Abstract
Background
Bats receive increasing attention in infectious disease studies, because of their well recognized status as reservoir species for various infectious agents. This is even more important, as bats with their capability of long distance dispersal and complex social structures are unique in the way microbes could be spread by these mammalian species. Nevertheless, infection studies in bats are predominantly limited to the identification of specific pathogens presenting a potential health threat to humans. But the impact of infectious agents on the individual host and their importance on bat mortality is largely unknown and has been neglected in most studies published to date.
Methodology/Principal Findings
Between 2002 and 2009, 486 deceased bats of 19 European species (family Vespertilionidae) were collected in different geographic regions in Germany. Most animals represented individual cases that have been incidentally found close to roosting sites or near human habitation in urban and urban-like environments. The bat carcasses were subjected to a post-mortem examination and investigated histo-pathologically, bacteriologically and virologically. Trauma and disease represented the most important causes of death in these bats. Comparative analysis of pathological findings and microbiological results show that microbial agents indeed have an impact on bats succumbing to infectious diseases, with fatal bacterial, viral and parasitic infections found in at least 12% of the bats investigated.
Conclusions/Significance
Our data demonstrate the importance of diseases and infectious agents as cause of death in European bat species. The clear seasonal and individual variations in disease prevalence and infection rates indicate that maternity colonies are more susceptible to infectious agents, underlining the possible important role of host physiology, immunity and roosting behavior as risk factors for infection of bats.
Introduction
Bats are among the most successful and diverse mammals on earth. Approximately 1230 chiropteran species are found on every continent except Antarctica and inhabit a multitude of diverse ecological niches [1]. Bats play essential roles in maintaining healthy ecosystems, as they act as plant pollinators, seed dispersers, and predators of populations of insects including harmful forest and agricultural pests [2]. Most bat species are listed in the IUCN Red list of endangered species and almost half of these are considered threatened or near-threatened [3]. To estimate and prevent further population declines, research has been primarily focused on bat biology, ecology and behavior, while disease aspects were largely neglected [4].
In the last two decades, the importance of chiropteran species as potential vectors of significant viral diseases especially in regard to zoonoses has received growing attention. Besides bat rabies that has been studied for more than half a century, extensive research efforts identified a large number of microbial agents [5] including important emerging zoonotic viruses detected in bats across the world [6]–[12]. However, most studies are limited to the identification of microorganisms detected and investigations regarding infectious diseases and causes of death in bats are sparse [13]–[16].
In Europe, research is predominantly focused on European bat lyssaviruses [17], [18] and coronaviruses [19], [20], but first indications of bat-pathogenic bacteria [13], [14], [21]–[23] and novel viruses [24], [25] isolated from deceased bats in Germany and Great Britain were found. In this study, we provide new data on infectious diseases in European bat species, considering factors likely to affect the susceptibility of bats to infectious agents including effects of seasonality, individual and species-specific heterogeneities, and possible intra- and inter-species transmission dynamics.
Materials and Methods
All bat species in Europe are strictly protected under the Flora-Fauna-Habitat Guidelines of the European Union (http://ec.europa.eu/environment/nature/legislation/habitatsdirective/index_en.htm) (92/43/EEC) and the Agreement on the Conservation of Populations of European Bats (www.eurobats.org) that prohibit invasive sampling of bats for research purposes. For the animals investigated in this study, carcasses of deceased bats found in Germany were kindly provided by bat researchers and bat rehabilitation centers of different federal states.
Study material
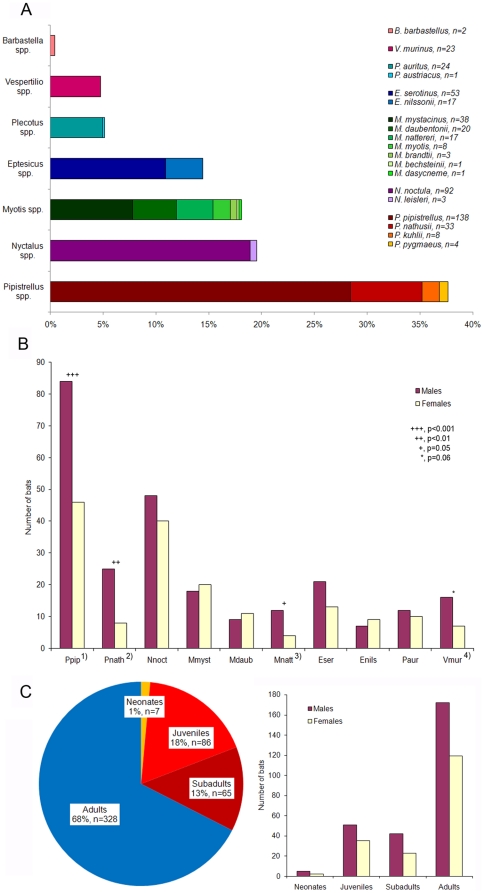
Between 2002 and 2009, a total of 486 deceased bats of 19 European vespertilionid species (i.e., Family Vespertilionidae) were investigated (Fig. 1A, [26]). The bat carcasses originated from 6 different geographic regions in Germany, i.e. Berlin greater metropolitan area (n = 223), Bavaria (n = 165), Brandenburg (n = 38), Lower Saxony (n = 36), Thuringia (n = 21), and Baden-Wuerttemberg (n = 3), and were collected by bat researchers and bat rehabilitation centers. Most animals represented individual cases that were found dead, injured or moribund near human habitation. Thus, the species composition in this study predominately reflected the urban and suburban bat fauna, which is characterized by a disproportionate abundance of a few bat species (Fig. 1A, [27], [28]). Two groups of 2 and 21 adult noctules (Nyctalus noctula), respectively, were collected from tree hibernacula destroyed during wood logging. A further group of 25 deceased adult N. noctula originated from a colony that was trapped in a rain pipe in December. Nine dead juvenile Pipistrellus pipistrellus were collected from a nursery roost.
Figure 1. Details on bats from Germany.
(A) Bat species distribution among the study sample (n = 486). (B) Male-to-female ratio (bat species >10 individuals). Footnotes: 1) Chi-square test, χ2 = 11.1, df = 1, p = 0.0009, 2) χ2 = 8.8, df = 1, p = 0.003, 3) χ2 = 4.0, df = 1, p = 0.05, 4) χ2 = 3.5, df = 1, p = 0.06. Abbreviations: Ppip, Pipistrellus pipistrellus; Pnath, Pipistrellus nathusii; Nnoc, Nyctalus noctula; Mmyst, Myotis mystacinus; Mdaub, Myotis daubentonii; Mnatt, Myotis nattereri; Eser, Eptesicus serotinus; Enils, Eptesicus nilssonii; Paur, Plecotus auritus; Vmur, Vespertilio murinus. (C) Age-sex distribution among the study sample (n = 486).
If bats died in care or had to be euthanized for animal welfare reasons, the carcasses were immediately stored at −20°C and were shipped to the Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany, for diagnostic investigations. Of all carcasses examined histo-pathologically, about 90% were suitable for bacteriological investigation. A lesser extend (43%) was also examined for selected viral agents at the Robert Koch Institute, Berlin, Germany. In addition, a brain sample of each animal was submitted to the Friedrich-Loeffler-Institute, Wusterhausen, Germany, for rabies diagnosis.
Pathological investigation
A full necropsy was performed on each bat and all macroscopic findings including ectoparasite infestation were recorded. For histo-pathological examination, small slices of multiple organ tissues (i.e., lung, liver, heart, kidney, adrenal gland, spleen, intestine, pancreas, brain, tongue, larynx, salivary gland and pectoral muscle) and tissues conspicuous for pathological changes were fixed in buffered 4% formalin, processed using standard methods and embedded in liquid paraffin. Sections were cut at 2–5 µm and routinely stained with hematoxylin-eosin (HE). In addition, special histological staining methods were used depending on microscopic findings, i.e. for the detection of bacteria (Gram or Giemsa staining), fungi (periodic acid Schiff or Grocott's Gomori methenamine silver nitrate staining), iron (Prussian blue stain), mineralization (von Kossa staining), connective and collagen tissue (trichrome staining). Details on pathological results are published elsewhere [26].
The causes of mortality were rigorously standardized with the primary cause of death identified for each bat as the most serious injury, disease or event subsequently fatal to the animal. To ensure independence of primary and contributing causes of death, the categorization was based on the severity of pathological findings.
Bacteriological investigation
Samples of lung, liver, heart and kidney, and tissues conspicuous for pathological changes (e.g. enlarged spleen) of 430 bats were plated onto Columbia (5% sheep blood), Chocolate, Gassner, and MacConkey agar (Oxoid, Germany) and were incubated at 37°C (Chocolate agar 5% CO2) for 24–48 h. Specific culture media and conditions for the isolation of Yersinia, Salmonella and anaerobic bacteria were used if appropriate. Primary identification of bacterial strains was based on colony morphology, hemolysis, Gram-staining, indol production, catalase and oxidase reaction. Bacterial species identification was carried out using the relevant commercial Api test system (bioMérieux, Germany). Additional conventional biochemical tests [29], [30] were applied to confirm Api test results where necessary. In case of ambiguous biochemical test results, 16S rDNA gene analysis was performed for final identification [23]. Salmonella isolates were characterized at the National Reference Laboratory for the Analysis and Testing of Zoonoses (Salmonella) at the Federal Institute for Risk Assessment, Berlin, Germany. Identification and characterization of Yersinia and Pasteurella species have been reported earlier [22], [23].
Virological investigation
Homogenized organ tissue of lung, liver, heart, kidney, spleen, brain and salivary gland of 210 bats were pooled for each individual and used for RNA/DNA extraction and further molecular analysis by generic PCR assays detecting flavi- [31], hanta- [32], corona- [33], and influenza A-viruses [34]. Also, PCR assays specific for 8 previously described herpesviruses [24] from European vespertilionid bats were used. For this purpose, RNA/DNA was isolated using the NucleoSpin® RNA II Kit (Macherey-Nagel, Germany) and the NucleoSpin® Tissue Kit (Macherey-Nagel), respectively, according to the manufacturer's instructions. Because of limitations in sample volume, for 180 out of the 210 bats PCR assays could only be applied for 4 different bat herpesviruses. Internal controls were used for all PCR assays to test for inhibition. For confirmation, all retrieved fragments of bat herpesvirus-specific PCR assays were checked for sequence identity to previously published isolates [24].
For detection of lyssavirus antigen in brain tissue the fluorescent antibody test (FAT) using a polyclonal antirabies conjugate (Sifin, Germany) was used [35]. FAT-positive brain tissues were subject of virus isolation in murine neuroblastoma cell culture (Na 42/13) using the Rabies Tissue Culture Infection Test (RTCIT) as described elsewhere [36]. Lyssaviruses isolated in cell culture were characterized using both a panel of 10 anti-nucleocapsid monoclonal antibodies (MAb) [37] and partial sequencing of a fragment of the nucleoprotein gene after RNA extraction using Trizol (Invitrogen, Germany) essentially as described [18].
Genetic identification of bat species
Genomic DNA was extracted from organ homogenates using the NucleoSpin® Tissue Kit (Macherey-Nagel) according to manufacturer's recommendations. Genetic identification of the bat species was performed by amplification and sequencing of a 241 bp fragment of the cytochrome B (cytB) gene [38] using primers FM up (5′- CCC CHC CHC AYA TYA ARC CMG ART GAT A -3′) and FM down (5′- TCR ACD GGN TGY CCT CCD ATT CAT GTT A -3′). In addition, for differentiation of the 2 distinct Pipistrellus species, P. pipistrellus and P. pygmaeus, a rapid multiplex PCR assay was performed as described by Kaňuch et al. [39] using primers PIP-F (5′- CTC ATT CAT TGA YCT ACC AGC -3′), PIP-R (5′- CAG CRA ATA GTA AAA TAA CTC C -3′) and Ppip-F (5′- CAT CTG TTT GGG ACT ACA GAT CC -3′).
Statistical analysis
The bat data were categorized in regard to different explanatory numeric and factor variables, e.g. bat species, sex and age class. The variable ‘age class’ ranked between 1 and 4 with increasing age (i.e. neonates, juveniles, subadults, and adults) and was used as numeric variable. For endoparasitic analysis, we defined a 3 level variable ‘bat size’ according to the body size of a certain bat species to reduce the degrees of freedom of the full model, i.e. large species (N. noctula, Eptesicus serotinus, and Vespertilio murinus), medium-sized species (E. nilssonii, Plecotus auritus, Myotis daubentonii, M. nattereri, and P. nathusii) and small species (P. pipistrellus, and M. mystacinus). To detect effects of seasonality, 4 different activity periods were specified according to the date of sampling, i.e. hibernation period (November to March), post-hibernation period (April/May), maternity period (June to August), and swarming period (September/October). As dependent binary variable for the respective models we either classified the mortality cause being disease or not (i.e. trauma), or the presence-absence of bacterial, ecto- and endoparasitic infections.
We formulated 4 different hypotheses to test for individual and species-specific differences in disease susceptibility and infection rates: (A) Disease-related mortality in bats is influenced by sex, age and species-specific differences, and degree of endoparasitic infection. (B) Bacterial infection in bats is influenced by sex, age and species-specific differences, occurrence of traumatic injuries and cat predation. (C) Ecto- or (D) endoparasitic infection in bats is affected by age, sex and species-specific differences. Seasonal effects were not analyzed because of too many missing data points. Because the long-term dataset was highly biased towards sampling procedure, preservation of bat carcasses and following diagnostic investigations, we split and filtered the full data into several subsets reflecting the different analyses (Table 1).
Table 1. Description of the data sets used for different analyses.
| Analysis | Data set(total n) | Sex(% males) | Age(% adults) | Bat species(total n) | |
| Full dataset | Bat samples | 486 | 55.6 | 67.5 | 19 |
| Subset 1 | Causes of death | 433a | 55.0 | 65.4 | 19 |
| GLMM: disease- vs. trauma-related mortality (A) | 289a | 55.0 | 65.7 | 17 | |
| Subset 2 | Bacteriological results | 430 | 58.4 | 65.3 | 18 |
| GLMM: bacterial infection vs. no infection (B) | 377a | 58.1 | 62.6 | 18 | |
| Subset 3 | Virological results | 210b | 56.7 | 64.3 | 16 |
| Subset 4 | Parasitological results | 433a | 55.0 | 65.4 | 19 |
| GLM: parasitic infection vs. no infection (C, D) | 402a | 54.7 | 65.2 | 10 |
GLMM, generalized linear mixed models with bat species included as random effect.
GLM, generalized linear models for datasets with bat species >10 individuals.
A–D: refers to the models analyzed on the different data sets (see chapter ‘Statistical analyses’).
To avoid overrepresentation of bat samples that were collected at the same time and location, a randomly selected individual of each group was included in the final dataset.
For detection of lyssavirus antigen, brain tissue of all 486 bats was tested.
All statistical analyses were performed using the R software V. 2.13.1 (R Development Core Team 2011, Vienna, Austria). We used the chi-square test for given probabilities to evaluate significant differences in the sex ratio among bats of different species. For hypotheses A and B, we used a generalized linear mixed modeling approach (binomial GLMM using function lmer in library lme4) with bat species included as random effect. This variable had not been significant as fixed effect (results not shown), but from other studies we can assume that there are species-specific differences in susceptibility of bats to certain infectious agents and therefore included it as random effect. We further used generalized linear models (GLM with logit link and binomial error structure; for datasets with bat species >10 individuals) to test for individual and species-specific differences in parasite infection rates (hypotheses C and D).
We created a full model for each hypothesis (A–D) to examine multiple and interaction effects of the specified variables. To select the final model variables, we used a stepwise backward algorithm (function stepAIC in library MASS) based on Akaike's information criterion (AIC) [40]. The ΔAIC of the final model was calculated relative to a random intercept model to demonstrate the effect size of the selected variables.
Results
Results of the diagnostic analyses follow the full data splitting into several subsets (see section ‘Statistical analysis’ in Material and Methods; Table 1).
Full dataset: Bat samples
All sampled bats belonged to 7 different genera (i.e. Pipistrellus, Nyctalus, Myotis, Eptesicus, Plecotus, Vespertilio, and Barbastella) and 19 European vespertilionid species (Fig. 1A). Three bat species, the common pipistrelle (P. pipistrellus, n = 138), the noctule bat (N. noctula, n = 92), and the serotine bat (E. serotinus, n = 53) constituted about 60% of all bat carcasses investigated in this study, whereas P. pygmaeus, Nyctalus leisleri, Myotis brandtii, M. bechsteinii, M. dasycneme, Plecotus austriacus and Barbastella barbastellus were represented in small numbers of 1 to 4 animals. The overall sex ratio was 1.5 males to 1 female with significant species-specific differences (Fig. 1B). Animals in their first year of life (neonates, juveniles, and subadults) represented one third (32.5%, n = 158) of bat samples (Fig. 1C).
Subset 1: Causes of death
Overall, we were able to assign a cause of death to 70% (n = 304) of bats investigated in this study. Two thirds of mortality were due to trauma (n = 145) or disease (n = 144), while almost 4% of bats had died of other non-infectious causes like pulmonary edema, dehydration and hypoglycemia (Table 2). In 30% (n = 129) no significant pathological findings could be found.
Table 2. Causes of mortality of bats from Germany.
| Age class | Sex class | |||||||
| Cause of death | n | % | Euthanasia | <1 Year | Adult | Female | Male | n.d. |
| Trauma | 145 | 33.5 | 54 | 41 | 104 | 55 | 87 | 3 |
| Unknown trauma cause | 71 | 16.5 | 29 | 19 | 52 | 33 | 36 | 3 |
| Cat predation | 66 | 15.3 | 23 | 19 | 47 | 18 | 47 | - |
| Roost destructiona | 2 | 0.5 | - | - | 2 | 2 | - | - |
| Trapped in rain pipea | 1 | 0.2 | - | - | 1 | - | 1 | - |
| Trapped in window | 1 | 0.2 | - | - | 1 | - | 1 | - |
| Trapped in lamp | 1 | 0.2 | - | 1 | - | - | 1 | - |
| Trapped in fly strip | 1 | 0.2 | - | 1 | - | 1 | - | - |
| Barbed wire injury | 1 | 0.2 | 1 | 1 | - | 1 | - | - |
| Smoke poisoning | 1 | 0.2 | 1 | - | 1 | - | 1 | - |
| Disease | 144 | 33.3 | 7 | 58 | 86 | 64 | 72 | 8 |
| Unknown etiology | 81 | 18.7 | 3 | 35 | 46 | 35 | 38 | 8 |
| Bacterial infection | 54 | 12.5 | 2 | 20 | 34 | 27 | 27 | - |
| Viral infectionb | 5 | 1.2 | 1 | 1 | 4 | 1 | 4 | - |
| Parasitic infection | 2 | 0.5 | - | - | 2 | - | 2 | - |
| Aspiration pneumonia | 1 | 0.2 | - | 1 | - | 1 | - | - |
| Bone deformation | 1 | 0.2 | 1 | 1 | - | - | 1 | - |
| Others | 15 | 3.4 | - | 6 | 9 | 6 | 9 | - |
| Pulmonary edema | 9 | 2.1 | - | 3 | 6 | 1 | 8 | - |
| Dehydration | 2 | 0.5 | - | - | 2 | 1 | 1 | - |
| Anemiac | 1 | 0.2 | - | - | 1 | 1 | - | - |
| Hyperthermiad | 1 | 0.2 | - | 1 | - | 1 | - | - |
| Hypothermia | 1 | 0.2 | - | 1 | - | 1 | - | - |
| Hypoglycemia | 1 | 0.2 | - | 1 | - | 1 | - | - |
| No significant findings | 129 | 29.8 | 1 | 45 | 84 | 33 | 70 | 26 |
| Total | 433 | 100 | 62 | 150 | 283 | 158 | 238 | 37 |
n.d., not determined.
A randomly selected individual of 3 different groups of adult Nyctalus noctula.
Adenovirus (bat AdV-2) [25] and European bat lyssavirus (EBLV-1) infection.
Due to severe tick infestation.
A randomly selected individual of a group of juvenile Pipistrellus pipistrellus.
Among the 145 traumatized bats, additional mild (n = 42), moderate (n = 28) and severe (n = 4) inflammatory organ changes were noted in one half (50.9%) of individuals, and 23% of the bats revealed bacterial (n = 19) and/or parasitic infections (n = 15) (Table 3). Of the 144 bats considered as dying of disease, fatal bacterial (n = 54), viral (n = 5) and parasitic infections (n = 2) were observed in 42%. Besides, amniotic fluid aspiration was noted in a neonate noctule bat (N. noctula), and a juvenile common pipistrelle (P. pipistrellus) was euthanized because of severe forearm bone deformation. The remaining 81 bats (56.3%) revealed moderate to severe pathological changes of unknown etiology or unconfirmed bacterial or viral cause (Table 2).
Table 3. Pathological findings and bacterial, viral and parasitic infections specified for the general causes of mortality, trauma and disease.
| Trauma | Disease | |||
| n | % | n | % | |
| Total number of bats | 145 | 33.5 | 144 | 33.3 |
| Pathological findings a | ||||
| Injuries | 136 | 93.8 | 37 | 25.7 |
| Inflammatory lesions | 74 | 51.0 | 124 | 86.1 |
| Non-inflammatory lesions | 1 | 0.7 | 20 | 13.9 |
| Spleen activation | 81 | 55.9 | 82 | 56.9 |
| Circulatory changes | 53 | 36.3 | 41 | 28.5 |
| Metabolic disorders | 10 | 6.8 | 12 | 8.3 |
| Bacterial infection | 19 | 13.0 | 54 | 37.5 |
| Viral infection b | - | - | 5 | 3.5 |
| Parasitic infection c | 15 | 10.3 | 14 | 9.7 |
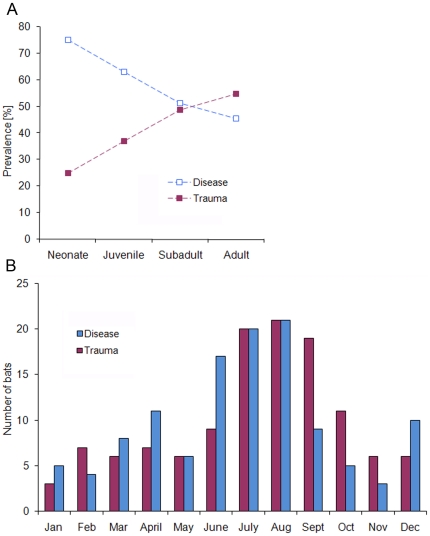
Based on the GLMM analysis, significant age- and sex-dependent differences (ΔAIC = 23.13) were detected between the general causes of mortality, disease and trauma (Table 4). The disease presence in bat samples decreased continuously with increasing age. Neonates and juveniles of both sexes were significantly more affected by disease than older age classes (Table 4; Fig. 2A). We also found a significant trend in disease-associated mortality between the sexes, with adult females displaying higher disease prevalence (52.5%) than males (36.4%) (Table 4). No significant association was observed between a certain cause of mortality (i.e. disease or trauma) and severity of endoparasitic infection (ΔAIC = 0.75, result not shown). The seasonal distribution of disease-related mortality cases (Fig. 2B) described a trimodal pattern, with peaks in spring (April), summer (June to August) and winter (December). The proportion of traumatized individuals also increased obviously during the summer months up to and including the swarming period, but was low during the rest of the year.
Table 4. Result of the final model variables corresponding to 4 different analyses: (A) disease- vs. trauma-related mortality, and presence-absence of (B) bacterial, (C) ecto- and (D) endoparasitic infection.
| Analysis | ΔAIC* | Variable | Factor level | Estimate | SE | z-value | p-value | |
| (A) | GLMM | 23.13 | Age class | −0.56 | 0.18 | −3.09 | 0.002 | |
| Sex (male) | −0.62 | 0.28 | −2.19 | 0.03 | ||||
| (B) | GLMM | 16.00 | Cat predation | 1.20 | 0.28 | 4.32 | <0.0001 | |
| (C) | GLM | 14.58 | Bat species | Nyctalus noctula | −0.30 | 0.30 | −1.02 | 0.3 |
| Myotis daubentonii | −1.10 | 0.52 | −2.13 | 0.03 | ||||
| Vespertilio murinus | −1.56 | 0.55 | −2.83 | 0.005 | ||||
| Eptesicus nilssonii | −2.01 | 0.75 | −2.68 | 0.007 | ||||
| Pipistrellus pipistrellus | −2.04 | 0.27 | −7.42 | <0.0001 | ||||
| Eptesicus serotinus | −2.06 | 0.43 | −4.75 | <0.0001 | ||||
| Plecotus auritus | −2.40 | 0.74 | −3.25 | 0.001 | ||||
| Pipistrellus nathusii | −2.74 | 0.73 | −3.76 | 0.0002 | ||||
| Myotis nattereri | −2.77 | 1.03 | −2.69 | 0.007 | ||||
| Myotis mystacinus | −2.90 | 0.73 | −3.98 | <0.0001 | ||||
| (D) | GLM | 24.95 | Age class | 0.43 | 0.15 | 2.88 | 0.004 | |
| Bat size | Large species | −0.18 | 0.18 | −0.99 | 0.3 | |||
| Medium-sized species | −1.30 | 0.23 | −5.64 | <0.0001 | ||||
| Small species | −1.29 | 0.19 | −6.86 | <0.0001 |
GLMM, generalized linear mixed models with bat species included as random effect.
GLM, generalized linear models for datasets with bat species >10 individuals.
AIC, Akaike's information criterion.
*ΔAIC of the final model relative to a random intercept model.
Figure 2. Age-dependent differences and seasonal variations among the general causes of mortality, disease and trauma.
(A) Age-specific prevalence. (B) Seasonal distribution of trauma- and disease-related mortality cases.
Subset 2: Bacteriological results
About 90% (n = 430) of bat samples were examined bacteriologically. Among these, 42 different bacterial genera with more than 53 bacterial species were identified (Table S1). Predominant bacteria isolated were Enterococcus faecalis (14.7%, n = 63), Hafnia alvei (11.2%, n = 48), Serratia liquefaciens (10%, n = 43), and Pasteurella multocida (7.7%, n = 29). In 37% (n = 157) of bats no bacterial growth was observed at all.
Comparative bacteriologic and histo-pathologic analysis identified 22 different bacterial species that were clearly associated with pathological lesions and/or systemic infection, found in 17% (n = 73) of bats investigated bacteriologically (Table 5). Members of the families Pasteurellaceae (above all P. multocida) (41.1%, n = 30), Enterobacteriaceae (various bacterial species) (28.8%, n = 21), and Streptococcaceae (above all Enterococcus spp.) (21.9%, n = 16) were predominant bacteria associated with disease. More than half (54.8%, n = 40) of bacterial infections were observed in bats with traumatic injuries. The GLMM analysis revealed low sex- and age-dependent differences in bacterial infection (ΔAIC = 1.97, result not shown). Female bats (21.9%) and adults (21.6%) showed marginally higher prevalence of bacterial disease compared to males (18.3%) and to other age classes (15.6%), respectively. However, we found a strong influence of cat predation (ΔAIC = 16) associated with bacterial infection in bats (Table 4).
Table 5. Bacteria associated with disease in bats from Germany.
| Bacteria | Bats | Clinical status* |
| Pasteurella multocida | 28 | Septicemia; pneumonia; pleuritis; peri-/epicarditis; myocarditis; nephritis; liver/spleen necroses; wound infection; abscess |
| Pasteurella multocida, Pasteurella species B | 1 | Septicemia; glossitis (bite wound infection); liver necrosis |
| Pasteurella pneumotropica, Vibrio spp. | 1 | Septicemia |
| Serratia liquefaciens | 5 | Systemic infection; pneumonia; wound infection |
| Serratia marcescens | 1 | Systemic infection; pneumonia |
| Enterobacter cancerogenus | 2 | Systemic infection; pneumonia |
| Enterobacter cancerogenus, Hafnia alvei | 1 | Peritonitis; pneumonia |
| Hafnia alvei | 1 | Systemic infection |
| Klebsiella oxytoca | 3 | Systemic infection; pneumonia |
| Klebsiella mobilis | 1 | Systemic infection; pneumonia |
| Escherichia coli | 2 | Systemic infection; pneumonia; nephritis; cystitis |
| Salmonella enterica serotype Typhimurium | 2 | Systemic infection; pneumonia; meningitis |
| Salmonella enterica serotype Enteritidis | 1 | Systemic infection; pneumonia, wound infection |
| Yersinia pseudotuberculosis | 1 | Systemic infection; pneumonia; liver/spleen necroses |
| Cedecea davisae | 1 | Pneumonia |
| Burkholderia sp. | 1 | Systemic infection |
| Enterococcus faecalis | 9 | Septicemia; pneumonia; endocarditis; abscess |
| Enterococcus faecium | 3 | Septicemia; pneumonia |
| Enterococcus faecalis, Enterococcus faecium | 2 | Septicemia; pneumonia; myocarditis; wound infection |
| Staphylococcus aureus | 3 | Septicemia |
| Staphylococcus aureus, Enterococcus faecalis | 1 | Septicemia; dermatitis |
| Aerococcus viridans | 1 | Systemic infection; pneumonia |
| Bacillus sp. | 1 | Pneumonia |
| Clostridium sordellii | 1 | Hemorrhagic enteritis |
*Histo-pathological findings described in more details elsewhere [26].
Subset 3: Virological results
Testing for human-pathogenic zoonotic viruses, no examined bat sample (0/210) was positive for influenza A virus, corona-, hanta- and flaviviruses, respectively. No inhibition of the PCR assays was notified. Out of 486 bats tested for rabies virus infection, 2 serotine bats (E. serotinus) were positive for lyssavirus by FAT and RTCIT. The viruses were identified as European bat lyssavirus type 1 (EBLV-1) sublineage a, both using MAbs and sequencing.
Applying bat herpesvirus-specific PCR assays, 63 out of 210 bats proved to be infected with 7 of the previously described 8 bat herpesviruses (Table 6). The highest prevalence of 65% (24/37) was observed for bat gammaherpesvirus 6 (BatGHV6) in common pipistrelle bats (P. pipistrellus), followed by bat gammaherpesvirus 5 (BatGHV5, 42.1%) in nathusius' pipistrelle bats (P. nathusii) and bat gammaherpesvirus 4 (BatGHV4, 33.8%) in noctule bats (N. noctula). Co-infection with different bat herpesviruses were recognized in 4 N. noctula (7.4%) infected with BatGHV3 and BatGHV4, and in one N. noctula (1.5%) infected with BatGHV4 and BatGHV5. BatGHV5 was not only detected in its initially specific host P. nathusii, but also in 3 other bat species, i.e. N. noctula, Myotis myotis and M. mystacinus. Although the prevalence of BatGHV3 (13.0%) and BatGHV4 (33.8%) differed significantly within its migrating host N. noctula, no difference was observed between the sexes. Two juvenile N. noctula were found to be infected with BatGHV4. Interestingly, for the sedentary bat species P. pipistrellus being infected with BatGHV6, a considerably higher prevalence was observed in 22 juvenile bats (72.7%) resulting in an overall prevalence of 65% also without difference between adult male and female bats.
Table 6. Bat herpesvirus infection in bats from Germany.
| Virus | Bat species | Total | Positive (%) | |
| Bat herpesviruses | 16 species | 210 | 63 | (30.0) |
| BatGHV1a | Eptesicus serotinus | 9 | 1 | (11.1) |
| BatGHV3a | Nyctalus noctula c | 54 | 7 | (13.0) |
| BatGHV4b | Nyctalus noctula c | 65 | 22 | (33.8) |
| BatGHV5b | Pipistrellus nathusii | 19 | 8 | (42.1) |
| Nyctalus noctula c | 65 | 1 | (1.5) | |
| Myotis myotis | 2 | 1 | n.d. | |
| Myotis mystacinus | 21 | 1 | (4.8) | |
| BatGHV6a | Pipistrellus pipistrellus | 37 | 24 | (64.9) |
| BatGHV7b | Plecotus auritus | 12 | 2 | (16.7) |
| BatBHV1b | Myotis nattereri | 2 | 1 | n.d. |
BatGHV, Bat gammaherpesvirus.
BatBHV, Bat betaherpesvirus.
Tested bats from a sample set containing 180 animals.
Tested bats from a sample set containing 210 animals.
Co-infection of different herpesviruses recognized.
n.d., not determined due to insufficient sample numbers.
Subset 4: Parasitological results
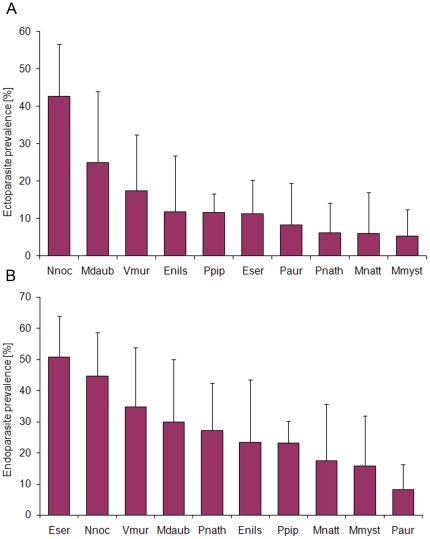
Ectoparasites (mites, fleas, and ticks) were noted in 14% (n = 62) of bats, but a potential bias in ectoparasite numbers collected from dead animals in comparison to ectoparasite abundance on live animals has to be taken in account. Female bats (17.1%) were slightly more infested by ectoparasites than males (14.7%), whereas in different age classes ectoparasite prevalence was almost balanced. The GLM analysis revealed significant species-specific differences in ectoparasite infestation (ΔAIC = 14.58, Table 4). Most bat species revealed low ectoparasite prevalence (range 5.3–11.8%), while almost 43% (n = 20) of N. noctula were infested with mites and/or fleas (Fig. 3A).
Figure 3. Species-specific parasite infection rates.
(A) Ecto- and (B) endoparasite prevalence in different European vespertilionid bat species. Error bars represent 95% binomial confidence intervals. Abbreviations: Nnoc, Nyctalus noctula; Mdaub, Myotis daubentonii; Vmur, Vespertilio murinus; Enils, Eptesicus nilssonii; Ppip, Pipistrellus pipistrellus; Eser, Eptesicus serotinus; Paur, Plecotus auritus; Pnath, Pipistrellus nathusii; Mnatt, Myotis nattereri; Mmyst, Myotis mystacinus.
Microscopic examination of organ tissues revealed endoparasitic infection in 29% (n = 124) of investigated bats, involving different protozoan (families Eimeriidae and Sarcocystidae) and helminth parasites (trematodes, cestodes, and nematodes). Helminthes were predominantly found in the gastro-intestinal tract of the bats, while in some animals, granulomatous organ lesions were associated with larval migration of nematode species. Based on the GLM analysis, clear age- and species-specific differences (ΔAIC = 24.95) were observed between infected and non-infected bats (Table 4). The prevalence of endoparasitic infection in bat samples increased significantly with increasing age, whereas the increase in prevalence was more rapid between juveniles and subadults (8.5%) compared to the older age classes (4.5%). Marginal differences were also observed between the sexes, with female bats showing slightly higher (30.4%) endoparasite prevalence than males (24.4%). Regarding species-specific differences, large bats like N. noctula, E. serotinus and V. murinus revealed higher endoparasite prevalence compared to individuals of medium-sized or small vespertilionid species (Table 4; Fig. 3B).
Discussion
Causes of death and disease dynamics in bats
This study was based on a passive surveillance sampling strategy that inherently influences the composition of bats sampled for diagnostic investigations [27] and might also effect the data presented on causes of death by ecological and anthropogenic impacts of urban landscapes [41]. Trauma and disease represented the most important causes of mortality in deceased bats from Germany, which differ from results of previous studies [13]–[15] where disease-related mortality often played a subordinate role. Young bats and adult females were significantly more affected by disease, indicating that sex- and age-related disease prevalence in bats are strongly correlated with the maternal season. This assumption is further supported by the distinct increase of disease-related mortality from June to August, which corresponds to the maternity period of Central European bat species. Similar seasonal prevalence patterns in bats have also been described for parasitic (e.g. [42]–[45]) and viral infections (e.g. [19], [46], [47]). In contrast, the increase of trauma-associated mortality cases from July to October resembles 4 major behavioral activity patterns of European bat species (i.e. weaning, mating, pre-hibernal fat storage, and migration) [48] and could therefore predispose bats to trauma. However, both seasonal peaks also coincide with time and locations where sick, injured or dead bats are more likely to be discovered as well as with the seasonal roosting behavior of bats adapted on urban habitats [27]. The additional seasonal peaks of disease-associated mortality corresponded to the post-hibernal and the early hibernal period of temperate zone bats. Currently, there is a lack of knowledge of bat immunology. It is known for other mammalian species that hibernation reduces the innate and adaptive immune response; likewise an increasing risk of infection could be assumed for hibernating bats [49]. With the start of the hibernation season, large aggregations of bats originating from various colonies might enhance the risk of spreading infectious agents similar to maternity colonies. Equally, the post-hibernal increase of disease-related mortality is suggestive for reduced immunity in association with prolonged fasting during hibernation.
Bacterial diseases and cat predation
Bacterial diseases have rarely been documented in bats. Pasteurella spp., here identified in 7% of bats, were the predominant bacterial pathogens reported in European bats and infection appears to be strongly correlated with cat predation [13], [14], [23], [26]. In our study, bacterial infections were confirmed in 17% of bats investigated bacteriologically. Most of these bacterial isolates represented opportunistic pathogens that usually do not harm the host unless the immune system is weakened [50] or preceding injury to natural host barriers (e.g. skin abrasion). Primary bacterial pathogens like Samonella enterica serovar Typhimurium, S. Enteritidis and Yersinia pseudotuberculosis [22] were identified in almost 12% of affected bats. Some of the bacterial species (e.g. Burkholderia sp., Cedecea davisae and Clostridium sordellii) are newly described in bats. Nevertheless, bacteriologic analyses can markedly be influenced by post-mortem bacterial invaders, freezing and storage of bat carcasses and the inability to detect certain bacteria by routine culture methods, resulting in some bacterial species that might have escaped detection.
We found a strong association between cat predation and bacterial infection in bats as almost one half of bats (44%) caught by cats were affected by bacterial disease. Various bacteria can be transmitted via cat bites [51]; hence bats attacked by cats are likely to succumb to bacterial infection even if non-fatal injuries were present. This relation has been proven for P. multocida infections in European bat species [13], [14], [23], [26]. On the other hand, bats already debilitated by disease might easier fall prey to predators like cats. Consequently, bats may also act as vectors for zoonotic pathogens, as domestic cats could pass these infectious agents on to humans. Such cross-species transmission events from bats to domestic animals are well documented [9], [52].
Virological investigations
For all tested human-pathogenic zoonotic viruses no infected bat could be detected in this study except lyssaviruses. Bat rabies is the only bat transmitted zoonosis in Europe that is known to have resulted in human cases [53]. Unlike in other mammals where lyssaviruses ultimately cause lethal rabies, in bats nonlethal lyssavirus infections may also lead to the development of immunity [47]. With the detection of EBLV-1 we confirm that this lyssavirus circulates among E. serotinus as previous studies showed [18]. In Germany, bat rabies is also caused by EBLV-2 and Bokeloh bat lyssavirus (BBLV) [54], [55], but while the latter was recently isolated from M. nattereri, EBLV-2 is associated with M. daubentonii and M. dasycneme [56]. The apparent absence of EBLV-2 and BBLV in the sampled bats is likely due to the fact that lyssavirus infections have a very low incidence in bat populations [18] and that the sample size was too limited, especially concerning the relevant species.
There is a high prevalence for herpesviruses in different insectivorous bat species in Germany (this study, [24]). Most of the previously described bat herpesviruses have been detected in low numbers in more than one bat species [24]. Here, we observed a high species-specific prevalence among herpesvirus-infected bats, indicating that a certain type of European bat herpesvirus is primarily associated with a single bat species. This is supported by BatGHV6 and BatGHV7 that were again only identified in their initial hosts P. pipistrellus and P. auritus (both sedentary), respectively, underlining the typical strong species-specificity of mammalian herpesviruses. However, species' range overlap and close inter-species contacts in bat roosts may result in cross-species transmission and could explain the observed overcoming of the species barrier (this study BatGHV5, [24]). Interspecies transmission have also been discussed for other mammalian herpesviruses, i.e. bovine and equine herpesviruses (e.g. [57], [58]). Furthermore, for RNA viruses (i.e. rabies virus) phylogenetic distance between different host species and overlap in geographic range have recently been demonstrated as strong predictors of host shifts and cross-species transmission in bats [59]. Some of the bat species (i.e. N. noctula, P. pipistrellus, and P. nathusii) in this study appear to be more susceptible to herpesvirus infection. In N. noctula, 3 different gammaherpesviruses (BatGHV3, 4, 5) with significant prevalence differences were recognized. Such type-specific differences in prevalence between the phylogenetically distant viruses BatGHV3 (13.0%) and BatGHV4 (33.8%) within one bat species indicates co-evolutionary virus-regulated mechanisms.
Differences in parasite prevalence
Parasite infestation in wildlife often occurs without clinical effects, but severe infection can reduce host fitness either in terms of survival or reproductive success [60]. Most data on infection dynamics in bats came from parasite studies focusing on individual and seasonal variations in ectoparasite prevalence (e.g. [43]–[45], [61]). Host density, roost preference and movement pattern seem to be important factors explaining individual and species-specific parasite infestation rates in bats [43]–[45]. In European vespertilionid species, female-biased parasite loads are most likely associated with host physiology and differences in roosting behavior [42], [44]. We also found species-specific seasonal variations in ectoparasitic infestation, with N. noctula and M. daubentonii showing higher ectoparasite prevalence in spring and autumn compared to the breeding season (data not shown), which is in accordance with Zahn and Rupp [43].
Additional findings of our parasite analyses are distinct variations in ecto- and endoparasite prevalence in relation to bat species. Bats primarily roosting in trees or nest boxes were more frequently infested with ectoparasites like N. noctula (43%) and M. daubentonii (25%) compared to other species (range 5–12%) investigated in this study. High ectoparasite loads have generally been described in bats preferring enclosed roosts like burrows and cavities [61], [62], suggesting that structural characteristics and the microclimate of roosting habitats influence ectoparasite survival and re-infection of bat hosts. This assumption is in accordance with results of Pearce and O'Shea [63] who found differences in ectoparasite prevalence and intensity in Eptesicus fuscus in relation to environmental factors (i.e. temperature and humidity) of different roost sites. In contrast to these results, the endoparasite prevalence in European vespertilionid bats seems to be correlated with the body size of the bat species [26]. Species-specific variations in diet and prey selection could possibly effect endoparasite prevalence in insectivorous bats [64], as larger bats feed on insects of a wider size range including hard-bodied prey [65], [66]. This assumption is supported by the clear prevalence increase in subadult and adult bats compared to low endoparasite infection rates in juveniles primarily feeding on milk.
Conclusion
A multitude of publications is restricted to pathogen presence or absence in different chiropteran species; here we demonstrate the impact of diseases and infectious agents on bats themselves. Alongside to trauma-associated mortality and undefined mortality cases, disease aspects represented one third of mortality causes in 486 investigated bats of 19 European vespertilionid species. By comparing pathology and bacteriology results, we were able to detect 22 different bacterial species that were clearly associated with disease in bats. At least 12% of all bats had died due to bacterial, viral and parasitic infections. Finally, we found clear seasonal and individual variations in disease prevalence and infection rates, indicating an increased susceptibility to infectious agents in female bats and juveniles during the maternity season. Our data emphasize and provide the basis for disease related studies in bat species on population level to elucidate the complexity of the ecology of infectious agents and host species likewise.
Supporting Information
Bacteria isolated from bats found in Germany.
(DOC)
Acknowledgments
The authors would like to thank Berliner Artenschutz Team–BAT-e.V., F. Brandes, I. Frey-Mann, H. Geiger, J. Haensel, J. Harder, L. Ittermann, M. Kistler, M. Kredler, S. Morgenroth, E. Mühlbach, K. Müller, R. Pfeiffer, W. Rietschel, S. Rosenau, R. Straub, G. Strauß, and W. and H. Zoels for providing the bat carcasses, to N. Jahn, C. Kohl, J. Kliemt, D. Krumnow, D. Lieckfeldt, P. Machnowska and M. Sonntag for their excellent lab work, and M. Grobbel and G.-A. Czirják for their assistance and helpful discussions. We are also grateful to 2 anonymous reviewers for their helpful suggestions and comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Adolf and Hildegard Isler-Stiftung, the FAZIT-Stiftung (fellowship to KM) and the Klara Samariter-Stiftung. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, et al. The status of the world's land and marine mammals: diversity, threat and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 2.Kunz TH, de Torrez EB, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Ann N Y Acad Sci. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- 3.Mickleburgh SP, Hutson AM, Racey PA. A review of the global conservation status of bats. Oryx. 2002;36:18–34. [Google Scholar]
- 4.Wibbelt G, Moore MS, Schountz T, Voigt CC. Emerging diseases in Chiroptera: why bats? Biol Lett. 2010;6:438–440. doi: 10.1098/rsbl.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wibbelt G, Speck S, Field H. Methods for assessing diseases in bats. In: Kunz TH, Parsons S, editors. Ecological and Behavioral Methods for the Study of Bats. 2nd Edition. The Johns Hopkins University Press; 2009. pp. 775–794. [Google Scholar]
- 6.Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 7.Chua KB, Koh CL, Hooi PS, Wee KF, Khong JH, et al. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002;4:145–151. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 8.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Shi Z, Yu M, Ren W, Smith C, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 10.Wong S, Lau S, Woo P, Yuen K-Y. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Towner JS, Amman BR, Sealy TK, Carroll SAR, Comer JA, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuzmin IV, Bozick B, Guagliardo SA, Kunkel R, Shak JR, et al. Bats, emerging infectious diseases, and the rabies paradigm revisited. Emerging Health Threats Journal. 2011;4:7159. doi: 10.3402/ehtj.v4i0.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson VR. Veterinary advances in the investigation of wildlife diseases in Britain. Res Vet Sci. 2000;69:11–16. doi: 10.1053/rvsc.2000.0384. [DOI] [PubMed] [Google Scholar]
- 14.Daffner B. Causes of morbidity and mortality in British bat species and prevalence of selected zoonotic pathogens. 2001. Thesis for MSc in Wild Animal Health, University of London.
- 15.Duignan P, Horner G, O'Keefe J. Infectious and emerging diseases of bats, and health status of bats in New Zealand. Surveillance. 2003;30:15–18. [Google Scholar]
- 16.Hajkova P, Pikula J. Veterinary treatment of evening bats (Vespertilionidae) in the Czech Republic. Vet Rec. 2007;161:139–140. doi: 10.1136/vr.161.4.139. [DOI] [PubMed] [Google Scholar]
- 17.Harris SL, Brookes SM, Jones G, Hutson AM, Racey PA, et al. European bat lyssaviruses: distribution, prevalence and implications for conservation. Biol Conserv. 2006;131:193–210. doi: 10.1016/j.biocon.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller T, Johnson N, Freuling CM, Fooks AS, Selhorst T, et al. Epidemiology of bat rabies in Germany. Arch Virol. 2007;152:273–288. doi: 10.1007/s00705-006-0853-5. [DOI] [PubMed] [Google Scholar]
- 19.Gloza-Rausch F, Ipsen A, Seebens A, Göttsche M, Panning M, et al. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg Infect Dis. 2008;14:626–631. doi: 10.3201/eid1404.071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rihtaric D, Hostnik P, Steyer A, Grom J, Toplak I. Identification of SARS-like coronavirus in horseshoe bats (Rhinolophus hipposideros) in Slovenia. Arch Virol. 2010;155:507–514. doi: 10.1007/s00705-010-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans NJ, Bown K, Timofte D, Simpson VR, Birtles RJ. Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg Infect Dis. 2009;15:1331–1333. doi: 10.3201/eid1508.090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mühldorfer K, Wibbelt G, Haensel J, Riehm J, Speck S. Yersinia species isolated from bats, Germany. Emerg Infec Dis. 2010;16:578–580. doi: 10.3201/eid1603.091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mühldorfer K, Schwarz S, Fickel J, Wibbelt G, Speck S. Genetic diversity of Pasteurella species isolated from European vespertilionid bats. Vet Microbiol. 2011;149:163–171. doi: 10.1016/j.vetmic.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Wibbelt G, Kurth A, Yasmum N, Bannert M, Nagel S, et al. Discovery of herpesviruses in bats. J Gen Virol. 2007;88:2651–2655. doi: 10.1099/vir.0.83045-0. [DOI] [PubMed] [Google Scholar]
- 25.Sonntag M, Mühldorfer K, Speck S, Wibbelt G, Kurth A. New adenovirus in bats, Germany. Emerg Infect Dis. 2009;15:2052–2055. doi: 10.3201/eid1512.090646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mühldorfer K, Speck S, Wibbelt G. Diseases in free-ranging bats from Germany. BMC Vet Res. 2011;7:61. doi: 10.1186/1746-6148-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaisler J, Zukal J, Rehak Z, Homolka M. Habitat preference and flight activity of bats in a city. J Zool Lond. 1998;244:439–445. [Google Scholar]
- 28.O'Shea TJ, Neubaum DJ, Neubaum MA, Cryan PM, Ellison LE, et al. Bat ecology and public health surveillance for rabies in an urbanizing region of Colorado. Urban Ecosystem. 2011 DOI 10.1007/s11252-011-0182-7. [Google Scholar]
- 29.Bisping W, Amtsberg G. Colour Atlas for the Diagnosis of Bacterial Pathogens in Animals. 1988. Paul Parey Scientific Publishers. Berlin, Hamburg.
- 30.Brenner DJ, Krieg NR, Staley JT. Bergey's Manual of Systematic Bacteriology. 2005. 2nd Edition. Springer, New York.
- 31.Sánchez-Seco MP, Rosario D, Domingo C, Hernández L, Valdés K, et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods. 2005;126:101–109. doi: 10.1016/j.jviromet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Klempa B, Fichet-Calvet E, Lecompte E, Auste B, Aniskin V, et al. Hantavirus in African wood mouse, Guinea. Emerg Infect Dis. 2006;12:838–840. doi: 10.3201/eid1205.051487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Souza Luna LK, Heiser V, Regamey N, Panning M, Drexler JF, et al. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. J Clin Microbiol. 2007;45:1049–1052. doi: 10.1128/JCM.02426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spackman E, Senne DA, Myers TJ, Bulaga JL, Garber LP, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean D, Abelseth MK. The fluorescent antibody test. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory Techniques in Rabies. 4th Edition. World Health Organization; 1996. pp. 88–95. [Google Scholar]
- 36.Webster WA, Casey GA. Virus isolation in neuroblastoma cell culture. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory Techniques in Rabies. 4th Edition. World Health Organization; 1996. pp. 96–103. [Google Scholar]
- 37.Schneider LG, Barnard BJH, Schneider HP. Application of monoclonal antibodies for epidemiological investigations and oral vaccination studies. In: Kuwert E, Mérieux C, Koprowski H, Bögel K, editors. Rabies in the Tropics. Springer, Berlin; 1985. pp. 47–59. [Google Scholar]
- 38.Linacre A, Lee JC. Species determination: the role and use of the cytochrome b gene. Methods Mol Biol. 2005;297:45–52. [PubMed] [Google Scholar]
- 39.Kaňuch P, Hájková P, Řehák Z, Bryja J. A rapid PCR-based test for species identification of two cryptic bats Pipistrellus pipistrellus and P. pygmaeus and its application on museum and dropping samples. Acta Chirop. 2007;9:277–282. [Google Scholar]
- 40.Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Akademiai Kiado, Budapest, UK; 1973. pp. 267–281. [Google Scholar]
- 41.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christe P, Arlettaz R, Vogel P. Variation of intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol Lett. 2000;3:207–212. [Google Scholar]
- 43.Zahn A, Rupp D. Ectoparasite load in European vespertilionid bats. J Zool Lond. 2004;262:383–391. [Google Scholar]
- 44.Lučan RK. Relationships between the parasitic mite Spinturnix andegavinus (Acari: Spinturnicidae) and its bat host, Myotis daubentonii (Chiroptera: Vespertilionidae): seasonal, sex- and age-related variation in infestation and possible impact of the parasite on the host condition and roosting behavior. Folia Parasitol. 2006;53:147–152. doi: 10.14411/fp.2006.019. [DOI] [PubMed] [Google Scholar]
- 45.Christe P, Glaizot O, Evanno G, Bruyndonckx N, Devevey G, et al. Host sex and ectoparasites choice: preference for, and higher survival on female host. J Anim Ecol. 2007;76:703–710. doi: 10.1111/j.1365-2656.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 46.Plowright RK, Field HE, Smith C, Divljan A, Palmer C, et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc Biol Sci. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George DB, Webb CT, Farnsworth ML, O'Shea TJ, Bowen RA, et al. Host and viral ecology determine bat rabies seasonality and maintenance. Proc Natl Acad Sci USA. 2011;108:10208–10213. doi: 10.1073/pnas.1010875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciechanowsky M, Zając T, Zielińska A, Dunajski R. Seasonal activity patterns of seven vespertilionid bat species in Polish lowlands. Acta Theriol. 2010;55:301–314. [Google Scholar]
- 49.Bouma HR, Carey HV, Kroese FGM. Hibernation: the immune system at rest? J Leukoc Biol. 2010;88:619–624. doi: 10.1189/jlb.0310174. [DOI] [PubMed] [Google Scholar]
- 50.Peterson JW. Bacterial Pathogenesis. In: Baron S, editor. Medical Microbiology. 4th Edition. Galveston (TX): University of Texas Medical Branch at Galveston. Chapter 7; 1996. [PubMed] [Google Scholar]
- 51.Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJC. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- 52.Dacheux L, Larrous F, Mailles A, Boisseleau D, Delmas O, et al. European bat lyssavirus transmission among cats, Europe. Emerg Infect Dis. 2009;15:280–284. doi: 10.3201/eid1502.080637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson N, Vos A, Freuling C, Tordo N, Fooks AR, et al. Human rabies due to lyssavirus infection of bat origin. Vet Microbiol. 2010;142:151–159. doi: 10.1016/j.vetmic.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Freuling C, Grossmann E, Conraths F, Schameitat A, Kliemt J, et al. First isolation of EBLV-2 in Germany. Vet Microbiol. 2008;131:26–34. doi: 10.1016/j.vetmic.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Freuling C, Beer M, Conraths FJ, Finke S, Hoffmann B, et al. Novel lyssavirus in Natterer's bat, Germany. Emerg Infect Dis. 2011;17:1519–1522. doi: 10.3201/eid1708.110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuzmin IV, Rupprecht CE. Bat rabies. In: Jackson AC, Wunner W, editors. Rabies 2nd Edition. Academic Press, New York; 2007. pp. 259–307. [Google Scholar]
- 57.Thiry E, Vercouter M, Dubuisson J, Barrat J, Sepulchre C, et al. Serological survey of herpesvirus infections in wild ruminants of France and Belgium. J Wildl Dis. 1988;24:268–273. doi: 10.7589/0090-3558-24.2.268. [DOI] [PubMed] [Google Scholar]
- 58.Schrenzel MD, Tucker TA, Donovan TA, Busch MDM, Wise AG, et al. New hosts for equine herpesvirus 9. Emerg Infect Dis. 2008;14:1616–1619. doi: 10.3201/eid1410.080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin IV, McCracken GF, et al. Host phylogeny constrains cross-species emergence of rabies virus in bats. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 60.Hart BL. Behavioral adaptations to parasites: an ethological approach. J Parasitol. 1992;78:256–265. [PubMed] [Google Scholar]
- 61.ter Hofstede HM, Fenton MB. Relationships between roost preferences, ectoparasite density, and grooming behaviour of neotropical bats. J Zool Lond. 2005;266:333–340. [Google Scholar]
- 62.Patterson BD, Dick CW, Dittmar K. Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). J Trop Ecol. 2007;23:177–189. [Google Scholar]
- 63.Pearce RD, O'Shea TJ. Ectoparasites in an urban population of big brown bats (Eptesicus fuscus) in Colorado. J Parasitol. 2007;93:518–530. doi: 10.1645/GE-973R.1. [DOI] [PubMed] [Google Scholar]
- 64.Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, et al. When parasites become prey : ecological and epidemiological significance of eating parasites. Trends Ecol Evol. 2010;25:362–371. doi: 10.1016/j.tree.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Aguirre LF, Herrel A, Van Damme R, Matthysen E. The implications of food hardness for diet in bats. Funct Ecol. 2003;17:201–212. [Google Scholar]
- 66.Feldhamer GA, Carter TC, Whitaker JO., Jr Prey consumed by eight species of insectivorous bats from Southern Illinois. Am Midl Nat. 2009;162:43–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacteria isolated from bats found in Germany.
(DOC)