Abstract
Animal studies have implicated the neuropeptides Prolactin (PRL) and Oxytocin (OT) in processes of maternal bonding and PRL has similarly been shown to play a role in the neurophysiology of fatherhood. Yet, very little is known on the involvement of PRL and OT in human fathering. Forty-three fathers and their firstborn infant were seen twice: in the second and sixth postpartum months. Paternal plasma PRL and OT were sampled at both time-points and analyzed with ELISA methods. At six months fathers were videotaped interacting with their child in social and exploratory play contexts and interactions were micro-analyzed for father–infant Affect Synchrony and father facilitation of child toy exploration. PRL and OT showed high individual stability across time and were correlated at the second observation. PRL was related to father– infant Coordinated Exploratory Play in the toy context whereas OT was associated with father–infant Affect Synchrony in the social context. Results point to the role of PRL and OT in the development of human fathering and underscore their differential relations with patterns of paternal care.
Keywords: Prolactin, Oxytocin, Fathers, Father-infant interactions, Affect Synchrony, Exploration, Transition to parenthood
Animal studies have demonstrated that the neuropeptides Prolactin (PRL) and Oxytocin (OT) act as important components of the neurobiological pathways underlying the initiation and maintenance of maternal behavior (Russell et al., 2001). PRL and OT are secreted from the pituitary via hypothalamic regulation (Gimpl and Fahrenholz, 2001) and both are involved in lactation (Svennersten-Sjaunja and Olsson, 2005). The administration of either PRL or OT to virgin rats quickly initiates the maternal behavior repertoire (Bridges et al., 1997; Mann and Bridges, 2001; Pedersen and Prange, 1979, 1985), and although PRL and OT are involved in a wide range of physiological functions (Gimpl and Fahrenholz, 2001; Grattan and Kokay, 2008) they are both theorized to facilitate physiological processes that assist maternal adaptation in the postpartum period (Grattan, 2001; Grattan and Kokay, 2008; Grattan et al., 2001; Uvnas-Moberg et al., 2001). However, although PRL and OT are considered as important components of the neuroendocrinology of fatherhood (Wynne-Edwards, 2001), their independent and combined role in the development of human paternal behavior has not been studied.
PRL has been examined in relation to fatherhood in a number of animal species (Storey et al., 2006; Wynne-Edwards, 2001; Ziegler, 2000). Administration of PRL to bird and fish fathers has shown to increase paternal behavior while antagonists to PRL reduced the displays of the paternal repertoire (Buntin et al., 1996; Kindler et al., 1991). In mammalian species, several studies have pointed to the associations between PRL and fatherhood (Schradin and Anzenberger, 1999). For instance, in the common marmoset and cotton-top tamarins, fathers had significantly higher peripheral PRL concentrations as compared to non-fathers (Dixson and George, 1982; Ziegler et al., 1996), suggesting that PRL is involved in the neuroendocrine systems that support the development of fatherhood.
In contrast to the large body of research on the links between PRL and fatherhood in animals, the involvement of PRL in the first months of human fatherhood received significantly less attention. Men and women were found to exhibit elevated levels of plasma PRL concentrations prior to childbirth as compared to the post-natal period and fathers who were most affected by infant cues had higher PRL levels (Storey et al., 2000). Fathers with higher PRL concentrations were more positive and alert to their infant cues (Fleming et al., 2002). In addition, PRL decreased when fathers were exposed to their firstborn infant's cry or held the infant in their arms for the first time. Interestingly, PRL levels increased after the same fathers held their second newborn (Delahunty et al., 2007), suggesting that there may be unique hormonal processes associated with the first period of fatherhood. These results complement those reported by Fleming et al. (2002), who showed that while listening to their infants' cries, experienced fathers showed a greater increase in PRL as compared to first-time fathers. On the other hand, among fathers of preschool-aged children, paternal PRL levels did not change after interaction with their child (Gray et al., 2007).
OT is involved in a wide range of social and affective processes in humans (Lee et al., 2009; Macdonald and Macdonald, 2010). Specifically, OT has been associated with processes related to maternal attachment and responsiveness to the infant (Carter, 1998; Feldman et al., 2007). Maternal OT release during breastfeeding increases feelings of calmness and sociability (Uvnas-Moberg, 1998) and decreases feelings that may hinder the mother's responsiveness to her infant, such as aggression, anxiety, guilt, or suspicion (Uvnas-Moberg et al., 1993; Uvnäs-Moberg et al., 1990). Furthermore, an increase in OT levels from the first to the third trimester of pregnancy was found to predict greater maternal bonding to her fetus (Levine et al., 2007), and variations in the OT receptor gene predicted maternal sensitivity (Bakermans-Kranenburg and Van IJzendoorn, 2008). OT was found to increase after parent–infant interaction sessions only among mothers whoexhibited high levels of affectionate touch and only among fathers who provided high levels of stimulatory contact, echoing research on the OT response in mothers and fathers in other mammals (Feldman et al., in press). However, although both PRL and OT are thought to provide the neuroendocrine basis of parental bonding, very little is known about the links between PRL and OT and between these two hormones and the development of paternal behavior in humans.
In light of the above, the main goal of the present longitudinal study was to examine the associations between PRL, OT, and the development of paternal behaviors in a group of first-time fathers. Fathers and their firstborn infants were seen in their home twice during the first six months of fatherhood: in the first weeks after childbirth and at 6 months postpartum and blood was drawn from fathers for the assessment of PRL and OT. During the second home visit, fathers were videotaped interacting with their infant in two interactive contexts, a social play and a toy exploration play. It was hypothesized that PRL and OT would be inter-related and would correlate with positive indicators of social bonding between father and infant, including gaze, vocalizations, positive affect, and tactile contact during the social context and with shared attention to toys and coordinated toy play in the toy context. Based on research demonstrating associations between OT and maternal behavior in social contexts, it was hypothesized that paternal OT would be more closely related to paternal behavior in the social context. Similarly, PRL has shown to play an important role in the development of paternal behavior in animals (Schradin and Anzenberger, 1999; Wynne-Edwards, 2001). In both humans and mammals, fathers tend to engage in exploratory behavior to a greater extent than mothers (Feldman, 2003; Lamb, 1976, 1977; Lonstein and De Vries, 1999; Parke and Sawin, 1976). It was thus expected that PRL would be associated with father's behavior in the exploratory context.
Methods and materials
Participants
Forty-three fathers and their firstborn infants, who were part of a larger project on the transition to parenthood, participated in both stages of the study. Fathers were all cohabitating with infant and mother, of middle class background, and completed at least high-school education. Fathers' age averaged 28.08 years (SD=4.19) and education averaged 15.39 years (SD=2.76) years. Infants' mean age at the first visit was 7.1 weeks (SD=2.11). Participants were seen again when the infant was approximately 6 months old (M=24.8 weeks, SD=4.38). Infants (20 girls and 23 boys) were all healthy firstborns of a singleton birth.
Hormones: Prolactin and Oxytocin
At both time-points of the study, fathers and infants were visited at home during the evening hours (4–8 PM). Participants first completed self-report measures that assessed a range of demographic and health variables (e.g., weight, height, smoking, and medication) and measures related to paternal stress and anxiety (state-trait anxiety and stress in parenting). Next, blood was drawn from antecubital veins into a 9 mL chilled vacutainer tubes that were supplemented with 400 KIU of Trasylol (Trasylol-Bayer, Germany) per 1 mL blood. Samples were kept ice-chilled for up to two hours before being centrifuged at4 °Cat 1000×gfor 15 min. Supernatants were collected and stored at − 70 °C until assayed. Fathers were asked to refrain from food intake for at least 30 min prior to blood draw.
To determine PRL concentrations plasma samples were defrosted to room temperature, immediately before analysis. PRL determination was done by Chemiluminescent Microparticle Immunoassay (CMIA) technology, measured by Architect i2000 system of ABBOT Diagnostic Division. The instrument measured relative light units, and the concentration of PRL was then calculated by using a 4 parameter logistic curve fit. All samples were measured by the same reagent Kit. The relevant intra-assay coefficient was 4.3%.
Determination of OT was performed using a commercial OT ELISA kit (Assay Design, MI, USA) as described in earlier studies (Carter, 2007; Feldman et al., 2007; Gordon et al., 2008; Levine et al., 2007). Measurements were performed in duplicate and the concentrations of samples were calculated using MatLab-7 according to relevant standard curves. The intra-assay and inter-assay coefficient were less than 12.4% and 14.5%, respectively.
Parenting behavior
In the second visit fathers were videotaped interacting with their infant in two contexts, social and exploratory, for 6 min each. In the social play, fathers were instructed to play with their infant as they normally do without using any toys. In the toy exploration session, fathers were asked to interact with the infant using toys they usually play with at home. Fathers positioned themselves and the infant in any way they wanted that would enable the continuous filming of both father and infant's faces. If the infant cried and could not be soothed or if the infant fell asleep during the home visit, interactions were not conducted. Thus, out of the sample of 43 fathers, only 29 dyads completed the social play and 34 completed the exploratory play.
Interactions were micro-coded on a computerized system (Noldus, The Vaggenigen, Netherlands), consistent with previous research on parent-infant interaction in social contexts (Feldman et al., 2004; Feldman, 2003; Feldman and Eidelman, 2004). Four non-verbal categories of parenting behavior were coded and each category included a set of mutually-exclusive codes (an “uncodable” code was added to each category in instances during which codes could not be determined). Categories and codes for both interactions videotaped were as follows: Parent Gaze – This category assessed the direction of the fathers' gaze and included the following codes: gaze to infant's face, gaze to infant's body, gaze to object or aspects of the environment, and gaze aversion, implying that father gazes away from the infant but gaze is not focused on other objects or the environment. Parent Affect – This category addressed the parent's expressed affect based on facial expression, body tone, movement, and other non-verbal signals and included the following three codes: positive, neutral, and negative. Parent Vocalizations: This category assessed the father's vocal output along the following four codes: “motherese” vocalizations, which are infant-directed speech that is high-pitched and typically includes sing-song vocalization, “typical” adult speech to the infant in normal range and regular rhythm, adult speech to other adult, and no speech. Parent Touch – This category assessed touch patterns between father and infant and included six codes: affectionate touch – father's loving touch such as hugging, kissing, stroking, or light pokes, touch of infant extremities – father touches infant's hand or feet, often with another object, functional touch – touch that has a functional goal such as wiping the infant's mouth, proprioceptive touch – includes touch that changes the infant's position in space, for instance, pulling the infant to a sitting position, stimulatory touch – touch that intends to stimulate and increase arousal, and no touch.
The toy exploration session was coded in line with previous research (Feldman et al., 2002) along five categories, each containing the following codes: Parent Gaze – to infant, to toy – when the infant is not looking at the same toy, to the environment, joint attention – father and infant gaze to the same toy simultaneously, and gaze aversion – father gazes away from the infant and gaze is not focused on other objects or the environment. Parent Affect – was coded similar to the social context as positive, neutral, and negative. Parent facilitation of infant toy exploration: This category addressed the ways in which father encouraged the infant's play with the toys and included five codes: presenting toy – father directing the infant's attention to the toy but not giving the toy to the infant, giving toy to infant – father places the toy in the infant's hand or gives him/her the toy to touch and handle by him/herself shared exploration of toy – father and infant engage in a shared manual exploration of the toy simultaneously while both are holding the toy, taking toy away from infant, and no toy exploration. Infant Toy Exploration: This category addressed the infant's engagement with the toy and included the following five codes: infant reaches for toy, infant explores the toy in one hand – for instance, the toy is on the infant's lap or in the father's hand and infant explores only with one hand, infant explores the toy with two hands – infant holds the toy with two hands and explores it for a brief period of shorter than 2 s, sustained exploration – infant holds the toy with two hands and explores the toy for at least 2 s with focused attention on the toy and visible interest, and no toy exploration – infant is not interested in the toy. Paternal Coordinated Exploratory Play – This category addressed the coordination between the child's readiness to explore and the father's facilitation of exploration and was coded as a binary variable (yes/no) whether the father introduced toys (in the form of presenting, handing, and shared exploration) when the infant's attention was focused on the toy or not. This code was used in the following analysis.
Only two variables from the aforementioned coded variables were used in the present study, consistent with our a-priori hypotheses. The Affect Synchrony construct was computed for the social context using conditional probabilities (that is, behaviors that co-occur in the two partners), consistent with previous research (Feldman and Eidelman, 2004; Feldman et al., 2007, 2002), as the sum of the proportions of time father was in social gaze, “motherese” type vocalizations, and positive affect given the infant was in positive vocalizations. The Father Coordinated Exploratory Play was the coded variable (see above) that addressed the father's presenting, handing, and jointly manipulating the toy when the infant's attention is directed on the toy. For this variable we computed both the proportion of time father was in Coordinated Exploratory Play and the mean duration of each Coordinated Exploratory Play episode.
Inter-rater reliability was conducted for 10% of the both types of interactions and averaged 98% (kappa = .86) for social play and for toy oriented play 98% (kappa = .85).
Results
PRL and OT: individual stability, change over time, and inter-relationship
Prior to data analysis we examined correlations between PRL and OT and potential confounding variables. No associations were found between PRL and OT with fathers' age, education, height, weight, smoking, or the use of medication, infant age in days, and time since last meal. Similarly, no significant correlations emerged between paternal PRL and OT with father's state or trait anxiety or stress in the parenting role. Five outliers greater than 3 SDs above the mean in OT concentrations were removed from following analyses and a single outlier higher than 3 SDs above the mean in PRL was removed.
Fig. 1 presents the distributions of PRL in fathers at the two study periods. Fig. 2 presents the mean levels of PRL and OT at the two time-points. PRL and OT levels were individually stable and showed high correlations between the two time-points: PRL, r=.88, p<.001, and OT, r=.87, p<.001.
Fig. 1.
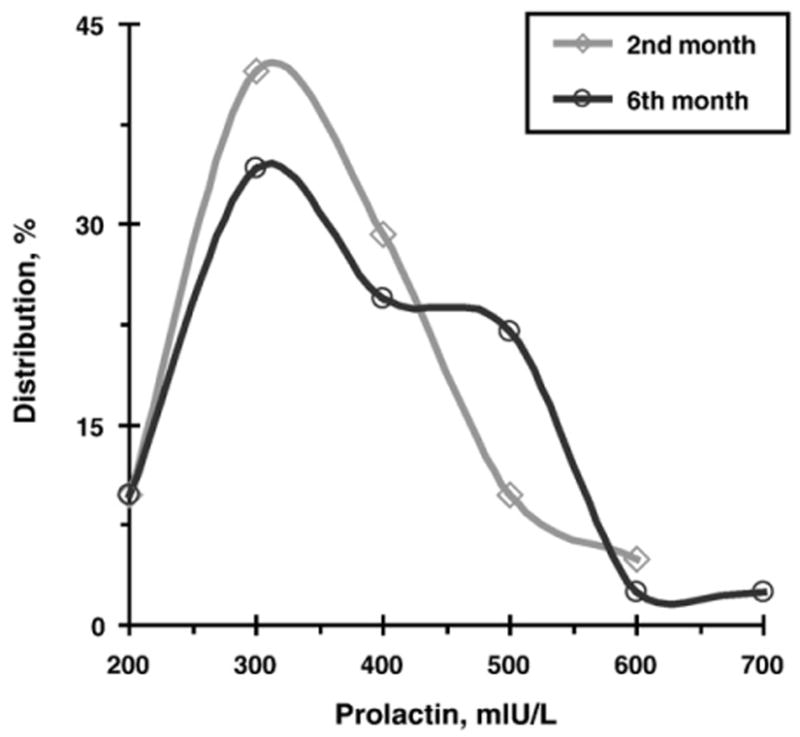
Distributions of fathers' Prolactin in second and sixth months following the birth of their first child.
Fig. 2.
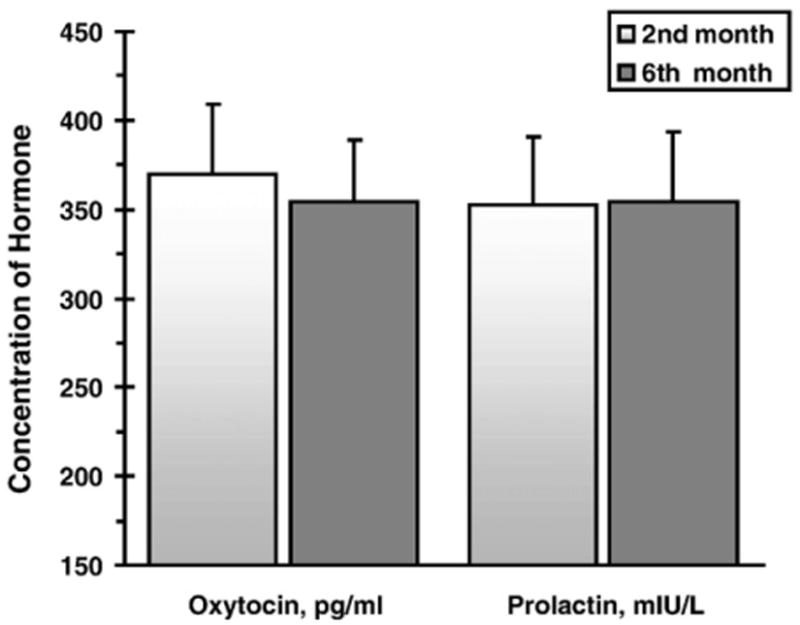
Mean (and SE) of fathers' Prolactin and Oxytocin at the second and sixth months following the birth of their first child.
Paired comparison t-tests assessed mean-level changes in OT and PRL across the two assessments. Both hormones did not show mean-level change over time: PRL: t (34)=.376, p>.1 and OT: t (37)= –.015, p>.1.
Pearson correlations were used to examine the associations between the two hormones. At the first time-point, PRL and OT were not significantly related, r=.14, p>.1. However, by the sixth month of fathering, PRL and OT showed medium-level correlations, r=.52, p<.005.
In light of the high level of individual stability in each hormone, PRL and OT were each averaged across the two time-points of the study. The mean level of the averaged PRL was 317.356 ng/mL, (SE=26.05) and the mean level of the averaged OT was 321.264 pg/mL (SE=20.30).
Hormones and paternal behavior
Paternal behaviors
The proportion of time out of the entire interaction fathers and infant spent in a state of Affect Synchrony in the social context was .22 (SD=.18). The proportion of time fathers spent in Coordinated Exploratory Play in the exploratory context was .39 (SD=.15). The mean duration of each episode of Coordinated Exploratory Play was on average 95.44 s (SD=54.63). Affect Synchrony and Coordinated Exploratory Play were not significantly correlated, r=−.097, p>.1.
PRL
The average PRL score was associated with the proportion of time and mean duration of each episode of Coordinated Exploratory Play with the infant during the toy context: r=.375, p<.05, and r=.548, p<.005 for proportion and mean duration respectively. PRL was only marginally related to Affect Synchrony between father and child during social play, r=−.244, p=.092.
OT
The averaged OT score was associated with the proportion of time father and infant spent in Affect Synchrony: r=.577, p<.005.
Predicting Affect Synchrony and Coordinated Exploratory Play
Two hierarchical regression equations were computed to predict Affect Synchrony and Coordinated Exploratory Play from PRL and OT, in order to assess the unique and combined contributions of the two hormones to the prediction of paternal behavior. Results are presented in Table 1.
Table 1.
Regression models predicting Affect Synchrony and Coordinated Exploratory Play from Prolactin and Oxytocin.
| Affect Synchrony | Coordinated Exploratory Play | |||||
|---|---|---|---|---|---|---|
| Beta | R2Change | FChange | Beta | R2Change | FChange | |
| Predictors | ||||||
| Prolactin | −.297 | .088 | 2.71 | .508** | .258 | 11.13** |
| Oxytocin | .542** | .294 | 12.84** | −.048 | .002 | .076 |
| R2 total=.382, F (2, 29) = 8.353, p<.005 | R2 total=.260, F (2, 33) = 5.44, p<.05 | |||||
p<.005.
As seen, both regression models were significant. Father–infant Affect Synchrony was uniquely predicted by OT, but PRL was unrelated to Affect Synchrony. On the other hand, paternal Coordinated Exploratory Play was uniquely predicted by PRL, whereas OT was unrelated to Coordinated Exploratory Play. Changing the order of entry in the regression did not meaningfully alter the results. In combination, the two hormones explained 38% and 26% of the variance in Affect Synchrony and Coordinated Exploratory Play respectively.
Discussion
The current study examined the relations between two neuropeptides with a well-known involvement in the initiation of motherhood and the development of fathering. Overall, the findings point to the links between both PRL and OT with paternal behavior in humans at the first period of fatherhood and are consistent with perspectives that suggest a common neuroendocrine pathway in the development of fathering and motheringin biparental species (Wynne-Edwards, 2001). Similar to the role of PRL and OT in processes related to maternal bonding (Carter, 1998; Feldman et al., 2007; Ross and Young, 2009; Russell et al., 2001), these hormones appear to be associated with typical paternal behaviors at the first months of fatherhood.
PRL and OT were each associated with a specific aspect of paternal behavior: PRL with father facilitation of the child's exploratory behavior and OT with the father–infant Affect Synchrony. Although PRL has been implicated in the development of paternal behavior in animals, this is the first study, to our knowledge, that demonstrates its specific involvement in the development of paternal behavior in human fathers. Recently, there has been a debate on whether or not PRL has a causal effect on the development of paternal behavior in mammals (Schradin, 2007; Wynne-Edwards and Timonin, 2007). Although results of the present longitudinal study could not imply causality in the associations between PRL and fatherhood, the findings may contribute to the discussion on the neurobiology of mammalian fathering by showing specific links between PRL and the typical behaviors of human fathers – the facilitation of child exploratory play (Lamb, 1977).
The high level of individual stability found for PRL and OT across the first six months of fatherhood provides validation for our assessment and may suggest that baseline levels of these two hormones represent a trait-like characteristic of the individual during the first period of becominga father. The levels of PRL and OT reported here are comparable to those determined previously using the same methodology in humans (Feldman et al., 2007; Gordon et al, 2008; Levine et al., 2007). The high stability of circulating PRL and OT may suggest that at the initial period of fatherhood these hormones may be stable biomarkers for some aspects of fathering and the father's typical interactive style. The fact that PRL and OT showed significant correlations only at the second assessment may point to a dynamic process in the neurobiological pathways underlying fatherhood. It is possible that as the father's daily encounters with his infant increase, hand in hand with the infant's growing social skills from the second to the sixth month of life, the PRL and OT systems reorganize and create new connections. Previous studies point to differences in PRL activity between experienced and first-time fathers (Delahunty et al., 2007; Fleming et al., 2002), whereas animal research in biparental species showed no difference in OT levels between first-time and experienced fathers (de Jong et al., 2009). Further research is thus required to fully understand the development of these hormones and their dynamic interplay during the first months of fatherhood.
PRL and OT were each related to a distinct aspect of the paternal behavioral repertoire that was expressed in a different interactive context. OT was related to Affect Synchrony during social play and PRL was associated with the father–infant Coordinated Exploratory Play during toy exploration. To date, the role of OT in parenting has mainly been studied in relation to maternal behavior. Maternal behavior is typically associated with more synchronized positive affect in a social context (Clarke-Stewart, 1978; Feldman, 2003). On the other hand, PRL was related to more optimal father facilitation of the infant's exploratory behavior, a style that has been associated with father– child play to a greater extent than mother–child play in both human and other mammals (Feldman, 2003; Lamb, 1976, 1977; Lonstein and De Vries, 1999; Parke and Sawin, 1976). Social and exploratory types of play have been described as two distinct types of parenting behavior that are stable across the first months of the infant's life (Bornstein and Tamis-LeMonda, 1990). Possibly, during the first months of fatherhood, PRL shows a closer association with the didactic exploratory style while OT is more closely linked with the social style. Further research is required to examine the neurohormonal underpinnings of the social and exploratory parenting styles across the child's first year and beyond among various normative and high-risk populations.
Parent–infant Affect Synchrony and Coordinated Exploratory Play are both markers of early parenting that are critical for the infant's cognitive, social, and emotional growth (Feldman, 2007). The infant's active exploration of the environment is among the central bi-products of a secure attachment relationship (Bowlby, 1969) that is facilitated by sensitive parenting (Ainsworth, 1989). The coordination of parent and infant's early non-verbal social signals into a process of Affect Synchrony plays an important role in the development of the social brain circuitry and contributes to the infant's ultimate growth (Feldman, 2007). Similarly, interventions that are targeted to increase sensitive synchronicity have been shown to increase attachment security (Bakermans-Kranenburg et al., 2003). As such, the findings that OT across the first six months of fatherhood predict father–infant synchrony and that PRL across the same period predict father's sensitive facilitation of infant exploration underscore the involvement of these neuropeptides in the development of adaptive fatherhood in humans and their potential contribution to more optimal infant development.
To our knowledge, the current study is the first to demonstrate associations between PRL, OT, and paternal behavior in humans. Thus, the findings need replication and much further research is required to validate, refine, and provide a conceptual framework for the links between PRL and fatherhood (Wynne-Edwards and Timonin, 2007). Our reliance on basal hormonal circulating levels is a possible limitation, and future research is required to measure hormonal reactivity that addresses the change from baseline levels to those observed following father–infant interactions in OT and PRL as well as in sex steroids, glucocorticoids, and vasopressin. Intranasal administration of OT or vasopressin may similarly shed further light on the role of neuropeptide hormones in human fathering and may provide a more comprehensive understanding of the neuroendocrine foundations of fatherhood in normative populations and under conditions of high risk, such as father absence, abuse, or neglect.
References
- Ainsworth MD. Attachments beyond infancy. Am Psychol. 1989;44:709–716. doi: 10.1037//0003-066x.44.4.709. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neur. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IMH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol Bull. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS. Activities and interactions of mothers and their firstborn infants in the first six months of life: covariation, stability, continuity, correspondence, and prediction. Child Dev. 1990;61:1206–1217. doi: 10.1111/j.1467-8624.1990.tb02854.x. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Basic Books; New York: 1969. [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology. 1997;138:756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Hnasko RM, Zuzick PH, Valentine DL, Scammell JG. Changes in bioactive prolactin-like activity in plasma and its relationship to incubation behavior in breeding ring doves. Gen Comp Endocrinol. 1996;102:221–232. doi: 10.1006/gcen.1996.0063. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Clarke-Stewart KA. And daddy makes three: the father's impact on mother and young child. Child Dev. 1978;49:466–478. [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Delahunty KM, McKay DW, Noseworthy DE, Storey AE. Prolactin responses to infant cues in men and women: effects of parental experience and recent infant contact. Horm Behav. 2007;51:213–220. doi: 10.1016/j.yhbeh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. Infant–mother and infant–father synchrony: the coregulation of positive arousal. Infant Ment Health J. 2003;24:1–23. [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Parent–infant synchrony and the social-emotional development of triplets. Dev Psychol. 2004;40:1133–1147. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (kangaroo care) promotes self-regulation in premature infants: sleep–wake cyclicity, arousal modulation, and sustained exploration. Dev Psychology. 2002;38:194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI, Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: a model for parent and child influences in a unique ecology. Child Dev. 2004;75:1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.01.013. in press. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–171. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol. 2008;20:752–763. doi: 10.1111/j.1365-2826.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Pi XJ, Andrews ZB, Augustine RA, Kokay IC, Summerfield MR, Todd B, Bunn SJ. Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm Behav. 2001;40:115–124. doi: 10.1006/hbeh.2001.1698. [DOI] [PubMed] [Google Scholar]
- Gray PB, Parkin JC, Samms-Vaughan ME. Hormonal correlates of human paternal interactions: a hospital-based investigation in urban Jamaica. Horm Behav. 2007;52:499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Kindler PM, Bahr JM, Gross MR, Philipp DP. Hormonal-regulation of parental care behavior in nesting male bluegills – do the effects of bromocriptine suggest a role for prolactin. Physiol Zool. 1991;64:310–322. [Google Scholar]
- Lamb ME. The role of the father: interactions between 8 month old children and their fathers and mothers. In: Lamb ME, editor. The Role of the Father in Child Development. Wiley & Sons; New York: 1976. [Google Scholar]
- Lamb ME. A re-examination of the infant social world. Hum Dev. 1977;20:65–85. doi: 10.1159/000271548. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal–fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Comparison of the parental behavior of pair-bonded female and male prairie voles (Microtus ochrogaster) Physiol Behav. 1999;66:33–40. doi: 10.1016/s0031-9384(98)00270-4. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Lactogenic hormone regulation of maternal behavior. Prog Brain Res. 2001;133:251–262. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- Parke RD, Sawin DB. The father's role in infancy: a re-evaluation. Fam Coord. 1976;25:365–371. [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Oxytocin and mothering behavior in the rat. Pharmacol Ther. 1985;28:287–302. doi: 10.1016/0163-7258(85)90056-7. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity – adaptive changes inbehavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Schradin C. Comments to K.E. Wynne-Edwards and M.E. Timonin 2007. Paternal care in rodents: weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care, Horm & Behav. 52:114-121. Horm Behav. 2007;52:557–9. doi: 10.1016/j.yhbeh.2007.03.018. author reply 560. [DOI] [PubMed] [Google Scholar]
- Schradin C, Anzenberger G. Prolactin, the hormone of paternity. News Physiol Sci. 1999;14:223–231. doi: 10.1152/physiologyonline.1999.14.6.223. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Storey AE, Delahunty KM, McKay DW, Walsh CJ, Wilhelm SI. Social and hormonal bases of individual differences in the parental behaviour of birds and mammals. Can J Exp Psychol. 2006;60:237–245. doi: 10.1037/cjep2006022. [DOI] [PubMed] [Google Scholar]
- Svennersten-Sjaunja K, Olsson K. Endocrinology of milk production. Domest Anim Endocrinol. 2005;29:241–258. doi: 10.1016/j.domaniend.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Arn I, Jonsson CO, Ek S, Nilsonne A. The relationships between personality traits and plasma gastrin, cholecystokinin, somato-statin, insulin, and oxytocin levels in healthy women. J Psychosom Res. 1993;37:581–588. doi: 10.1016/0022-3999(93)90052-h. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Johansson B, Lupoli B, Svennersten-Sjaunja K. Oxytocin facilitates behavioural, metabolic and physiological adaptations during lactation. Appl Anim Behav Sci. 2001;72:225–234. doi: 10.1016/s0168-1591(01)00112-5. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Widstrom AM, Nissen E, Bjorvell H. Personality traits in women 4 days post partum and their correlation with plasma levels of oxytocin and prolactin. J Psychosomatic Obstet Gynecol. 1990;11 [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Ziegler TE. Hormones associated with non-maternal infant care: a review of mammalian and avian studies. Folia Primatol (Basel) 2000;71:6–21. doi: 10.1159/000021726. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wegner FH, Snowdon CT. Hormonal responses to parental and nonparental conditions in male cotton-top tamarins, Saguinus oedipus, a New World primate. Horm Behav. 1996;30:287–297. doi: 10.1006/hbeh.1996.0035. [DOI] [PubMed] [Google Scholar]


