Abstract
Background
Numerous studies in Chinese populations have evaluated the association between the A-6G and A-20C polymorphisms in the promoter region of angiotensinogen gene and hypertension. However, the results remain conflicting. We carried out a meta-analysis for these associations.
Methods and Results
Case–control studies in Chinese and English publications were identified by searching the MEDLINE, EMBASE, CNKI, Wanfang, CBM, and VIP databases. The random-effects model was applied for dichotomous outcomes to combine the results of the individual studies. We finally selected 24 studies containing 5932 hypertensive patients and 5231 normotensive controls. Overall, we found significant association between the A-6G polymorphism and the decreased risk of hypertension in the dominant genetic model (AA+AG vs. GG: P = 0.001, OR = 0.71, 95%CI 0.57–0.87, Pheterogeneity = 0.96). The A-20C polymorphism was significantly associated with the increased risk for hypertension in the allele comparison (C vs. A: P = 0.03, OR = 1.14, 95%CI 1.02–1.27, Pheterogeneity = 0.92) and recessive genetic model (CC vs. CA+AA: P = 0.005, OR = 1.71, 95%CI 1.18–2.48, Pheterogeneity = 0.99). In the subgroup analysis by ethnicity, significant association was also found among Han Chinese for both A-6G and A-20C polymorphisms. A borderline significantly decreased risk of hypertension between A-6G and Chinese Mongolian was seen in the allele comparison (A vs. G: P = 0.05, OR = 0.79, 95%CI 0.62–1.00, Pheterogeneity = 0.84).
Conclusion
Our meta-analysis indicated significant association between angiotensinogen promoter polymorphisms and hypertension in the Chinese populations, especially in Han Chinese.
Introduction
Essential hypertension (EH), the major section (over 95%) of hypertension, represents a serious health problem all over the world. In China, hypertension affects more than 18.8% of the adult population, and a total of 170 million people suffer from this disease [1]. Hypertension has a multi-factorial origin arising from an interaction between susceptibility genes and environmental factors [2]. It is noteworthy that about 20% to 60% of the inter-individual variation of blood pressure (BP) is determined by heritable factors [3]. As a consequence, many potential genes involved in blood pressure regulation have been screened and recognized as candidates for hypertension.
The renin-angiotensin-aldosterone system (RAAS) plays a crucial role in the maintenance of BP [4]. Among the system, the angiotensinogen (AGT) is a liver protein that interplays with renin to produce angiotensin I, the prohormone of angiotensin II, which is the major effector molecule of RAAS. The human AGT gene is located on chromosome 1 (1q42–q43) and contains 5 exons [5]. Many variants in the AGT gene can modify the plasma AGT concentration that is directly linked with arterial blood pressure [6]. At present, we paid particular attention to the rs5051 (A-6G) and rs5050 (A-20C) single nucleotide polymorphisms (SNPs) in the promoter region, which both were reported to influence AGT transcriptional activity and then plasma AGT [6]. Haplotype analysis has revealed that the two SNPs, A-6G and A-20C, were in strong linkage disequilibrium [7].
In a 2008 meta-analysis by Pereira et al. [8], the relationship between hypertension and the A-6G and A-20C polymorphisms has been evaluated in European Caucasian subjects. However, no meta-analysis of these specific genetic assocations has been conducted in Chinese populations so far. The genetic background difference between the two ethnic groups may lead to different conclusions. In addition, the published results of Chinese case-control studies for both polymorphisms remained unsettled. Some studies implied that the A-6G polymorphism [9]–[12] as well as A-20C polymorphism [13] in the AGT gene were associated with the increased or even reduced risk of EH in Chinese, whereas most studies [14]–[24] still provided equivocal or largely negative evidence for this relationship. Taken together, we decided to perform a carefully designed meta-analysis from all eligible case-control studies, in order to clarify the role of the A-6G and A-20C polymorphisms in hypertension among the Chinese populations.
Materials and Methods
Identification and eligibility of relevant studies
To search for all the studies that examined the association of the A-6G and A-20C polymorphisms with hypertension in Chinese, we conducted a computerized literature search of the PubMed, EMBASE, CNKI (China Nation Knowledge Infrastructure Platform), Wanfang, CBM (China Biological Medicine Database) and VIP databases (up to May 2011), using the following keywords and subject terms: ‘AGT or Angiotensinogen’, ‘polymorphism’, ‘hypertension’ and ‘Chinese or China or Taiwanese or Taiwan’. We only included studies published in Chinese or in English. References of retrieved articles were also screened. When a report overlapped with another publication, to prevent data duplication, only the more detailed one was kept. If an article reported results on different ethnic sub-populations, each sub-population was treated as separate study in our meta-analysis. We used the following inclusion criteria for a study to be included in the meta-analysis: (a) studies investigating the association of the A-6G or A-20C polymorphisms with hypertension in Chinese individuals, (b) use of an unrelated case–control design (family-based study design with linkage considerations was excluded), (c) available genotype frequency, (d) the genotype distribution of the control population must be in Hardy–Weinberg equilibrium (HWE) and (e) hypertension defined as systolic blood pressure (SBP)≥140 mmHg and/or diastolic blood pressure (DBP)≥90 mmHg and/or treatment with anti-hypertensive medication. If the genotype frequency was not reported, we contacted the original authors by e-mail in order to obtain the missing data.
Data extraction
Two authors (W. Gu and J. Liu) independently reviewed and extracted the data needed. Disagreements were resolved through discussion among the authors to achieve a consensus. The following information was abstracted from each study: first author, year of publication, racial background and resident region of study population, diagnostic criteria, matching, source of samples, genotype detecting method of each study, number of cases and controls, distribution of genotypes and alleles in both case and control groups.
Statistical analysis
Odds ratios (OR) corresponding to 95% confidence interval (CI) was applied to measure the strength of the association of A-6G and A-20C with hypertension as case–control studies were used, and OR was calculated according to the method of Woolf [25]. We examined the association between allele A of A-6G and hypertension (A vs. G), the dominant genetic model (AA+AG vs. GG), and the recessive genetic model (AA vs. AG+GG). The same method was applied to analyze the A-20C polymorphism. In our study, only the random-effects model using the DerSimonian and Laird's method was employed to bring the individual effect-size estimates together in Review-Manager 5.0.25 software [26]. We then performed a chi-square-based Q statistic test to assess the between-study heterogeneity [27]. Heterogeneity was considered significant for P<0.10 because of the low power of the statistic. The inconsistency index I2 was also calculated to evaluate the variation which was caused by heterogeneity rather than by chance, and higher values of the index indicate the existence of heterogeneity [28]. The significance of the pooled OR was determined by the Z test and a P value of <0.05 was considered significant. For each genetic comparison, subgroup analysis according to racial descent was considered for Han Chinese and non-Han Chinese minorities to estimate ethnic-specific OR. In addition, subgroup analysis according to gender was also carried out. Each subgroup had at least two independent studies.
When unexpected heterogeneity was present, sensitivity analysis was performed to examine specific sensitivity of the findings. This analysis was conducted by examining and recalculating the pooled association sizes and joint values of P in homogeneous subgroups, as well as after excluding studies one by one.
Publication bias was investigated by funnel plot, in which the standard error of the log (OR) of each study was plotted against its OR. An asymmetric plot suggested possible publication bias. Funnel-plot asymmetry was assessed by the method of Egger's linear regression test [29]. We performed a t-test to determine the significance of the intercept, and a P-value of <0.05 was considered significant. HWE was tested by the chi-square-test for goodness of fit based on a web program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). All statistical analyses were performed using ReviewManager 5.0.25 (Oxford, England) and the software Stata version 10.0 (Stata Corporation, College Station, Texas, USA). All P-values were two-sided.
Results
Selection of studies
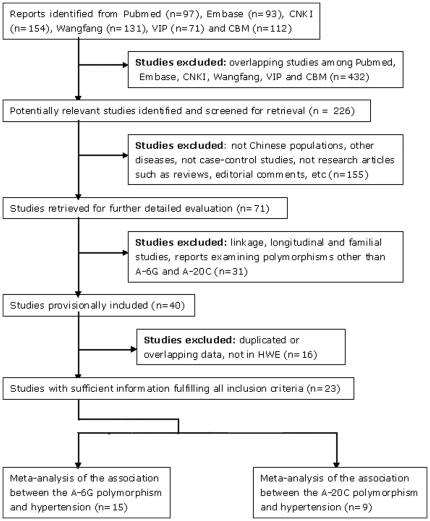
After literature search and selection applying our inclusion criteria, we identified a total of 27 relevant articles (40 studies) [7]–[24], [30]–[38]. Among the 40 eligible studies, seven studies [13], [23], [30]–[34] were excluded because they shared the same or overlapping data with others. Moreover, eight studies [13]–[14], [22], [24], [32], [35]–[37] with control groups deviating from HWE were deleted. Finally, 15 studies [7], [9]–[12], [14]–[21], [38] containing 3442 hypertensive patients and 3058 controls for A-6G, as well as 9 studies [7], [11], [15], [17], [20]–[24] containing 2490 hypertensive patients and 2173 controls for A-20C, were collected as being appropriate for the meta-analysis (Figure 1). Thereof, Ge et al. [16], Wang et al. [18] Yue et al. [21] and Liu et al. [38], were four unpublished theses acquired from medical doctorate dissertation database, which was one public sub-database shared by CNKI and Wanfang databases. One paper by Liu et al. [12] provided data on subjects of two Chinese minorities: Tibetan and Yi. Thus, the two minorities were treated as separate studies. For the Yi population, the samples (both cases and controls) were selected only from male individuals. The characteristics of the included studies were summarized in Table 1. Genotype distributions of the control population met HWE for all qualified studies (P>0.05). The flowchart summarizing the process of study selection and reasons for exclusion was presented in Figure 1.
Figure 1. The flowchart of selection of studies and specific reasons for exclusion from the meta-analysis.
Table 1. Detailed characteristics of eligible studies considered in the meta-analysis.
| First author | Year | Ethnicity | Region | Single-nucleotide polymorphism | Diagnostic criteria | Matching | Source | Method |
| Ge [16] | 2000 | Tibetan | Tibet | A-6G | SBP≥140, DBP≥90 | Yes | P-B | PCR-RFLP |
| Hu [14] | 2007 | Mongolian | Inner Mongolian | A-6G | SBP≥140, DBP≥90 | Yes | P-B | Sequencing technique |
| Jiang [17] | 2009 | Han | Jiangsu | A-6G, A-20C | SBP≥140, DBP≥90 | Yes | P-B | TaqMan - PCR |
| Kong [15] | 2002 | Han | Henan | A-6G, A-20C | SBP≥160, DBP≥95 | Yes | H-B | PCR-RFLP |
| Liu [38] | 2002 | Han | Shanghai | A-6G | SBP≥140, DBP≥90 | No | H-B | Sequencing technique |
| Li [22] | 2004 | Kazakh | Xinjiang | A-20C | SBP≥140, DBP≥90 | Yes1 | P-B | PCR-SSCP |
| Liu [12] | 2001 | Tibetan | Tibet | A-6G | SBP>140, DBP>90 | Yes | P-B | PCR-RFLP |
| Liu [12] | 2001 | Yi | Sichuan | A-6G | SBP>140, DBP>90 | Yes | P-B | PCR-RFLP |
| Qi [7] | 2008 | Han | Beijing | A-6G, A-20C | SBP≥140, DBP≥90 | Yes1 | P-B | PCR-RFLP |
| Wang [9] | 2002 | Amis | Taiwan | A-6G | SBP≥140, DBP≥90 | Yes | H-B | Sequencing technique |
| Wang [18] | 2003 | Kazakh | Xinjiang | A-6G | SBP≥160, DBP≥95 | Yes | P-B | MS-PCR |
| Wang [11] | 2007 | Li | Hainan | A-6G, A-20C | SBP≥140, DBP≥90 | Yes | H-B | Sequencing technique |
| Wu [10] | 2004 | Han | Taiwan | A-6G | SBP≥140, DBP≥90 | Yes1 | H-B | Sequencing technique |
| Yang [23] | 2000 | Tibetan | Tibet | A-20C | SBP≥140, DBP≥90 | Yes | P-B | PCR-RFLP |
| Yao [19] | 2010 | Bai | Yunnan | A-6G | SBP≥140, DBP≥90 | No | H-B | PCR-RFLP |
| Ying [20] | 2010 | Mongolian | Inner Mongolian | A-6G, A-20C | SBP≥140, DBP≥90 | No | P-B | PCR-RFLP |
| Yue [21] | 2008 | Han | Hebei | A-6G, A-20C | SBP≥140, DBP≥90 | Yes2 | P-B | PCR-RFLP |
| Liu [24] | 2004 | Han | Shanghai | A-20C | SBP>140, DBP>90 | No | H-B | Sequencing technique |
Abbreviations: SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); P-B, population-based study; H-B, hospital-based study; PCR-RFLP, polymerase chain reaction and restriction fragment length polymorphism; PCR-SSCP, polymerase chain reaction and single strand conformation polymorphism; MS-PCR, mutagenically separated polymerase chain reaction; Yes, age- and gender- matched, Yes1, gender-matched, Yes2, age-matched.
Association between the AGT A-6G polymorphism and hypertension
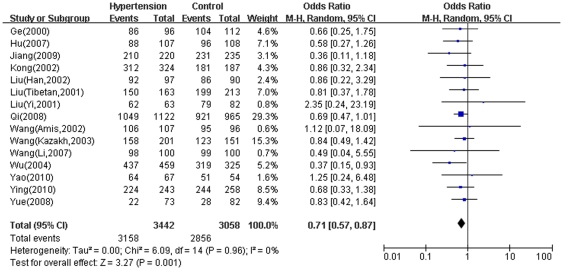
The distribution of AGT A-6G genotypes and alleles in the individual studies was listed in Table 2. The pooled overall frequency of the -6A allele in the Chinese populations was 75.09% in hypertensive cases and 76.60% in normotensive controls. The main results of the meta-analysis about A-6G and the heterogeneity test were presented in Table S1. For all subjects, in the allele comparison and recessive genetic model, there were no significant association between the A-6G polymorphism and hypertension (A vs. G: P = 0.08, OR = 0.9, 95%CI 0.8–1.01, Pheterogeneity = 0.09, I2 = 35%. AA vs. AG+GG: P = 0.4, OR = 0.93, 95%CI 0.8–1.09, Pheterogeneity = 0.04, I2 = 43%) (Table S1). However, we detected significantly reduced risk of hypertension in the dominant genetic model (AA+AG vs. GG: P = 0.001, OR = 0.71, 95%CI 0.57–0.87, Pheterogeneity = 0.96, I2 = 0) (Figure 2).
Table 2. Sample size, the distribution of A-6G genotypes and allele frequencies, and P-values of HWE.
| Sample size | AA(genotype) | AG(genotype) | GG(genotype) | A allele frequency (%) | HWE(P* value) | ||||||
| First author | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Controls |
| Ge [16] | 96 | 112 | 36 | 49 | 50 | 55 | 10 | 8 | 63.54 | 68.30 | 0.1558 |
| Hu [14] | 107 | 108 | 53 | 59 | 35 | 37 | 19 | 12 | 65.89 | 71.76 | 0.1078 |
| Jiang [17] | 220 | 235 | 136 | 169 | 74 | 62 | 10 | 4 | 78.64 | 85.11 | 0.5325 |
| Kong [15] | 324 | 187 | 200 | 104 | 112 | 77 | 12 | 6 | 79.01 | 76.20 | 0.0641 |
| Liu(Han) [38] | 97 | 90 | 65 | 59 | 27 | 27 | 5 | 4 | 80.93 | 80.56 | 0.6878 |
| Liu(Tibetan) [12] | 163 | 213 | 64 | 102 | 86 | 97 | 13 | 14 | 65.64 | 70.66 | 0.2927 |
| Liu(Yi) [12] | 63 | 82 | 47 | 52 | 15 | 27 | 1 | 3 | 86.51 | 79.88 | 0.8259 |
| Qi [7] | 1122 | 965 | 671 | 608 | 378 | 313 | 73 | 44 | 76.65 | 79.22 | 0.6470 |
| Wang(Amis) [9] | 107 | 96 | 89 | 65 | 17 | 30 | 1 | 1 | 91.12 | 83.33 | 0.2207 |
| Wang(Kazakh) [18] | 201 | 151 | 77 | 52 | 81 | 71 | 43 | 28 | 58.46 | 57.95 | 0.6550 |
| Wang(Li) [11] | 100 | 100 | 83 | 89 | 15 | 10 | 2 | 1 | 90.50 | 94.00 | 0.2565 |
| Wu [10] | 459 | 325 | 316 | 229 | 121 | 90 | 22 | 6 | 82.03 | 84.31 | 0.4010 |
| Yao [19] | 67 | 54 | 33 | 30 | 31 | 21 | 3 | 3 | 73.49 | 75.00 | 0.7855 |
| Ying [20] | 243 | 258 | 138 | 161 | 86 | 83 | 19 | 14 | 74.49 | 78.49 | 0.4473 |
| Yue [21] | 73 | 82 | 3 | 1 | 19 | 27 | 51 | 54 | 80.77 | 82.32 | 0.2354 |
Abbreviations: HWE, Hardy–Weinberg equilibrium.
*The P-value of HWE determined by the χ2 test.
Figure 2. Meta-analysis for the overall association between the A-6G polymorphism and hypertension under the dominant genetic model.
Figure 2 shows that the -6A allele carrier (AA+AG) can reduce the risk of hypertension compared to the homozygous GG genotype carriers.
In the subgroup analysis by ethnicity, studies were categorized into three groups: Han Chinese, Tibetan and Mongolian. The number of these subpopulations was as follows: six studies involved Han Chinese subjects (2295 cases and 1884 controls), two studies involved Chinese Mongolian (350 cases and 366 controls) and two studies involved Chinese Tibetan (259 cases and 325 controls). The -6A allele had a much higher representation in cases and controls of Han Chinese (76.54% and 77.92%, respectively) than that of Tibetan (64.87 and 69.84%, respectively) and of Mongolian (71.86 and 76.5%, respectively). In Han Chinese population, significant association between the A-6G polymorphism and the decreased risk for hypertension was observed in the dominant genetic model (AA+AG vs. GG: P = 0.005, OR = 0.66, 95%CI 0.50–0.88, Pheterogeneity = 0.64, I2 = 0)(Table S1). In the allele comparison and recessive genetic model, no evidence of association was found (Table S1). For the subgroups of non-Han Chinese minorities, we found no significant association in any genetic models in the population of Tibetan (Table S1). For Mongolian, a borderline decreased risk of hypertension was seen for A allele carriers compared with G allele carriers (A vs. G: P = 0.05, OR = 0.79, 95%CI 0.62–1.00, Pheterogeneity = 0.84, I2 = 31%) (Table S1). There was no significant association found in other genetic models conducted using Mongolian (Table S1).
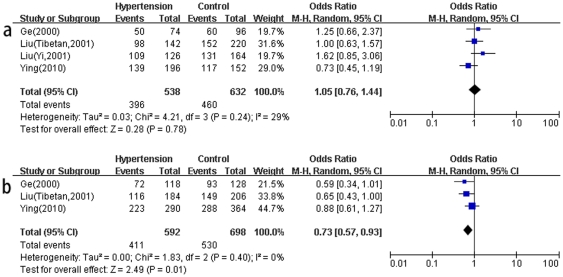
Considering the fact that the sex discrepancy might bias the overall association, a further subgroup analysis was conducted according to gender. As reflected in Table 3, four studies (3 articles) provided data for males and females, respectively. Accordingly, a total of 269 hypertension patients and 316 controls for males, as well as 296 hypertension patients and 349 controls for females, were investigated. In male subjects, the lack of significant association was found between A-6G and hypertension in all genetic models (A vs. G: P = 0.78, OR = 1.05, 95%CI 0.76–1.44, Pheterogeneity = 0.24, I2 = 29%) (Figure 3a). However, in female subjects, the A-6G polymorphism displayed significantly reduced risk for hypertension in the allele comparison (A vs. G: P = 0.01, OR = 0.73, 95%CI 0.57–0.93, Pheterogeneity = 0.40, I2 = 0%) (Figure 3b) and recessive genetic model (AA vs. AG+GG: P = 0.02, OR = 0.69, 95%CI 0.50–0.95, Pheterogeneity = 0.49, I2 = 0%).
Table 3. The characteristics of all included studies for the A-6G polymorphism in the sex-specific subgroup analysis.
| Genotype(Number, M/F) | Blood pressure | |||||||||
| First author | Ethnicity | Status | Number, M/F | AA | AG | GG | Age, year | BMI, kg/m2 | SBP, mmHg | DBP, mmHg |
| Ge [16] | Tibetan | Cases | 37/59 | 16/20 | 18/32 | 3/7 | 49.53±11.4 | 23.34±3.99 | 159.74±23.15 | 105.96±10.83 |
| Controls | 48/64 | 17/32 | 26/29 | 5/3 | 47.98±12.07 | 21.54±3.11 | 116.38±16.66 | 78.21±9.94 | ||
| Liu(Tibetan) [12] | Tibetan | Cases | 71/92 | 30/34 | 38/48 | 3/10 | 48±12 | 24±4 | 164±20 | 105±12 |
| Controls | 110/103 | 52/50 | 48/49 | 10/4 | 46±10 | 22±3 | 117±14 | 78±9 | ||
| Liu(Yi) [12] | Yi | Cases | 63/0 | 47/0 | 15/0 | 1/0 | 51±12 | 25±4 | 158±30 | 103±16 |
| Controls | 82/0 | 52/0 | 27/0 | 3/0 | 49±6 | 21±3 | 112±8 | 73±6 | ||
| Ying [20] | Mongolian | Cases | 98/145 | 52/86 | 35/51 | 11/8 | 53.5±11.3 | 23.2±4.1 | 159.4±24.2 | 98.3±11.3 |
| Controls | 76/182 | 45/116 | 27/56 | 4/10 | 50.3±9.5 | 22.1±3.2 | 119.6±10.8 | 78.1±6.3 | ||
Abbreviations: M/F, males/females; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Values, mean±s.d.
Figure 3. Meta-analysis for the association between the A-6G polymorphism and hypertension under allele comparison (A vs. G) in the subgroup by sex.
Figure 3a shows that the A-6G polymorphism is not associated with hypertension in men. Figure 3b shows that the -6 A allele carrier can reduce the risk of hypertension in women compared to the -6G allele carrier.
Association between the AGT A-20C polymorphism and hypertension
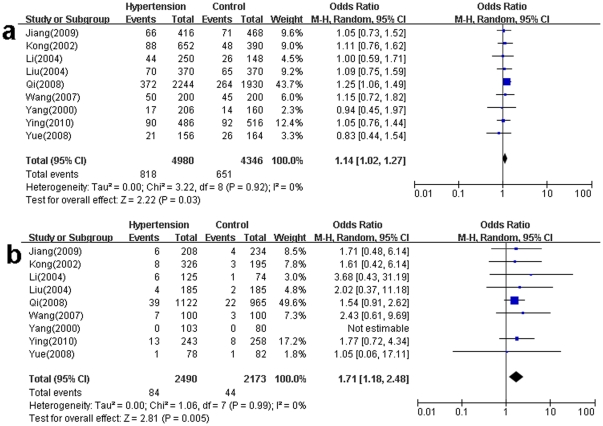
The distribution of AGT A-20C genotypes and alleles in the individual studies was showed in Table 4. The overall prevalence of the -20C allele in the Chinese populations was 16.43% in cases and 14.98% in controls. The main results of the meta-analysis about A-20C and the heterogeneity test were presented in Table S1. Overall, the significant increased risk of hypertension could be found in the allele comparison (C vs. A: P = 0.03, OR = 1.14, 95%CI 1.02–1.27, Pheterogeneity = 0.92, I2 = 0) (Figure 4a) and recessive genetic model (CC vs. CA+AA: P = 0.005, OR = 1.71, 95%CI 1.18–2.48, Pheterogeneity = 0.99, I2 = 0) (Figure 4b). There was no significant association found in the dominant genetic model (CC+CA vs. AA: P = 0.14, OR = 1.10, 95%CI 0.97–1.25, Pheterogeneity = 0.83, I2 = 0) (Table S1).
Table 4. Sample size, the distribution of A-20C genotypes and allele frequencies, and P-values of HWE.
| Sample size | CC(genotype) | CA(genotype) | AA(genotype) | C alleleFrequency(%) | HWE(P* value) | ||||||
| First author | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Controls |
| Jiang [17] | 208 | 234 | 6 | 4 | 54 | 63 | 148 | 167 | 15.87 | 15.17 | 0.4815 |
| Kong [15] | 326 | 195 | 8 | 3 | 72 | 42 | 246 | 150 | 13.50 | 12.31 | 0.9756 |
| Li [22] | 125 | 74 | 6 | 1 | 32 | 24 | 87 | 49 | 17.60 | 17.57 | 0.3028 |
| Liu [24] | 185 | 185 | 4 | 2 | 62 | 55 | 119 | 128 | 18.92 | 15.95 | 0.1380 |
| Qi [7] | 1122 | 965 | 39 | 22 | 294 | 220 | 789 | 723 | 16.58 | 13.68 | 0.2823 |
| Wang [11] | 100 | 100 | 7 | 3 | 36 | 39 | 57 | 58 | 25.00 | 22.50 | 0.2367 |
| Yang [23] | 103 | 80 | 0 | 0 | 17 | 14 | 86 | 66 | 8.25 | 8.75 | 0.3910 |
| Ying [20] | 243 | 258 | 13 | 8 | 64 | 76 | 166 | 174 | 18.52 | 17.83 | 0.9317 |
| Yue [21] | 78 | 82 | 1 | 1 | 19 | 24 | 58 | 57 | 13.46 | 15.85 | 0.3798 |
Abbreviations: HWE, Hardy–Weinberg equilibrium.
*The P-value of HWE determined by the χ2 test.
Figure 4. Meta-analysis for the overall association between the A-20C polymorphism and hypertension under various genetic contrasts.
Figure 4a shows that the C allele carrier can increase the risk of hypertension compared to the A allele carrier. Figure 4b shows that the homozygous CC genotype carriers can increase the risk of hypertension compared to the A allele carrier (CA+AA).
Due to the limited studies of non-Han Chinese minorities, the subgroup analysis by ethnicity was only performed for Han Chinese. Specifically, there were five studies dealing with Han Chinese (1919 cases and 1661 controls), and only one study considered Kazakh, Li, Tibetan and Mongolian populations. In the Han population, we found significantly elevated risk of hypertension with the A-20C polymorphism in the allele comparison (C vs. A: P = 0.02, OR = 1.17, 95%CI 1.02–1.33, Pheterogeneity = 0.67, I2 = 0) and recessive genetic model (CC vs. CA+AA: P = 0.04, OR = 1.58, 95%CI 1.02–2.45, Pheterogeneity = 1.00, I2 = 0) (Table S1). No positive association was obtained in the dominant genetic model (Table S1). Finally, because only one article [20] provided data for males and females, respectively, we could not perform an additional subgroup analysis based on gender.
Sensitivity analysis
Significant between-study heterogeneity only existed among all studies in the meta-analysis of the A-6G polymorphism. Sensitivity analyses were conducted by sequentially removing a single study each time to find out the origin of heterogeneity. As a result, the heterogeneity no longer existed for the A-6G polymorphism when four studies were excluded (Liu (Yi) et al. [12]: A vs. G, Pheterogeneity = 0.15; Kong et al. [15]: A vs. G, Pheterogeneity = 0.16; Jiang et al. [17]: A vs. G, Pheterogeneity = 0.16; Wang (Amis) et al. [9]: A vs. G, Pheterogeneity = 0.37, AA vs. AG+GG, Pheterogeneity = 0.23). In addition, our analysis showed that the corresponding pooled ORs were materially altered with the sequential removal of these four studies (data not shown). The findings revealed that these independent studies might be the main cause of heterogeneity across all subjects.
Publication bias
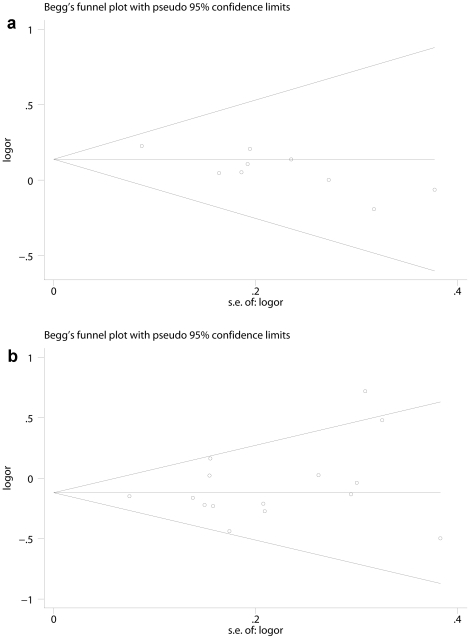
The Egger's test and Begg's funnel plot were applied for allele comparison to asses the publication bias of the literatures. As indicated by the Egger's test and funnel plot, there was no publication bias for the A-6G polymorphism (t = 0.98, P = 0.347 for A vs. G) (Figure 5a), and a possibility of publication bias for the A-20C polymorphism (t = −3.88, P = 0.006 for C vs. A) (Figure 5b).
Figure 5. Begg's funnel plot analysis to detect publication bias.
Figure 5a shows the funnel plot for allele comparison (A vs. G) of the A-6G polymorphism. Figure 5b shows the funnel plot for allele comparison (C vs. A) of the A-20C polymorphism.
Discussion
The literature examining the relationship between the A-6G and A-20C polymorphisms and hypertension in the Chinese populations abounds in small studies reporting controversial findings. No clear consensus has yet been reached. Therefore, we restricted our research to Chinese populations and did a meta-analysis with 24 studies totaling 5932 hypertensive patients and 5231 normotensive controls to form a more precise estimation of their association. To our knowledge, this was one of the largest meta-analysis to date investigating the association of angiotensinogen promoter polymorphisms with hypertension in Chinese. In the current study, we found that the A allele of the A-6G polymorphism was associated with a significant decrease in the risk of hypertension in all subject, and the C allele of A-20C could increase the risk of hypertension. These positive associations should be treated with caution as the P values obtained were reported without correction for multiple testing.
In 1997, Inoue et al. [39] were the first to provide direct evidence for increased transcriptional activity of the -6A variant as compared with the -6G variant in the AGT promoter in a hepatocyte cell line. Since then, much effort has been made to pursue the possible association of the A-6G polymorphism with hypertension. Hegele RA et al. [40] observed that the presence of the AGT -6A variant tended to be associated with higher systolic BP in Canadian Oji-Cree. This positive result was partly confirmed by Ishigami T et al. [41] who studied on Japanese population. A more recent research by Jain S et al. [42] showed that transgenic mice containing -6A haplotype have increased plasma AGT level and increased blood pressure as compared to -6G haplotype, and also reported higher expression of AGT mRNA in Caucasian hypertensive patients carrying the same allele. Moreover, Pereira TV's meta-analysis [8] that came out in 2007 found that A-6G have a higher yet nonsignificant risk for hypertension in Asian populations. The Asian participants included in the analysis were not all from Chinese population, which meant genetic background between this article and our study was different. Nevertheless, these previous findings were inconsistent with the result of our meta-analysis, which was very interesting and exhibited a protective effect on hypertension in the Chinese populations. There were many possible explanations that could be put forward to account for the inconsistency, and ethnic specificity as well as population structure might be the most important potential confounding factors. An obvious supporter was a Chinese case-control study by Liu et al. [12] also generating a protective effect of the A-6G variant on hypertension. It was necessary to perform a meta-analysis in a genetically well-defined population.
Regarding the A-20C polymorphism, a report by Zhao et al. [43] showed that the -20C allele enhanced basal promoter activity on transient transfection in human hepatoma cells (HepG2) as compared with the -20A allele. In human subjects, DR Velez et al. [44] found that this polymorphism seemed to be involved in hypertension in white women. A Chinese case-control article [13] also displayed that the -20C variant could apparently increase the risk of hypertension in the Taiwanese population. Then the results of a 2008 meta-analysis [8] and our meta-analysis verified that the A-20C polymorphism might be major genetic predisposing factor for hypertension in the Chinese populations. Furthermore, with a tight linkage disequilibrium (LD) between the M235T and A-20C polymorphisms, our results about the relationship between A-20C and EH are consistent with previous results by Ji et al. [45] in 2010, which reported that the M235T variant increases the risk of essential hypertension in the Chinese populations (OR = 1.54, 95%CI 1.16–2.03, P = 0.002). An interaction between the A-6G and A–20C variants was biologically plausible [32], [46] because these 2 variants were located in 2 distinct regulatory elements of the core promoter in AGT gene. Subsequently, this interaction between them might affect the transcription of the gene and/or the stability of the resulting mRNA, and in turn play an important role in the pathogenesis of hypertension. Niu et al. [32] observed that the A-6G/A-20C polymorphism was significantly associated with hypertension, which might be attributed to a strong synergistic effect of these two polymorphisms.
China is a huge multi-ethnic country with 56 identified ethnic groups. Among these groups, Han Chinese is the largest ethnic group, making up over 93% of the total population [47]. In the subgroup analysis, we divided studies into two subgroups: Han Chinese and non-Han Chinese minorities. Significant association was identified in Han Chinese for both A-6G and A-20C polymorphisms, which were in accordance with the results in the overall population. For non-Han Chinese minorities, owing to the limited studies and population numbers, only a marginal significant association between A-6G and hypertension was seen in Chinese Mongolian. More studies based on larger population are required to reach more obvious conclusion in different minorities. In another subgroup analysis by gender, the significantly decreased risk of hypertension was associated with the A-6G polymorphism in Chinese women, but not in Chinese men. However, in the view of the relatively small sample size, the conclusion in this sex-specific analysis might be unreliable and must be considered cautiously. Furthermore, no extra subgroup analysis according to gender was undertaken for the A-20C polymorphism because the eligible studies were scarce in number. In the sensitivity analysis, four studies (Wang (Amis) et al. [9], Liu (Yi) et al. [12], Kong et al. [15] and Jiang et al. [17]) were considered as the origin of the heterogeneity. When these articles were deleted, significant association could be found.
The positive findings about A-20C should be interpreted in light of the fact that publication bias was detected. The statistical test and funnel plot inspection in the meta- analysis have indicated the potential for such bias. Publication bias is a relatively common phenomenon in clinical literature [48]–[50], perhaps because positive results have a better chance of being accepted for publication than small studies with non-significant or negative findings. Therefore, conclusions based on these published work might be misleading [51]. In the analysis of the A-20C polymorphism, we conducted a comprehensive search of the published literature and thought it unlikely that many important papers would have been overlooked, but despite this effort, there was still a possibility of publication bias. Thus, the positive findings for A-20C have to be regarded as preliminary.
Several limitations of our meta-analysis need to be noted. First, because of a small number of available studies, we failed to perform additional subgroup analysis in other minority populations (such as Bai), and by gender for the A-20C polymorphism. Second, publication bias was present, and might distort the final conclusion. Third, due to the lack of original data, an evaluation of potential interactions such as gene-gene or gene-environment was not considered in this meta-analysis, which might confound our results.
In conclusion, our meta-analysis suggested significant association between the A-6G and A-20C polymorphisms and hypertension in the Chinese populations, particularly in Han Chinese. More large–scale studies, and especially studies stratified for different minorities and different sexes, should be performed to further elucidate the association between the A-6G and A-20C polymorphisms and hypertension in the Chinese populations.
Supporting Information
The PRISMA checklist for this meta-analysis.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was financially supported by the National High Technology Research and Development Program (2008AA02Z441) (http://www.most.gov.cn/eng/programmes1/200610/t20061009_36225.htm), and the National Eleventh Five-year Plan Program (2008BAI52B03) (http://www.most.gov.cn/eng/programmes1/index.htm) from the Ministry of Science and Technology in People's Republic of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kong LZ, Hu SS. Report on cardiovascular disease in China. Beijing: Encyclopedia of China Publishing House; 2005. pp. 86–88. [Google Scholar]
- 2.O'Shaughnessy KM. The genetics of essential hypertension. Br J Clin Pharmacol. 2001;51:5–11. doi: 10.1046/j.1365-2125.2001.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz TW, Spence MA. Genetics of essential hypertension. Am J Med. 1993;94:77–84. doi: 10.1016/0002-9343(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 4.Perazella MA, Setaro JF. Renin–angiotensin–aldosterone system: fundamental aspects and clinical implications in renal and cardiovascular disorders. J Nucl Cardiol. 2003;10:184–196. doi: 10.1067/mnc.2003.392. [DOI] [PubMed] [Google Scholar]
- 5.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 6.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 7.Qi Y, Niu W, Zhou W, Hou S, Qiu C. Correlation between angiotensinogen gene polymorphisms and essential hypertension in Chinese population. Hypertens Res. 2008;22:147–150. doi: 10.1038/sj.jhh.1002282. [DOI] [PubMed] [Google Scholar]
- 8.Pereira TV, Nunes AC, Rudnicki M, Yamada Y, Pereira AC, et al. Meta-Analysis of the association of 4 angiotensinogen polymorphisms with essential hypertension: a role beyond M235T? Hypertension. 2008;51:778–783. doi: 10.1161/HYPERTENSIONAHA.107.100370. [DOI] [PubMed] [Google Scholar]
- 9.Wang JH, Lin CM, Wang LS, Lai NS, Chen DY, et al. Association between molecular variants of the angiotensinogen gene and hypertension in Amis tribes of eastern Taiwan. J Formos Med Assoc. 2002;101:181–188. [PubMed] [Google Scholar]
- 10.Wu SJ, Chiang FT, Chen WJ, Liu PH, Hsu KL, et al. Three single-nucleotide polymorphisms of the angiotensinogen gene and susceptibility to hypertension: single locus genotype vs. haplotype analysis. Physiol Genomics. 2004;17:79–86. doi: 10.1152/physiolgenomics.00133.2003. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Chen ZB, Jin SJ, Su QJ. Correlation between angiotensinogen gene and primary hypertension with cerebral infarction in the Li nationality of China. Neurosci Bull. 2007;23:287–292. doi: 10.1007/s12264-007-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Qin W, Hou S, Shan G, Zhuo M, et al. A-6G variant of the angiotensinogen gene and essential hypertension in Han, Tibetan, and Yi populations. Hypertens Res. 2001;24:159–163. doi: 10.1291/hypres.24.159. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CT, Fallin D, Chiang FT, Hwang JJ, Lai LP, et al. Angiotensinogen gene haplotype and hypertension: interaction With ACE gene I allele. Hypertension. 2003;41:9–15. doi: 10.1161/01.hyp.0000045080.28739.12. [DOI] [PubMed] [Google Scholar]
- 14.Hu RL, Zhao SG, Niu GM, Zhang CY, Hu RL, et al. Relationship between the mononucleotide polymorphism of angiotensinogen gene at 5′ end promoter A-20C and A-6G and essential hypertension in Mongol nationality. Zhongguo Zu Zhi Gong Cheng Yang Jiu Yu Lin Chuang Kang Fu. 2007;11:5865–5868. [Article in Chinese] [Google Scholar]
- 15.Kong XD, Zhang SZ, Yang YX, Zheng KQ, Tong Y. The relationship between haplotypes of angiotensinogen gene and essential hypertension. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2002;19:488–490. [Article in Chinese] [PubMed] [Google Scholar]
- 16.Ge SLB. Renin–angiotensin system gene polymorphisms in essential hypertension in Tibetan population. Beijing: Zhong Guo Xie He Yi Ke Da Xue; 1995. 175 [Article in Chinese] [Google Scholar]
- 17.Jiang X, Sheng H, Li J, Xun P, Cheng Y, et al. Association between renin–angiotensin system gene polymorphism and essential hypertension: a community-based study. J Hum Hypertens. 2009;23:176–181. doi: 10.1038/jhh.2008.123. [DOI] [PubMed] [Google Scholar]
- 18.Wang XF. Four polymorphisms of renin-angiotensin system in essential hypertension in a kazakh genetic isolate. Wulumuqi: Xin Jiang Yi Ke Da Xue; 2003. 116 [Article in Chinese] [Google Scholar]
- 19.Yao CY, Yin XY, Wu LX. Association between the A-6G variant in 5′ upstream core promoter of angiotension gene and essential hypertension in Bai population. Shi Yong Yi Xue Za Zhi. 2010;26:3514–3516. [Article in Chinese] [Google Scholar]
- 20.Ying CQ, Wang YH, Wu ZL, Fang MW, Wang J, et al. Association of the renin gene polymorphism, three angiotensinogen gene polymorphisms and the haplotypes with essential hypertension in the Mongolian population. Clin Exp Hypertens. 2010;32:293–300. doi: 10.3109/10641960903443517. [DOI] [PubMed] [Google Scholar]
- 21.Yue YH. The study of associations between single nucleotide polymorphisms of angiotension gene and renin gene and cerebralinfarction. Shijiazhuang: He Bei Yi Ke Da Xue; 2008. 62 [Article in Chinese] [Google Scholar]
- 22.Li NF, Zhou L, Wu WD, Shi Y, Wang XL, et al. The relationship between the variants in 5′ upstream core promoter A(-6)G and A(-20)C of angiotension gene and essential hypertension in Kazakans of Xinjiang. Zhongguo Yi Xue Yi Chuan Xue Za Zhi. 2004;21:23–28. [Article in Chinese] [PubMed] [Google Scholar]
- 23.Yang C, Qiu CC, Lu SD, Cen WJ, Zhuo M, et al. Association analysis of variants in the core promoter region of angiotensinogen gene with essential hypertension in Tibetan population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2000;17:149–152. [Article in Chinese] [PubMed] [Google Scholar]
- 24.Liu Y, Jin W, Jiang ZW, Zhang KX, Sheng HH, et al. Relationship between six single nucleotide polymorphisms of angiotensinogen gene and essential hypertension. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:116–119. [Article in Chinese] [PubMed] [Google Scholar]
- 25.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 26.Munafò MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Qiu CC, Zhou WY, Zheng Y, Hou SQ, et al. Gene polymorphism of the renin-angiotensin system in essential hypertension. Chin Med J (Engl) 1999;112:115–120. [PubMed] [Google Scholar]
- 31.Niu WQ, Qi Y, Hou SQ, Zhai XY, Zhou WY, et al. Haplotype-based association of the renin-angiotensin-aldosterone system genes polymorphisms with essential hypertension among Han Chinese: the Fangshan study. J Hypertens. 2009;27:1384–1391. doi: 10.1097/HJH.0b013e32832b7e0d. [DOI] [PubMed] [Google Scholar]
- 32.Niu WQ, Qi Y, Cen WJ, Cui CY, Zhuoma C, et al. Genetic polymorphisms of angiotensinogen and essential hypertension in a Tibetan population. Hypertens Res. 2007;30:1129–1137. doi: 10.1291/hypres.30.1129. [DOI] [PubMed] [Google Scholar]
- 33.Wang GY, Wang YH, Xu Q, Tong WJ, Gu ML, et al. Associations between RAS gene polymorphisms, environmental factors and hypertension in Mongolian people. Eur J Epidemiol. 2006;21:287–92. doi: 10.1007/s10654-005-6006-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang GY, Wang YH, Xu Q, Tong WJ, Qin CC, et al. Genetic gene polymorphisms and interactions of hypertension in Mongolian people. Zhongguo Gong Gong Wei Sheng. 2006;22:1332–1333. [Article in Chinese] [Google Scholar]
- 35.Wang Z, Zhao H, Yuan HL, Xie JX, Guo SX, et al. The association between the G-6A allele of angiotensinogen gene core promoter element 1 (AGCEI) and hypertension in Kazakans of Xinjiang. Zhongguo Xin Xue Guan Bing Za Zhi. 2006;4:116–119. [Article in Chinese] [Google Scholar]
- 36.Chen XD, Wang SM, Wang XF, Lv M, Jin L. Study on the association of predisposing genes with essential hypertension among Kazakhs ethnic group in Xinjiang. Zhonghua Liu Xing bing Xue Za Zhi. 2008;29:752–756. [Article in Chinese] [PubMed] [Google Scholar]
- 37.Qin JH, Liu ZY, Wu DP, Zhu N, Zhou XM, et al. Genotyping the –6A/G functional polymorphism in the core promoter region of angiotensinogen gene by microchip electrophoresis. Electrophoresis. 2005;26:219–224. doi: 10.1002/elps.200406158. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y. Association studies of angiotensinogen gene and cardiocerebrovascular disease. Shanghai: Shang Hai Di Er Yi Ke Da Xue; 2002. 51 [Article in Chinese] [Google Scholar]
- 39.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1998;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegele RA, Harris SB, Hanley AJ, Sun F, Connelly PW, et al. -6A promoter variant of angiotensinogen and blood pressure variation in Canadian Oji-Cree. J Hum Genet. 1998;43:37–41. doi: 10.1007/s100380050034. [DOI] [PubMed] [Google Scholar]
- 41.Ishigami T, Tamura K, Fujita T, Kobayashi I, Hibi K, et al. Angiotensinogen gene polymorphism near transcription start site and blood pressure: role of a T-to-C transition at intron I. Hypertension. 1999;34:430–434. doi: 10.1161/01.hyp.34.3.430. [DOI] [PubMed] [Google Scholar]
- 42.Jain S, Tillinger A, Mopidevi B, Pandey VG, Chauhan CK, et al. Transgenic mice with -6A haplotype of the human angiotensinogen gene have increased blood pressure compared with -6G haplotype. J Biol Chem. 2010;285:41172–41186. doi: 10.1074/jbc.M110.167585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao YY, Zhou J, Narayanan CS, Cui Y, Kumar A. Role of C/A polymorphism at -20 on the expression of human angiotensinogen gene. Hypertension. 1999;33:108–115. doi: 10.1161/01.hyp.33.1.108. [DOI] [PubMed] [Google Scholar]
- 44.Velez DR, Guruju M, Vinukonda G, Prater A, Kumar A, et al. Angiotensinogen promoter sequence variants in essential hypertension. Am J Hypertens. 2006;19:1278–1285. doi: 10.1016/j.amjhyper.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Ji LD, Zhang LN, Shen P, Wang P, Zhang YM, et al. Association of angiotensinogen gene M235T and angiotensin-converting enzyme gene I/D polymorphisms with essential hypertension in Han Chinese population: a meta-analysis. J Hypertens. 2010;28:419–428. doi: 10.1097/HJH.0b013e32833456b9. [DOI] [PubMed] [Google Scholar]
- 46.Hilgers KF, Delles C, Veelken R, Schmieder RE. Angiotensinogen gene core promoter variants and non-modulating hypertension. Hypertension. 2001;38:1250–1254. doi: 10.1161/hy1201.096545. [DOI] [PubMed] [Google Scholar]
- 47.Cavalli-Sforza LL. The Chinese human genome diversity project. Proc Natl Acad Sci USA. 1998;95:11501–11503. doi: 10.1073/pnas.95.20.11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalmers I. Underreporting research is scientific misconduct. JAMA. 1990;263:1405–1408. [PubMed] [Google Scholar]
- 49.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 50.Niu W, Qi Y. Association of α-adducin and G-protein β3 genetic polymorphisms with hypertension: a meta-analysis of Chinese populations. Plos One. 2011;6:e17052. doi: 10.1371/journal.pone.0017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egger M, Smith GD. Misleading meta-analysis. BMJ. 1995;310:752–754. doi: 10.1136/bmj.310.6982.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PRISMA checklist for this meta-analysis.
(DOC)