Abstract
Diabodies are non-covalent dimers of single chain antibody fragments (scFvs) that retain the avidity of intact IgG but have more favorable blood clearance than intact IgGs. Radiometals offer a wide range of half lives and emissions for matching imaging and therapy requirements and provide facile labeling of chelate-antibody conjugates. However, due to their high retention and metabolism in the kidney, use of radiometal labeled diabodies can be problematic for both imaging and therapy.
Methods
Having previously shown that 111In-DOTA-PEG3400-anti-CEA-diabody has similarly high tumor uptake and retention and less than 50% as much kidney uptake and retention as non-PEGylated diabody, we synthesized a similar derivative for an anti-TAG-72-diabody. We also reduced the molecular size of the polydispersed PEG3400 to monodispersed PEG27 and PEG12 (nominal masses of 1188 and 528, respectively). We performed biodistributions of their DOTA conjugates radiolabeled with 125I, 111In, or 64Cu in tumor bearing athymic mice.
Results
Addition of PEG3400 to the diabody reduced kidney uptake to a level (≈10 %ID/g) comparable to that obtained with radiometal labeled intact IgG. The PEG27 and PEG12 diabody conjugates also demonstrated low kidney uptake without reduction of tumor uptake or tumor to blood ratios. When radiolabeled with 64Cu, the DOTA-PEG12- and PEG27-diabody conjugates gave high contrast PET images of colon cancer xenografts in athymic mice.
Conclusion
PEGylated diabodies may be a valuable platform for delivery of radionuclides and other agents to tumors.
Keywords: Diabody, CC49, TAG-72, DOTA, Cu-64, polyethylene glycol, PET
INTRODUCTION
To be effective tumor imaging agents, targeted radiolabeled antibodies must provide both high tumor uptake and high tumor to blood ratios (1, 2). At one extreme, monoclonal antibodies (150 kDa) have a relatively slow blood clearance (t1/2 β = 48-72 h), allowing ample time for accumulation in the tumor, but suffer from low tumor to blood ratios. At the other extreme, single chain (sc) Fv recombinant fragments (25 kDa) are rapidly cleared from the blood (t1/2 β = 0.5-2.0 h) resulting in high tumor to blood ratios but low overall accumulation of radioactivity in tumor (3). After analyzing a series of radioiodinated recombinant antibody fragments (4), we and others concluded that simultaneous achievement of high tumor uptake and high tumor to blood ratio requires a bivalent antibody of size 50-80 kD (1, 3, 4) with diabodies (noncovalent dimers of size 55 kDa) as one platform fulfilling these criteria. For example 124I-labeled diabodies have performed well in a pre-clinical model of PET imaging (5). However, a drawback to radioiodinated antibodies is that they may be rapidly metabolized in tissues, including tumor, especially if the antigen undergoes internalization upon antibody binding. Radiometal-labeled recombinant antibody fragments offer a significant improvement in tumor to blood ratio, but this is offset by high kidney retention. Thus, systemic excretion of radiometal metabolites is slow compared to radioiodine metabolic products. We have identified 111In-DOTA-ε-lysine as the metabolic endpoint product in the kidney for 111In-DOTA anti-CEA antibody fragments (6).
We and others have sought to improve the utility of radiometal labeled recombinant antibody fragments by decreasing kidney uptake/retention. In one approach kidney uptake is partially blocked by the administration of intravenous L- or D-Lysine (7, 8). Alternatively, we have designed unique metabolizable linkers between the antibody and DOTA that have decreased kidney retention up to four-fold compared with NHS-DOTA conjugates (9). A third approach is to decrease kidney retention of antibody fragments by increasing their apparent molecular weight by PEGylation (10). This approach was successfully applied to anti-CEA F(ab’) fragment A5B7 by conjugating it to MPEG6000, which increased tumor retention and serum half life by 6- and 5-fold, respectively (11). In the case of anti-mucin scFv CC49/218 conjugated to PEG5000, a 14-fold increase in serum half life was observed (12). In the latter study, the authors concluded that greater improvements were observed with increasing size of PEG polymers rather than total amount of PEG, which often led to decreased immunoreactivity of the conjugate. In another study, investigators engineered an anti-MUC-1 dimeric scFv-SH (di-scFv, 55 kDa) with C-terminal cysteine residues for site specific conjugation to maleimide-PEGs of different sizes (13). They concluded that conjugation yields needed improvement prior to clinical application. Interestingly, a recent report (14) evaluated a 40 kDa branched chain PEG conjugated to a bispecific diabody that bound covalently to CEA and CD3 and showed that PEGylation doubled tumor uptake and increased circulation time (t1/2β of 50 h). Along the same line of investigation, Leong et al. (15) utilized the natural thiol group of an engineered Fab’ fragment to IL-8 for conjugation to maleimide-PEGs of different sizes. They found that conjugation to maleimide PEG 10 kDa resulted in an apparent molecular size of 180 kDa, comparable to the size of an intact antibody. Importantly, blood clearance studies revealed a t1/2β of 44 h compared to 3 h for the unmodified Fab’. Also, Yang et al. (10) conjugated maleimide PEGs of different sizes to an anti-TNF-α scFv (half the size of a diabody) with a cysteine engineered into the linker region or C-terminus. They found that conjugation to maleimide PEG (20 kDa) gave a blood clearance half life similar to intact IgG but offered no conclusion regarding the effect of the PEG modification on kidney retention.
Recently, we have shown that adding PEG3400 to 111In-DOTA-anti-CEA diabody reduced kidney uptake at 24-48 h from over 200 %ID/g for the non-PEGylated diabody to 50 %ID/g, while preserving high tumor uptake (16). However, further reduction in kidney uptake is required. To study diabody dependence of DOTA-PEG3400 conjugation, we generated and PEGylated an anti-TAG-72-diabody for which kidney retention was reduced to levels (10 %ID/g at 1-24 h) usually seen with intact IgG. This suggested that kidney uptake is a function of both apparent molecular size (as modified by PEG) and other less-defined factors intrinsic to the diabody (e.g., modification of pI has been shown to influence kidney uptake (17, 18)).
Having reduced the kidney uptake for radiometal labeled diabody by conjugation to PEG3400, we sought to further investigate the approach by using monodispersed DOTA-PEG conjugates. We synthesized DOTA-PEG-27-Cys-VS and DOTA-PEG-12-Cys-VS, where Cys-VS = vinyl sulfone conjugated to the thiol of cysteine, and conjugated these monodispersed PEGs to amino groups on the diabody at pH 9.0, a reaction we have previous described (19). The conjugates were characterized by mass spectrometry, IEF, and size exclusion chromatography. Biodistributions of 111In-labeled conjugates in athymic mice bearing LS-174T colon tumor xenografts gave tumor uptakes of 40-45 %ID/g, tumor/blood ratios of 8-10 and a kidney uptake of 10 %ID/g at 24 h. Furthermore, PET imaging with 64Cu-labeled conjugates provided high-contrast tumor images within 24 h after injection (tumor to blood = 8:1).
EXPERIMENTAL PROCEDURES
Materials, radiolabels, mass spectrometry
LS-174T cells were obtained from ATCC and maintained as previously described (16). 1,4,7,10-Tetraazacyclododecane-1,4,7-tris(t-butyl acetate)-10-acetic acid was obtained from Macrocyclics, Inc., Dallas, TX. NHS-PEG3400-VS (Cat No 4M5B0F02) was purchased from Nektar (San Carlos, CA). N-FMOC-amido-dPEG-27 acid was obtained from Novabiochem (EMD Biosciences, San Diego, CA) and N-FMOC-amido-dPEG-12 acid was purchased from Quanta Biodesign Ltd (Powell, OH). 111In chloride was from Amersham, 64Cu from Washington University School of Medicine, St. Louis, MO., and 125I from Perkin Elmer. Mass spectra were recorded on an Agilent 6520 Q-TOF LC/MS.
Construction, cloning, expression and purification of the anti-TAG-72 diabody
The anti-TAG72 diabody (AVP04-07) was based on V-domains from the parent CC49 antibody and derivative scFv fragments. The gene had codons optimized for E. coli expression with the orientation VH-VL joined by a G4S linker and included a C-terminal His6 tail. AVP04-07 was subcloned into E. coli BL21 (DE3)(F– ompT hsdSB(rB- mB-) gal dcm (DE3)) (Novagen) and produced in an 18L fermentor. Bacterial lysate cleared by centrifugation (16,000 × g, 30 min) and filtration at 0.45 μm was purified using a three-step procedure (HisTrap, HiTrap SP HP, and Superdex 75 prep chromatography) on an AKTA Purifier 10 (GE Healthcare, Uppsala, Sweden).
Analytical ultracentrifugation
Sedimentation velocity experiments were conducted using a Beckman model XL-I analytical ultracentrifuge (Fullerton, CA, USA) at 20°C. Data were collected at A290nm in continuous mode, using a time interval of 300 sec and a step-size of 0.003cm, without averaging. Solvent density (1.00499 g/mL at 20°C), solvent viscosity (1.0214 cp), and partial specific volume for AVP04-07 were computed using the program SEDNTERP. Sedimentation velocity data were fitted to a continuous size-distribution model using the program SEDFIT.
Immunoreactivity and isoelectric focusing
Binding of AVP04-07 to the TAG-72 antigen was determined using bovine submaxillary mucin (BSM, Aldrich-Sigma (St. Louis, MO) in a column shift assay (Superdex 200). Isoelectric focusing gel electrophoresis was run either on an IEF Precast gel (pH 3 – 10) (Novex) or on a Pharmacia PhastGel as per the manufacturer’s instructions.
Conjugation of AVP04-07
NHS-DOTA was conjugated to AVP04-07 diabody as previously described (16). NHS-PEG3400-VS was conjugated to AVP04-07 diabody at a molar ratio of 30:1 and pH 6.0 as previously described (19). The conjugate was reacted with Cysteineamido-DOTA (20) at a molar ratio of 50. N-FMOC-amido-PEG-27-acid was coupled to Cys-polystyrene Wang resin, cleaved and purified by RP-HPLC, reacted with a 10 molar excess of vinyl sulfone and repurified by RP-HPLC. DOTA-PEG-27-Cys-VS was reacted with AVP04-07 diabody at a molar ratio of 50. Synthesis and conjugation of DOTA-PEG12-Cys-VS to diabody at molar ratios of 20:1 and 50:1 were performed as described above.
Radiolabeling of AVP04-07 and its conjugates
Radioiodination of AVP04-07 and its conjugates with 125I (Perkin Elmer) was performed using the Iodogen method (21). Na 125I (5-10 μL, 26 mBq) was added to 200 μg AVP04-07 in a tube pre-coated with 20 μg Iodogen (Pierce), and incubated at RT for 3 min. Radiolabeling yields were 80-100%. Radiometal labeling of DOTA-AVP04-07 was performed using 111InCl3 (0.15 mBq/μg) or 64CuCl2 (0.37 mBq/μg) as previously described (19). Radiolabeling yields were typically 70-90%. The radiolabeled material was purified on Superdex-75 or 200 columns.
LS-174T Xenograft Model
Female, athymic nu/nu mice (Charles River Laboratories), 6 – 8 weeks old, were injected with LS-174T cells (106) s.c. in the flank. After 10 days mice were injected i.v. with a mixture (200 μl) of 370 kBq of 125I- and 150 kBq of 111In-labelled AVP04-07 (2-6 μg of total protein) for biodistribution studies. Mice were euthanized at various time points: tumor, blood and major organs were collected, weighed and counted with correction of for crossover of 111In counts into the 125I channel. Time-activity curves were corrected for radioactive decay and presented as percentage of injected dose/g tissue.
PET imaging
Tumor-bearing mice were injected i.v. with 64Cu-labeled diabody or diabody-PEG conjugates and imaged at 1, 4 , 21-22 and 45-46 h with a small-animal PET scanner (microPET Model R4; Siemens/CTIMI, Knoxville, TN). Mice anesthetized with isoflurane, were scanned for 20 min for the 1 and 4 h time points, 45 min at 21-22 h and 60 min at 45-46 h. Data were sorted into two-dimensional sinograms using the Fourier rebinning method and corrected for intrascan radiodecay, detector non-uniformity and random coincidence noise. Images were reconstructed by the iterative ordered subsets expectation maximization (OSEM) method (4 iterations, 16 subsets).
Comparison of Imaging Characteristics Among Diabody and Derivatives
An Imaging Figure of Merit (IFOM) was applied to both to planar and PET images (1, 2, 16). The IFOM at time t after injection is defined as:
with CV0 being the coefficient of variation of the difference in tumor and background counts (z), ε the detector efficiency factor, λ the decay constant, A0, the total injected activity and Vol the tumor volume. Uptake (%ID/g) was denoted as uT (tumor) and uB (background), respectively. In plotting this function, we set the product of CV0, ε, A0 and Vol equal to unity. The only sources of variation are tumor uptake, background tissue uptake and physical decay. Note that IFOM(t) < 0 implies that background tissue (blood or kidney) has greater uptake than tumor at time t.
RESULTS
Characterization of AVP04-07 Diabody
AVP04-07 diabody expressed in E. coli and purified by a three-step method eluted as a single species on gel filtration (Suppl Fig 1A) as a monodispersed dimer with an apparent molecular mass of 52.5 kDa by analytical untracentrifugation (Suppl Fig 1B). It had >99% immunoreactivity as judged by a column shift assay (Suppl Fig 1C).
Biodistribution of 111In-DOTA- and 125I- AVP04-07 in an Athymic Mouse LS-174T Xenograft Model
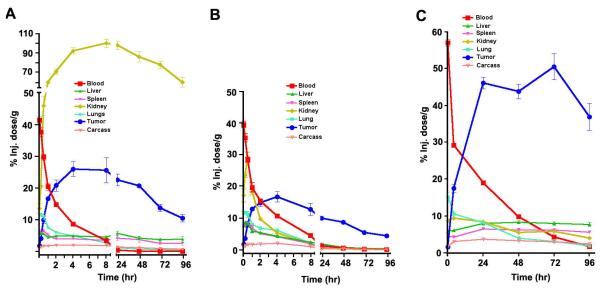
125I-DOTA-AVP04-07 and 111In-DOTA-AVP04-07 had similar blood clearances, with about 50% of radiolabel removed by 1h post-injection and 10% in circulation at 4 h (Fig. 1A, B). Kidney uptake was high (100 %ID/g at 24 h) for 111In-labeled, but not for 125I-labeled diabody, demonstrating that kidney was the major route of clearance. For the 111In-labeled diabody there was significant tumor uptake, with over 25 %ID/g observed as early as 4 h post injection and more than 20 %ID/g retained at 48 h. Tumor to blood ratio for 111In-AVP04-07 was >50:1 at 24 h. 125I-labeled AVP04-07 gave tumor uptakes of 17 and 10 %ID/g at 4 and 48 h respectively.
Figure 1. Biodistributions of unconjugated and DOTA-Cys-VS-PEG3400 conjugated AVP04-07.
Biodistributions were measured in athymic mice bearing LS-174T xenografts as described in Methods. A. 111In-labeled non-PEGylated diabody. B. 125I-labeled intact diabody. C. 111In-labeled PEG3400 conjugated diabody.
Synthesis and biodistribution of DOTA-Cys-VS-PEG3400-AVP04-07
Since PEGylation reduces kidney uptake of radiometal labeled diabodies, we first conjugated AVP04 -07 to DOTA-Cys-VS-PEG3400-NHS as previously described for a anti-CEA diabody (16). VS-PEG3400-NHS was conjugated to surface lysines of the diabody (via active ester), followed by reaction with DOTA-Cys (20). Analysis by SDS and IEF gel electrophoresis indicate an expected shift to lower pI (IEF gel, Suppl Fig 2A, lane 5) as well as a shift to higher apparent molecular size due to the addition of PEG3400 (SDS gel, Suppl Fig 2B, lane 5). The conjugate was radiolabeled with 111InCl3, chromatographed on Superdex 75 and compared to unconjugated AVP04-07 (Suppl Fig. 2A-B). The apparent molecular weight of the PEG3400 derivative was 105 kDa, versus 52.5 kDa. The increase can be attributed to the effect of PEGylation on Stokes radius and is similar to the shift in size we observed for PEG3400 derivatized anti-CEA diabody (16).
The biodistribution of 111In-AVP04-07-PEG3400 is shown in Fig 1C. Kidney uptake was 98 %ID/g at 24 h for non-PEGylated diabody (Fig 1A) and 8.4 %ID/g at 24 h for PEGylated diabody (Fig 1C). The large reduction of kidney uptake with PEGylation was accompanied by an increase in tumor uptake from 22 to 46 %ID/g at 24 h and a reduction in tumor/blood ratio (> 46:1 to 2:1 at 24 h). The increase in tumor retention is evidently due to the prolonged blood clearance of PEGylated diabody (t1/2β = 36 h) versus non-PEGylated diabody (t1/2β = 18 h). The reduction in kidney uptake was much greater than previously observed with 111In-DOTA-Cys-VS-PEG3400 anti-CEA-diabody (16). Possible explanations for the observed differences will be discussed later.
Synthesis and biodistribution of a monodispersed PEG27 AVP04-07conjugate
Although conjugation of AVP04-07 to a PEG3400 moiety achieved reduced kidney uptake, PEG3400 would have major drawbacks as a clinical product, due to its inherent polydispersity and the consequent inability to manufacture products that are easy to characterize chemically. Several monodispersed PEG building blocks are commercially available that can be converted into heterobifunctional reagents using peptide synthesis methodology. Thus, we first synthesized a DOTA-PEG27-Cys-VS derivative as summarized in Suppl Scheme 1. Second, we conjugated DOTA-PEG27-Cys-VS to surface lysines of the diabody at pH 9.0 at a molar ratio of 50:1 (22). When the conjugate was characterized by IEF and SDS gel electrophoresis (Suppl Fig 2), the expected shifts in pI and molecular size were observed. The 111In (Fig 2, green trace) and 125I radiolabeled products were purified on Superdex 75, the major peak collected and biodistribution studies performed. Inspection of the chromatogram and comparison to non-PEGylated diabody reveal a shift in apparent molecular size.
Figure 2. Size exclusion chromatography of radiolabeled non-PEGylated AVP04-07 diabody and its PEG conjugates.
All samples were radiolabeled with 111In and run on a Superdex 75 size exclusion column as described in Methods (radioactivity in arbitrary units). Black: DOTA- non-PEGylated diabody conjugate. Red: DOTA-Cys-VS-PEG3400-diabody conjugate. Green: DOTA-PEG27-Cys-VS-diabody conjugate. Blue: DOTA-PEG12-Cys-VS-diabody conjugate.
Biodistribution results are shown in Fig 3A-B. Despite the lower molecular weight of the PEG27 derivative compared to the PEG3400 derivative, nearly equivalent results were obtained (compare with Fig 1C). At 24 h, kidney uptake of 111In-labeled conjugate was 8.3 %ID/g, tumor uptake 49 %ID/g, and the tumor to blood ratio 4.2:1. Given the encouraging reduction in kidney uptake for the PEG27 derivative, we decided to synthesize and test the analogous PEG12 derivative.
Figure 3. Biodistributions of DOTA-PEG27-Cys-VS-AVP04-07 and DOTA-PEG12-Cys-VS-AVP04-07.
Biodistributions were measured in athymic mice bearing LS-174T xenografts as described in Methods. A. 111In-labeled PEG27 conjugate. B. 125I-labeled PEG27 conjugate. C. 111In-labeled PEG12 conjugate. D. 125I-labeled PEG12 conjugate.
Synthesis and biodistribution of monodispersed PEG12 AVP04-07
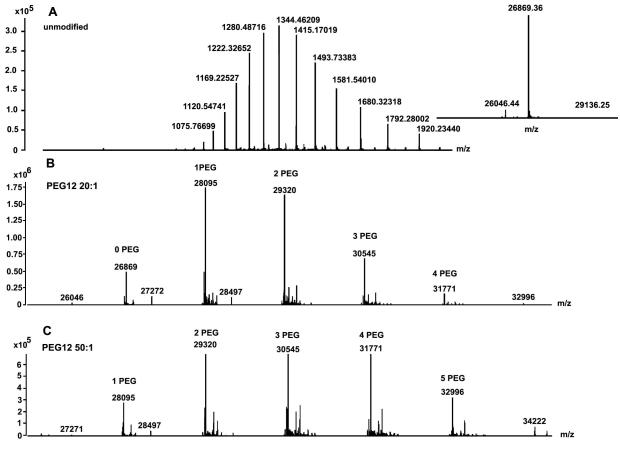
We synthesized DOTA-PEG12-Cys-VS (Suppl Scheme 1) and conjugated it to AVP04-07 at pH 9.0 using molar ratios of 20:1 and 50:1. Unmodified AVP04-07 and the two conjugates were analyzed by high-resolution nanospray mass spectrometry to determine their degrees of substitution (Fig 4A-C). The unmodified diabody gave a series of m/z species that, when deconvoluted, had a calculated mass of 26,869 (Fig 4A), in good agreement with that predicted by the amino acid sequence. For conjugation to DOTA-PEG12-Cys-VS at a molar ratio of 20:1, the deconvoluted peaks all differ from each other by multiples of 1225 mass units (Fig 4B), as expected for conjugation with DOTA-PEG12-Cys-VS. The mass differences are the same for the 50:1 molar ratio conjugation, but are shifted to a higher degree of substitution (Fig 4C). Using peak heights as an estimate, we calculate an average of 1.7 PEGs per monomer for the 20:1 conjugate, and an average of 3.0 PEGs for the 50:1 conjugate. When each conjugate was test labeled with 111In (178 mBq/mg), the 20:1 conjugate gave 8.3% incorporation and the 50:1 92% incorporation of radiolabel.
Figure 4. High resolution nanospray mass spectrometry analysis of PEG12 AVP04-07 conjugates.
A. Raw mass spectrum for non-PEGylated diabody (inset: deconvoluted spectrum). B. Deconvolved spectrum for PEG12 conjugate 20:1. C. Deconvolved spectrum for PEG12 conjugate 50:1.
Biodistribution data for 111In- and 125I-labeled DOTA-PEG12-Cys-VS-AVP04-07 were similar to those for the PEG3400 and PEG27 derivatives (Fig 3C-D). Notably, kidney uptake was initially higher (13.4 %ID/g) for the PEG12 than the PEG27 conjugate, but fell to lower levels (6.1 %ID/g) by 96 h, while tumor uptake and blood clearance are nearly identical for PEG12 and PEG27 conjugates.
64Cu PET imaging of DOTA-PEG conjugates of AVP04-07
PET imaging of 64Cu-labeled AVP04-07 (Fig 5A) demonstrated relatively modest tumor uptake and very high kidney uptake over 2 days for the non-PEGylated diabody. In contrast, the PEG12 and 27 conjugates showed relatively little kidney uptake throughout and high tumor uptake as early as 21-22h (Fig 5B-C). The measured tumor uptake for PEG12 was 49 ± 3 %ID/g at 46 h with a tumor to blood ratio of (9 ± 4):1. These results are similar to those for the 111In-labeled conjugate and suggest that choice of radiometal has little effect on the biodistribution, an observation we made previously for an anti-CEA diabody (16).
Figure 5. Serial PET imaging of 64Cu AVP04-07 and PEGylated AVP04-07 in athymic mice peripherally xenografted with TAG-72 expressing LS-174T colon tumors.
Images are PET anterior-view maximum intensity projections (MIPs) normalized to reflect radiodecay-corrected relative image intensity per unit injected activity. Labels in red, green, yellow and turquoise respectively show %ID/g in blood (heart), liver, kidney and tumor as measured by direct assay following the final scan. A: 64Cu-AVP04-07. B: 64Cu-PEG12 AVP04-07 C: 64Cu PEG27 AVP04-07.
Comparative Imaging Characteristics of 64Cu- and 111In-Labeled AVP04-07and Its DOTA-PEG conjugates
In order to compare different PEGylated versions of AVP04-07 radiolabeled with either 111In or 64Cu, we examined two parameters- (a) absolute tumor uptake and (b) tumor to non-tumor ratios using an Imaging Figure of Merit (23). The biodistribution data were also replotted on the same graph for comparisons (Suppl Fig 3). The PEGylated versions outperform the non-PEGylated diabody in terms of both absolute tumor uptake and tumor to blood ratios for both 111In and 64Cu. The IFOM plots presented in Suppl Fig 4 predict best imaging with either the 64Cu-labeled non-PEGylated diabody at 10 h or the 64Cu-labeled PEG12 version at 20 h. With 111In, imaging is optimal with the PEG12 conjugate at 30-40 h. However, when the background tissue is kidney, non-PEGylated diabody shows negative contrast out to 50 h due to high renal uptake and retention of radiolabel. All PEGylated diabodies had similar image quality through 50 h with a slight edge to the PEG27 conjugate when imaged at 10-15 h for 64Cu and 20-30 h for 111In.
DISCUSSION
The search for an effective radiolabeled antibody based imaging agent has focused on antibody fragments for their high tumor to blood ratios and on PET imaging for its high sensitivity and quantitative results. We chose the diabody construct because it is the smallest bivalent recombinant antibody that can be produced directly in large quantities (24). However, if renal clearance of diabody is too rapid, the absolute tumor uptake will be low. In the case of radiometals such as 64Cu where tumor retention is greater than for radioiodine labels, its advantage is offset by prolonged kidney retention. Use of 124I (5) avoids the kidney uptake issue due to metabolism and redistribution, but at the expense of lower tumor uptake.
In this study our goal was to optimize the clearance parameters for 64Cu which has an appropriate half-life (12 h), comparable to that of the diabody itself. PEGylation of diabody was selected because it has biodistributions similar to whole IgG or F(ab’)2 fragments but has the advantages that it is engineered from a single gene rather than two and does not need to be treated with a protease to reduce its molecular size. We previously approached this problem by conjugating a DOTA-PEG3400 derivative to an anti-CEA diabody, and although kidney uptake was reduced from 200 to 50 %ID/g at 24 h, we were unable to lower it to the levels (10-12 %ID/g at 24-48 h) seen with intact anti-CEA IgG (21, 25). Factors that could explain the remaining high kidney uptake included the pI of the diabody (17, 18), the effect of binding to circulating antigen, or some unforeseen result of the conjugation chemistry. To gain insight into the problem, we selected a different diabody that, like anti-CEA diabody, shows strong tumor targeting in athymic mice bearing LS-174T xenografts. The anti-TAG-72 antibody CC49 and a diabody derivative of CC49 have been previously described (26-29) and CC49 intact IgG has been used in many clinical studies (25, 30, 31), making CC49 a good choice for a comparative study. Using an hexahistidine-tagged, E. coli produced version of the CC49 diabody, namely AVP04-07, we found that an absolute tumor uptake in the LS-174T mouse model at 24 h of 25 %ID/g (Fig 1A), superior to that previously reported for a (scFv)2 version of CC49 (4 %ID/g) (32).
For purposes of comparison, our first PEG construct (DOTA-Cys-VS-PEG3400) was identical to the one used with anti-CEA diabody (16). DOTA-Cys-VS-PEG3400 is a linear, bifunctional, polydispersed PEG that attaches to amino groups of diabodies via an active ester at one end and to DOTA-Cys via vinyl sulfone at the other end. In contrast to anti-CEA diabody, kidney uptake was significantly lower (10-12 %ID/g) than normally seen for intact IgGs. It is unlikely that the reduction in kidney uptake was due either to the conjugation chemistry or the nature of the PEG agent (identical in both the anti-CEA and anti-CC49 cases); however, the reduction in kidney uptake could be due to either the final pI of the DOTA-PEG-diabody conjugate or binding to circulating antigen. Regarding the pI, both antibodies have a net negative charge after conjugation. Although negatively charged proteins are cleared less rapidly by the kidney compared to positively charged proteins (18), the difference in pIs between the anti-CEA and anti-CC49 diabodies seems insufficient to account for their rather large differences in kidney uptake. Likewise, the role of circulating antigen in the kidney seems an unlikely explanation. While it is well known that CEA is cleared rapidly by Kupffer cells in the liver (33), thus explaining the accumulation of 111In-DOTA-anti-CEA IgG in liver (34, 35), there is no obvious connection to the kidney. While less is known about clearance of TAG-72, this high molecular weight acidic mucin is, like CEA, released into circulation and its binding to diabody may be expected to protect the diabody from kidney filtration. The main argument against this idea is that only the PEGylated diabody has reduced kidney accumulation. Thus, further studies will have to be performed to determine the mechanism(s) causing the observed differences between the PEGylated anti-CEA and PEGylated anti-TAG-72 diabodies.
Although PEGylation is an attractive method for reducing kidney accumulation, over-substitution may inhibit immunoreactivity (10-12, 36). In the case of anti-CEA diabody, it was necessary to limit the number of PEG3400s to about one per monomer (16). This fact, plus the polydispersed nature of PEG3400, prompted us to evaluate the effect of smaller DOTA-PEGs on kidney uptake. In the case of DOTA-PEG12-Cys-VS-diabody, the degree of substitution was 3 PEG moieties per monomer as determined by high-resolution nanospray mass spectrometry. Notably, the biodistribution results for 111In-labeled PEG12 conjugate were similar to both the PEG3400 and PEG27 conjugates, indicating that even PEGs of modest molecular size are efficient in reducing kidney uptake.
Based on the satisfactory biodistribution results with 111In, we went on to demonstrate high quality PET imaging of tumors both with 64Cu-labeled PEG12 and PEG27 conjugates by 24 h, a time point that is well matched with the half-lives of both blood clearance and radionuclide.
All of the DOTA-PEG-diabody conjugates had dramatically reduced kidney uptake compared to non-PEGylated diabody and were similar among themselves with regard to biodistribution (Fig 1 and Suppl Fig 3). In order to further refine the comparison among the derivatives, we employed a previously developed Imaging Figure of Merit (4, 16, 23, 37). The analysis indicates modest advantages for the PEG12 conjugate when tumor-to-blood ratio is of primary concern, and for the PEG27 conjugate when tumor-to-kidney contrast is the chief criterion. Based on their excellent tumor and minimal kidney uptakes, we predict that these monodispersed DOTA-PEGylated diabody conjugates will be suitable for human imaging of TAG-72-positive tumors. Further, we suggest that PEGylated diabodies may be an ideal platform for delivering other conjugated payloads to tumor.
Supplementary Material
Supplemental Figure 1. Characterization of AVP04-07 Diabody. AVP04-07 expressed in E. coli was purified to homogeneity as indicated by a single elution peak on Superdex 200 gel filtration (A) and was further shown to be both dimeric and monodispersed by analytical ultracentrifugation (B), with an apparent molecular weight of 52 kDa. AVP04-07 diabody was able to bind antigen in vitro as shown by reduced elution time (i.e.; increases in apparent molecular weight) of diabody-antigen complexes (C, black solid line) when compared to AVP04-07 alone (C, grey dotted line) and antigen alone (C, gray solid line).
Supplemental Figure 2. Isoelectric focusing and SDS gel electrophoresis of AVP04-07 and its PEG conjugates. A. Isoelectric focusing: 1, non-PEGylated diabody; 2, PEG27 conjugate, 50:1 (ratio of PEGylating agent to diabody); 3, PEG12 conjugate, 20:1 (ratio of PEGylating agent to diabody); 4, PEG12 conjugate, 50:1 (ratio of PEGylating agent to diabody); 5, PEG3400 conjugate, 30:1 (ratio of PEGylating agent to diabody). B. SDS gel electrophoresis: 1, intact diabody; 2, PEG27 conjugate; 3, PEG12 conjugate; 4, PEG12 conjugate; 5, PEG3400 conjugate.
Supplemental Figure 3. Comparative blood, tumor, kidney and liver curves for 111In-DOTA labeled PEGylated diabody derivatives and intact diabody. A. Blood clearance curves. B. Tumor uptake curves. C. Kidney uptake curves. D. Liver uptake curves. Red: intact diabody. Magenta: PEG3400 diabody conjugate, 15:1 molar ratio. Black: PEG3400 diabody conjugate, 60:1 molar ratio. Blue: PEG27 diabody conjugate. Green: PEG12 diabody conjugate.
Supplemental Fig 4. IFOM plots for tumor vs. blood and tumor vs. kidney for non-PEGylated diabody and its PEGylated conjugates. A. Tumor vs. Blood, 64Cu-labeled AVP04-07 and its derivatives. B. Tumor vs. kidney, 64Cu-labeled AVP04-07 and its derivatives. C. Tumor vs. blood, 111In-labeled AVP04-07 and its derivatives. D. Tumor vs. kidney, 111In-labeled AVP04-07 and its derivatives. Dark Blue: unmodified diabody. Light Blue: PEG3400 diabody conjugate. Yellow: PEG27 diabody conjugate. Magenta: PEG12 diabody conjugate.
Supplemental Scheme 1. Synthesis of DOTA-PEG-Cys-VS. FMOC-amido-PEG-acid was conjugated to S-t-butyl cysteine on Wang resin using standard activation chemistry (DCC/HOBt). The FMOC was removed with piperazine and conjugated to DO3AtBuAc using standard activation chemistry (DCC/HOBt). The product was removed from the resin with TFA, purified by reverse phase HPLC, reacted with excess vinyl sulfone in DMF, and repurified by reverse phase HPLC.
REFERENCES
- 1.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008 May-Jun;14(3):191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 2.Williams LE, Wu AM, Yazaki PJ, et al. Numerical selection of optimal tumor imaging agents with application to engineered antibodies. Cancer Biother Radiopharm. 2001 Feb;16(1):25–35. doi: 10.1089/108497801750095989. [DOI] [PubMed] [Google Scholar]
- 3.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005 Sep;23(9):1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 4.Williams LE, Lopatin G, Kaplan DD, Liu A, Wong JY. Update on Selection of Optimal Radiopharmaceuticals for Clinical Trials. Cancer Biother Radiopharm. 2008 Dec 27; doi: 10.1089/cbr.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundaresan G, Yazaki PJ, Shively JE, et al. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med. 2003 Dec;44(12):1962–1969. [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SW, Li L, Williams LE, Anderson A-L, Raubitschek AA, Shively JE. Metabolism and renal clearance of 111In-labeled DOTA conjugated antibody fragments. Bioconj Chem. 2001;12:264–270. doi: 10.1021/bc0000987. [DOI] [PubMed] [Google Scholar]
- 7.Olafsen T, Kenanova VE, Sundaresan G, et al. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005 Jul 1;65(13):5907–5916. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998 Feb;25(2):201–212. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Olafsen T, Anderson AL, Wu A, Raubitschek AA, Shively JE. Reduction of kidney uptake in radiometal labeled peptide linkers conjugated to recombinant antibody fragments. Site-specific conjugation of DOTA-peptides to a Cys-diabody. Bioconjug Chem. 2002 Sep-Oct;13(5):985–995. doi: 10.1021/bc025565u. [DOI] [PubMed] [Google Scholar]
- 10.Yang K, Basu A, Wang M, et al. Tailoring structure-function and pharmacokinetic properties of single-chain Fv proteins by site-specific PEGylation. Protein Eng. 2003 Oct;16(10):761–770. doi: 10.1093/protein/gzg093. [DOI] [PubMed] [Google Scholar]
- 11.Delgado C, Pedley RB, Herraez A, et al. Enhanced tumour specificity of an anti-carcinoembrionic antigen Fab’ fragment by poly(ethylene glycol) (PEG) modification. Br J Cancer. 1996 Jan;73(2):175–182. doi: 10.1038/bjc.1996.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LS, Conover C, Shi C, Whitlow M, Filpula D. Prolonged circulating lives of single-chain Fv proteins conjugated with polyethylene glycol: a comparison of conjugation chemistries and compounds. Bioconjug Chem. 1999 Nov-Dec;10(6):973–981. doi: 10.1021/bc990076o. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht H, Denardo GL, Denardo SJ. Development of anti-MUC1 di-scFvs for molecular targeting of epithelial cancers, such as breast and prostate cancers. Q J Nucl Med Mol Imaging. 2007 Dec;51(4):304–313. [PubMed] [Google Scholar]
- 14.Stork R, Campigna E, Robert B, Muller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem. 2009 Sep 18;284(38):25612–25619. doi: 10.1074/jbc.M109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong SR, DeForge L, Presta L, et al. Adapting pharmacokinetic properties of a humanized anti-interleukin-8 antibody for therapeutic applications using site-specific pegylation. Cytokine. 2001 Nov 7;16(3):106–119. doi: 10.1006/cyto.2001.0936. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Yazaki PJ, Anderson AL, et al. Improved biodistribution and radioimmunoimaging with poly(ethylene glycol)-DOTA-conjugated anti-CEA diabody. Bioconjug Chem. 2006 Jan-Feb;17(1):68–76. doi: 10.1021/bc0502614. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Le N, Kim IS, et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 1999 Jan 15;59(2):422–430. [PubMed] [Google Scholar]
- 18.Kim I, Kobayashi H, Yoo TM, et al. Lowering of pI by acylation improves the renal uptake of 99mTc-labeled anti-Tac dsFv: effect of different acylating reagents. Nucl Med Biol. 2002 Nov;29(8):795–801. doi: 10.1016/s0969-8051(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Bading J, Yazaki PJ, et al. A versatile bifunctional chelate for radiolabeling humanized anti-CEA antibody with In-111 and Cu-64 at either thiol or amino groups: PET imaging of CEA-positive tumors with whole antibodies. Bioconjug Chem. 2008 Jan;19(1):89–96. doi: 10.1021/bc700161p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis MR, Shively JE. Maleimidocysteineamido-DOTA derivatives: new reagents for radiometal chelate conjugation to antibody sulfhydryl groups undergo pH-dependent cleavage reactions. Bioconj Chem. 1998;9:72–86. doi: 10.1021/bc970136v. [DOI] [PubMed] [Google Scholar]
- 21.Yazaki PJ, Wu AM, Tsai SW, et al. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001 Mar-Apr;12(2):220–228. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Tsai SW, Anderson AL, Keire DA, Raubitschek AA, Shively JE. Vinyl sulfone bifunctional derivatives of DOTA allow sulfhydryl- or amino-directed coupling to antibodies. Conjugates retain immunoreactivity and have similar biodistributions. Bioconjug Chem. 2002 Jan-Feb;13(1):110–115. doi: 10.1021/bc015535b. [DOI] [PubMed] [Google Scholar]
- 23.Williams LE, Liu A, Wu AM, et al. Figures of merit (FOMs) for imaging and therapy using monoclonal antibodies. Med Phys. 1995 Dec;22(12):2025–2027. doi: 10.1118/1.597646. [DOI] [PubMed] [Google Scholar]
- 24.Yazaki PJ, Shively L, Clark C, et al. Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J Immunol Methods. 2001 Jul 1;253(1-2):195–208. doi: 10.1016/s0022-1759(01)00388-x. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez RD, Huh WK, Khazaeli MB, et al. A Phase I study of combined modality (90)Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002 Sep;8(9):2806–2811. [PubMed] [Google Scholar]
- 26.Santos AD, Kashmiri SV, Hand PH, Schlom J, Padlan EA. Generation and characterization of a single gene-encoded single-chain-tetravalent antitumor antibody. Clin Cancer Res. 1999 Oct;5(10 Suppl):3118s–3123s. [PubMed] [Google Scholar]
- 27.Chauhan SC, Jain M, Moore ED, et al. Pharmacokinetics and biodistribution of 177Lu-labeled multivalent single-chain Fv construct of the pancarcinoma monoclonal antibody CC49. Eur J Nucl Med Mol Imaging. 2005 Mar;32(3):264–273. doi: 10.1007/s00259-004-1664-0. [DOI] [PubMed] [Google Scholar]
- 28.Wittel UA, Jain M, Goel A, Chauhan SC, Colcher D, Batra SK. The in vivo characteristics of genetically engineered divalent and tetravalent single-chain antibody constructs. Nucl Med Biol. 2005 Feb;32(2):157–164. doi: 10.1016/j.nucmedbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Goel A, Baranowska-Kortylewicz J, Hinrichs SH, et al. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv’s: novel imaging agents for rapid in vivo localization of human colon carcinoma. J Nucl Med. 2001 Oct;42(10):1519–1527. [PubMed] [Google Scholar]
- 30.Tempero M, Leichner P, Baranowska-Kortylewicz J, et al. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000 Aug;6(8):3095–3102. [PubMed] [Google Scholar]
- 31.Meredith RF, Bueschen AJ, Khazaeli MB, et al. Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med. 1994 Jun;35(6):1017–1022. [PubMed] [Google Scholar]
- 32.Beresford GW, Pavlinkova G, Booth BJ, Batra SK, Colcher D. Binding characteristics and tumor targeting of a covalently linked divalent CC49 single-chain antibody. Int J Cancer. 1999 Jun 11;81(6):911–917. doi: 10.1002/(sici)1097-0215(19990611)81:6<911::aid-ijc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Gangopadhyay A, Bajenova O, Kelly TM, Thomas P. Carcinoembryonic antigen induces cytokine expression in Kuppfer cells: implications for hepatic metastasis from colorectal cancer. Cancer Res. 1996 Oct 15;56(20):4805–4810. [PubMed] [Google Scholar]
- 34.Gangopadhyay A, Thomas P. Processing of carcinoembryonic antigen by Kupffer cells: recognition of a penta-peptide sequence. Arch Biochem Biophys. 1996 Oct 1;334(1):151–157. doi: 10.1006/abbi.1996.0440. [DOI] [PubMed] [Google Scholar]
- 35.Jessup JM, Petrick AT, Toth CA, et al. Carcinoembryonic antigen: enhancement of liver colonisation through retention of human colorectal carcinoma cells. Br J Cancer. 1993 Mar;67(3):464–470. doi: 10.1038/bjc.1993.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natarajan A, Xiong CY, Albrecht H, DeNardo GL, DeNardo SJ. Characterization of site-specific ScFv PEGylation for tumor-targeting pharmaceuticals. Bioconjug Chem. 2005 Jan-Feb;16(1):113–121. doi: 10.1021/bc0498121. [DOI] [PubMed] [Google Scholar]
- 37.Cai W, Olafsen T, Zhang X, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007 Feb;48(2):304–310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Characterization of AVP04-07 Diabody. AVP04-07 expressed in E. coli was purified to homogeneity as indicated by a single elution peak on Superdex 200 gel filtration (A) and was further shown to be both dimeric and monodispersed by analytical ultracentrifugation (B), with an apparent molecular weight of 52 kDa. AVP04-07 diabody was able to bind antigen in vitro as shown by reduced elution time (i.e.; increases in apparent molecular weight) of diabody-antigen complexes (C, black solid line) when compared to AVP04-07 alone (C, grey dotted line) and antigen alone (C, gray solid line).
Supplemental Figure 2. Isoelectric focusing and SDS gel electrophoresis of AVP04-07 and its PEG conjugates. A. Isoelectric focusing: 1, non-PEGylated diabody; 2, PEG27 conjugate, 50:1 (ratio of PEGylating agent to diabody); 3, PEG12 conjugate, 20:1 (ratio of PEGylating agent to diabody); 4, PEG12 conjugate, 50:1 (ratio of PEGylating agent to diabody); 5, PEG3400 conjugate, 30:1 (ratio of PEGylating agent to diabody). B. SDS gel electrophoresis: 1, intact diabody; 2, PEG27 conjugate; 3, PEG12 conjugate; 4, PEG12 conjugate; 5, PEG3400 conjugate.
Supplemental Figure 3. Comparative blood, tumor, kidney and liver curves for 111In-DOTA labeled PEGylated diabody derivatives and intact diabody. A. Blood clearance curves. B. Tumor uptake curves. C. Kidney uptake curves. D. Liver uptake curves. Red: intact diabody. Magenta: PEG3400 diabody conjugate, 15:1 molar ratio. Black: PEG3400 diabody conjugate, 60:1 molar ratio. Blue: PEG27 diabody conjugate. Green: PEG12 diabody conjugate.
Supplemental Fig 4. IFOM plots for tumor vs. blood and tumor vs. kidney for non-PEGylated diabody and its PEGylated conjugates. A. Tumor vs. Blood, 64Cu-labeled AVP04-07 and its derivatives. B. Tumor vs. kidney, 64Cu-labeled AVP04-07 and its derivatives. C. Tumor vs. blood, 111In-labeled AVP04-07 and its derivatives. D. Tumor vs. kidney, 111In-labeled AVP04-07 and its derivatives. Dark Blue: unmodified diabody. Light Blue: PEG3400 diabody conjugate. Yellow: PEG27 diabody conjugate. Magenta: PEG12 diabody conjugate.
Supplemental Scheme 1. Synthesis of DOTA-PEG-Cys-VS. FMOC-amido-PEG-acid was conjugated to S-t-butyl cysteine on Wang resin using standard activation chemistry (DCC/HOBt). The FMOC was removed with piperazine and conjugated to DO3AtBuAc using standard activation chemistry (DCC/HOBt). The product was removed from the resin with TFA, purified by reverse phase HPLC, reacted with excess vinyl sulfone in DMF, and repurified by reverse phase HPLC.