Abstract
Culex tarsalis is a superior horizontal and vertical vector of West Nile virus (WNV) compared with Culex salinarius. Culex salinarius transmitted WNV genotype NY99 (CT 2741-99 strain) horizontally to suckling mice at significantly lower rates than Cx. tarsalis on Days 8, 9, 10, and 12 post-infection, and Cx. salinarius transmitted WNV genotype NY99 to offspring at a lower vertical transmission infection rate than Cx. tarsalis. Culex tarsalis transmitted WNV genotypes NY99 and WN02 (CT S0084-08 strain) with equal efficiency. Daily percent horizontal transmission of genotype NY99 by Cx. tarsalis-infected per os and by intra-thoracic infection was not significantly different from daily transmission of genotype WN02 from Days 5–23 and Days 2–9 post-infection, respectively. Our findings do not support the previously published hypothesis that genotype NY99 was replaced in the New World by WN02 because of a shorter extrinsic incubation of WN02.
Introduction
West Nile virus (family Flaviviridae, genus Flavivirus, WNV) in the northeastern United States amplifies during the summer months in an enzootic cycle by horizontal transmission and survives the winter months in non-blood-fed females infected by vertical transmission.1–3 Culex pipiens pipiens L. is the primary vector, but numerous isolations of WNV also have been made from other species.4 Culex salinarius Coquillett, which feeds on mammals and birds from New England to New Mexico and is frequently infected with WNV,4–10 is a likely bridge vector for transferring WNV from birds to humans.4,11 In the western United States, Culex tarsalis Coquillett is the likely primary vector in rural agricultural environments,12 and species of the Cx. pipiens complex and Cx. salinarius may be the important vectors in urban ecosystems.6,7,12
Culex tarsalis has been reported to have a relatively short WNV extrinsic incubation period,12 to vertically transmit WNV,13,14 and, along with Cx. pipiens,15 to transmit the relatively newly recognized WN02 genotype more quickly than the original NY99 genotype.16 The extrinsic incubation period of the WN02 genotype was up to 2 days shorter in Cx. pipiens and 4 days shorter in Cx. tarsalis than that of the NY99 genotype and led the authors to suggest that genotype WN02 displaced genotype NY99 in North America by leading to higher infection rates of genotype WN02 in avian hosts. We examined this hypothesis by comparing the horizontal post-infection (pi) transmission rates at daily time points for WNV strains of genotype NY99 and genotype WN02 from Connecticut in Cx. tarsalis that probed or fed on suckling mice. We also compared the pi daily transmission rate for WNV strain genotype NY99 in Cx. salinarius, which had not previously been extensively studied as a vector of WNV and which we thought would have a contrasting extrinsic incubation period to Cx. tarsalis.11 Additionally, we report the vertical transmission of WNV genotype NY99 by Cx. salinarius and Cx. tarsalis and the vertical transmission of WNV genotype WN02 by Cx. tarsalis.
Materials and Methods
Mosquito colonies.
The colony of Cx. salinarius was established in 2003 from specimens collected in Stratford, CT (41°10′41″N, 73°07′34″W) by procedures published previously.17 The Cx. tarsalis colony was established by William K. Reisen, University of California, Davis, from specimens collected from Coachella Valley, CA in 2005. Culex tarsalis collected from Coachella Valley in 2001 were reported to be most efficient at transmitting WNV after a 14-day incubation period.12 This Cx. tarsalis colony was obtained from the U.S. Department of Agriculture/Agricultural Research Service (USDA/ARS) Laboratory, Gainesville, FL. Colonies of both species were kept at 25°C and a photoperiod of 16:8 (L: D) hr.
West Nile virus.
The CT 2741-99 strain of WNV from Cx. p. pipiens is a NY99 genotype.18 Strain CT S0084-08 was from Cx. p. pipiens collected September 16, 2008 in Stratford, CT and is a WN02 genotype. The two strains were sequenced at the DNA Analysis Facility on Science Hill at Yale University. Primers used were WN1101-GATGAATATGGAGGCGGCCA and WN1816A-CCGACGTCAACTTGACAGTG.19 Genotype NY99 strain CT 2741-99 (GenBank accession JN182684) differed from genotype WN02 strain CT S0084-08 (GenBank accession JN182683) by a U to C substitution at position 1442, resulting in a Val to Ala amino acid substitution at position 159 of the E protein, and by silent changes A to G at position 1482 and C to U at position 1629. The WNV CT 2741-99 strain was passed three times and the CT S0084-08 strain two times through Vero cells and either mixed 1:1 with fresh heparinized hamster blood as described previously,20 or frozen virus of each strain was added to Vero cell growth medium and mixed 1:1 with fresh mouse blood to produce approximately equal titers. Complete genomes of WNV strains CT 2741-99 (GenBank accession no. AF206518) and CT S0084-08 (GenBank accession no. HM488170) have been published.21
Per os infection of Cx. salinarius and Cx. tarsalis.
Seven- to 10-day-old females of each species were deprived of raisins for 1 d before being allowed to feed on an infectious mixture of hamster or mouse blood and WNV through a membrane feeder (Hemotek 5W1, Discovery Workshops, Accrington, UK) placed on top of each cage. Each engorged female was transferred into a 296-mL numbered clear plastic cup covered with netting and kept at 26°C and a photoperiod of 16:8 (L:D) hr. Approximately 25 mL of hay infusion was added to each cup for deposition of eggs. A raisin was provided to each female before and after oviposition. Each female that had fed on infectious blood was examined daily, and when it died or was killed, it was frozen at -80°C and later tested for virus in Vero cells.
Laboratory animals.
Suckling mice from pregnant CD-1 mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Suckling mice were tattooed and exposed to individual female mosquitoes as described previously.20 A tattooed suckling mouse, 2–4 d old, was securely placed over the netting of a cup containing a single mosquito. The suckling mouse was later returned to its mother. A non-tattooed mouse that was not exposed to a mosquito served as a negative control for each litter. Suckling mice were examined daily for signs of illness. Sick mice were euthanized with CO2 and frozen at -80°C. The missing suckling mice, presumably eaten by their mothers, were not included in the analysis of extrinsic incubation. All live mice on Day ≥ 4 pi exposed to Cx. salinarius were euthanized with CO2 and frozen at -80°C. Suckling mice exposed to Cx. tarsalis were euthanized on Days 3 or 4 pi. Brain tissue was removed from each suckling mouse and triturated in phosphate-buffered saline (PBS) containing gelatin (0.5% gelatin, 30% rabbit serum, antibiotic, and antimycotic). This mixture was centrifuged at 5°C, and 100 µL of the supernatant was transferred onto a confluent layer of 1- and 2-day-old Vero cells for attempted isolation of virus. Reverse transcription-polymerase chain reaction (RT-PCR) was used to confirm all isolates.22 Isolation of WNV from suckling mice was evidence of transmission. Groups of mice with an infected untreated control suckling mouse were not included in the analysis.
The Connecticut Agricultural Experiment Station's Animal Care and Use Committee approved the procedures for handling animals.
Comparison of horizontal transmission of West Nile virus genotype NY99 by Cx. salinarius and Cx. tarsalis at 26°C.
Females of each species were exposed daily to suckling mice on Days 6 through 30 pi. Infectious hamster blood fed to Cx. salinarius contained 5.9–28.0 × 105 plaque-forming units (PFU)/mL, and infectious hamster blood fed to Cx. tarsalis contained 1.7–3.2 × 108 PFU/mL.23 These quantities of WNV were within the range of PFU reported in experimentally infected birds.24 Suckling mice and mosquitoes were tested for WNV as described previously.
Vertical transmission relative to days pi.
Eggs were collected on days pi from female Cx. salinarius infected with genotype NY99 and from Cx. tarsalis infected with genotypes NY99 and WN02. Egg rafts were from females that had fed relatively recently on the membrane and from females that had fed on the membrane and later on suckling mice. Egg rafts were each transferred into a 16-mL glass vial (Wheaton, Science Products, Millville, NJ) containing 10-mL distilled water and 0.02 g of rodent chow. Upon hatching, F1 larvae were reared at 26°C and a photoperiod of 16:8 (L:D) hr in clear plastic, disposable 540-mL containers and fed rodent chow as needed. Larvae were reared in groups of 40–50 per container. Adults were separated according to sex, frozen at -80°C, and processed for virus isolation in Vero cells in groups of 25 to determine if vertical transmission had occurred.
Comparison of transmission of West Nile virus genotypes NY99 and WN02 by Cx. tarsalis at 26°C.
Females, which were infected with WNV genotypes NY99 or WN02, were exposed daily to suckling mice from Day 5 pi until Day 23 pi. An observer carefully monitored each mosquito during a 2-hr exposure period and recorded the mosquito as probed, fed, or not probed. Culex females transmit WNV after probing without ingesting blood.25 Suckling mice that were not probed or fed upon by a mosquito were not included in the analysis. The titers of infectious mouse blood were 2.25–23.0 × 105 PFU/mL for genotype NY99 and 2.0–22.0 × 105 PFU/mL for genotype WN02. Suckling mice and mosquitoes were tested for WNV as described previously. Mosquitoes that tested negative were not included in the analysis.
Comparison of transmission of West Nile virus genotypes NY99 and WN02 by Cx. tarsalis at 26°C after thoracic microinjection of virus.
The stains of genotype NY99 and genotype WN02 were titrated in Cx. tarsalis females by injection of virus into the thorax.26
The dose of each strain was determined by 10-fold serial dilutions (i.e., 10-4, 10-5, 10-6, and 10-7) in PBS. The mosquitoes (10 in each group) were inoculated in the thorax by microinjection with 300 nL of diluted virus. On Day 6 pi, the mosquitoes were killed in dry ice, total RNA was isolated, and the viral load was determined by quantitative polymerase chain reaction (qPCR). Infected mosquitoes were homogenized in 350-μL RLT Buffer (RNeasy Mini kit, Qiagen) with a Pestle Grinder System (Fisher Scientific, Pittsburgh, PA). Total RNA was isolated, and cDNA was transcripted for qPCR detection. The specific RNA of the WNV-E gene was quantified by Taqman qPCR. The primers and probe were: WNV-F: TTCTCGAAGGCGACAGCTG; WNV-R: CCGCCTCCATATTCATCATC; WNV-Probe: FAM6-ATGTCTAAGGACAAGCCTACCATC-TRAMA. The C. quinquefasciatus actin (CPIJ012570) gene was used as an internal control in qPCR detection: Actin-F: CCCCCTGAGCGCAAGTACT; Actin-R: CGTCGTATTCCTGCTTGGAGAT; Actin-Probe: FAM6-TGGCCTCGCTGTCCACCTTCCA-TRAMA. The qPCR was performed in a Bio-Rad iQ5 Cycler with a Taqman Universal PCR Master Mix system (Applied Biosystems, Foster City, CA). The PCR mixtures were preincubated at 50°C for 2 min in the thermal cycle, denatured at 95°C for 10 min, and cycled 40 times at 95°C for 30 sec and 60°C for 1 min. There were no nucleotide differences in the PCR amplified gene of genotypes NY99 (1019–1099 bp, GenBank accession no. AF206518) and WN02 (1037–1117 bp, GenBank accession no. HM488170).21 These data indicate that the PCR efficiency for the two genotypes should be the same. The standard curves were measured by 10-fold dilutions of WNV E gene (PCR amplified fragments) and the C. quinquefasciatus actin gene.
The 50% mosquito infective dose (MID50) was estimated by Reed-Muench.27 Equivalent quantities of virus of the two strains were then made, and 300 nL of each strain containing 1,000 MID50 virus was injected into each female mosquito. Female mosquitoes injected with genotype NY99 or genotype WN02 were allowed to feed or probe on suckling mice as described previously on Days 2–9 after injection with WNV to determine if there were differences in extrinsic incubation between the two strains. Suckling mice were returned to their mothers after exposure to female mosquitoes, and each was euthanized on Day 3 after exposure to a female mosquito. Female mosquitoes and suckling mice were tested for WNV as described previously.
Statistics.
The vertical transmission rate and the vertical minimal infection rate were determined using previously described methods.28 The vertical minimal infection rate was calculated using the formula for bias corrected maximum likelihood estimate.29 Fisher's exact test was used to compare infection and daily horizontal transmission rates of Cx. salinarius and Cx. tarsalis and the infection and daily horizontal transmission rates of the two WNV genotypes by Cx. tarsalis. Only females that tested positive for WNV were included in the analyses.
Results
Comparison of horizontal transmission of WNV genotype NY99 by Cx. salinarius and Cx. tarsalis relative to days pi.
Horizontal transmission of WNV genotype NY99 by Cx. salinarius to suckling mice was recorded in < 2% of the females that fed on Days 6 and 7 pi (Figure 1). Percent infection increased to 17–47% on Days 10–12 pi and then to 69–80% on Days 13–17 pi. Thereafter, until Day 30 pi, percent transmission ranged from 80% to 100%. Females that were 42, 43, 47, and 48 days pi also transmitted virus to suckling mice (data not shown in Figure 1). In contrast, transmission of WNV genotype NY99 by Cx. tarsalis increased from 5% on Day 6 pi to 67% on Day 9 pi to 77–100% on Days 11–30 pi with the exception of 63% transmitting on Day 14 pi. A significantly higher percentage of Cx. tarsalis kept at 26°C transmitted WNV genotype NY99 to suckling mice than did Cx. salinarius during Days pi 8, 9, 10, and 12. Seventy-eight percent (N = 1,526 fed females) and 80% (N = 594 fed females) of Cx. salinarius and Cx. tarsalis that fed on WNV genotype NY99 were infected, respectively. Infection rates were not significantly different (Fisher exact test, degrees-of-freedom [df] = 1, P > 0.05).
Figure 1.
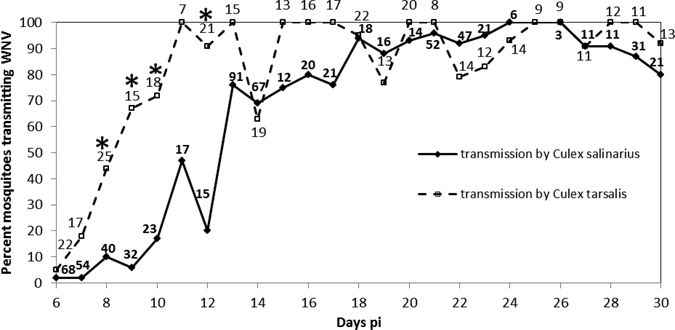
Percent Cx. salinarius and Cx. tarsalis horizontally transmitting WNV genotype NY99 at 26°C to suckling mice on days 6–30 pi. The number of infected mosquitoes that fed each day is shown above or below each data point. *Differences in transmission between the two species at specific time-points pi significant at P < 0.05, df = 1, Fisher exact test.
Vertical transmission by Cx. salinarius and Cx. tarsalis.
One of 222-infected Cx. salinarius females that laid eggs vertically transmitted WNV genotype NY99 for a vertical transmission rate of 0.45%. Virus was isolated from one pool of 25 F1 females from an egg raft that was laid 43 days pi. The female that vertically transmitted WNV had previously horizontally transmitted WNV to suckling mice on three occasions. In all, 48,101 F1 adults (24,308 males and 23,793 females) from infected females were tested. The vertical minimal infection rate was 0.04 WNV-infected mosquitoes/1,000 mosquito specimens tested.
Fifteen of 379-infected Cx. tarsalis females vertically transmitted WNV genotype NY99 and one of 57 infected females vertically transmitted WNV genotype WN02 to F1 adults for vertical transmission rates of 3.69% and 1.75%, respectively. Seven, 7, and 1 females transmitted WNV genotype NY99 to progeny in egg rafts 1, 2, and 3, respectively. Two, 8, and 5 females vertically transmitted on Days 5 and 8, 10–16, and 24–30 pi, respectively. The female infected with WNV genotype WN02 transmitted virus to her second egg raft on Day 22 pi. One pool of 25 males and one pool of 25 females were infected.
A total of 28,372 and 5,450 F1 adults from Cx. tarsalis females infected with genotypes NY99 and WN02, respectively, were tested for WNV. The vertical minimal infection rates were 0.96 WNV-infected genotype NY99 mosquitoes per 1,000 mosquito specimens tested and 0.37 WNV-infected genotype WN02 mosquitoes per 1,000 mosquito specimens tested.
Comparison of horizontal transmission of WNV genotype NY99 and genotype WN02 by Cx. tarsalis relative to days pi.
The percent of females that became infected with WNV after feeding on infectious blood containing genotypes NY99 and WN02 were 56% (N = 189 fed females) and 59% (N = 209 fed females), respectively. Infection rates were not significantly different (Fisher exact test, df = 1, P > 0.05). Horizontal transmission of genotype NY99 increased from 15% on Day 6 pi to 54% on Day 9 pi to 70–100% on Days 12–23 pi (Figure 2). Similar horizontal transmission results were obtained with genotype WN02. Significant differences in transmission were not noted in the two strains on any of the days from 5 to 23 pi (Fisher exact test, df = 1, P > 0.05).
Figure 2.
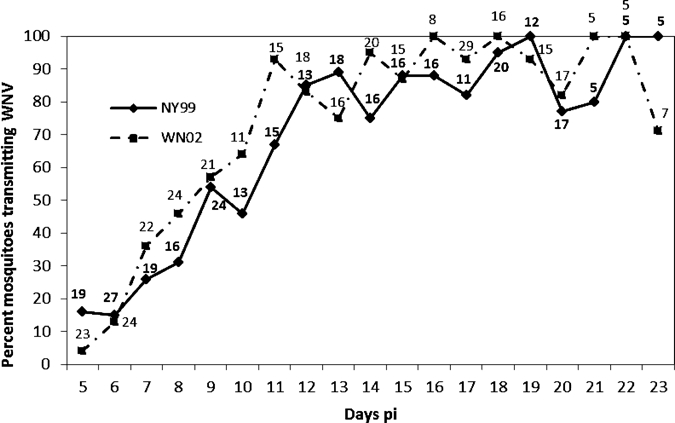
Percent Cx. tarsalis horizontally transmitting WNV genotypes NY99 and WN02 to suckling mice on days 5–23 pi at 26°C. The number of infected mosquitoes that probed or fed each day is shown above or below each data point. There were no significant differences in transmission between the two strains at any of the time points pi at P > 0.05, df = 1, Fisher exact test.
Horizontal transmission of WNV genotypes NY99 and WN02 by Cx. tarsalis injected with virus was similar (Table 1). Significant differences in transmission were not observed in the two strains on any of the days from 2 to 9 pi (Fisher exact test, df = 1, P > 0.05).
Table 1.
Horizontal extrinsic incubation at 26°C in Culex tarsalis injected with 300 nL of WNV genotype NY99 and WNV genotype WN02 containing 1,000 mosquito infective dose50*
| Days pi | Viral strain injected | |
|---|---|---|
| NY99 | WN02 | |
| No. infected mice/no. mice probed or fed upon (%) | No. infected mice/no. mice probed or fed upon (%) | |
| 2 | 1/8 (12.5) | 0/4 (0) |
| 3 | 7/12 (58.3) | 3/10 (30) |
| 4 | 13/14 (92.9) | 9/10 (90) |
| 5 | 16/16 (100) | 16/18 (88.9) |
| 6 | 11/12 (91.7) | 8/11 (72.7) |
| 7 | 2/2 (100) | 1/1 (100) |
| 8 | 5/5 (100) | 2/2 (100) |
| 9 | 7/7 (100) | 1/1 (100) |
There were no significant differences in transmission between the two stains at any of the days pi (P > 0.05, df = 1, Fisher exact test).
Discussion
Culex salinarius is a relatively abundant species in the northeastern United States. It feeds readily on mammals, including humans and on birds,5 and is naturally infected with WNV.4,30 Although Sardelis and others11 reported an estimated 34% transmission rate of WNV by Cx. salinarius on Day 14 pi at 26°C, our transmission values were higher and similar to those reported for Cx. pipiens.20,31 The extrinsic incubation periods for transmission of WNV for both Cx. salinarius and Cx. pipiens were longer than what we and others reported for Cx. tarsalis.12,32,33 Although the concentration of virus ingested by Cx. tarsalis was greater than by Cx. salinarius in our experiments, the infection rates in both species were relatively high and not significantly different from one another, indicating that the viral meals for both species were well above the threshold levels of infection. On the basis of our results, Cx. salinarius, although not as efficient as Cx. tarsalis, is as efficient as Cx. p. pipiens in transmitting WNV in the northeastern United States, and although minimum field infection rates of WNV have been reported to be lower for Cx. salinarius than for Cx. pipiens,4,30 the opportunistic feeding of Cx. salinarius on mammals, compared with Cx. pipiens,5,10 likely results in a greater percentage of females feeding on humans. It is noteworthy that in urban areas of New Mexico in the southwestern United States, WNV infection rates were relatively high in Cx. salinarius and in Cx. pipiens quinquefasciatus Say compared with the rates reported for Cx. tarsalis.7
Vertical transmission of WNV has been reported in eight species.2,3,13,14,34–38 We now document Cx. salinarius to also vertically transmit WNV. The 0.45% vertical transmission rate and the vertical minimal infection rate of 0.04/1,000 mosquitoes tested, although lower than those reported for Cx. p. pipiens, suggest to us that WNV could survive winter in non-blood-fed, vertically infected females of Cx. salinarius as previously reported for Cx. p. pipiens and possibly contribute to the field infection rate during the summer.2,3 This ability to vertically transmit and to horizontally transmit WNV similarly to Cx p. pipiens suggests to us that Cx. salinarius may have a role in maintaining this virus in the northeastern United States.
The minimum incubation for vertical transmission of strain WNV genotype NY99 is shorter in Cx. tarsalis than in Cx. p. pipiens at 26°C. Transmission occurred in Cx. tarsalis females as early as 5 and 8 days pi during deposition of the first egg raft, whereas vertical transmission in Cx. p. pipiens did not occur until the second egg raft was laid 13 or more days pi.20 Higher rates of vertical transmission by Cx. tarsalis occurred 10 days or later pi. Vertical transmission likely enables WNV to survive in Cx. tarsalis, as it does in Cx. p. pipiens, during winter in the northern part of its geographical range and likely enhances the field infection rates during the warm months of the year.
The original NY99 genotype of WNV was replaced by genotype WN02 throughout the United States in the early 2000s.15,39,40 The WN02 strains consistently differed by a Val to Ala amino acid substitution in the E protein at position 159. The WNV strains of genotype WN02 were reported to infect a larger proportion of Culex and to have shorter extrinsic incubation periods than did females infected with genotype strains of NY99.15,16 A more recent study reported transmission of the WN02 genotype to be advantageously increased in Cx. pipiens with increasing temperature and time compared with the NY99 genotype.41 The authors of these studies suggested that the increased transmission efficiency, resulting from the decreased extrinsic incubation period in Culex infected with genotype WN02 strains compared with those infected with NY99 genotype strains, was the likely reason for the displacement of the original genotype.
The initial descriptions of the WN02 genotype were made from birds and mosquitoes collected in June and July 2002 in Houston, TX where Cx p. quinquefasciatus is relatively abundant,19 and in 2002 and 2003 from mosquitoes, birds, and mammals in New York State where Cx. p. pipiens is relatively abundant, and Cx. tarsalis is essentially absent.15 We examined the hypothesis that genotype WN02 has a shorter incubation period in Culex compared with NY99 by exposing the western United States mosquito vector of WNV Cx. tarsalis infected with Connecticut genotypes NY99 or WN02 to suckling mice at specific time points pi. Isolation was attempted from each mouse that had been probed or fed upon by infected Cx. tarsalis. Even though the initial reports of genotype WN02 were made in species other than Cx. tarsalis, we selected Cx. tarsalis as the model in our studies because 1) our initial studies confirmed that Cx. tarsalis is a superior vector of WNV,12,33 2) we thought Cx. tarsalis would more readily probe and feed on suckling mice during the day than Cx. p. pipiens, and 3) the extrinsic incubation period of genotype WN02 was reported to be up to 4 days shorter than genotype NY99 in Cx. tarsalis.16
Our studies, which used a different strain of Cx. tarsalis and different genotype NY99 and WN02 strains than reported by Moudy and others,16 did not confirm a shorter extrinsic incubation period of genotype WN02 compared with genotype NY99 in Cx. tarsalis. We detected no significant differences at daily time points in transmission between the two genotypes from Days 5 through 23 pi when infected per os or from Days 2–9 when infected by intra-thoracic injection. Our percentages of transmission following per os infection, based upon natural transmission of WNV from Cx. tarsalis to suckling mice and subsequent isolation of virus, were considerably higher than those reported by Moudy and others.16 Their transmission rates at 27°C for genotype NY99 on Days 7, 9, and 14 pi were 0%, 2%, and 12%, respectively, in contrast to our transmission rates at 26°C on Days 7, 9, and 14 pi of 26%, 54%, and 75%, respectively. Others also have reported relatively high rates of transmission of genotype NY99 by Cx. tarsalis at 14 days pi or less.12,32 Although our transmission data following intra-thoracic inoculation were consistent with Moudy and others,16 our data from per os infections suggest that the replacement of genotype NY99 by WN02 was the result of factors other than increased transmission efficiency of WN02 by overcoming the midgut barrier. Representative mutations have yet to be identified that might explain the selective or adaptive processes for replacement of the original NY99 genotype by the WN02 genotype in North America.
ACKNOWLEDGMENTS
We thank Bonnie Hamid, Angela Bransfield, Michael Misencik, Heidi Stuber, Tanya Petruff, and Michael Vasil for technical assistance. Philip M. Armstrong compared sequences of the CT S0084-08 (genotype WN02) and CT 2741-99 (genotype NY99) strains. Sandra Allen, USDA/ARS, Gainesville, FL, provided the colony of Culex tarsalis.
Footnotes
Financial support: The research was supported in part by USDA Specific cooperative agreement 58-6615-1-218 and by Laboratory Capacity for Infectious Diseases cooperative agreement U50/CCU116806-01-1 from the Centers for Disease Control and Prevention.
Authors' addresses: John F. Anderson, Department of Entomology and Center for Vector Biology and Zoonotic Diseases, The Connecticut Agricultural Experiment Station, New Haven, CT, E-mail: John.F.Anderson@ct.gov. Andy J. Main, Department of Biology, American University in Cairo, Cairo, Egypt, E-mail: andymain@aucegypt.edu. Gong Cheng, Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, E-mail: chenggong2006@gmail.com. Francis J. Ferrandino, Department of Plant Pathology, The Connecticut Agricultural Experiment Station, New Haven, CT, E-mail: Francis.Ferrandino@ct.gov. Erol Fikrig, Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, E-mail: erol.fikrig@yale.edu; Erol Fikrig is an Investigator with the Howard Hughes Medical Institute, The Connecticut Agricultural Experiment Station, New Haven, CT.
References
- 1.Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K, Lanciotti RS. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- 4.Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- 5.Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 6.DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, LaBeau EM, Hatton ES, Glass GE. Emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Gande Valley. J Med Entomol. 2006;43:594–599. doi: 10.1603/0022-2585(2006)43[594:eownvi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, LaBeau EM, Hatton ES, Roberts CM, Glass GE. Urban habitat evaluation for West Nile virus surveillance in mosquitoes in Albuquerque, New Mexico. J Am Mosq Control Assoc. 2007;23:153–160. doi: 10.2987/8756-971x(2007)23[153:uhefwn]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 9.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- 14.Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, O'Connor P, Carney R, Cahoon-Young B, Shafii M, Brault AC. Overwintering of West Nile virus in southern California. J Med Entomol. 2006;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am J Trop Med Hyg. 2004;71:493–500. [PubMed] [Google Scholar]
- 16.Moudy RM, Meola MA, Morin LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- 17.Wallis RC, Whitman L. Colonization of Culex salinarius in the laboratory. Mosq News. 1968;28:366–368. [Google Scholar]
- 18.Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, Garmendia AE, Van Kruiningen HJ. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 19.Beasley DW, Davis CT, Guzman H, Vanlandingham DL, Travassos da Rosa AP, Parsons RE, Higgs S, Tesh RB, Barrett AD. Limited evolution of West Nile virus has occurred during its southwesterly spread in the United States. Virology. 2003;309:190–195. doi: 10.1016/s0042-6822(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae) J Med Entomol. 2008;45:445–451. doi: 10.1603/0022-2585(2008)45[445:eipfha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong PM, Vossbrinck CR, Andreadis TG, Anderson JF, Pesko KN, Newman RM, Lennon NJ, Birren BW, Ebel GD, Henn MR. Molecular evolution of West Nile virus in a temperate region: Connecticut, USA 1999–2008. Virology. 2011;417:203–210. doi: 10.1016/j.virol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaty BJ, Calisher CH, Shope RE. In: Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Sixth edition. Schmidt NJ, Emmons RW, editors. Washington, DC: American Public Health Association; 1989. pp. 797–855. (Arboviruses). [Google Scholar]
- 24.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng G, Cox J, Wang P, Krishnan MN, Dai J, Qian F, Anderson JF, Fikrig E. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzi M. Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum Biol. 1950;22:151–190. [PubMed] [Google Scholar]
- 28.Turell MJ. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 127–152. [Google Scholar]
- 29.Biggerstaff BJ. PooledInfRate, Version 3.0: a Microsoft Excel add-in to compute prevalence estimates from pooled samples. Fort Collins, CO: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 30.Anderson JF, Andreadis TG, Main AJ, Kline DL. Prevalence of West Nile virus in tree canopy-inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg. 2004;71:112–119. [PubMed] [Google Scholar]
- 31.Dohm DJ, O'Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 34.Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae) J Med Entomol. 2002;39:640–644. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- 35.Baqar S, Hayes CG, Murphy JR, Watts DM. Vertical transmission of West Nile virus by Culex and Aedes species of mosquitoes. Am J Trop Med Hyg. 1993;48:757–762. doi: 10.4269/ajtmh.1993.48.757. [DOI] [PubMed] [Google Scholar]
- 36.Miller BR, Nasci RS, Godsey MS, Savage HM, Lutwama JJ, Lanciotti RS, Peters CJ. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley Province, Kenya. Am J Trop Med Hyg. 2000;62:240–246. doi: 10.4269/ajtmh.2000.62.240. [DOI] [PubMed] [Google Scholar]
- 37.Phillips RA, Christensen K. Field-caught Culex erythrothorax larvae found naturally infected with West Nile virus in Grand County, Utah. J Am Mosq Control Assoc. 2006;22:561–562. doi: 10.2987/8756-971X(2006)22[561:FCELFN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Tesh RB. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Am J Trop Med Hyg. 1980;29:657–666. doi: 10.4269/ajtmh.1980.29.657. [DOI] [PubMed] [Google Scholar]
- 39.Davis CT, Beasley DW, Guzman H, Raj P, D'Anton M, Novak RJ, Unnasch TR, Tesh RB, Barrett ADT. Genetic variation among temporally and geographically distinct West Nile virus isolates, United States, 2001, 2002. Emerg Infect Dis. 2003;9:1423–1429. doi: 10.3201/eid0911.030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett AD. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]


