sanchez-Perales C, Vazquez E, Garcia-Cortes MJ, et al. Ischaemic stroke in incident dialysis patients. Nephrology Dialysis Transplantation. 2010;25(10):3343–3348. doi: 10.1093/ndt/gfq220. [DOI] [PubMed] [Google Scholar]
- 45.Combe C, Albert J, Bragg-Gresham J, et al. The Burden of Amputation Among Hemodialysis Patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) American Journal of Kidney Disease. 2009;54(4):680–692. doi: 10.1053/j.ajkd.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 46.Shah D, Polkinhorne K, Pellicano R, Kerr P. Are traditional risk factors valid for assessing cardiovascular risk in end-stage renal failure patients? Nephrology. 2008;13(8):667–671. doi: 10.1111/j.1440-1797.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 47.Schwaiger JP, Neyer U, Sprenger-Mahr H, et al. A simple score predicts future cardiovascular events in an inception cohort of dialysis patients. Kidney Int. 2006;70(3):543–548. doi: 10.1038/sj.ki.5001589. [DOI] [PubMed] [Google Scholar]
- 48.Huybrechts KF, Caro JJ, London GM. Modeling the implications of changes in vascular calcification in patients on hemodialysis. Kidney Int. 2005;67(4):1532–1538. doi: 10.1111/j.1523-1755.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 49.Zoccali C, Mallamaci F, Parlongo S, et al. Plasma Norepinephrine Predicts Survival and Incident Cardiovascular Events in Patients With End-Stage Renal Disease. Circulation. 2002;105(11):1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 50.Cressman M, Heyka R, Paganini E, O'Neil J, Skibinski C, Hoff H. Lipoprotein(a) is an independent risk factor for cardiovascular disease in hemodialysis patients. Circulation. 1992;86(2):475–482. doi: 10.1161/01.cir.86.2.475. [DOI] [PubMed] [Google Scholar]
- 51.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors Associated with Discrepancies between Self-Reports on Cigarette Smoking and Measured Serum Cotinine Levels among Persons Aged 17 Years or Older : Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2001;153(8):807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 52.Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 53.Florescu AM, Ferrence RP, Einarson TP, Selby PMD, Soldin OPMBA, Koren GMD. Methods for Quantification of Exposure to Cigarette Smoking and Environmental Tobacco Smoke: Focus on Developmental Toxicology. Therapeutic Drug Monitoring. 2009;31(1):14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.