Abstract
We report the results of a study conducted during 1990–2006 with 89 cases of American visceral leishmaniasis in Chiapas State in southeastern Mexico and a seroprevalence study performed with 726 persons and 224 dogs that lived near cases of American visceral leishmaniasis. Clinical aspects, epidemiologic profiles, and risk factors are described. Most cases were in children ≤ 5 years of age, the prevalence of seropositive persons was 77%. The main risk factors associated with this disease were having 1–3 rooms in a house compared with ≥ 4 rooms, having a roof that was not made of cement, and having domestic animals. In contrast, only 19% of dogs were seropositive, suggesting that this species is not important in the transmission cycle of Leishmania. These data indicate that active transmission is taking place in the central valley of Chiapas State, Mexico, in communities located < 1,000 meters above sea level near the Grijalva River.
Introduction
Leishmaniasis is a parasitic disease caused by a protozoa that belongs to the genus Leishmania. The disease is transmitted to humans and domestic or wild animals by the bite of Lutzomyia sand flies. Clinical manifestations of leishmaniasis vary from local cutaneous, diffuse cutaneous, mucocutaneous, to a visceral disease.1 American visceral leishmaniasis (AVL) has been recorded from Mexico to north of Argentina mostly in semi-arid areas;1,2 the highest incidence is in northeastern Brazil.3 American cutaneous leishmaniasis in Mexico is caused by Leishmania chagasi and the main vector is Lutzomyia longipalpis.4,5
Until 1989, only eight cases of AVL were reported in Mexico; all of them were in the Balsas River basin, which includes the states of Guerrero, Puebla, Morelos, and Oaxaca.6–10 In Chiapas State, the first case was documented in 1990 at the Regional Hospital of Tuxtla Gutierrez, the capital city of the state, which is located in the Grijalva River basin. An increase in cases in several municipalities was observed in subsequent years. Actions to establish control and surveillance of the disease were initiated by the Vector Borne Disease Control Department. Little is known about the transmission and the risk factors associated with the disease in Chiapas. We describe the clinical aspects, epidemiologic profiles, and risk factors of this new disease focus.
Materials and Methods
Study site.
The state of Chiapas is located in southeastern Mexico. Its climate is tropical and rainy, but the central valley is dry in the northern region and humid in the southern region; it has an annual average rainfall of 1,810 mm. The temperature range is 28–30°C, and vegetation is mainly tropical deciduous forest. Communities in Chiapas are found from sea level to the highlands. The first part of this study describes cases detected and recorded during 1990–2006 in Chiapas from patients who were given a diagnosis at the Institute of Epidemiological Diagnosis and Reference (InDRE) and treated at second-level hospitals in the capital city of Chiapas; all cases were reported to the Vector Borne Disease Control Department according to the Mexican Official Guidelines for Vector Borne Diseases (NOM).11,12
Data and sample collection.
The second part of this study describes prospective field work conducted during 2000–2002, in which the prevalence of antibodies against Leishmania in 726 persons without a history of AVL were studied mainly in eight communities in which cases of AVL were identified. Samples were taken from every house along three blocks surrounding each case, taking into account the short flight range of sand flies (approximately 1,000 meters). All participants or their parent or guardian were informed about the objectives of the study and were included only after providing oral consent.
Approximately 5 mL of peripheral blood were collected from each person. Data including age, sex, and present symptoms related to AVL, building materials for the household, connection of walls and roof, rooms per house, number of bedrooms, households, persons per bedroom, electricity, type of light (white or yellow) and location of a television in the house, domestic animals, farm animals, and presence of adjacent corral and chicken breeding were documented.
Dog census and sampling.
The third part of the study included 224 dogs; all belonged to the families included in the human survey. Age, sex, living quarters during the day and at night, signs related to the disease, veterinary visits, and frequency of bathing were recorded. After owner acceptance, 3 mL of blood were obtained from the femoral vein for antibody detection. The collection of dog samples was also performed during 2002–2006.
This work complies with the current health laws of Mexico, and was approved by the Ethics and Research Committees of the Hospital General Dr. Manuel Gea Gonzalez and InDRE.
Laboratory diagnosis.
Criteria used for diagnosis of human cases were based in clinical, epidemiologic, and laboratory aspects that included bone marrow aspirate, liver or spleen biopsy, and serologic analysis by immunofluorescence antibody test (IFAT). Serologic detection of antibodies against Leishmania by IFAT was performed using as antigen a combination of six local strains: Leishmania mexicana (MHOM/MX/92/AG, diffuse cutaneous leishmaniasis; MHOM/MX/88/HRC GS, diffuse cutaneous leishmaniasis; MHOM/MX/88/HRC MC, localized cutaneous leishmaniasis; MHOM/MX/94/INDRE BFC, localized cutaneous leishmaniasis; and L. chagasi (MHOM/MX/93/INDRE BP, AVL, and MCAN/MX/97/INDRE TRAC, AVL).
Promastigotes were harvested in the stationary phase, washed three times in phosphate-buffered saline (PBS), pH 7.2, and resuspended in 1% PBS buffered-formalin. Ten microliters of a 6 × 105 parasites/mL suspension were distributed in 10-well immunofluorescence slides. Slides were air-dried for 24 hours at room temperature and stored at –20°C until use. Patient and control serum samples were incubated in serial two-fold dilutions from 1:2 to 1:1,024 for 30 minutes at 37°C. After three washes in PBS, antibodies were identified with protein A–fluorescein isothiocyanate conjugate (Invitrogen Corporation, Camarillo, CA) by incubation for 30 minutes at 37°C and a 1:100 dilution of in 0.01% Evans blue for counterstaining. Slides were washed, covered with buffered glycerin, pH 7.5, and a coverslip, and examined the same day by using a fluorescence microscope (Carl Zeiss, Obercochen, Germany). The IFAT results were considered positive when a ≥ 1:16 dilution of serum was fluorescent.
Persons with signs and symptoms of suspected AVL, as defined in NOM, were subjected to the following diagnosis algorithm: each person was hospitalized and samples for laboratory diagnosis were sent to InDRE in Mexico City. Results were returned after a few days by fax or telephone. On the basis of laboratory results, clinicians provided treatment. In the medical file, every case contained information on age, sex, diagnosis, treatment outcome (cured or died), and residence and location of the patient or the parents and the infected child when the infection became symptomatic. In some cases, patients that did not come for follow-up were contacted in their homes to provide the treatment outcome.
For the first part of the study, parasite identification in bone marrow aspirates and biopsy specimens of spleen or lymph nodes was conducted by staining with Giemsa. Bone marrow aspirates were obtained only from five patients from whom the clinician did not provide a serum sample for the IFAT. On the basis of the decision of the clinician, two patients had laboratory diagnosis obtained only by testing spleen and lymph node biopsy specimens.
For the serologic study, the IFAT was performed as stated above by using ≥ 1:4 as cutoff for human samples and ≥ 1:2 for dogs. Although the area where this study was conducted is not considered endemic for Trypanosoma cruzi because the vector had not been found since it requires more humidity for its development, every sample was also tested to discriminate T. cruzi infection at InDRE by using an in-house enzyme-linked immunosorbent assay with a crude extract of T. cruzi epimastigotes from isolates from Mexico and the Pathozyme® Chagas OD147 Kit (Omega Diagnostics Ltd., Alva, Scotland) to identify any false-positive results. All samples used for human and canine analysis showed negative results in the second test for T. cruzi.
Statistical analysis.
Characteristics of cases and factors included in the serologic study were compared by univariate analysis for estimating crude odds ratios and 95% confidence intervals with the Mantel-Haenszel chi-square test and Epi-Info version 6.0 (Centers for Disease Control and Prevention, Atlanta, GA). Variables that were significantly associated with P values ≤ 0.10 were included in a backwards stepwise multivariate logistic regression analysis. The least significant variables were removed for the final model by using SPSS version 17.0 (SPSS, Inc., Chicago, IL).
Results
A total of 89 AVL patients were detected during 1990–2006; 60% were male. Their residence is shown in Figure 1. Of these patients, 78% were from the central valley of Chiapas, mainly from Tuxtla Gutierrez, Chiapa de Corzo, and Venustiano Carranza (municipalities 101, 27, and 106, respectively). Five patients were found in the communities outside the map. The number of cases per year is shown in Figure 2. An average of five cases were detected yearly. Two years had 13 and 14 new cases, and one year (2001) did not have any new reported cases. The absence of cases in 2001 was likely the result of interruption of the state control program in that year. The distribution of patients by age and sex is shown in Figure 3. Interestingly, 84% of the patients were ≤ 5 years of age and 11% of the children were < 1 year of age. All patients came from their communities to second-level hospitals in Tuxtla Gutierrez. No data were found in medical records regarding previous visits to health centers. Clinical findings for all 89 patients (all patients had fever, hepatomegaly, splenomegaly, and pallor) are summarized in Table 1. Regarding laboratory tests,13 77 of 89 patients had an IFAT performed and 24 of these patients also had a bone marrow aspirate obtained on the basis of a medical decision by the clinician. Results of the IFAT were positive in 92% of the samples, and parasites were identified in 90% of bone marrow aspirates and in the two biopsy specimens.
Figure 1.
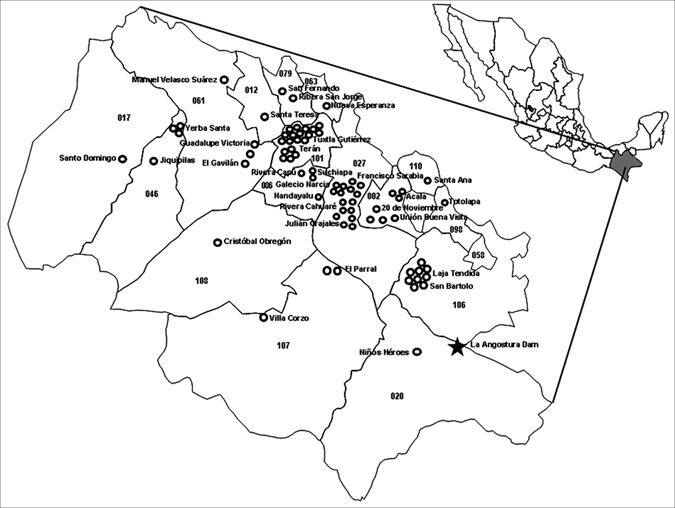
Central part of Chiapas State, Mexico showing cases of American visceral leishmaniasis (circles). Names indicate communities and numbers indicate municipalities where cases were found.
Figure 2.
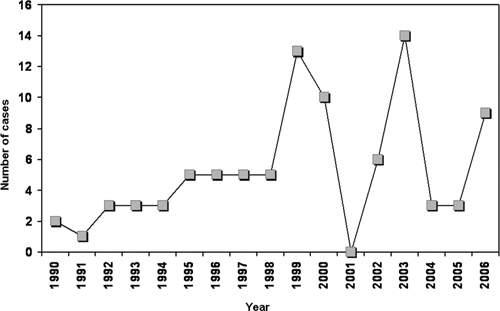
Annual distribution of cases of American visceral leishmaniasis Chiapas, Mexico, 1990–2006.
Figure 3.
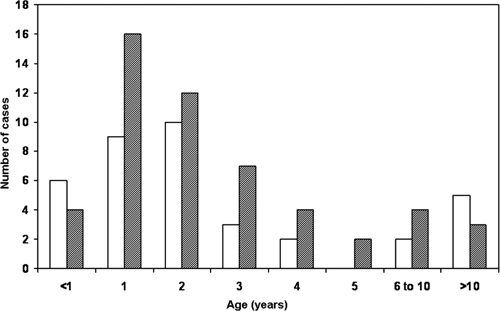
Age and sex of persons with American visceral leishmaniasis, Chiapass State, Mexico, during 1990–2006. Squares = males and stippled squares = females.
Table 1.
Signs and symptoms found in 89 patients with American visceral leishmaniasis in Chiapas State, Mexico
| Sign/symptom | No. patients | % |
|---|---|---|
| Fever | 89 | 100 |
| Hepatomegaly | 89 | 100 |
| Splenomegaly | 89 | 100 |
| Pallor | 89 | 100 |
| Weight loss | 69 | 78 |
| Abdominal pain | 57 | 64 |
| Vomit | 44 | 49 |
| Diarrhea | 28 | 31 |
| Diaphoresis | 18 | 20 |
| Jaundice | 12 | 13 |
| Petechias | 3 | 3 |
For all patients, meglumine antimonite, 60 mg/kg/day (Glucantime; Aventis Pharma, Bridgewater, NJ), which is equivalent to 20 mg of antimony, was administered at the hospital by parenteral route for 20 days. Eleven patients (12%) with suspected AVL were treated on the basis of epidemiologic data because they did not provide samples or results of laboratory diagnosis were negative. Five patients were cured and six were lost to follow-up, as were six from the group that was given laboratory diagnoses. The mortality rate was 35% in the first eight years; only 2 patients (3.5%) died thereafter. The overall mortality rate was 11.7% (9 of 77). Statistical analysis showed significant differences in age, sex, detection period, and sanitary jurisdiction (Table 2).
Table 2.
Demographic characteristics of the patients with American visceral leishmaniasis in Chiapas State, Mexico*
| Characteristic | No. (%) | OR (95% CI) | P |
|---|---|---|---|
| Age, years | |||
| ≤ 5 | 75 (85.2) | 33.28 (13.5–84.5) | < 0.0001 |
| > 5 | 13 (14.8) | ||
| Sex | |||
| M | 53 (59.6) | 2.17 (1.1–4.1) | 0.01 |
| F | 36 (40.4) | ||
| Detection period | |||
| 1999–2006 | 57 (64.0) | 3.17 (1.7–6.1) | 0.0002 |
| 1990–1998 | |||
| Sanitary jurisdiction | |||
| Tuxtla | 69 (77.5) | 11.9 (5.6–25.8) | <0.0001 |
| Others | 20 (22.5) | ||
OR = odds ratio; CI = confidence interval.
For the human serologic study, unadjusted odds ratios for possible risk factors are shown in Table 3. Results indicate that the type of walls, roof, and floor (other than cement), number of rooms per house (1–3), number of bedrooms (1–3), persons per bedroom (≥ 4), not having a television or having it in the bedroom, owning domestic animals, and farm animals were significant risk factors by univariate analysis. The final model of the backwards stepwise multivariate logistic regression analysis is shown in Table 4. Significant risk factors for seropositivity in multivariate analysis were identified. Some factors that were significant risk factors by univariate analysis were not significant risk factors by multivariate analysis. For the canine survey, age and sex are shown in Table 5. Most (72.3%) of the dogs were male and up to one year of age (44.3%). Univariate analysis was performed that compared positive and negative dogs by age, sex, living quarters during the day and at night, veterinary visits, and how many times it was bathed; no significant association was found.
Table 3.
Comparison of persons seropositive and seronegative for American visceral leishmaniasis in Chiapas State, Mexico, by possible risk factors in an univariate analysis*
| Risk factor | No. (%) positive | No. (%) negative | Mantel-Haenszel χ2 | OR (95% CI) | P |
|---|---|---|---|---|---|
| Age, years | |||||
| < 1–5 | 67 (12.1) | 21 (12.6) | 16.50 | – | 0.086 |
| 6–10 | 110 (19.9) | 27 (16.2) | |||
| 11–15 | 84 (15.2) | 18 (10.8) | |||
| 16–20 | 31 (5.6) | 21 (12.6) | |||
| 21–25 | 39 (7.1) | 15 (9.0) | |||
| 26–30 | 50 (9.0) | 13 (7.8) | |||
| 31–35 | 42 (7.6) | 16 (9.6) | |||
| 36–40 | 29 (5.2) | 8 (4.8) | |||
| 41–45 | 14 (2.5) | 8 (4.8) | |||
| 46–50 | 21 (3.8) | 6 (3.6) | |||
| ≥ 51 | 66 (11.9) | 14 (8.4) | |||
| Sex | |||||
| F | 336 (60.5) | 98 (57.6) | 0.45 | 1.13 (0.78–1.62) | 0.500 |
| M | 219 (39.5) | 72 (42.4) | |||
| Walls of house | |||||
| Other | 176 (35.0) | 21 (22.3) | 5.73 | 1.87 (1.08–3.25) | 0.017 |
| Brick | 327 (65.0) | 73 (67.0) | |||
| Roof of house | |||||
| Other | 419 (85.0) | 66 (71.0 | 10.77 | 2.32 (1.34–3.98) | 0.001 |
| Cement | 74 (15.0) | 27 (29.0) | |||
| Floor of house | |||||
| Other | 99 (20.0) | 8 (8.6) | 6.78 | 2.65 (1.19–6.12) | 0.009 |
| Cement | 397 (80.0) | 85 (91.4) | |||
| Separation of roof/wall | |||||
| Yes | 337 (66.5) | 57 (59.4) | 1.79 | 1.36 (0.85–2.17) | 0.1808 |
| No | 170 (33.5) | 39 (40.6) | |||
| Rooms per house | |||||
| 1–3 | 398 (81.6) | 61 (64.2) | 14.27 | 2.46 (1.49–4.08) | 0.00016 |
| ≥ 4 | 90 (18.4) | 34 (35.8) | |||
| Persons per house | |||||
| ≥ 4 | 424 (81.9) | 78 (75.0) | 4.30 | 1.58 (1.00–2.48) | 0.0381 |
| 1–3 | 94 (18.1) | 26 (25.0) | |||
| No. bedrooms | |||||
| 1–3 | 479 (98.2) | 88 (92.6) | 9.08 | 4.23 (1.38–12.81) | 0.0025 |
| ≥ 4 | 9 (1.8) | 7 (7.4) | |||
| Persons per bedroom | |||||
| ≥ 4 | 257 (52.7) | 34 (35.8) | 9.04 | 2.00 (1.24–3.23) | 0.0026 |
| 1–3 | 231 (47.3) | 61 (64.2) | |||
| Electricity | |||||
| Yes | 494 (97.8) | 89 (97.8) | 0.00 | 1.01 (0.11–4.74) | 0.9906 |
| No | 11 (2.2) | 2 (2.2) | |||
| Light type | |||||
| Yellow | 329 (66.1) | 55 (59.8) | 1.35 | 1.31 (0.81–2.12) | 0.2459 |
| White | 169 (33.9) | 37 (40.2) | |||
| Owns television | |||||
| No | 105 (20.6) | 5 (5.1) | 13.33 | 4.83 (1.84–13.85) | 0.00023 |
| Yes | 404 (79.4) | 93 (94.9) | |||
| Television location | |||||
| Bedroom | 82 (22.7) | 10 (12.2) | 4.44 | 2.11 (1.00–4.56) | 0.0351 |
| Living room | 280 (77.3) | 72 (87.8) | |||
| Owns domestic animals | |||||
| Yes | 399 (81.3) | 57 (65.5) | 10.98 | 2.28 (1.35–3.86) | 0.00091 |
| No | 92 (18.7) | 30 (34.5) | |||
| Owns farm animals | |||||
| Yes | 429 (86.0) | 69 (72.6) | 10.46 | 2.31 (1.33–3.99) | 0.0012 |
| No | 70 (14.0) | 26 (27.4) | |||
| Owns chickens | |||||
| No | 49 (11.4) | 5 (7.2) | 1.06 | 1.67 (0.60–4.89) | 0.3036 |
| Yes | 381 (88.6) | 64 (92.8) | |||
| Owns horses | |||||
| Yes | 118 (27.4) | 13 (18.8) | 2.27 | 1.63 (0.83–3.26) | 0.1321 |
| No | 312 (72.6) | 56 (81.2) | |||
| Owns pigs | |||||
| No | 314 (73.0) | 48 (69.6) | 0.36 | 1.18 (0.65–2.13) | 0.5506 |
| Yes | 116 (27.0) | 21 (30.4) | |||
| Adjacent corral | |||||
| No | 151 (35.3) | 16 (23.5) | 3.62 | 1.77 (0.95–3.36) | 0.0570 |
| Yes | 277 (64.7) | 52 (76.5) | |||
| Domestic fowl | |||||
| No | 103 (34.2) | 6 (26.1) | 0.63 | 1.47 (0.53–4.33) | 0.427 |
| Yes | 198 (65.8) | 17 (73.9) | |||
OR = odds ratio; CI = confidence interval.
Table 4.
Risk factors associated to seropositivity in patients with American visceral leishmaniasis in Chiapas State, Mexico, defined by multivariate analysis using a logistic regression model*
| Variables | Regression coefficient | SE | OR (95% CI) | P |
|---|---|---|---|---|
| One to three rooms per house | 0.904 | 0.266 | 2.5 (1.47–4.16) | < 0.001 |
| Roof of house (other than cement) | 0.642 | 0.302 | 1.9 (1.05–3.43) | 0.033 |
| Presence of domestic animals | 0.583 | 0.278 | 1.8 (1.04–3.09) | 0.036 |
OR = adjusted odds ratio; CI = confidence interval.
Table 5.
Characteristics of dogs in Chiapas State, Mexico
| Age, years/sex | No. positive | % | No. negative | % | Total | % Total |
|---|---|---|---|---|---|---|
| 0–1 | 13 | 46.4 | 49 | 43.8 | 62 | 44.3 |
| 1.1–2 | 5 | 17.9 | 26 | 23.2 | 31 | 22.1 |
| 2.1–3 | 4 | 14.3 | 13 | 11.6 | 17 | 12.1 |
| 3.1–4 | 6 | 21.4 | 10 | 8.9 | 16 | 11.4 |
| 4.1–5 | 0 | 0.0 | 3 | 2.7 | 3 | 2.1 |
| 5.1–6 | 0 | 0.0 | 4 | 3.6 | 4 | 2.9 |
| 6.1–7 | 0 | 0.0 | 5 | 4.5 | 5 | 3.6 |
| 7.1–10 | 0 | 0.0 | 2 | 1.8 | 2 | 1.4 |
| M | 21 | 72.4 | 81 | 72.3 | 102 | 72.3 |
| F | 8 | 27.6 | 31 | 27.7 | 39 | 27.7 |
In the entire serologic study, the global prevalence was 77% in humans and 18% in dogs. The highest prevalence for humans (99.3%) was in the community of Rio Jordan (Venustiano Carranza municipality). The highest prevalence for dogs (48%) was in the community of Barrio Santa Cruz (La Concordia municipality) (Table 6).
Table 6.
Serorevalence of leishmaniasis in humans and dogs in a stable transmission zone in Chiapas State, Mexico
| Municipality (no.) | Community | Human samples | Dog samples | ||||
|---|---|---|---|---|---|---|---|
| Total | No. positive | Prevalence, % | Total | No. positive | Prevalence, % | ||
| Tuxtla Gutierrez (101) | El Cebollal | 111 | 88 | 79.28 | 45 | 3 | 6.67 |
| Patria Nueva | 53 | 8 | 15.09 | ||||
| Chiapa de Corzo (027) | Ribera Cahuare | 11 | 6 | 54.55 | 82 | 14 | 17.07 |
| Venustiano Carranza (106) | Paraiso del Grijalva | 121 | 119 | 98.35 | 22 | 3 | 13.64 |
| Rio Jordan | 139 | 138 | 99.28 | 18 | 2 | 11.11 | |
| San Bartolome | 60 | 45 | 75.00 | 15 | 3 | 20.00 | |
| La Concordia (020) | Barrio La Candelaria | 100 | 72 | 72.00 | 19 | 7 | 36.84 |
| Barrio Santa Cruz | 117 | 78 | 66.67 | 23 | 11 | 47.83 | |
| Acala (002), San Fernando (079), and Suchiapa (086) | Several communities | 14 | 2 | 14.29 | |||
| Total | 726 | 556 | 76.58 | 224 | 43 | 19.20 | |
Discussion
This study provides results of an analysis of AVL in Chiapas, Mexico. Furthermore, it identifies associations of sociodemographic factors with antibodies against Leishmania in humans and dogs in Chiapas. The much higher mortality rate found in the first half of the study was likely caused by suboptimal management of patients related to lack of knowledge of the disease. It is evident that the group affected most often by AVL is young children, a finding that is consistent with others areas to which visceral leishmaniasis is endemic14–16 because the risk for being a patient in children less than five years of age was 33 times higher than in older persons. The high susceptibility to infection can be explained by the absence of long-term immunity in infants less than one year of age, which generates reduced levels of immunity in childhood.17–19 In Brazil, infections, hemorrhages, and severe anemia are responsible for most deaths caused by leishmaniasis, and late diagnosis, young age at disease onset, and malnutrition are important contributing mortality factors.18
None of these elements was stated in the medical records of the cases of Chiapas. However, poverty is a contributing factor that may influence malnutrition and low immunity in the patients,20 and the communities in this study had populations with poor resources, poor education, and difficult access to health services. Statistical differences in sex were also found, indicating that males had a higher risk for disease than females. When the periods of detection were compared, a significant difference was found in 1999–2006, suggesting that the disease incidence is increasing, mainly in the sanitary jurisdiction of Tuxtla where the cases were found. When we considered case distribution, we found that active transmission occurred in communities 50–900 meters above sea level in areas adjacent to the Grijalva River and the Angostura Dam, where conditions are suitable for sand fly development.
Nevertheless, five cases were found in the highlands of Chiapas, where the climate is cold and the vector is absent. These five cases were in persons likely infected in the region of the Angostura Dam, where their parents worked for several months and to which their families moved, which is a common practice in field labor. Communities where cases were found were mostly villages located on the outskirts of large towns, where nearby forested areas facilitate transmission, establishing a direct link between sylvatic and urban cycles of AVL. This finding is consistent with urban transmission documented in some major cities.21–23
Furthermore, the prevalence of seropositive persons was high (77%) because sampling took place in the blocks surrounding houses of patients with AVL. Interestingly, 1–3 rooms in the house, as opposed to ≥ 4, or having a roof not made of cement had a significant impact on the presence of antibodies against Leishmania. This finding may be related to poor and crowded households, to which sand flies are particularly attracted by the concentration of human odor and CO2.24 The latter observation is also true for domestic animals because their presence was statistically associated with AVL.
Contrary to what was expected, a much lower positivity was found in dogs living in the same areas compared with other countries with zoonotic cycles, such as Brazil and southern Europe, where reported canine infection rates are as high as 35–80%.25–27 The low prevalence we report may indicate that dogs are not as highly susceptible to bites of the main AVL vector, Lu. longipalpis in Mexico, as they are elsewhere.5 Alternatively, the low prevalence can be the result of high mortality rates in infected dogs because of the disease, inadequate nutrition, or habitat conditions. However, the rate of acquiring new dogs in these communities is high, given that most (66.4%) of the dogs found were not yet two years of age. The low frequency of antibodies in dogs suggests that humans are the main reservoir in this area because some reports have proposed anthroponotic transmission in areas considered zoonotic.21,26 Additionally, wild and domestic animals other than dogs may also serves as reservoirs in the establishment of the zoonotic cycle, as was described for goats in Nepal,28 Didelphis marsupialis in Colombia,29 and several rodents and domestic animals in Brazil.30 However, contradictory results have been reported because the presence of cattle or buffaloes is associated with lower risk for acquiring the disease in Bangladesh and Nepal,31,32 suggesting that these animals are a preferred blood source. However, an alternative explanation is that large animals indicate a higher socioeconomic status and therefore a better nutritional condition that may prevent the progression from subclinical infection to clinical visceral leishmaniasis.
In conclusion, active transmission of AVL is occurring in the central valley of Chiapas, Mexico. Eighty-nine cases of AVL were reported over a 16-year period. A seroprevalence study of 726 persons and 224 dogs showed that the prevalence of AVL in humans was high; only 19% dogs were seropositive. These results suggest that dogs are not important in the transmission cycle of Leishmania. We also identify risk factors associated with AVL in this region of Mexico.
Footnotes
Financial support: This study was partially supported by grant I30103-B from the Consejo Nacional de Ciencia y Tecnología.
Authors' addresses: Jorge A. Pastor-Santiago, Departamento de Prevención y Control de Enfermedades Transmitidas por Vectores, Unidad Administrativa, Edificio C, Segundo Piso, Colonia Maya, C.P. 29007, Tuxtla Gutiérrez, Chiapas, México, E-mail: albertosan.pas@gmail.com. Susana Chávez-López and Carmen Guzmán-Bracho, Departamento de Parasitología, Instituto de Diagnóstico y Referencia Epidemiológicos, SSA, Carpio 470 2° Piso, Colonia Santo Tomas, C.P. 11340, México City, México, E-mails: sus_bte@yahoo.com.mx and cguzman@salud.gob.mx. Ana Flisser, Departamento de Microbiología y Parasitología, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México, E-mail: flisser@servidor.unam.mx. Angélica Olivo-Díaz, Departamento de Biología Molecular e Histocompatibilidad, Hospital General Dr. Manuel Gea González, SSA, Calzada de Tlalpan 4800, Colonia Sección XVI, C.P. 14080, México City, México, E-mail: aolivod@yahoo.com.
References
- 1.Velasco-Castrejón O. Las leishmaniasis en México. Rev Latinoam Microbiol. 1987;29:119–126. [PubMed] [Google Scholar]
- 2.Arias JR, Beltrán F, Desjeux P, Walton B. Epidemiología y Control de las Leishmaniasis en las Américas, por País o Territorio. Cuaderno Técnico: OPS; 1996. p. 44. [Google Scholar]
- 3.Braga RR, Lainson R, Shaw JJ, Ryan L, Silveira FT. Leishmaniasis in Brazil. XXII: Characterization of Leishmania from man, dogs and the sandfly Lutzomyia longipalpis (Lutz & Neiva, 1912) isolated during an outbreak of visceral leishmaniasis in Santarem, Para State. Trans R Soc Trop Med Hyg. 1986;80:143–145. doi: 10.1016/0035-9203(86)90214-2. [DOI] [PubMed] [Google Scholar]
- 4.Velasco O, Guzmán-Bracho C, Ibáñez-Bernal S, Rivas B. In: Enfermedades Tropicales en México. Diagnóstico, Tratamiento y Distribución Geográfica, México. Valdespino JL, Velasco O, Escobar A, Del Río A, Ibáñez-Bernal S, Magos C, editors. Mexico City: Secretaría de Salud, INDRE; 1994. pp. 293–308. (Leishmaniasis). [Google Scholar]
- 5.Ibáñez-Bernal S, Rodríguez-Domínguez G, Gómez-Hernández CH, Ricardez-Esquinca JR. First Record of Lutzomyia evansi (Nuñez-Tovar 1924) in México (Diptera: Psychodidae, Phlebotominae) Mem Inst Oswaldo Cruz. 2004;99:127–129. doi: 10.1590/s0074-02762004000200002. [DOI] [PubMed] [Google Scholar]
- 6.Baez-Villaseñor J, Ruiloba J, Rojas E, Treviño A, Campillo C. Presentación de un caso de kala-azar. Rev Invest Clin México. 1952;4:57–78. [PubMed] [Google Scholar]
- 7.Aguirre A, Biagi F, Hernández-Nieto A. Segundo caso autóctono de kala-azar en México. Leishmaniasis visceral. Bol Med Hosp Infant Mex. 1963;20:317–333. [PubMed] [Google Scholar]
- 8.Biagi FF, Tay J. Observaciones sobre un nuevo foco endémico de kala-azar en México. Rev Fac Med UNAM. 1963;5:7–11. [Google Scholar]
- 9.Biagi F, López R, De Biagi AM. El kala-azar en México; problema ecológico por estudiar. Rev Inst Salubr Enferm Trop Mex. 1965;25:3–12. [PubMed] [Google Scholar]
- 10.Quiñones A, Galindo PL, Halabe J, Butrón-Pérez L, Velasco O, Lisfshitz A. Leishmaniasis visceral (kala-azar). Informe de un adulto mexicano. Rev Med IMSS (Mex) 1989;27:49–52. [Google Scholar]
- 11.SSA . Norma Oficial Mexicana de Emergencia para la Vigilancia, Prevención y Control de Enfermedades Transmitidas por Vector. 1999. NOM-EM-001-SSA2-1999. [Google Scholar]
- 12.SSA . Norma Oficial Mexicana para la Vigilancia Epidemiológica, Prevención y Control de Enfermedades Transmitidas por Vector. 2002. NOM-032-SSA2-2002. [Google Scholar]
- 13.Olivo-Díaz A, Chávez LS, Guzmán BC. Manual de Procedimientos de Técnicas Diagnósticas del Laboratorio de Leishmaniosis. Mexico City: CENAVECE-InDRE; 2006. [Google Scholar]
- 14.Dursun O, Erişir S, Yeşilipek A. Visceral childhood leishmaniasis in southern Turkey: experience of twenty years. Turk J Pediatr. 2009;51:1–5. [PubMed] [Google Scholar]
- 15.Mueller Y, Mbulamberi DB, Odermatt P, Hoffmann A, Loutan L, Chappuis F. Risk factors for in-hospital mortality of visceral leishmaniasis patients in eastern Uganda. Trop Med Int Health. 2009;14:910–917. doi: 10.1111/j.1365-3156.2009.02305.x. [DOI] [PubMed] [Google Scholar]
- 16.Rey LC, Martins CV, Ribeiro HB, Lima AA. American visceral leishmaniasis (kala-azar) in hospitalized children from an endemic area. J Pediatr (Rio J) 2005;81:73–78. [PubMed] [Google Scholar]
- 17.Schaefer KU, Kurtzhals JA, Sherwood JA, Githure JI, Kager PA, Muller AS. Epidemiology and clinical manifestations of visceral and cutaneous leishmaniasis in Baringo District, Rift Valley, Kenya. A literature review. Trop Geogr Med. 1994;46:129–133. [PubMed] [Google Scholar]
- 18.Queiroz MJ, Alves JG, Correia JB. Visceral leishmaniasis: clinical and epidemiological features of children in an endemic area. J Pediatr (Rio J) 2004;80:141–146. [PubMed] [Google Scholar]
- 19.Shamsizadeh A, Nikfar R, Maraghi S, Zaker N. Pediatric visceral leishmanisis in the south west part of Iran: a study of 215 cases. Pak J Med Sci. 2006;22:461–464. [Google Scholar]
- 20.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Costa CH, Pereira HF, Araújo MV. Epidemia de leishmaniose visceral no estado do Piauí, Brazil, 1980–1986. Rev. Saude. Publica. Sao Paulo. 1990;24:361–372. doi: 10.1590/s0034-89101990000500003. [DOI] [PubMed] [Google Scholar]
- 22.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 23.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 24.Pinto MC, Campbell-Lendrum DH, Lozovei AL, Teodoro U, Davies CR. Phlebotomine sandfly responses to carbon dioxide and human odour in the field. Med Vet Entomol. 2001;15:132–139. doi: 10.1046/j.1365-2915.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 25.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis. 2002;186:1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- 26.Dietze R, Barros GB, Teixeira L, Harris J, Michelson K, Falqueto A, Corey R. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis. 1997;25:1240–1242. doi: 10.1086/516096. [DOI] [PubMed] [Google Scholar]
- 27.Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol. 2001;39:560–563. doi: 10.1128/JCM.39.2.560-563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattarai NR, Van der Auwera G, Rijal S, Picado A, Speybroeck N, Khanal B, De Doncker S, Das ML, Ostyn B, Davies C, Coosemans M, Berkvens D, Boelaert M, Dujardin JC. Domestic animals and epidemiology of visceral leishmaniasis, Nepal. Emerg Infect Dis. 2010;16:231–237. doi: 10.3201/eid1602.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travi BL, Jaramillo C, Montoya J, Segura I, Zea A, Goncalves A, Velez ID. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am J Trop Med Hyg. 1994;50:557–565. doi: 10.4269/ajtmh.1994.50.557. [DOI] [PubMed] [Google Scholar]
- 30.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136:1915–1934. doi: 10.1017/S0031182009991156. [DOI] [PubMed] [Google Scholar]
- 31.Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Haque R, Kurkjian K, Vaz LE, Begum M, Akter T, Cetre-Sossah CB, Ahluwalia IB, Dotson E, Secor WE, Breiman RF, Maguire JH. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bern C, Joshi AB, Jha SN, Das ML, Hightower A, Thakur GD, Bista MB. Factors associated with visceral leishmaniasis in Nepal: bed-net use is strongly protective. Am J Trop Med Hyg. 2000;63:184–188. doi: 10.4269/ajtmh.2000.63.184. [DOI] [PubMed] [Google Scholar]


