Abstract
The safety of artemether-lumefantrine in patients with acute, uncomplicated Plasmodium falciparum malaria was investigated prospectively using the auditory brainstem response (ABR) and pure-tone thresholds. Secondary outcomes included polymerase chain reaction-corrected cure rates. Patients were randomly assigned in a 3:1:1 ratio to either artemether-lumefantrine (N = 159), atovaquone-proguanil (N = 53), or artesunate-mefloquine (N = 53). The null hypothesis (primary outcome), claiming that the percentage of patients with a baseline to Day-7 ABR Wave III latency increase of > 0.30 msec is ≥ 15% after administration of artemether-lumefantrine, was rejected; 2.6% of patients (95% confidence interval: 0.7–6.6) exceeded 0.30 msec, i.e., significantly below 15% (P < 0.0001). A model-based analysis found no apparent relationship between drug exposure and ABR change. In all three groups, average improvements (2–4 dB) in pure-tone thresholds were observed, and polymerase chain reaction-corrected cure rates were > 95% to Day 42. The results support the continued safe and efficacious use of artemether-lumefantrine in uncomplicated falciparum malaria.
Introduction
Malaria is one of the most significant global health problems, particularly as resistance to older antimalarial drugs is increasing. In 2006, the World Health Organization (WHO) recommended the use of artemisinin-based combination therapy (ACT), such as artemether-lumefantrine (A-L), as the first-line treatment of uncomplicated Plasmodium falciparum malaria in all endemic areas.1 In 2004, A-L (Coartem) became the first fixed-dose ACT to be prequalified by the WHO, and received approval from the U.S. Food and Drug Administration in 2009. Currently, it is registered in almost 90 countries worldwide2; however, concerns have been raised about the possibility of artemisinin-related neurotoxicity affecting hearing. In some animal models, the brainstem structures involved in auditory processing and vestibular reflexes may be adversely affected under certain dosing regimens of artemisinin derivatives.3–5 Several clinical and pathological studies have attempted to determine whether similar adverse effects occur in humans exposed to artemisinins,6–8 but no evidence indicating brainstem lesions has been found to date. Rapid absorption and elimination with oral drug administration and the short duration of treatment at therapeutic doses likely would not favor neurotoxic effects of artemisinin derivatives.
One retrospective longitudinal study reported pure-tone threshold changes in construction site workers from Mozambique who had been treated for malaria with A-L, versus a comparison group who had not had malaria or been treated for malaria.9 However, interpretation of the results was confounded by the lack of statistical correction for multiple comparisons, occupational noise exposure, and possible effects of malaria on hearing. In addition, the design was not prospective and lacked active controls.
The study of drug-related damage to the auditory system is a relatively new area of research, and audiological testing has not been a routine part of assessing drug safety. Against this background, we performed a randomized, prospective study to confirm the safety and efficacy of A-L in patients with uncomplicated P. falciparum malaria. The primary aim was to assess the safety of A-L based on auditory brainstem response (ABR) and pure-tone threshold findings. Secondary outcomes included Day 28 and Day 42 polymerase chain reaction (PCR)-corrected cure rates.
Methods
Study population.
The study was performed between May 2007 and November 2008 in Tumaco, state of Nariño, a city on the Colombian Pacific coast where malaria is endemic. Patients, at least 12 years of age, with microscopically confirmed acute uncomplicated P. falciparum malaria or mixed infection (parasite density between 1,000 and 100,000/μL blood) and a history of fever were eligible to enter the study. Patients were excluded if they showed signs and symptoms indicative of severe or complicated malaria according to WHO criteria.10 Patients who did not have normal hearing or met specific audiological exclusion criteria (Table 1) were also excluded. Other exclusion criteria included pregnancy or breastfeeding, abnormal cardiac function, either known congenital or known acquired prolongation of the QTc interval, any other clinical condition, or were using any drugs known to influence cardiac function, history of serious side-effects related to any of the study drugs or related compounds, serious underlying disease, history of psychiatric disorders, convulsion or splenectomy, presence of severe vomiting, or any other medical or physical condition that could interfere with the study objectives.
Table 1.
Clinical trial audiological exclusion criteria
| Ingestion within the previous 2 months: Mefloquine, aminoglycoside antibiotics, halofantrine, artemether-lumefantrine. |
| Ingestion within the previous 2 weeks: Quinine, chloroquine, any other antimalarial drug, aspirin, loop diuretics, macrolide antibiotics. |
| History of any drug-related hearing impairment or prior middle or inner ear surgery. |
| Current ear infection or discharge. |
| Untreatable impaction of cerumen with occlusion of the external auditory canal. |
| Currently wearing a hearing aid. |
| Abnormal tympanometry in either or both ears defined as Type B (“flat”) or Type C (“negative”) with peak ear pressure more negative than −120 dPA; a static compliance outside the range of 0.3–1.4 mL was considered abnormal. |
| Abnormal audiogram: Pure-tone air conduction thresholds in either or both ears (≥ 25 dB at 3 contiguous frequencies) or aural asymmetry (≥ 25 dB right-left difference at any frequency or ≥ 20 dB right-left difference at 2 contiguous frequencies or ≥ 15 dB right-left difference at 3 contiguous frequencies). |
| Abnormal auditory brainstem response: Absence of identifiable, measurable waveforms (Wave I, III, and V) after 2 replicable runs in either or both ears or greater than 0.20 msec difference between the right and left ear Wave V latencies. |
| Unable to cooperate with testing. |
Before enrollment, patients gave written informed consent to participate after being informed about the study and its risks and benefits. For adolescents, written consent was obtained from the parents or legal guardians. The trial protocol was approved by local institutional review boards (Comité Corporativo de Etica en Investigación, Fundación Santa Fe de Bogotá, Bogotá; Comité de Etica Médica, Hospital San Andrés, Tumaco; Instituto Nacional de Vigilancia de Medicamentos y Alimentos, Bogotá). This study is registered with ClinicalTrials.gov as NCT00444106.
Study design, treatments, and procedures.
This was an open-label (i.e., investigator and patients were unblinded), randomized, single-center, parallel group study. Key personnel involved in assessments of the primary objective were blinded. Patients were randomly assigned in a 3:1:1 ratio to receive either A-L (Coartem, Novartis, Basel, Switzerland) administered twice daily for 3 days, atovaquone-proguanil (A-P; Malarone, GlaxoSmithkline, Harlow, UK) given once daily for 3 days, or artesunate plus mefloquine (A-M; free combination of Plasmotrim and Mephaquin, Mepha, Aesch, Switzerland) taking artesunate once daily for 3 days with mefloquine on Days 2 and 3. Patients took their study medication hospitalized under supervised conditions together with chocolate milk. The study participants were dosed according to their body weight:
-
•
Artemether-Lumefantrine: 40 mg of artemether and 240 mg of lumefantrine (15–24 kg) or 60 mg/360 mg (25–34 kg) or 80 mg/480 mg (≥ 35 kg) at 0, 8, 24, 36, 48, and 60 hours.
-
•
Atovaquone-Proguanil: 250 mg of atovaquone and 100 mg of proguanil (11–20 kg) or 500 mg/200 mg (21–30 kg) or 750 mg/300 mg (31–40 kg) or 1000 mg/400 mg (> 40 kg) once daily.
-
•
Artesunate plus mefloquine: 4 mg/kg/day of artesunate and 25 mg/kg of mefloquine (15 mg/kg on Day 2 and 10 mg/kg on the Day 3) administered on a once daily regimen.
If vomiting occurred within 30 minutes after intake, the entire dose was to be administered again. If vomiting occurred between 30 and 60 minutes then half of the dose was replaced. Patients unable to tolerate the study medication after three attempts had their medication changed. In case of premature discontinuation of study medication, the patient stayed in the study and follow-up visits for efficacy were to be performed according to the scheduled study visits. Patients developing severe malaria or danger signs10 were to be hospitalized with subsequent administration of rescue therapy. Rescue therapy according to local treatment guidelines was also to be administered to subjects with early or late treatment failures11 and in a case of intolerance to trial drugs. Administration of primaquine in patients diagnosed with gametocytes was not recommended before study Day 7.
Patients were enrolled consecutively and admitted to the study center for the 3 treatment days, and then followed up on an outpatient basis until study Day 42 (i.e., site visits on Days 7, 14, 28, and 42). Audiological assessments were made at baseline (before initiation of treatment), Day 3 (1 hour after last dose of study medication), and at Days 7, 28, and 42. Patients who discontinued the study prematurely had a final audiological examination at study termination. Vital signs and hematology parameters were assessed at baseline, during hospitalization, and each follow-up visit. At every follow-up visit, potential adverse events (AEs) were assessed for severity and association with study medication. A Data Safety Monitoring Board (DSMB) was established to review accumulating auditory brainstem response and pure-tone threshold data as well as relevant AE data to determine if there is a signal of elevated risk of any nature based on safety data. The DSMB included a malaria expert, an expert in clinical trials, and an otologist, who is an expert in audiological assessments.
Giemsa-stained thick blood films were examined before initiation of treatment, and then daily until negative or at least up to Day 7, and then at each follow-up visit. Readings were performed locally. Two qualified microscopists independently read the slides. To distinguish between recrudescence and recurrence, blood samples for PCR analysis were collected from every patient at baseline and on Days 14, 28, and 42. The PCR determinations were processed centrally on all recurrent parasitemia using a standard protocol.12 Patients without paired PCR results or ambiguous results were classified as treatment failures. Malaria cure was defined as clearance of asexual parasitemia within 7 days of initiation of trial treatment, without subsequent recrudescence (i.e., by Day 14, 28, or 42).
Audiological tests.
The audiological battery included otoscopy, tympanometry, pure-tone air conduction thresholds, and ABR. All equipment was calibrated to the appropriate American National Standards Institute (ANSI) specifications. The audiological evaluations were performed in a quiet test environment at < 60 dB external noise. First, the audiological technician performed otoscopy using a standard medical otoscope with disposable tips. Any cerumen noted was removed before continuing the evaluation. After otoscopic examination, tympanometry was performed. A probe tone was presented while varying the air pressure in the external ear canal. The mobility of the tympanic membrane was evaluated by observing the amplitude of the compliance peak. A clinical audiometer with insert earphones was used to obtain air conduction thresholds in both ears (250, 500, 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hz) using the modified Hughson-Westlake ascending procedure.13 The strict auditory inclusion criteria and complete audiological examination before obtaining ABR waveforms were designed to exclude patients with other auditory factors that contribute to changes in ABR latencies. The fact that patients had a normal auditory response contributes to the strength of the study and to conclusions based on the ABR waveform latency changes.
ABR testing was performed using a validated clinical auditory evoked potential system (Bio-Logic AEP, Bio-Logic Systems Corp., Mundelein, IL). Care was taken to avoid external noise or electrical/magnetic interference with the ABR recordings. Patients were asked to lie on a flat couch and rested for 5 minutes before testing was initiated. Gold surface electrodes were applied to the vertex, both mastoids and the forehead. Electrode impedance was maintained at ≤ 3,000 ohms per electrode and ≤ 1,000 ohms between electrodes. A rarefaction click-stimulus delivered by insert earphones at 21.1 clicks/second at 70 dB nHL was used to elicit the auditory brainstem response. The contralateral ear was masked using white noise at 40 dB nHL. At least two complete passes per ear were made to ensure replicability of the waveforms. Waveform analysis and labeling of Waves I, III, and V were performed by the audiological technician at the testing site, assisted by the Bio-Logic software peak picking algorithm. The latencies and placement of the peak markings for each of the waves were first reviewed by an audiologist in Bogotá, and a second review was done by an audiologist at the House Ear Institute (HEI), Los Angeles, CA. The House Ear Institute audiologist had access to the primary data collection files from the Bio-Logic software, enabling a detailed check on testing procedure. The HEI review also confirmed adherence to the testing protocol. Because of a potential unblinding issue during this initial review, a second independent review of ABR waveform latencies (Waves I, III, and V) assessed at baseline and Day 7 was performed under fully blinded conditions by an experienced ABR researcher from the University of California, Los Angeles, Division of Head and Neck Surgery. Patients with abnormal tympanograms or pure-tone thresholds (see Table 1 for definitions) were excluded from ABR analysis as were patients with inconclusive ABR tests (e.g., no measurable or reproducible waveforms).
The audiological technicians performing the audiological battery at the study site were trained by an experienced audiologist located in Colombia, who received ABR training at the House Ear Institute in advance of the study. The investigational site audiological testing area and testing techniques were also reviewed by the audiologist.
Drug levels and pharmacokinetic assessments.
Blood samples were taken from all patients in the A-L and A-M arms. For lumefantrine measurements, blood sampling occurred at pre-dose and in each of the following time windows: 0–48, 49–72, 73–120, and 121–240 hours after the first dose. For the determination of artemether and its active metabolite dihydroartemisinin (DHA) in plasma, a single blood sample was collected at 1 hour after the last A-L dose. Blood samples for the measurement of mefloquine were drawn at pre-dose and at 6, 14, 24, 38, 96, and 672 hours post first dose. A single blood sample was collected at 1 hour after the last A-M dose to quantify artesunate and DHA concentrations in plasma. All blood samples were taken by venipuncture into heparin-coated tubes. After centrifugation, aliquots of plasma were taken and frozen at −70°C until analysis. Drug plasma levels were determined independent of audiological test results.
Artemether and DHA were measured in plasma using a reversed-phase high-performance liquid chromatography (HPLC) with atmospheric pressure chemical ionization and mass spectrometry (MS) detection, with a limit of quantification (LOQ) of 5.0 ng/mL (with modifications from Souppart14). Lumefantrine was measured in plasma by HPLC with tandem mass spectrometry (MS/MS) detection; the LOQ was 50 ng/mL. The within-study assay validation showed an assay precision (percent coefficient of variation [%CV]) of 5.0% to 6.6%, with a deviation (bias) of −1.2% to 1.0% of nominal concentrations (0.1, 2.0, and 16.0 μg/mL). Mefloquine plasma concentrations were quantified by HPLC; the LOQ was 40 ng/mL15; the method used for the determination of artesunate in plasma was an LC/MS/MS method with a LOQ of 1 ng/mL. The within-study assay validation for this method showed an assay precision (%CV) of 5.6% to 7.0%, with a deviation (bias) of −8.1% to −1.5% of nominal concentrations (30, 160, and 800 ng/mL).
Pharmacokinetic parameters of lumefantrine were determined by a compartmental pharmacokinetic population model in the Nonlinear Mixed Effects Modeling (NONMEM) software (version 6). Descriptive statistics was used to summarize artemether, artesunate and DHA plasma concentrations. The pharmacokinetic parameters of mefloquine were computed using non-compartmental methods (WinNonlin Pro, Version 5.2, Pharsight Corporation, Mountain View, CA). The relationship between drug exposure and ABR change, and between exposure and body weight, was investigated by exploratory methods, linear regression analysis, and linear mixed models.
Study endpoints.
The primary objective of the trial was to demonstrate the safety of A-L in patients with acute, uncomplicated P. falciparum malaria by testing the null hypothesis that the percentage of patients with Day 7 ABR Wave III latency increases over baseline of > 0.30 msec is ≥ 15% in the A-L group. Secondary objectives were to assess the changes in pure-tone threshold and ABR Wave I and V latencies over the study course. Efficacy was another secondary objective of this study. The PCR-adjusted parasitological cure rates at Days 14, 28, and 42 were the key efficacy parameters. Exploratory endpoints included tolerability assessment of the three treatment regimens by analyzing incidence rates of AEs, determination of the relationship between audiological changes and drug exposure, and changes in auditory brainstem latencies with a non-ACT antimalarial (A-P) and another ACT combination (A-M).
Discussion on the study design and primary endpoint.
An open-label design was used because of the different dosing schedules of the three study treatments. To achieve blinding of the investigator and patients would have required patients in each treatment arm to be supplied with two sets of dummy medication in addition to their own active treatment, leading to a complicated dosing schedule and a considerable study medication burden. Because of the potential for high inter-center variability in administration of the audiological tests, only one study center with highly trained technicians was used. The addition of A-P and A-M arms allowed gathering information on the rate of abnormal ABR wave latencies for these drugs. These data may help to design potential new trials involving these antimalarials. Moreover, the addition of these two treatment arms assured blinding of the audiological technician. The ABR evaluation examines nerve conduction along the auditory pathways from the cochlea to the brainstem, and is the gold standard for detection of damage to the participating neural structures.16 The primary endpoint of the study was based on ABR Wave III latency, as it was expected to be the most sensitive parameter to detect any artemisinin derivative-induced toxicity.17,18 In the field of auditory evoked potentials, a change from baseline in an ABR Wave III peak latency in either or both ears of > 0.30 msec is widely considered to be clinically significant (not definitive, but usually prompting further evaluation) and potentially biologically significant in individual subjects. It is the most widely used latency change value in the clinical use of ABR for disease screening.19–22 Based on clinical and experimental experience, a 15% incidence rate of subjects showing such an ABR abnormality is considered to represent a clinically relevant rate for suspicion of systematic damage to the auditory brainstem (M. Don, HEI, CA, personal communication, November 2, 2004).
Statistical analysis.
The primary safety variable, the rate of patients with Day-7 ABR Wave III latency changes of > 0.30 msec (assessed by the fully blinded audiologist) was analyzed for the per-protocol (PP) population, defined as all randomized patients who took at least 80% of the entire recommended dose and had a valid baseline and Day-7 ABR Wave III latency evaluation, and did not use any medication having an ototoxic effect. The null hypothesis test was performed using a one-sided, exact test for a single proportion as provided in PROC FREQ of the Statistical Analysis System (SAS Institute, Inc., Cary, NC) with a level of significance at 5%. Supporting one-sample, two-sided 95% confidence intervals (CIs) and one-sample, one-sided (upper) 95% CIs were constructed on binomial data to determine the precision of the observed data based on the sample size. All one-sample CIs were based on the exact Pearson-Clopper limits. All asymptotic CIs were generated for informational purposes. The safety population included all patients who received at least one dose of study drug and had at least one post-baseline safety assessment.
To describe more accurately ABR changes in secondary analyses, latency shifts occurring in Wave V, and at other time points than Day 7 alone were taken into consideration. Both Waves III and V are thought to be generated primarily in the brainstem, whereas Wave I is thought to arise from the distal portion of the auditory nerve.16 A clinically relevant change in one ear was defined as an ABR latency shift from baseline > 0.30 msec occurring after the patient had received the treatment full course, observed at Day 3 and/or Day 7, and sustained at all subsequent readings (regardless of initial or blinded reading) through Day 42 in the same wave latency (Wave III or V).
Efficacy was assessed for the full analysis set (FAS), thereby following intention-to-treat principles. The FAS was defined as all randomized patients who had confirmed P. falciparum malaria at baseline, and who had at least one dose of study drug with at least one relevant post-baseline efficacy assessment. Efficacy analyses comprised calculation of PCR-adjusted parasitological cure rates as well as further efficacy variables related to parasite reduction. Exact Pearson-Clopper two-sided 95% CIs were constructed for all three treatment groups. Comparisons between the treatment groups were pairwise and considered exploratory. Two-sided 95% CIs on the difference in proportions were constructed using the Wilson score limits. No P-values were generated as the study was not formally powered to perform such hypothesis testing.
Using the nQuery software (Statistical Solutions Ltd., Boston, MA), based on an assumed Day-7 ABR Wave III latency change rate of 7.5% under the alternative hypothesis, a one-sided test, and a type I error of 5%, 150 A-L patients were needed to have ∼90% power to reject the null hypothesis of a Day-7 ABR Wave III latency change rate ≥ 15%. Because it was expected that at most 5% of the randomized patients would be excluded from the PP analysis, it was planned to enroll ∼159 A-L patients. Approximately 53 patients were planned to be randomized into each of the A-P and A-M treatment arm to achieve a 3:1:1 randomization ratio. In terms of efficacy, a minimum sample size of 50 patients has been suggested by the WHO for clinical trials.11
Results
In total, 542 patients were screened, of which 265 patients fulfilled the inclusion criteria. Approximately 50% of the screening failures did not have normal hearing or met specific audiological exclusion criteria (Table 1). The eligible patients were randomized to treatment groups (159 to A-L, 53 to A-P, 53 to A-M). Four (1.5%) patients discontinued the study prematurely (A-L: 2, A-P: 1, A-M: 1), most of them being lost to follow-up. No patient was excluded from the FAS or safety population. A total of 246 (92.8%) subjects (A-L: 151, A-P: 50, A-M: 45) had acceptable ABR latency data and were included in the PP population used for the primary safety analysis.
Demographic and clinical characteristics were typical for the population investigated and comparable between treatment groups. This applies to both the safety population (Table 2) and the Day-7 PP population (data not shown). There was an almost balanced gender distribution and approximately one-third of the patients were younger than 18 years of age. Baseline values for ABR Wave III latency were virtually identical between treatment groups. Specifically, mean values for A-L, A-P, and A-M patients were 3.86, 3.89, and 3.86 msec for the right ear and 3.85, 3.88, and 3.82 msec for the left ear, respectively. All randomized patients took their scheduled doses of study medication. Vomiting of study drug was rare and occurred in four patients in the A-M group. These patients received the replacement dose. Over the 3-day treatment period, the mean (range) actual doses of study medication in mg/kg were 8.0 (4.4–13.3) for artemether, 48.0 (26.2–80.0) for lumefantrine, 51.2 (30.9–73.2) for atovaquone, 20.5 (12.4–29.3) for proguanil, 9.4 (5.5–15.0) for artesunate, and 23.1 (13.6–31.3) for mefloquine considering the safety population. Dosages for the Day-7 PP population were virtually identical.
Table 2.
Baseline characteristics of enrolled patients (safety population)*
| Artemether-lumefantrineN = 159 | Atovaquone-proguanilN = 53 | Artesunate-mefloquineN = 53 | |
|---|---|---|---|
| Sex - n (%) | |||
| Male | 96 (60.4) | 34 (64.2) | 31 (58.5) |
| Race - n (%) | |||
| Black | 132 (83.0) | 42 (79.2) | 44 (83.0) |
| Hispanic | 27 (17.0) | 11 (20.8) | 9 (17.0) |
| Mean Age (yr) | 25.6 | 25.1 | 25.2 |
| SD | 11.6 | 11.2 | 11.3 |
| Range | 12–56 | 12–53 | 12–56 |
| Age category - n (%) | |||
| < 18 years | 51 (32.1) | 21 (39.6) | 18 (34.0) |
| 18 to < 65 years | 108 (67.9) | 32 (60.4) | 35 (66.0) |
| Mean weight (kg) | 62.8 | 60.3 | 65.2 |
| SD | 14.4 | 14.5 | 15.4 |
| Range | 30.0–110.0 | 30.0–97.0 | 35.0–110.0 |
| Weight category - n (%) | |||
| ≥ 70 kg | 50 (31.4) | 18 (34.0) | 19 (35.8) |
| BMI ≥ 25 kg/m2 | 50 (31.4) | 11 (20.8) | 21 (39.6) |
| Mean body temperature (°C) | 37.8 | 37.7 | 37.8 |
| SD | 0.6 | 0.7 | 0.6 |
| Range | 36.5–40.0 | 36.5–40.0 | 36.0–40.0 |
| Median parasite density, asexual forms (/μL) | 3925 | 3864 | 4620 |
| Range | 1,008–44,744 | 1,030–31,124 | 1,012–35,112 |
BMI = body mass index; SD = standard deviation.
Auditory brainstem response.
The rate of ABR Wave III latency that increased > 0.30 msec over the study course is shown for the PP populations in Table 3. The percentage of patients with ABR Wave III latency changes > 0.30 msec from baseline to Day 7 was 2.6% (95% CI: 0.7–6.6) in the A-L group (4 patients), and thus statistically significantly below 15% (P < 0.0001). The null hypothesis was rejected. The incidence rates of ABR Wave III latency changes of > 0.30 msec assessed on Days 3, 28, and 42 were also low with only small differences between treatment groups (Table 3). Mean absolute ABR Wave III latency changes from baseline to Day 7 were very small in all three treatment groups. The maximum mean absolute change from baseline ranged from −0.04 msec (95% CI: −0.08 to 0.01) in the A-M group to 0.01 msec (95% CI: −0.01 to 0.03) in the A-L group (Day-7 PP population); this was far below the 0.30 msec considered clinically significant. Likewise, mean changes at other assessments were also small (range: −0.05 to 0.02 msec) with no consistent trends or differences among treatments. Patients with ABR Wave III latency increases > 0.30 msec tended to have short baseline latencies. There were no discernable changes in mean ABR Wave I and V latencies in any group (data not shown).
Table 3.
Incidence rate of ABR Wave III latency changes > 0.30 msec from baseline over time (per protocol populations)*
| Artemether-lumefantrine | Atovaquone-proguanil | Artesunate-mefloquine | |
|---|---|---|---|
| Day 3 | N = 145 | N = 50 | N = 45 |
| 4 (2.8) | 0 | 0 | |
| [0.8–6.9] | [0.0–7.1] | [0.0–7.9] | |
| Day 7 (primary endpoint)† | N = 151 | N = 50 | N = 45 |
| 4 (2.6)‡ | 0 | 0 | |
| [0.7–6.6] | [0.0–7.1] | [0.0–7.9] | |
| Day 28 | N = 143 | N = 50 | N = 46 |
| 6 (4.2) | 1 (2.0) | 0 | |
| [1.6–8.9] | [0.1–10.6] | [0.0–7.7] | |
| Day 42 | N = 138 | N = 49 | N = 47 |
| 4 (2.9) | 0 | 1 (2.1) | |
| [0.8–7.3] | [0.0–7.3] | [0.1–11.3] |
Data are presented as n (%) [Exact Pearson-Clopper 95% confidence interval].
ABR = auditory brainstem response; N = number of patients in the respective per-protocol population
Based on a review by an independent audiologist (fully blinded).
P < 0.0001 for the one-sided null hypothesis that the incidence rate of ABR Wave III latency change is ≥ 15% in the artemether-lumefantrine group.
Pure-tone air conduction threshold.
No notable changes were observed for any treatment group at any frequency. In all three groups, small improvements (2–4 dB) in pure-tone air conduction threshold average (i.e., apparent hearing improvement with treatment) were recorded, with no differences between treatments (Table 4). Of note, the A-L patients showing ABR Wave III latency changes > 0.30 msec had only small changes from baseline to final observation in their pure-tone threshold across ears.
Table 4.
Pure-tone air conduction threshold average* at baseline and change over time (per-protocol populations)
| Artemether-lumefantrine | Atovaquone-proguanil | Artesunate-mefloquine | |
|---|---|---|---|
| Baseline | N = 148 | N = 51 | N = 47 |
| Right ear | 12.2 [11.4, 13.0] | 12.0 [10.5, 13.6] | 12.7 [11.2, 14.2] |
| Left ear | 11.4 [10.5, 12.3] | 11.3 [9.9, 12.7] | 12.5 [10.8, 14.3] |
| Day 3 | N = 145 | N = 50 | N = 45 |
| Right ear | −2.5 [−3.1, −1.9] | −2.4 [−3.6, −1.2] | −1.9 [−3.0, −0.7] |
| Left ear | −1.2 [−1.8, −0.5] | −1.5 [−2.6, −0.3] | −1.2 [−2.2, −0.1] |
| Day 7 | N = 143 | N = 49 | N = 46 |
| Right ear | −2.2 [−2.9, −1.5] | −2.6 [−4.0,−1.1] | −2.6 [−3.9, −1.3] |
| Left ear | −1.7 [−2.4, −0.9] | −1.3 [−2.8, 0.2] | −1.4 [−2.8, −0.1] |
| Day 28 | N = 143 | N = 50 | N = 46 |
| Right ear | −2.7 [−3.5, −1.9] | −2.6 [−4.2, −1.0] | −3.6 [−4.8, −2.3] |
| Left ear | −2.0 [−2.8, −1.1] | −1.8 [−3.0, −0.5] | −2.5 [−4.3, −0.7] |
| Day 42 | N = 138 | N = 49 | N = 47 |
| Right ear | −3.0 [−3.8, −2.2] | −3.3 [−4.9, −1.7] | −3.1 [−4.2, −1.9] |
| Left ear | −1.5 [−2.7, −0.4] | −2.1 [−3.5, −0.6] | −3.0 [−4.7, −1.3] |
Average of the pure-tone thresholds for the frequencies 500, 1000, 2000, and 3000 Hz.
Data are presented as mean change from baseline (dB) [95% CI]; none of the average changes exceed the measurement error for pure-tone thresholds.
CI = confidence interval; N = number of patients in the respective per-protocol population.
Post-hoc analysis.
Ten (6.3%), 1 (1.9%), and 1 (2.0%) patients in the A-L, the A-P, and in the A-M groups, respectively, experienced changes in one ear in the ABR Wave III or V latency, from baseline > 0.30 msec after Day 3 but not later than Day 7 (regardless of initial or blinded reading), which were sustained at all subsequent readings through Day 42 in the same wave (Wave III or V). The 95% CIs (Exact Pearson-Clopper 95% CI) were wide and highly overlapping across treatment groups ([3.1–11.3], [0.0–10.1], [0.1–10.9] for the A-L, the A-P, and in the A-M groups, respectively). However, the 10 patients treated with A-L had no change in their pure-tone threshold (i.e., pure-tone audiometry [PTA] threshold > 30 dB) at anytime in the tested ear (data not shown).
Tolerability and safety.
All three treatments were generally well tolerated by the study participants, with the majority of AEs being of mild severity. Only one serious AE (SAE) was reported in one patient of the A-M group who experienced respiratory distress syndrome on Day 4. This SAE was considered unrelated to study drug, and the subject recovered upon therapy at the same day of occurrence. There were no AEs leading to discontinuation of study drug or requiring dose adjustment or study drug interruption. No patient died. The lowest AE rate was observed with A-L, mainly caused by fewer AEs related to the gastrointestinal tract (A-L: 7.5%, A-P: 22.6%, A-M: 35.8%) and central nervous system disorders (A-L: 8.2%, A-P: 20.8%, A-M: 35.8%). In particular, vomiting, dizziness, and headache were distinctly less frequent with A-L compared with the two other treatments (Table 5). Changes of hematology parameters (Table 6) and vital signs were in accordance with malaria recovery, without notable differences between treatment groups.
Table 5.
Adverse events occurring in more than 5% of patients in any treatment group, irrespective of cause (safety population)*
| Artemether-lumefantrineN = 159 | Atovaquone-proguanilN = 53 | Artesunate-mefloquineN = 53 | |
|---|---|---|---|
| Patients with AEs | 46 (28.9) | 25 (47.2) | 36 (67.9) |
| Dizziness | 9 (5.7) | 5 (9.4) | 14 (26.4) |
| Pyrexia | 6 (3.8) | 4 (7.5) | 2 (3.8) |
| Headache | 5 (3.1) | 7 (13.2) | 7 (13.2) |
| Diarrhea | 3 (1.9) | 2 (3.8) | 7 (13.2) |
| Abdominal pain | 3 (1.9) | 2 (3.8) | 4 (7.5) |
| Vomiting | 2 (1.3) | 9 (17.0) | 15 (28.3) |
| Insomnia | 0 | 0 | 4 (7.5) |
Data are presented as n (%); AEs are listed according to decreasing frequency in the artemether-lumefantrine group.
AE = adverse event.
Table 6.
Change from baseline in hematology parameters over time (safety population)*
| Day 3 | Day 7 | Day 28 | Day 42 | |
|---|---|---|---|---|
| Hemoglobin (g/L) | ||||
| A-L (N = 157–159) | −5.9 ± 10.2 | −6.3 ± 11.5 | 0.6 ± 14.8 | 3.7 ± 16.1 |
| A-P (N = 52–53) | −8.0 ± 10.5 | −8.9 ± 12.6 | 0.6 ± 15.9 | 1.8 ± 19.1 |
| A-M (N = 52–53) | −4.8 ± 8.8 | −5.1 ± 10.7 | 3.4 ± 12.7 | 3.9 ± 13.7 |
| Hematocrit (%) | ||||
| A-L (N = 157–159) | −1.9 ± 3.4 | −2.0 ± 3.7 | −0.3 ± 5.0 | 0.7 ± 5.1 |
| A-P (N = 51–53) | −2.4 ± 2.6 | −3.2 ± 3.4 | −0.4 ± 5.2 | 0.5 ± 6.1 |
| A-M (N = 52–53) | −1.0 ± 2.9 | −0.8 ± 3.7 | 1.6 ± 4.4 | 2.0 ± 4.5 |
| WBC (total) (109/L) | ||||
| A-L (N = 157–159) | −0.3 ± 2.3 | 1.5 ± 2.6 | 1.5 ± 2.7 | 1.5 ± 3.2 |
| A-P (N = 52–53) | −0.3 ± 1.8 | 2.4 ± 2.8 | 2.2 ± 2.4 | 2.5 ± 2.9 |
| A-M (N = 52–53) | 0.1 ± 2.4 | 2.6 ± 2.6 | 1.6 ± 2.9 | 2.1 ± 3.2 |
All values are presented as mean ± standard deviation.
A-L = artemether-lumefantrine; A-P = atovaquone-proguanil; A-M = artesunate plus mefloquine; N = number of patients with assessments; WBC = white blood cells.
Efficacy.
The PCR-corrected cure rates were high up to Day 42, regardless of body mass index (< 25 kg/m2 or ≥ 25 kg/m2) or body weight (< 70 kg or ≥ 70 kg), with only minor differences between treatment groups (Tables 7 and 8). For three patients in the A-L group, no blood smear data were available at Day 42; those were classified as having a recrudescence. Uncorrected cure rates were similar to the PCR-corrected rates. Specifically, uncorrected Day-42 cure rates for A-L, A-P, and A-M were 97.5%, 96.2%, and 98.1%, respectively. A rapid clearance of asexual parasite forms was observed in the A-L and A-M groups. At 48 hours after treatment initiation, 98.1%, 63.5%, and 100% of patients in the A-L, A-P, and A-M groups, respectively, had microscopically negative blood smears. Likewise, mean percent parasite reduction at 24 hours was higher with A-L (−94.5%) and A-M (−97.5%) compared with A-P (−50.2%). No patient had fever by Day 4. Plasmodium falciparum gametocytes after Day 8 were observed in all three treatment groups, but were less frequent in the A-L group (3 patients, 1.9%) and the A-M group (1 patient, 1.9%) than with A-P (6 patients, 11.3%). No patient required rescue therapy.
Table 7.
Polymerase chain reaction-corrected cure rates over time (full analysis set)*
| Artemether-lumefantrineN = 159 | Atovaquone-proguanilN = 53 | Artesunate-mefloquineN = 53 | |
|---|---|---|---|
| Day 14 | 158 (99.4) | 53 (100) | 52 (98.1) |
| [96.5–100] | [93.3–100] | [89.9–100] | |
| Day 28 | 157 (98.7) | 52 (98.1) | 52 (98.1) |
| [95.5–99.8] | [89.9–100] | [89.9–100] | |
| Day 42 | 155 (97.5)† | 51 (98.1)‡ | 52 (98.1) |
| [93.7–99.3] | [89.7–100] | [89.9–100] |
Data are presented as n (%) [95% CI]. Exact Pearson-Clopper limits.
1 recrudescence and 3 patients with missing blood smear data.
N = 52.
CI = confidence interval.
Table 8.
Polymerase chain reaction-corrected cure rates by body mass index and body weight on day 42 (full analysis set)*
| Artemether-lumefantrine | Atovaquone-proguanil | Artesunate-mefloquine | |
|---|---|---|---|
| BMI < 25 kg/m2 | N = 109 | N = 42 | N = 32 |
| 106 (97.2) | 40 (97.6)† | 32 (100) | |
| [92.2–99.4] | [87.1–99.9] | [89.1–100] | |
| BMI ≥ 25 kg/m2 | N = 50 | N = 11 | N = 21 |
| 49 (98.0) | 11 (100) | 20 (95.2) | |
| [89.4–99.9] | [71.5–100] | [76.2–99.9] | |
| Weight < 70 kg | N = 109 | N = 35 | N = 34 |
| 106 (97.2) | 33 (97.1)‡ | 34 (100) | |
| [92.2–99.4] | [84.7–99.9] | [89.7–100] | |
| Weight ≥ 70 kg | N = 50 | N = 18 | N = 19 |
| 49 (98.0) | 18 (100) | 18 (94.7) | |
| [89.4–99.9] | [81.5–100] | [74.0–99.9] |
Data are presented as n (%) [95% CI]. Exact Pearson-Clopper limits.
BMI = body mass index; CI = confidence interval
N = 41.
N = 34.
Relationship between drug exposure and ABR change.
Descriptive graphical exploration and linear regression analysis showed no significant relationship between exposure to lumefantrine, artemether, or DHA and ABR Wave III latency change (based on Day-7 PP population) (Figure 1). Likewise, ABR latency changes on Day 3 did not show a relationship to drug exposure (data not shown). The four patients in the A-L group with an ABR Wave III latency change > 0.30 msec from baseline were spread among the exposure range for model-derived lumefantrine maximum plasma concentration (Cmax) and area under the curve as well as for observed lumefantrine Cmax, artemether, and DHA concentrations. Similarly, no evidence of a relationship between artesunate, DHA, or mefloquine exposure and ABR changes was found in the A-M treated patients (data not shown). A comparable drug exposure was achieved across body weight groups after administration of A-L (data not shown).
Figure 1.
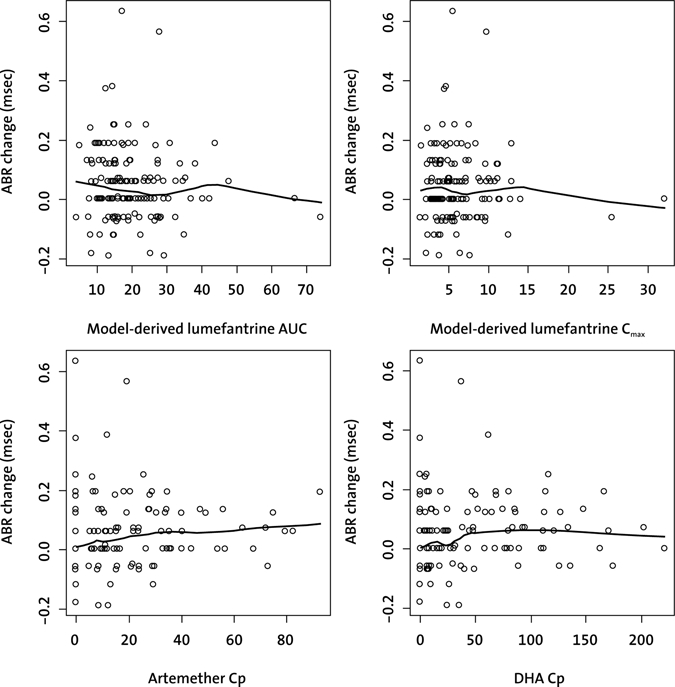
ABR Wave III latency change (msec) from baseline to Day 7 versus artemether, DHA, and lumefantrine exposure. The largest ABR change of both ears was considered for presentation. The black line represents the LOWESS smoother showing the general trend of the exposure—ABR change relationship. ABR = auditory brainstem response; AUC = area under the plasma concentration-time curve (mg.d/L); Cmax = maximum plasma concentration (mg/L); DHA = dihydroartemisinin; Cp = plasma concentration at 1 hour after the last artemether-lumefantrine dose (ng/mL).
Discussion
Following the observation that high doses of artemisinin derivatives may adversely affect the brainstem structures, some of which may involve the auditory pathway in animal models, a number of studies in humans have been performed. These studies have mainly been retrospective and focused on pure-tone threshold data for determining auditory pathway integrity; they neither analyzed the threshold data appropriately nor used adequate controls. Here, we report the results of a prospective randomized trial assessing the effects of A-L on auditory brainstem function in patients with acute P. falciparum malaria. The study was designed in such a way that if ≥ 15% of patients had a Day-7 Wave III latency changes > 0.30 msec, it would be reasonable to suspect that at least some of these cases represented auditory brainstem pathway impairment. The primary analysis of the percentage of patients with Day-7 ABR Wave III latency changes > 0.30 msec from baseline showed that A-L was not associated with an elevated rate of auditory brainstem wave latencies. The four patients with ABR Wave III latency increases > 0.30 msec tended to have shorter baseline latencies. None of the latency increases were sustained and bilateral and associated with significant PTA threshold deteriorations (PTA threshold > 30 dB23) (data not shown). Similarly, the 10 patients treated with A-L with a sustained change in ABR latency from baseline > 0.30 msec noted in the post-hoc analysis had no significant deteriorations in their PTA threshold (PTA threshold > 30 dB23) over time (data not shown).
The observed changes from baseline in the four subjects with ABR Wave III latency increases > 0.30 msec likely do not indicate toxicity of artemisinin derivatives, broadly defined. This conclusion is supported by the absence of signs for a relationship between drug exposures (either of the analytes) and ABR Wave III changes in both artemisinin-containing treatment groups, and by the observation that no patient receiving artesunate plus mefloquine revealed Day-7 ABR Wave III latency increases of > 0.30 msec. Additional analyses on Wave I, III, and V latencies showed little or no changes over the study period, and pure-tone thresholds did not reveal significant changes, further supporting that A-L does not adversely affect the auditory brainstem pathway.
The small improvements in pure-tone thresholds from baseline are a common finding when testing very ill patients, who are subsequently treated, feel better, and are more able to concentrate. These changes support the conclusion that the drugs did not adversely affect the auditory pathway.
Our results were in accordance with previous findings. Two case-control studies in which audiological measurements including ABR were performed in patients exposed to several courses of artemisinin derivatives reported no differences in the test results between cases and controls.7,24 A more recent case-control study along the Thailand-Myanmar border performed pure-tone threshold and ABR tests in subjects treated with A-L within the previous 5 years and found no evidence of auditory brainstem impairment attributable to A-L.25 Similarly, a pure-tone threshold and ABR study in a limited number of healthy volunteers with experimentally induced malaria, treated with A-L, found no evidence of a detrimental drug effect on the auditory system.26 In a randomized study in malaria patients from Ethiopia, pure-tone thresholds revealed statistically significant temporary threshold shift only in the quinine group, but this effect did not appear to be clinically significant. No hearing loss was observed in the A-L or A-P groups. There was no evidence of drug-induced brainstem impairment using ABR measurements.27 Another prospective study assessed the effects on auditory functioning of malaria patients from the Thai-Burmese border after a standard 3-day oral dose of artesunate combined with mefloquine. Neither pure-tone thresholds nor ABR tests showed clinical evidence of auditory impairment 7 days after receiving the first dose of study medication. No patient showed a shift in ABR Wave III peak latency change > 0.30 msec.22
Administration of A-L was well tolerated in this study. A favorable AE profile was observed, particularly if compared with A-M. Although the study was not powered for this comparison, it corroborates previous comparative trials in adolescents and adults with falciparum malaria.28
The efficacy of A-L was excellent, with PCR-corrected cure rates > 95% up to Day 42, including patients with a body mass index of ≥ 25 kg/m2. These results were in accordance with those of previous randomized studies reporting Day-42 cure-rate data with A-L in adolescents and adults from areas of multi-drug resistance, and applying a similarly conservative approach for analysis.29,30
In conclusion, over a 6-week observation period, A-L administered twice daily for three consecutive days was well tolerated and highly efficacious for the treatment of acute uncomplicated P. falciparum malaria. Neither ABR latencies nor pure-tone thresholds showed clinical or statistical evidence of an effect of A-L on auditory functioning, confirming previous studies. The absence of clinically relevant adverse effects of A-L on brainstem auditory pathways supports its continued safe use in uncomplicated falciparum malaria.
ACKNOWLEDGMENTS
We thank the study population and local staff, without whom this trial could not have taken place. We are also grateful to the following people for their help and support in conducting this study: Lina Restrepo, Liliana Ortiz, Zulema Ortiz, Augusto Peñaranda, Olga Helena Henao (Fundación Santa Fe de Bogotá, Bogotá, Colombia); Javier Molineros, Fausto Tenorio, Nefer Lopez (Hospital San Andrés, Tumaco, Colombia); Maria Vargas, Heidi Frazier, Manuel Don (House Ear Institute [HEI], Los Angeles, CA); Yolanda Alarcon, Angelica Rusinque (Novartis Ltd, Bogotá, Colombia); Kim Andriano, Patricia Ibarra de Palacios, Gabriela Mani-Caplazi, Anne Claire Marrast, Fiyinfolu Oladiran, Mailis Virtanen, (Novartis Pharma AG, Basel, Switzerland). We also thank Feiko Ter Kuile (School of Tropical Medicine, Liverpool, UK) and Wendy Mack (University of Southern California, Los Angeles, CA) for their participation in the Data Safety Monitoring Board. Hans-Peter Beck from the Swiss Tropical and Public Health Institute performed the PCR-analyses. Statistical analysis was carried out by DATAMAP GmbH, Freiburg, Germany. We especially acknowledge the dedicated work of Martina Wibberg, Carmen Wiesmann, and Jürgen Lilienthal. The House Ear Institute thanks Bio-Logic Systems Corporation for assistance with the auditory brainstem response equipment and software, and ensuring the equipment remained in good condition. Drafting of the manuscript was done by Edgar A. Mueller, 3P Consulting; the authors were responsible for critical revisions of the manuscript and for important intellectual content.
Footnotes
Financial support: This study was supported by Novartis Pharma Ltd., Basel, Switzerland.
Disclosure: Marc Cousin, Verena Walter, Gilbert Lefèvre, and Oliver Sander are employees of Novartis Pharma Ltd.
Authors' addresses: Gabriel Carrasquilla and Clemencia Barón, Centro de Estudios e Investigación en Salud-CEIS, Fundación Santa Fe de Bogotá, Bogotá, DC, Colombia, E-mails: Gabriel.Carrasquilla@fsfb.org.co and cbaron@cable.net.co. Edwin M. Monsell, Department of Otolaryngology-Head and Neck Surgery, Wayne State University, Detroit, MI, E-mail: emonsell@med.wayne.edu. Marc Cousin, Established Medicines DF/Tropical Medicines, Novartis Pharma AG, Basel, Switzerland, E-mail: marc.cousin@novartis.com. Verena Walter, Integrated Information Sciences, Novartis Pharma AG, Basel, Switzerland, E-mail: verena.walter@novartis.com. Gilbert Lefèvre, Translational Sciences, Novartis Pharma AG, Basel, Switzerland, E-mail: gilbert.lefevre@novartis.com. Oliver Sander, Modeling and Simulation, CHBS, Novartis Pharma AG, Basel, Switzerland, E-mail: oliver.sander@novartis.com. Laurel M. Fisher, House Ear Institute (HEI), Los Angeles, CA, E-mail: lfisher@hei.org.
Reprint requests: Gabriel Carrasquilla, Centro de Estudios e Investigación en Salud-CEIS, Fundación Santa Fe de Bogotá, Carrera 7B No. 123-90, Bogotá, DC, Colombia.
References
- 1.World Health Organization Guidelines for the Treatment of Malaria. Second edition. 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf Available at. Accessed January 20, 2011.
- 2.Premji ZG. Coartem: the journey to the clinic. Malar J. 2009;8((Suppl 1)):S3. doi: 10.1186/1475-2875-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer TG, Peggins JO, Grate SJ, Petras JM, Levine BS, Weina PJ, Swearengen J, Heiffer MH, Schuster BG. Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg. 1994;88((Suppl 1)):S33–S36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 4.Brewer TG, Grate SJ, Peggins JO, Weina PJ, Petras JM, Levine BS, Heiffer MH, Schuster BG. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 5.Petras JM, Kyle DE, Gettayacamin M, Young GD, Bauman RA, Webster HK, Corcoran KD, Peggins JO, Vane MA, Brewer TG. Arteether: risks of two-week administration in Macaca mulatta. Am J Trop Med Hyg. 1997;56:390–396. doi: 10.4269/ajtmh.1997.56.390. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro IR, Olliaro P. Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med Trop. 1998;58((Suppl 3)):50–53. [PubMed] [Google Scholar]
- 7.Kissinger E, Hien TT, Hung NT, Nam ND, Tuyen NL, Dinh BV, Mann C, Phu NH, Loc PP, Simpson JA, White NJ, Farrar JJ. Clinical and neurophysiological study of the effects of multiple doses of artemisinin on brain-stem function in Vietnamese patients. Am J Trop Med Hyg. 2000;63:48–55. doi: 10.4269/ajtmh.2000.63.48. [DOI] [PubMed] [Google Scholar]
- 8.Hien TT, Turner GD, Mai NT, Phu NH, Bethell D, Blakemore WF, Cavanagh JB, Dayan A, Medana I, Weller RO, Day NP, White NJ. Neuropathological assessment of artemether-treated severe malaria. Lancet. 2003;362:295–296. doi: 10.1016/s0140-6736(03)13974-8. [DOI] [PubMed] [Google Scholar]
- 9.Toovey S, Jamieson A. Audiometric changes associated with the treatment of uncomplicated falciparum malaria with co-artemether. Trans R Soc Trop Med Hyg. 2004;98:261–267. doi: 10.1016/j.trstmh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Management of Severe Malaria: A Practical Handbook. Second edition. 2000. http://www.rollbackmalaria.org/docs/hbsm.pdf Available at. Accessed January 20, 2011. [Google Scholar]
- 11.World Health Organization Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. 2003. http://whqlibdoc.who.int/hq/2003/WHO_HTM_RBM_2003.50.pdf Available at. Accessed January 20, 2011.
- 12.Felger I, Beck HP. In: Malaria Methods and Protocols: Methods in Molecular Medicine. Doolan D, editor. Totawa, NJ: Humana Press; 2002. pp. 117–129. (Genotyping of Plasmodium falciparum: PCR-RFLP analysis). [DOI] [PubMed] [Google Scholar]
- 13.Carhart R, Jerger J. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord. 1959;24:330–345. [Google Scholar]
- 14.Souppart C, Gauducheau N, Sandrenan N, Richard F. Development and validation of a high-performance liquid chromatography-mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:195–203. doi: 10.1016/s1570-0232(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 15.Bergqvist Y, Hellgren U, Churchill FC. High-performance liquid chromatographic assay for the simultaneous monitoring of mefloquine and its acid metabolite in biological samples using protein precipitation and ion-pair extraction. J Chromatogr A. 1988;432:252–263. doi: 10.1016/s0378-4347(00)80650-7. [DOI] [PubMed] [Google Scholar]
- 16.Burkard RF, Don M, Eggermont JJ. Auditory Evoked Potentials: Basic Principles and Clinical Applications. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- 17.Fausti SA, Flick CL, Bobal AM, Ellingson RM, Henry JA, Mitchell CR. Comparison of ABR stimuli for the early detection of ototoxicity: conventional clicks compared with high frequency clicks and single frequency tone bursts. J Am Acad Audiol. 2003;14:239–250. [PubMed] [Google Scholar]
- 18.Don M, Kwong B. In: Handbook of Clinical Audiology. Fifth edition. Katz J, editor. New York: Lippincott Williams & Wilkins; 2002. pp. 274–297. (Auditory brainstem response: differential diagnosis). [Google Scholar]
- 19.Hecox K, Galambos R. Brain stem auditory evoked responses in human infants and adults. Arch Otolaryngol. 1974;99:30–33. doi: 10.1001/archotol.1974.00780030034006. [DOI] [PubMed] [Google Scholar]
- 20.Clemis JD, Mc Gee T. Brain stem electric response audiometry in the differential diagnosis of acoustic tumors. Laryngoscope. 1979;89:31–42. doi: 10.1288/00005537-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bergholtz L. Normative data in clinical ABR. Scand Audiol Suppl. 1981;13:75–81. [PubMed] [Google Scholar]
- 22.Carrara VI, Phyo AP, Nwee P, Soe M, Htoo H, Arunkamomkiri J, Singhasivanon P, Nosten F. Auditory assessment of patients with acute uncomplicated Plasmodium falciparum malaria treated with three-day mefloquine-artesunate on the north-western border of Thailand. Malar J. 2008;7:233. doi: 10.1186/1475-2875-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Academy of Otolaryngology-Head and Neck Surgery Foundation Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma) Otolaryngo Head Neck Surg. 1995;113:179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 24.Van Vugt M, Angus BJ, Price RN, Mann C, Simpson JA, Poletto C, Htoo SE, Looareesuwan S, White NJ, Nosten F. A case-control auditory evaluation of patients treated with artemisinin derivatives for multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 2000;62:65–69. doi: 10.4269/ajtmh.2000.62.65. [DOI] [PubMed] [Google Scholar]
- 25.Hutagalung R, Htoo H, Nwee P, Arunkamomkiri J, Zwang J, Carrara VI, Ashley E, Singhasivanon P, White NJ, Nosten F. A case-control auditory evaluation of patients treated with artemether-lumefantrine. Am J Trop Med Hyg. 2006;74:211–214. [PubMed] [Google Scholar]
- 26.McCall MB, Beynon AJ, Mylanus EA, van der Ven AJ, Sauerwein RW. No hearing loss associated with the use of artemether-lumefantrine to treat experimental human malaria. Trans R Soc Trop Med Hyg. 2006;100:1098–1104. doi: 10.1016/j.trstmh.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Gürkov R, Eshetu T, Miranda IB, Berens-Riha N, Mamo Y, Girma T, Krause E, Schmidt M, Hempel JM, Löscher T. Ototoxicity of artemether/lumefantrine in the treatment of falciparum malaria: a randomized trial. Malar J. 2008;7:179. doi: 10.1186/1475-2875-7-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller EA, van Vugt M, Kirch W, Andriano K, Hunt P, de Palacios PI. Efficacy and safety of the six-dose regimen of artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in adolescents and adults: a pooled analysis of individual patient data from randomized clinical trials. Acta Trop. 2006;100:41–53. doi: 10.1016/j.actatropica.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Mayxay M, Khanthavong M, Lindegårdh N, Keola S, Barends M, Pongvongsa T, Yapom R, Annerberg A, Phompida S, Phetsouvanh R, White NJ, Newton PN. Randomized comparison of chloroquine plus sulfadoxine-pyrimethamine versus artesunate plus mefloquine versus artemether-lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People's Democratic Republic. Clin Infect Dis. 2004;39:1139–1147. doi: 10.1086/424512. [DOI] [PubMed] [Google Scholar]
- 30.Hutagalung R, Paiphun L, Ashley EA, McGready R, Brockman A, Thwai KL, Singhasivanon P, Jelinek T, White NJ, Nosten FH. A randomized trial of artemether-lumefantrine versus mefloquine-artesunate for the treatment of uncomplicated multi-drug resistant Plasmodium falciparum on the western border of Thailand. Malar J. 2005;4:46. doi: 10.1186/1475-2875-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]


