Abstract
The goal of this study was to present an overview of human infections with Capillaria philippinensis, a new emerging parasite in Upper Egypt. The study included 21 inpatients who had been admitted to the Assiut University Hospital. Patients suffered from intermittent abdominal pain, borborygmi, chronic diarrhea lasting for several weeks, and marked weight loss. Hypoalbuminemia and low serum levels of potassium, calcium, and sodium were detected in most patients. A stool examination was performed using direct smears and the formalin-ether concentration method. Intact adult worms and/or eggs were evaluated using a light microscope and processed for scanning electron microscopy. The examination by light microscopy illustrated the general morphology of different stages. Using scanning electron microscopy, intestinal villi were found partially covering the cuticle of the adult worms, which provided evidence for the invasion of adult worms into the jejunal mucosa. Two distinct types of eggs, thick-shelled and thin-shelled, were identified and measured.
Introduction
Human intestinal capillariasis is a rare parasitosis that is caused by Capillaria philippinensis, a tiny nematode first documented in the Philippines in 1963 by Chitwood and others.1 Subsequently, more than 2,000 cases of intestinal capillariasis have been reported in the Philippines and Thailand, and sporadic cases have been reported in Korea, Japan, Taiwan, India, Iran, Italy, the United Arab Emirates, Spain, and the United Kingdom, which indicates that this infection is widespread.2,3
In Egypt, the first case of intestinal capillariasis was reported by Youssef and others4 in a 41-year-old female from Cairo, the second case was a 38-year-old female living in Lower Egypt.5 Many subsequent cases have been reported from different parts of Egypt, including Cairo6,7 and the Menouf area.3,8
In Upper Egypt, the first reported case was a female patient in the Assiut Governorate,9 and this was followed by a case of two sisters from the El Menia Governorate.10
Intestinal capillariasis is a life-threatening disease in humans that causes severe enteropathy.2 Worms can be found in the lumen, the mucosa, or in the crypts of Lieberkuhn in the human jejunum. The most common pathological features are the thickening of the intestinal wall, the prominence of the vessels, the atrophy of the crypts, and the flattening of the villi.11 The infestation of the small bowel by this parasite results in the severe derangement of intestinal functions, which leads to weight loss, chronic (continuous or intermittent) diarrhea, abdominal pain, borborygmi, muscle wasting, cachexia, weakness, and edema. Laboratory examinations of infected individuals have also showed low levels of potassium and albumin in the blood, the malabsorption of fats and sugars, and the severe reduction in the levels of proteins and electrolytes.12,13
A diagnosis of capillariasis is based on the recovery of eggs, larvae, and/or adult worms from the stool of patients, although multiple stool samples may be required for an early diagnosis in some cases caused by the sporadic excretion of eggs in the feces.14 However, these diagnoses are difficult in non-endemic areas, as this remains an unknown disease among many physicians and laboratory workers.15 In addition, small intestinal aspirations or biopsies may be necessary to confirm the presence of a C. philippinensis infection.11
Although this parasite has been extensively studied over the past 30 years, the only existing description of this nematode is that originally made by Chitwood and others.1 Moravec16 has recently redescribed the adults and eggs of this parasite and has renamed it Paracapillaria philippinensis to replace the longstanding and well-known name C. philippinensis. The classification of this nematode species in this new genus and subgenus was because of recent taxonomic advances.
This study aimed to provide a more detailed view of the rapidly emerging human infections with C. philippinensis in the Assiut Governorate and in Upper Egypt. A detailed description of the different stages detected during the course of the disease may make it easier for laboratory technicians and doctors to identify the parasite and to compare the morphometric data on C. philippinensis in Upper Egypt with other data collected in different countries to identify any adaptive changes in the parasite.
Material and Methods
This study evaluated 21 patients who were admitted to either the Assiut University Hospital in the Department of Gastroenterology and Tropical Diseases (19 cases) or the Pediatric University Hospital (2 cases). These patients had symptoms of chronic diarrhea, abdominal pain, borborygmi, and marked weight loss and cachexia. There were two cases with specific symptoms, one case with a bilateral pleural effusion, a bilateral lower limb edema, moderate ascites, and massive scrotal edema, and another comatose case with generalized edema.
A full history was taken from each patient or the patient's relatives (including age, sex, occupation, address, duration of the illness, food habits, traveling history, and family history). A physical examination was performed on each patient, and chest x-rays and abdominal ultrasonography were also performed on all patients.
Laboratory investigations were performed on samples taken from each patient (these included a urine analysis, a complete blood count, and a serum albumin and serum electrolyte analysis). Stool sample analyses for each patient were carried out twice by the hospital laboratory. The first analysis revealed the presence of Hymenolepis nana eggs in the stools of two patients, Ancylostoma duodenale eggs from one patient, Giardia lamblia cysts from one patient, and Entamoeba coli cysts from one patient. However, the second analysis revealed no parasites in the stool samples of any of the patients.
The patients fresh stool samples were sent to the Parasitology Department in the Faculty of Medicine at Assiut University. Here, these cases were diagnosed as intestinal capillariasis, as no other intestinal parasites were detected in the stool samples.
Parasitological Analysis
A direct smear examination and the formalin-ether concentration technique (three subsequent slides) were performed on each stool sample to detect the presence of C. philippinensis eggs, larvae, and/or adults. These stool examinations were repeated three times over a 1- to 2-day interval when the first examination was negative. The remaining fecal specimens were then processed for scanning electron microscopy (SEM).
The patients (except the comatose one) were each given a single dose of levamisole† (Ketrax, 2.5 mg/kg), which is an antihelmenthic drug that primarily targets nematode nicotinergic acetylcholine receptors and causes paralysis in the muscles of the adult worms17 to mediate the expulsion of intact adult worms in the stool.18 For the next 24 hours after drug administration, stool samples were collected and examined, and the expelled worms were isolated using pasture pipettes. The worms were washed several times in a saline solution, fixed, and stored in 70% ethanol. Before examination, the worms were transferred to 4% formalin and were cleared with glycerin.16 The average measurements of the 20 adult worms were made using a light microscope that was equipped with a calibrated micrometer eyepiece.
For SEM, the worms and eggs were treated for 2 hours with 1% osmium tetroxide at room temperature. After fixation, the samples were rinsed three times with a phosphate buffer solution for 15 min. The specimens were dehydrated using ascending grades of aqueous ethanol (30%, 50%, 70%, and 90%), followed by critical point drying and sputter coating with gold in the sputter-coating apparatus for 6 min. The prepared specimens were examined at the Scanning Electron Microscope Unit of Assiut University with a JEOL-JSM-5400 LV microscope (JEOL, Japan).
Upper gastrointestinal endoscopic examinations were performed on all patients, and intestinal biopsies were taken from the duodenum and the jejunum for histopathological examination. Some patients received colonoscopies.
An examination of the biopsy tissues using SEM was conducted at the Scanning Electron Microscope Unit of Assiut University.
An anti-helminthic treatment was administered to adults (albendazole 200 mg/day) in two daily doses for 3 weeks, and the two children were given doses of 100 mg/day. The follow-up on the patients included a stool examination every month for 6 months following the chemotherapy and supportive treatments.
The intestines and the muscles of fish commonly consumed by patients were examined.
Two types of wild freshwater fish, the bolty (Oreochromis niloticus) and the karmout (catfish, Clarias species), were used for experimental infections. Ten bolty and 10 karmout fish, which weighed between 100 and 150 grams, were purchased directly from fishermen in the Assiut Governorate, and these fish were maintained in a tank at the Department of Parasitology. Stool samples that contained eggs or eggs and larvae were concentrated and washed in a phosphate buffered saline (PBS) solution. For the samples that contained larvae, one part of the sample was added directly to water, whereas the other parts as well as those samples only containing eggs were embryonated in dechlorinated water for 5 days at room temperature. The eggs were examined on Day 5, and the viability of the larvae was confirmed by the detection of their movement inside the eggs. Embryonated eggs were then mixed with food and added to the water in the tank after the fish had been denied food for one day. The fish were killed at 7 days (4 fish), 14 days (3 fish), and 21 days (3 fish) post-infection. The intestines were eviscerated and opened on a Petri dish containing PBS and were examined for the presence of larvae using a stereoscope and a light microscope.19,20
Results
Twenty-one cases of C. philippinensis were diagnosed in the Assiut Governorate from May 2007 to January 2009. These diagnoses were made by experienced parasitologists in the Parasitology Department of the Faculty of Medicine at Assuit University.
Clinical data.
The majority of the cases were of females ranging from 25 to 50 years of age, although two of the cases were of 9-year-old boys. Of these cases, 18 were from the Assiut Governorate, 2 were from the El Menia Governorate, and 1 (a male case) was from the Aswan Governorate. The majority of the female patients were housewives, although one was a schoolteacher. One of the male patients worked in the fishing industry with his father, and occasionally, they ate grilled fish on the fishing boat. There was no history of raw fish consumption for any of the patients, and there was no history of traveling abroad, although one patient had a history of traveling to the Fayoum Governorate (a northern part of Upper Egypt). In addition, the patients did not provide family histories that described similar conditions.
The duration of the illness varied from 3 to 18 months and involved chronic diarrhea.
Each of the cases showed clinical symptoms suggestive of C. philippinensis infection, including intermittent abdominal pain, borborygmi, chronic (continuous or intermittent) diarrhea for several weeks, marked weight loss (up to 10 kg in some patients), and lower leg edema of varying degrees. One patient was comatose.
Indications of muscle wasting and weakness were reported in most of the patients. The detailed clinical data are shown in Table 1.
Table 1.
Clinical data of capillariasis patients in Upper Egypt
| Case no. | Date of admission | Occupation | Age (year) | Parasites diagnosed at first stool examination at hospital | Duration from onset to diagnosis (m) | Chronic diarrhea | Abdominal borborygmi | Body weight loss in kilograms | Anemia g/dL | Eosinophil ratio | Hypo- albuminemia g/dL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | May 2007 | Housewife | 32 | – | 12 | + | + | 10 | 9 | 7 | 0.9 |
| 2 | June 2007 | Housewife | 29 | – | 10 | + | + | 8 | 8 | 7 | 0.9 |
| 3 | June 2007 | Housewife | 35 | Hymenolepis nana egg | 3 | + | + | – | 12 | 4 | 2 |
| 4 | July 2007 | Housewife | 39 | – | 7 | + | + | 6 | 10.5 | 6 | 1.5 |
| 5 | July 2007 | Teacher* | 50 | – | 18 | + | + | 10 | 7 | 7 | 0.5 |
| 6 | Aug. 2007 | Housewife | 25 | – | 3 | + | + | – | 11 | 5 | 2 |
| 7 | September 2007 | Housewife | 40 | Giardia lamblia cyst | 5 | + | + | – | 11 | 4 | 1.9 |
| 8 | September 2007 | Housewife | 36 | – | 9 | + | + | 8 | 8 | 7 | 1.6 |
| 9 | November 2007 | Housewife | 25 | – | 8 | + | + | 7 | 10.5 | 5 | 1.8 |
| 10 | December 2007 | Male child | 9 | Entamoeba coli cyst | 6 | + | + | 7 | 10 | 4 | 0.8 |
| 11 | March 2008 | Housewife | 46 | – | 11 | + | + | 9 | 9 | 7 | 0.8 |
| 12 | April 2008 | Housewife | 42 | – | 9 | + | + | 6 | 10 | 6 | 0.9 |
| 13 | April 2008 | Housewife | 35 | – | 6 | + | + | – | 11 | 5 | 1.8 |
| 14 | May 2008 | Housewife | 35 | – | 4 | + | + | – | 11 | 5 | 1.8 |
| 15 | May 2008 | Housewife | 28 | – | 3 | + | + | – | 12 | 4 | 1.8 |
| 16 | June 2008 | Housewife | 36 | – | 6 | + | + | – | 12 | 6 | 2 |
| 17 | June 2008 | Housewife | 44 | – | 10 | + | + | 7 | 8 | 7 | 0.8 |
| 18 | July 2008 | Housewife | 42 | Ancylostoma duodenale egg | 3 | + | + | – | 11 | 4 | 2 |
| 19 | September 2008 | Male child (fisherman) | 9 | Hymenolepis nana egg | 5 | + | + | 5 | 10.5 | 3 | 1.5 |
| 20 | September 2008 | Housewife | 25 | – | 14 | + | + | 10 | 7 | 6 | 1 |
| 21 | January 2009 | Housewife | 25 | – | 8 | + | + | 9 | 10 | 7 | 1 |
This case was diagnosed by the histopathological examination following an upper gastrointestinal endoscopic biopsy, and the patient died just after starting treatment.
The laboratory findings revealed the presence of anemia in 10 cases (hemoglobin level of 10 mg/dL or less) and an eosinophil ratio of 6–8% in 11 cases.
Hypoalbuminemia (albumin levels of 0.5–2.0 mg/dL) and low serum levels of potassium (1.45 mmol/L), sodium (< 130 mmol/I), and calcium (3.5–5 mg/dL) were detected in each of the cases.
Abdominal ultrasonography revealed the existence of distended, small intestinal loops with thickened walls in each of the patients. Ascites and a pleural effusion were present in one female comatose patient and in one male child.
Diagnostic measures (parasitological diagnosis).
-
•
The diagnosis was based on the presence of C. philippinensis eggs (Figure 1A) in the stools of the majority of patients, although one patient had eggs, larvae, and adults in the stool (Figure 1B). Charcot-Leyden crystals were detected in the stools of most patients, and they varied in number and size. The number of these crystals was generally found to be higher when the clinical manifestation was more severe and the number of eggs in the stool was higher. However, these crystals were not detected in the stools of the two male children (Figure 1C).
-
•
The gastroduodenoscopies revealed segmented, erythematous swelling, and dilatation of the proximal jejunal mucosa and thickening of the small intestinal folds (suggesting malabsorption).
-
•
The histopathological studies revealed the presence of atrophic flattened villi; a hypertrophic crypt layer with a moderate infiltration of eosinophils, lymphocytes, polymorphonuclear leukocytes, and plasma cells; and eosinophilic granulomata. Numerous worm sections were found in the jejunal mucosa (Figure 1D). The cross-sectioned worms had cuticular body walls, and stichocytes were present in the pseudocoelomic body cavity. Chains of stichocytes were found in the semi-thin sections of the obliquely sectioned worms.
-
•
A transmission electron microscopy examination of the biopsy tissues found adult worms in the epithelial tunnels, but they did not penetrate the basal lamina. The cells that were in direct contact with the worms showed evidence of degeneration, including swollen mitochondria and distended rough endoplasmic reticulum (Figure 1E). Atrophy of the villous surface of the intestinal wall was also found.
-
•
After treatment, all of the patients were cured. They returned to their normal weights within a few months, and relapses were not observed within the 3–6 months duration following the chemotherapy and the supportive treatments. Only one female patient died; she had begun to receive her medication at the time of death.
-
•
An examination of the commonly consumed types of fish, including bolty (O. niloticus), boury (Mugil), and karmout (cat fish, Clarias species), did not reveal the presence of C. philippinensis at any stage.
-
•
The experimental trial infections of the fresh water fish with C. philippinensis failed, as no larvae were detected in the intestines.
Figure 1.
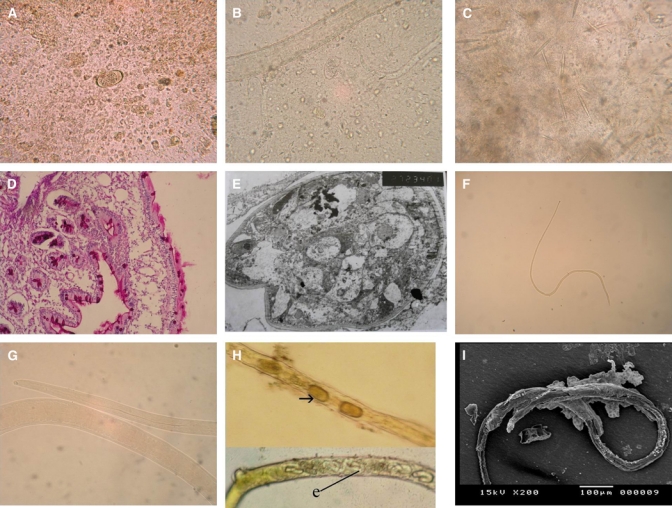
A, Translucent or yellowish, barrel-shaped, and thin-shelled Capillaria philippinensis eggs with rounded mucoid plugs protruding from both poles (×400). B, Adults, larvae, and distorted eggs in the stool of the same patient (×400). C, Charcot-Leyden crystals in the stool; they varied in number and size (×400). D, Jejunal biopsy showing numerous worm sections invading the mucosa (×400). E, Transmission electron microscopy of the jejunal mucosa showing the adult worm, which appears to lie in direct contact with an epithelial cell cytoplasm, with swollen mitochondria, distended rough endoplasmic reticulum, and atrophy of the villous surface of the intestinal wall. F, Capillaria philippinensis adult female; a: anterior end and p: posterior end (×40). G, Capillaria philippinensis esophagus; m: muscular portion and c: cellular portion (×400). H, The uterus contained either thin (e) or thick-shelled (arrow) eggs that were arranged in one row in the direction of the vulva (×200). I, SEM of an adult female with an envelope-like membrane covering most of the body surface.
Morphology of adult female worms.
Examinations using light microscopy.
The adult female was found to be a small, fine nematode. Its body length ranged from 2.8 to 3.4 mm, and the maximum width at the posterior part of the body was between 33.6 and 37.7 μm (Figure 1F). The length of the entire esophagus measured 1.3–1.4 mm; the esophagus was composed of a short muscular portion of 111–192 μm in length and a long cellular portion of 1.1–1.2 mm in length, which was formed from a single row of stichocytes with indistinct nuclei. The stichocytes on the posterior portion appeared somewhat larger than those on the anterior portion (Figure 1G). The vulva was located 24–36 μm posterior to the esophagus. The uterus contained either thin- or thick-shelled eggs with defined or ill-defined embryos. The eggs were arranged in one row, and some were found embryonating into larvated eggs in the direction of the vulva (Figure 1H). The posterior end of the body was rounded, and the anus was distinctly subterminal.
Examinations using SEM.
The adult female appeared slender and had a hair-like, envelope-like membrane that covered most of the body surface (Figure 1I). There were bunches of intestinal villi covering the worms in many areas (Figure 2J). The cuticle was deeply striated transversely and longitudinally and had a small zigzag cuticular expansion that extended the entire body length and terminated near the posterior end (Figure 2K).
Figure 2.
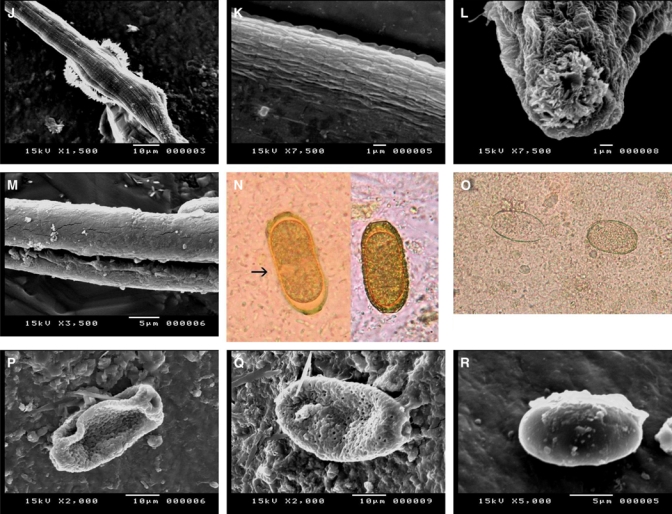
J, SEM showing bunches of intestinal villi covering the worms in many places. K, SEM showing a striated cuticle both transversely and longitudinally and a small zigzag cuticular expansion. L, SEM showing the anterior end, a mouth opening plugged with intestinal villi and wrinkled, backwardly directed ridges. M, SEM showing a wide, long cleft on the undersurface of the body of a worm. N, Yellowish-brown, barrel- or slipper-shaped thick-shelled eggs (arrow) with inconspicuous flattened bipolar plugs (×1,000). O, Abnormally swollen eggs with the loss of one or both mucoid plugs (×1,000). P, SEM showing the typical elongated Capillaria egg with deeply concave polar plugs and a large circular ridge connecting both poles at each side of the egg with the distinct coarse, dense net-like structure. Q, SEM showing the swollen peanut-shaped eggs with an irregular orange peel-like surface and slightly protruding plugs. R, SEM showing swollen, thin-shelled eggs with a smooth surface and non-apparent polar plugs.
At the anterior end, there was a moderately wide mouth opening that had wrinkled and backward ridges radiating from it (Figure 2L). A wide longitudinal cleft was seen on the worm's body (Figure 2M).
Morphology of the eggs.
Examinations using light microscopy.
Two types of C. philippinensis eggs were seen:
-
I.
The thick-shelled eggs were barrel-shaped and had a yellowish-brown color. They measured between 40 and 48 μm in length and 17 and 20 μm in width, and these eggs had inconspicuous flattened bipolar plugs that were 2–3.6 μm long and 9–12 μm wide. The wall thickness was 2–3 μm. The egg shells appeared to have the following three layers: an outer layer that was fine and densely pitted, a middle layer that was thick and radially striated, and an inner hyaline layer. These eggs most often contained a single cell stage embryo that filled most of the egg but that left a small and indistinct clear space between the egg shell and the embryo. There was a slight concavity to the middle third of some of the eggs, which gave them a slipper-shaped appearance (Figure 2N).
-
II.
The thin-shelled eggs were also barrel shaped and measured between 38 and 45.6 μm in length and 18 and 20 μm in width. These eggs were translucent or yellowish in color, and they had single cell-stage embryos with two characteristically rounded mucoid plugs protruding from both of the poles (Figure 1A).
Regardless of egg shell thickness, abnormally swollen eggs with the loss of one or both of the mucoid plugs were observed. These eggs appeared unembryonated (unfertilized) and contained degenerated cells or refractile granules that partially or completely filled the inner space of the egg (Figure 2O).
By SEM.
Two distinctly peanut-shaped thick-shelled eggs were detected, and these had either the typical elongated or swollen appearance. The Capillaria egg with the elongated appearance had deeply concave polar plugs and a large circular ridge, which connected both of the poles at one side of the egg to a distinct coarse and dense net-like structure (Figure 2P). The swollen peanut-shaped egg had an irregular orange peel-like surface and scattered holes of different sizes and slightly protruding plugs (Figure 2Q).
The thin-shelled eggs appeared swollen with a smooth surface, and polar plugs at each end were not apparent (Figure 2R).
Discussion
It has been clearly demonstrated that the severity of intestinal capillariasis cases has grown in Egypt and that Egypt is the only country outside of the endemic area with a relatively high number of reported cases.21
In this study, we described and reported the first series of patients with C. philippinensis infections outside of endemic areas, as previous authors have reported only on single or a small number of cases. The cases reported in our study presented within a year and a half of each other, which is unusual because these cases had previously appeared only sporadically. This cluster of cases suggests that C. philippinensis infections have spread across Egypt and may also indicate the presence of a suitable natural intermediate host. These results appear to answer the question posed by Ahmed and others7 as to whether C. philippinensis infection will spread further across Egypt.
The actual prevalence of this parasite is likely greater than it has been estimated to be, but physician incognizance and technician inexperience at clinical laboratories as well as the availability of over-the-counter anti-helminthic and intestinal antiseptics have hindered its identification.13 These inconsistencies explain why each of the cases in this study was diagnosed only by the laboratory of the parasitology department. It remains unclear how this disease, which is endemic in the Far East, began appearing in Egypt and other Middle East countries, although it is possible that some of the imported species of fresh water fish from the Far East were infected. It has been suggested that some fish-eating migratory birds may acquire this infection by consuming infected fish along their migratory pathways and that the birds could then excrete eggs to infect fish in other remote areas.21 However, Ahmed and others7 disproved this theory because birds of the Far East migrate to Europe, and it would be unlikely that they could have transmitted this parasite to Egypt. Furthermore, if this theory was true, the northern parts of Egypt should be more greatly affected than Upper Egypt. Additional hypotheses have suggested that imported fish are the more likely source of this infection in Egypt, as most of the frozen fish sold at Egyptian markets are imported from the Far East. However, because this fish is frozen at very low temperatures for a lengthy amount of time before consumption makes this theory difficult to accept. All of the cases detected in this study were from middle-aged females (25–50 years of age). In addition, most of these individuals were housewives of the lower middle class, although one woman was a schoolteacher. The age of these female patients indicates that they are active housewives who manage the household responsibilities including food preparation.21 The infected individuals denied eating raw fish, which is not an Egyptian custom. However, almost all of the patients included in this study revealed a history of eating bolty (O. niloticus), which is a popular fish with a reasonable price. Other types of fish, such as boury (Mugil) and karmout (catfish, Clarias species), were also consumed. The preponderance of female cases may be related to the role of these individuals in the preparation and cooking of the fish for the family, as they would have greater exposure to raw, infected fish than would males. However, this hypothesis would not be supported by the infection pattern of endemic areas in the Philippines and Taiwan, where the disease is more prevalent among middle-aged men who work in agricultural activities besides fishing and routinely eat the freshly trapped fish.12,21
Each of the cases in this study presented with various degrees of intestinal manifestations, which included intermittent abdominal pain, borborygmi, chronic (continuous or intermittent) diarrhea lasting several weeks, and marked weight loss (up to 10 kg). In addition, these patients had various degrees of painless lower leg edema, although two cases had manifestations of generalized edema (pleural effusion, ascites), and one male child experienced scrotal edema. These symptom data are in agreement with those of previous studies, which have documented the absence of asymptomatic patients and patients with only mild symptoms. Although a family history is very important in this type of study because family members usually consume the same meals, the presence of more than one infected family member was not significant in this study. This result may be because the infectious larvae are found in the fish gut and that infections typically result from individuals who have eviscerated fish and carry the infectious organisms under their fingernails.21
Although mebendazole has been the drug of choice since 1975,22 albendazole administered in two doses of 200 mg/day for at least 3 weeks was the drug used for treatment of patients in this study. The improvement was rapid and remarkable in all of the patients, and there were no recurrences and only one death. The one female patient who died had begun to receive her medication, and her death was a result of the irreversible effects of electrolyte loss (hypokalemia) that resulted in heart failure and the septicemia that was a consequence of a secondary bacterial infection. The patients in this study had hyponatremia, hypocalcaemia, hypokalemia, anemia, and hypoalbumina, which was similar to the conditions of patients in previous studies.7,12,21
The most common method used to diagnose intestinal capillariasis is a stool examination, and usually only a single analysis is needed to detect the parasite.7,12 In this study, the stool analysis was critical for a diagnosis in each of the cases. These cases were diagnosed in a laboratory at a parasitology department by experienced medical personnel following their misdiagnosis by other laboratories. The presence of Charcot-Leyden crystals in the stool samples of each of the patients supported the diagnosis of intestinal capillariasis, and the number of these crystals correlated with the intensity of the infection. In particular, the number of crystals was greater in patients with severe symptoms and in stool specimens with a larger number of eggs (average was 8–20 per high-power field). Furthermore, these crystals were not detected in the stools of the two male children who received albendazole before they were diagnosed, which may have changed their immune responses. Many potential early cases of this infection needed to be differentiated from other diseases with identical clinical manifestations, such as Crohn's disease, TB, enteritis, and various intestinal malignancies. As a result, these cases were evaluated using a long list of sophisticated measures that were not diagnostic for intestinal capillariasis but did reveal important features that improved the knowledge of the pathogenicity of this parasitic disease. These additional diagnostic measures included a full blood panel, abdominal ultrasonography (revealing pleural effusion and ascites in some patients), upper endoscopies (gastroduodenoscopies that revealed malabsorption), and histopathological examinations of the duodenal and jejunal biopsies (revealing atrophic and flattened villi, inflammatory cell infiltration, and numerous sections of the adult worm in the epithelial layer). In addition to these findings, the SEM examination of the biopsy tissues revealed evidence of degeneration, swollen mitochondria, and distended rough endoplasmic reticulum in the cells in direct contact with the worm.
An experimental infection of bolty (O. niloticus) and karmout (catfish, Clarias species) fish with eggs and/or larvae was attempted without success. These two types of fish were chosen because they are commonly consumed in the area, are sold at a low price, and are available in most areas of Egypt. El-Dib and others21 conducted an experimental infection of O. niloticus and had an identical result. These authors suggested that it would be necessary to repeat the experiment using greater numbers of fish and fish species.
The observations of the features and measurements of the adult female worms made using light microscopy in this study corresponded to a degree to the original descriptions of C. philippinensis made by Chitwood and others1 and the redescription made by Moravec.16 Although the samples in this study were collected from the same host and habitat, the parasites detected had measurements that were smaller than those reported.
In this study, levamisole was given to patients, and it was very effective at expelling the adult worms from all of the patients. In the studies by Mahmoud and others18 and Attia,23 this drug was also used to expel other nematodes such as A. duodenale. This current study has provided a comprehensive description of the adult female worms and has illustrated their internal structures. Unfortunately, no male specimens were detected during this study.
The use of SEM has allowed for the visualization of additional morphological features, such as the structure of the cephalic end and the striations in the cuticle, which are not usually visible using light microscopy and have not been mentioned in previous descriptions. The unexplained tight obscure membrane that covered nearly the entire surface of the adult worms may have been the result of an intense inflammatory reaction by the immune system of the host. Bunches of intestinal villi that covered the cuticle and plugged the mouth opening may be related to the mucosal penetration that is commonly initiated by the worm and that manifests as severe diarrhea.
This study confirmed previous observations concerning the presence of two morphologically distinct eggs, which include the thick-shelled eggs with two distinct forms (typical peanut-shaped and swollen peanut-shaped) and the thin-shelled eggs. These eggs were also detected in the studies from Cross,19 Anderson,24 and Moravec.16
The size of both the thick- and the thin-shelled eggs was smaller than that found by Moravec16 but was slightly larger than those found by Cross19 and Khalifa and others.9 In general, the thin-shelled eggs were smaller than the thick-shelled eggs, and this was also recorded in the study from Moravec.16
This study found thin-shelled eggs with protruding polar plugs, whereas all previous studies have observed polar plugs without protrusions.9,16,23 The thin shell most likely enables the larvated eggs to hatch in utero or in the lumen of the host gut to facilitate autoinfection and subsequent hyperinfection.
In a recent study from Sukontason and others25 using SEM, both thick- and thin-shelled eggs were described for the life cycle of this nematode. Two distinct morphological shapes of peanut-shaped thick-shelled eggs were detected, and these consisted of the typical elongated egg and the swollen egg. The typical elongated Paracapillaria eggs detected in this study were characterized by unique features such as the presence of two deep concave polar plugs, the large circular ridge that connected both poles on each side of the eggs, and the coarsely pitted network on the outer surface. An intermediate-shaped egg that appeared to have a partial beam-like network on the surface of the egg was observed by Sukontason and others,25 but this shape was not observed in this study.
A previous study by Wharton26 of the egg shells of closely related species found that the shell of Capillaria hepatica eggs was composed of the following three layers: an inner lipid layer, a middle chitinous layer, and an outer vitelline layer. According to Sukontason and others,25 the thick egg shell of P. philippinensis seen using SEM represented the vitelline layer, and the entire beam-like network that was connected to the pillars represented the chitinous layer. However, in this study, the coarse, dense pitted network formed by the chitinous layer resembled the component of the C. hepatica egg shell shown using transmission electron microscopy by Grigonis and Solomon17 and Inatomi.27
Two forms of swollen peanut-shaped eggs were observed; one had an irregular orange peel-like surface, whereas the other had a smooth surface with non-apparent polar plugs. The latter form was not described by Sukontason and others.25
In conclusion, this study has produced some crucial results. First, the diagnosis of intestinal capillariasis is commonly delayed and generally requires the experience of medical parasitologists. Therefore, we have emphasized that clinicians in this area should remain highly alert to recognize the signs of this treatable infection. Furthermore, the number of cases of infection with this parasite may far exceed that estimated for the Assuit Governorate and Upper Egypt, and middle-aged females are more likely to be infected than males caused by the handling and the cleaning of fish. In addition, this study has described in detail the structures of the adult female worms and the eggs of C. philippinensis using light and SEM. Moreover, this description has provided evidence for autoinfection and hyperinfection, which occur during the life cycle of this species within the host's intestine and lead to the aggressive pathogenesis and the clinical features of infected patients.
Further studies need to be conducted to investigate the routes of infection and the reservoir hosts of C. philippinensis in Upper Egypt and in the Assiut Governate, and proper control measures should be implemented to prevent the spread of intestinal capillariasis.
Footnotes
Authors' addresses: Rasha A. H. Attia, Mohammed E. M. Tolba, Doaa A. Yones, Hanaa Y. Bakir, and Hanan E. M. Eldeek, Department of Parasitology, Faculty of Medicine, Assiut University, Egypt, E-mails: rashaattia@gmail.com, essa3eg@yahoo.com, doaayones@gmail.com, hanaabakeer@yahoo.com, and hananhmo@yahoo.com. Shereef Kamel, Department of Gastroenterology and Tropical Diseases, Faculty of Medicine, Assiut University, Egypt, E-mail: kamel_sherif@yahoo.com.
The drug was not meant for treatment as it only causes paralysis of adult worms, which could be hidden in mucosa or threading enterocytes. Treatment is described later.
References
- 1.Chitwood MB, Valesquez C, Salazar NG. Capillaria philippinensis sp. n. (Nematoda: Trichinellida), from the intestine of man in the Philippines. J Parasitol. 1968;54:368–371. [PubMed] [Google Scholar]
- 2.Cross JH. In: Capillariasis. Zoonoses: Biology, Clinical Practice, and Public Health Control. First edition. Palmer SR, Soulsby L, Simpson DI, editors. New York: Oxford University Press; 1998. pp. 759–772. [Google Scholar]
- 3.Austin DN, Mikhail MG, Chiodini PL, Murray-Lyon LM. Intestinal capillariasis acquired in Egypt. Eur J Gastroenterol Hepatol. 1999;11:935–936. doi: 10.1097/00042737-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Youssef FG, Mikhail EM, Mansour NS. Intestinal capillariasis in Egypt: a case report. Am J Trop Med Hyg. 1989;40:195–196. doi: 10.4269/ajtmh.1989.40.195. [DOI] [PubMed] [Google Scholar]
- 5.Mansour NS, Anis MH, Mikhail EM. Human intestinal capillariasis in Egypt. Trans R Soc Trop Med Hyg. 1990;84:114. doi: 10.1016/0035-9203(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 6.El-Dib NA, Farrag AJ, Salama HM, Salama ZA, Mostafa SM. A new case of intestinal capillariasis in Egypt. J Trop Med Hyg. 1992;2:75–76. [Google Scholar]
- 7.Ahmed L, EL-Dib NA, EL-Boraey Y, Ibrahim M. Capillaria philippinensis: an emerging parasite causing severe diarrhea in Egypt. J Egypt Soc Parasitol. 1999;29:483–493. [PubMed] [Google Scholar]
- 8.Anis MH, Shafik H, Mansour NS, Moody A. Intestinal capillariasis as a cause of chronic diarrhea in Egypt. J Egypt Soc Parasitol. 1998;28:143–147. [PubMed] [Google Scholar]
- 9.Khalifa RM, Sakla A, Hassan AA. Capillaria philippinensis–a human intestinal nematode newly introduced to Upper Egypt. Helminthologia. 2000;37:23–27. [Google Scholar]
- 10.El-Karaksy H, El-Shabrawi M, Mohsen N, Kotb M, El-Koofy N, El-Deeb N. Capillaria philippinensis a cause of fatal diarrhea in one of two infected Egyptian sisters. J Trop Pediatr. 2004;50:57–60. doi: 10.1093/tropej/50.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Sangcha A, Wongsaensook A, Kularbkaew C, Sawanyawisuth K, Sukeepaisarn W, Mairiang P. The endoscopic-pathologic findings in intestinal capillariais: a case report. J Med Assoc Thai. 2007;90:175–178. [PubMed] [Google Scholar]
- 12.Lu LH, Lin MR, Choi WM, Hwang KP, Hsu YH, Bair MJ, Liu JD, Wang TE, Liu TP, Chung WC. Human intestinal capillariasis (Capillaria philippinensis) in Taiwan. Am J Trop Med Hyg. 2006;74:810–813. [PubMed] [Google Scholar]
- 13.Saichua P, Choosak N, Natthawut K. Human intestinal capillariasis in Thailand. World J Gastroenterol. 2008;14:506–510. doi: 10.3748/wjg.14.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bair MJ, Hwang KP, Wang TE, Liou TC. Clinical features of human intestinal capillariasis in Taiwan. World J Gastroenterol. 2004;10:2391–2393. doi: 10.3748/wjg.v10.i16.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong ST, Kim YT, Choe G, MinCho SH, Kook J, Chai JY, Lee SH. Two cases of intestinal capillariasis in Korea. Korean J Parasitol. 1994;32:43–48. doi: 10.3347/kjp.1994.32.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Moravec F. Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings. J Parasitol. 2001;87:161–164. doi: 10.1645/0022-3395(2001)087[0161:RASSOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Grigonis JR, Solomon GB. Capillaria hepatica: fine structure of the egg shell. Exp Parasitol. 1976;40:286–297. doi: 10.1016/0014-4894(76)90093-x. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud MS, Abou Gamra MM, Elkhayat MM. Ancylostoma duodenale infection: a study of serum immunoglobulinG4 response to the excretory secretory antigen of adult worm. J Egypt Soc Parasitol. 2005;35:1–17. [PubMed] [Google Scholar]
- 19.Cross JH. Intestinal capillariasis. Clin Microbiol Rev. 1992;5:120–129. doi: 10.1128/cmr.5.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Dib NA, Ahmed JA, El-Arousy M, Mahmoud MA, Garo K. Parasitological aspects of Capillaria philippinensis recovered from Egyptian cases. J Egypt Soc Parasitol. 1999;29:139–147. [PubMed] [Google Scholar]
- 21.El-Dib NA, Doss WH. Intestinal capillariasis in Egypt. Epidemiological background. J Egypt Soc Parasitol. 2002;32:145–154. [PubMed] [Google Scholar]
- 22.Cross J, Basaca-Sevilla V. Intestinal capillariasis: current concepts, laboratory diagnosis and chemotherapy. Asian J Clin Sci Monograph. 1986;7:63–67. [Google Scholar]
- 23.Attia RA. Studies on some parasitic nematodes which infect man. MD Thesis. Assiut University; Egypt: 2006. [Google Scholar]
- 24.Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission. Second edition. Oxon, UK: CABI Publishing; 2000. pp. 143–180. [Google Scholar]
- 25.Sukontason KL, Sukontason K, Piangjai SR. Ultrastructure of eggs Paracapillaria (Capillaria) philippinensis and evidence related to its life cycle. Micron. 2006;37:87–90. doi: 10.1016/j.micron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Wharton D. Nematode egg-shells. Parasitology. 1980;81:447–463. doi: 10.1017/s003118200005616x. [DOI] [PubMed] [Google Scholar]
- 27.Inatomi S. Submicroscopic structure of the egg shell of helminthes III. A study on Capillaria hepatica. Acta Med Okayama. 1960;14:261–264. [Google Scholar]


