Abstract
The title compound, C22H26O9, crystallizes with two independent molecules in the asymmetric unit in which the dihedral angles between the two benzene rings are 21.4 (2) and 5.1 (2)°. An intramolecular O—H⋯O hydrogen bond occurs in each molecule. Intermolecular C—H⋯O hydrogen bonds stabilize the crystal structure.
Related literature
For the biological activity of flavonoids, see: Jung et al. (2006 ▶); Ong & Khoo (1996 ▶); Vessal et al. (2003 ▶); Sousa et al. (2004 ▶). For bond-length data, see: Allen et al. (1987 ▶); Chu et al. (2004 ▶); Zhang et al. (2011 ▶). For the preparation, see: Duan et al. (2006 ▶).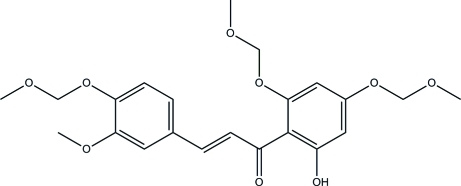
Experimental
Crystal data
C22H26O9
M r = 434.43
Monoclinic,

a = 12.008 (3) Å
b = 13.016 (4) Å
c = 13.663 (4) Å
β = 97.154 (4)°
V = 2119.0 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 113 K
0.24 × 0.22 × 0.18 mm
Data collection
Rigaku Saturn CCD area-detector diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku/MSC, 2009) ▶ T min = 0.975, T max = 0.981
22288 measured reflections
5259 independent reflections
4715 reflections with I > 2σ(I)
R int = 0.045
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.076
S = 1.03
5259 reflections
569 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.18 e Å−3
Data collection: CrystalClear-SM Expert (Rigaku/MSC, 2009) ▶; cell refinement: CrystalClear-SM Expert ▶; data reduction: CrystalClear-SM Expert ▶; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041213/hg5103sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041213/hg5103Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811041213/hg5103Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H5⋯O6 | 0.84 | 1.75 | 2.506 (2) | 148 |
| O14—H14⋯O15 | 0.84 | 1.72 | 2.475 (2) | 148 |
| C8—H8C⋯O8i | 0.98 | 2.57 | 3.312 (3) | 132 |
| C9—H9A⋯O5ii | 0.99 | 2.52 | 3.444 (3) | 155 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
supplementary crystallographic information
Comment
Several flavonoids, such as Hesperidin and naringin(Jung et al., 2006), myricetin (Ong & Khoo, 1996), quercetin (Vessal et al., 2003), Kaempferol-3,7-O-(r)-dirhamnoside (Sousa et al., 2004), have been reported to treat diabetes. We synthesized a series of 5,7-dihydroxy flavonoids. The vitro antidiabetic activity experiment showed that most of the flavonoids showed a remarkable in vitro anti-diabetic activity. The title compound, (E)-1-(2-hydroxy-4,6-bis(methoxymethoxy)phenyl)-3- (3-methoxy-4-(methoxymethoxy)phenyl)prop-2-en-1-one was prepared as an intermediate.
In title compound, C22H26O9, crystallizes with two independent molecules in the asymmetric unit. All bond lengths and angles in the molecular are normal (Allen et al., 1987) and in a good agreement with those reported previously (Chu et al., 2004; Zhang et al., 2011). The dihedral angle between two phenyl ring (C1—C6 and C14—C19; C23—C28 and C36—C41) are 21.4 (2)° and 5.1 (2) °, respectively. The C—H···O intermolecular hydrogen bonds stabilized the crystal structure (Table 1).
Experimental
A round-bottomed flask was charged with 1.52 g (10 mmol) of 4-hydroxy-3-methoxybenzaldehyde, 9.66 g (70 mmol) of K2CO3, 805 mg (10 mmol) of chloromethyl methyl ether (MOMCl) and 30 ml of anhydrous acetone, and the mixture was stirred at room temperature for four hours. The reaction mixture was filtered, and evaporated to afford 3-methoxy-4-(methoxymethoxy)benzaldehyde. Dropwise chloromethyl methyl ether (4.83 g, 6 mmol) was added to a mixture of 2,4,6-trihydroxyacetophenone (503.0 mg, 3 mmol) and K2CO3 (2.89 g, 21 mmol) in anhydrous acetone (30 ml). The mixture was heated at reflux for 1.5 h, filtered, and evaporated to afford 2-hydroxy-4,6-dimethoxymethoxyacetophenone. Then 3-methoxy-4-(methoxymethoxy)benzaldehyde (589 mg, 3.0 mmol) in 60% NaOH aqua (8 ml) and MeOH (15 ml) was added and stirred for 24 h. The resulting mixture was poured into cold 2 M HCl (40 ml), and then extracted with three 20-ml portions of EtOAc. The organic layer was washed with water and saturated brine, dried over MgSO4, and evaporated to afford the title compound via recrystallization from EtOH (Duan et al., 2006). Single crystals suitable for X-ray diffraction were obtained from slow evaporation of a solution of the pure title compound in ethanol at room temperature.
Refinement
All H atoms were found on difference maps, with C—H = 0.95–0.99, O—H = 0.84 Å and included in the final cycles of refinement using a riding model, with Uiso(H) = 1.2Ueq(C) and 1.5Ueq(C, O) for the methyl and hydroxyl H atoms.
Figures
Fig. 1.
View of the title compound, with displacement ellipsoids drawn at the 40% probability level.
Crystal data
| C22H26O9 | F(000) = 920 |
| Mr = 434.43 | Dx = 1.362 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 7434 reflections |
| a = 12.008 (3) Å | θ = 1.7–27.9° |
| b = 13.016 (4) Å | µ = 0.11 mm−1 |
| c = 13.663 (4) Å | T = 113 K |
| β = 97.154 (4)° | Block, orange |
| V = 2119.0 (10) Å3 | 0.24 × 0.22 × 0.18 mm |
| Z = 4 |
Data collection
| Rigaku Saturn CCD area-detector diffractometer | 5259 independent reflections |
| Radiation source: rotating anode | 4715 reflections with I > 2σ(I) |
| multilayer | Rint = 0.045 |
| Detector resolution: 14.63 pixels mm-1 | θmax = 27.9°, θmin = 1.7° |
| ω and φ scans | h = −14→15 |
| Absorption correction: multi-scan (CrystalClear; Rigaku/MSC, 2009) | k = −17→17 |
| Tmin = 0.975, Tmax = 0.981 | l = −17→17 |
| 22288 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.076 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0347P)2] where P = (Fo2 + 2Fc2)/3 |
| 5259 reflections | (Δ/σ)max = 0.001 |
| 569 parameters | Δρmax = 0.21 e Å−3 |
| 1 restraint | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.22434 (13) | 0.90191 (12) | 0.50952 (12) | 0.0273 (4) | |
| O2 | 0.14482 (13) | 0.95752 (11) | 0.35274 (11) | 0.0244 (4) | |
| O3 | 0.58068 (13) | 0.88250 (11) | 0.69967 (11) | 0.0249 (4) | |
| O4 | 0.46961 (13) | 0.78830 (13) | 0.79761 (12) | 0.0312 (4) | |
| O5 | 0.48367 (14) | 1.17704 (12) | 0.49837 (12) | 0.0302 (4) | |
| H5 | 0.5482 | 1.1951 | 0.5233 | 0.045* | |
| O6 | 0.66802 (13) | 1.16766 (12) | 0.60636 (11) | 0.0267 (4) | |
| O7 | 0.91164 (14) | 0.91335 (12) | 1.08562 (11) | 0.0273 (4) | |
| O8 | 1.09693 (14) | 1.01811 (12) | 1.11830 (11) | 0.0257 (4) | |
| O9 | 1.19143 (13) | 1.17603 (12) | 1.14491 (11) | 0.0266 (4) | |
| O10 | 0.18533 (13) | 0.81450 (12) | 0.00995 (11) | 0.0265 (4) | |
| O11 | 0.15303 (14) | 0.77679 (11) | −0.15985 (12) | 0.0275 (4) | |
| O12 | 0.49745 (13) | 0.80118 (12) | 0.25640 (11) | 0.0273 (4) | |
| O13 | 0.44653 (14) | 0.97448 (11) | 0.26498 (12) | 0.0296 (4) | |
| O14 | 0.46845 (14) | 0.56582 (13) | −0.01679 (12) | 0.0329 (4) | |
| H14 | 0.5328 | 0.5489 | 0.0095 | 0.049* | |
| O15 | 0.64358 (14) | 0.57331 (13) | 0.09948 (12) | 0.0360 (4) | |
| O16 | 0.89706 (13) | 0.82460 (12) | 0.57367 (11) | 0.0248 (4) | |
| O17 | 1.08189 (13) | 0.71974 (11) | 0.60496 (11) | 0.0233 (4) | |
| O18 | 1.18426 (13) | 0.56566 (12) | 0.63086 (11) | 0.0266 (4) | |
| C1 | 0.53421 (18) | 1.03548 (16) | 0.60884 (15) | 0.0195 (5) | |
| C2 | 0.45869 (19) | 1.08459 (16) | 0.53524 (16) | 0.0213 (5) | |
| C3 | 0.35580 (19) | 1.04253 (17) | 0.49855 (16) | 0.0222 (5) | |
| H3 | 0.3074 | 1.0772 | 0.4489 | 0.027* | |
| C4 | 0.32511 (19) | 0.94969 (17) | 0.53531 (16) | 0.0225 (5) | |
| C5 | 0.39895 (19) | 0.89474 (17) | 0.60430 (16) | 0.0244 (5) | |
| H5A | 0.3777 | 0.8297 | 0.6274 | 0.029* | |
| C6 | 0.50153 (18) | 0.93550 (17) | 0.63790 (16) | 0.0218 (5) | |
| C7 | 0.13674 (19) | 0.95890 (18) | 0.45297 (17) | 0.0255 (5) | |
| H7A | 0.0633 | 0.9298 | 0.4645 | 0.031* | |
| H7B | 0.1394 | 1.0310 | 0.4762 | 0.031* | |
| C8 | 0.1145 (2) | 0.85967 (17) | 0.30924 (18) | 0.0297 (6) | |
| H8A | 0.0405 | 0.8394 | 0.3259 | 0.045* | |
| H8B | 0.1703 | 0.8082 | 0.3347 | 0.045* | |
| H8C | 0.1120 | 0.8645 | 0.2374 | 0.045* | |
| C9 | 0.5511 (2) | 0.78293 (17) | 0.73444 (17) | 0.0277 (5) | |
| H9A | 0.5233 | 0.7394 | 0.6772 | 0.033* | |
| H9B | 0.6190 | 0.7498 | 0.7692 | 0.033* | |
| C10 | 0.5096 (2) | 0.8409 (2) | 0.8875 (2) | 0.0481 (8) | |
| H10A | 0.5307 | 0.9113 | 0.8724 | 0.072* | |
| H10B | 0.5751 | 0.8047 | 0.9210 | 0.072* | |
| H10C | 0.4501 | 0.8426 | 0.9306 | 0.072* | |
| C11 | 0.63687 (19) | 1.08955 (17) | 0.64962 (16) | 0.0214 (5) | |
| C12 | 0.70300 (18) | 1.05763 (16) | 0.74328 (16) | 0.0211 (5) | |
| H12 | 0.6728 | 1.0086 | 0.7842 | 0.025* | |
| C13 | 0.80475 (18) | 1.09697 (16) | 0.77090 (16) | 0.0207 (5) | |
| H13 | 0.8301 | 1.1467 | 0.7278 | 0.025* | |
| C14 | 0.88206 (18) | 1.07296 (17) | 0.85929 (16) | 0.0203 (5) | |
| C15 | 0.85724 (19) | 0.99989 (16) | 0.92922 (16) | 0.0212 (5) | |
| H15 | 0.7888 | 0.9626 | 0.9184 | 0.025* | |
| C16 | 0.9308 (2) | 0.98154 (16) | 1.01337 (16) | 0.0205 (5) | |
| C17 | 1.03260 (19) | 1.03632 (16) | 1.03000 (16) | 0.0211 (5) | |
| C18 | 1.05886 (19) | 1.10596 (16) | 0.95928 (16) | 0.0221 (5) | |
| H18 | 1.1287 | 1.1411 | 0.9683 | 0.027* | |
| C19 | 0.98402 (18) | 1.12444 (17) | 0.87596 (16) | 0.0222 (5) | |
| H19 | 1.0026 | 1.1734 | 0.8290 | 0.027* | |
| C20 | 0.8059 (2) | 0.86135 (18) | 1.07342 (18) | 0.0313 (6) | |
| H20A | 0.7979 | 0.8226 | 1.0114 | 0.047* | |
| H20B | 0.7451 | 0.9118 | 1.0718 | 0.047* | |
| H20C | 0.8021 | 0.8140 | 1.1287 | 0.047* | |
| C21 | 1.2022 (2) | 1.07013 (18) | 1.13626 (17) | 0.0269 (5) | |
| H21A | 1.2443 | 1.0430 | 1.1977 | 0.032* | |
| H21B | 1.2466 | 1.0551 | 1.0815 | 0.032* | |
| C22 | 1.1447 (2) | 1.20680 (19) | 1.23220 (17) | 0.0318 (6) | |
| H22A | 1.1861 | 1.1735 | 1.2899 | 0.048* | |
| H22B | 1.0656 | 1.1863 | 1.2265 | 0.048* | |
| H22C | 1.1505 | 1.2816 | 1.2397 | 0.048* | |
| C23 | 0.49240 (19) | 0.68245 (17) | 0.12260 (16) | 0.0224 (5) | |
| C24 | 0.42976 (19) | 0.64374 (17) | 0.03440 (16) | 0.0230 (5) | |
| C25 | 0.32671 (19) | 0.68403 (17) | −0.00400 (16) | 0.0236 (5) | |
| H25 | 0.2863 | 0.6555 | −0.0619 | 0.028* | |
| C26 | 0.28369 (19) | 0.76586 (17) | 0.04285 (16) | 0.0227 (5) | |
| C27 | 0.34081 (18) | 0.80713 (17) | 0.12906 (16) | 0.0228 (5) | |
| H27 | 0.3101 | 0.8640 | 0.1600 | 0.027* | |
| C28 | 0.44134 (18) | 0.76566 (16) | 0.16925 (16) | 0.0215 (5) | |
| C29 | 0.11434 (19) | 0.76794 (18) | −0.06884 (17) | 0.0265 (5) | |
| H29A | 0.1061 | 0.6941 | −0.0537 | 0.032* | |
| H29B | 0.0390 | 0.7997 | −0.0729 | 0.032* | |
| C30 | 0.1521 (2) | 0.88016 (18) | −0.19537 (19) | 0.0395 (7) | |
| H30A | 0.2100 | 0.9201 | −0.1551 | 0.059* | |
| H30B | 0.0784 | 0.9109 | −0.1912 | 0.059* | |
| H30C | 0.1674 | 0.8803 | −0.2642 | 0.059* | |
| C31 | 0.4462 (2) | 0.87896 (17) | 0.31042 (17) | 0.0269 (5) | |
| H31A | 0.3678 | 0.8589 | 0.3164 | 0.032* | |
| H31B | 0.4871 | 0.8839 | 0.3778 | 0.032* | |
| C32 | 0.5560 (2) | 1.0166 (2) | 0.2683 (2) | 0.0408 (7) | |
| H32A | 0.6041 | 0.9689 | 0.2373 | 0.061* | |
| H32B | 0.5875 | 1.0279 | 0.3372 | 0.061* | |
| H32C | 0.5521 | 1.0822 | 0.2328 | 0.061* | |
| C33 | 0.60378 (19) | 0.63850 (17) | 0.15390 (16) | 0.0235 (5) | |
| C34 | 0.67431 (19) | 0.66787 (17) | 0.24565 (17) | 0.0243 (5) | |
| H34 | 0.6443 | 0.7116 | 0.2915 | 0.029* | |
| C35 | 0.77910 (18) | 0.63422 (16) | 0.26537 (16) | 0.0207 (5) | |
| H35 | 0.8046 | 0.5895 | 0.2180 | 0.025* | |
| C36 | 0.85976 (18) | 0.65812 (16) | 0.35166 (16) | 0.0191 (5) | |
| C37 | 0.96138 (18) | 0.60702 (16) | 0.36697 (16) | 0.0210 (5) | |
| H37 | 0.9787 | 0.5577 | 0.3199 | 0.025* | |
| C38 | 1.03903 (18) | 0.62596 (17) | 0.44958 (16) | 0.0206 (5) | |
| H38 | 1.1084 | 0.5900 | 0.4582 | 0.025* | |
| C39 | 1.01467 (18) | 0.69756 (16) | 0.51928 (16) | 0.0192 (5) | |
| C40 | 0.91248 (18) | 0.75263 (15) | 0.50337 (16) | 0.0190 (5) | |
| C41 | 0.83640 (19) | 0.73288 (16) | 0.42099 (16) | 0.0204 (5) | |
| H41 | 0.7678 | 0.7700 | 0.4110 | 0.024* | |
| C42 | 0.7928 (2) | 0.87817 (18) | 0.56172 (17) | 0.0300 (6) | |
| H42A | 0.7310 | 0.8287 | 0.5595 | 0.045* | |
| H42B | 0.7859 | 0.9175 | 0.5001 | 0.045* | |
| H42C | 0.7898 | 0.9251 | 0.6174 | 0.045* | |
| C43 | 1.18969 (18) | 0.67200 (18) | 0.61951 (17) | 0.0249 (5) | |
| H43A | 1.2300 | 0.6875 | 0.5623 | 0.030* | |
| H43B | 1.2336 | 0.7018 | 0.6789 | 0.030* | |
| C44 | 1.1416 (2) | 0.53416 (19) | 0.71952 (18) | 0.0299 (6) | |
| H44A | 1.0617 | 0.5514 | 0.7151 | 0.045* | |
| H44B | 1.1827 | 0.5698 | 0.7761 | 0.045* | |
| H44C | 1.1513 | 0.4598 | 0.7280 | 0.045* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0205 (9) | 0.0293 (9) | 0.0301 (9) | −0.0058 (7) | −0.0044 (7) | 0.0054 (7) |
| O2 | 0.0271 (9) | 0.0210 (8) | 0.0239 (9) | −0.0014 (7) | −0.0013 (7) | 0.0003 (6) |
| O3 | 0.0236 (9) | 0.0231 (8) | 0.0272 (9) | 0.0009 (6) | −0.0002 (7) | 0.0071 (6) |
| O4 | 0.0299 (9) | 0.0339 (9) | 0.0309 (10) | −0.0061 (8) | 0.0076 (8) | 0.0061 (7) |
| O5 | 0.0312 (10) | 0.0249 (8) | 0.0324 (10) | −0.0075 (7) | −0.0049 (8) | 0.0089 (7) |
| O6 | 0.0249 (9) | 0.0274 (8) | 0.0272 (9) | −0.0059 (7) | 0.0009 (7) | 0.0063 (7) |
| O7 | 0.0338 (10) | 0.0255 (9) | 0.0217 (9) | −0.0054 (7) | 0.0003 (8) | 0.0053 (7) |
| O8 | 0.0266 (9) | 0.0271 (9) | 0.0215 (9) | −0.0012 (7) | −0.0046 (7) | 0.0001 (7) |
| O9 | 0.0287 (9) | 0.0263 (8) | 0.0242 (9) | −0.0023 (7) | 0.0012 (7) | −0.0024 (7) |
| O10 | 0.0210 (9) | 0.0304 (9) | 0.0266 (9) | 0.0054 (7) | −0.0035 (7) | −0.0024 (7) |
| O11 | 0.0361 (10) | 0.0220 (8) | 0.0234 (9) | 0.0031 (7) | −0.0002 (7) | 0.0016 (7) |
| O12 | 0.0292 (9) | 0.0286 (9) | 0.0227 (8) | 0.0087 (7) | −0.0023 (7) | −0.0068 (7) |
| O13 | 0.0269 (10) | 0.0278 (9) | 0.0332 (10) | 0.0035 (7) | −0.0001 (8) | −0.0038 (7) |
| O14 | 0.0334 (11) | 0.0320 (9) | 0.0309 (10) | 0.0127 (8) | −0.0054 (8) | −0.0098 (8) |
| O15 | 0.0318 (10) | 0.0381 (10) | 0.0354 (10) | 0.0125 (8) | −0.0064 (8) | −0.0142 (8) |
| O16 | 0.0291 (9) | 0.0213 (8) | 0.0248 (9) | 0.0044 (7) | 0.0060 (7) | −0.0048 (7) |
| O17 | 0.0226 (9) | 0.0259 (8) | 0.0202 (8) | 0.0005 (7) | −0.0015 (7) | −0.0016 (6) |
| O18 | 0.0278 (10) | 0.0280 (9) | 0.0239 (9) | 0.0033 (7) | 0.0023 (7) | 0.0001 (7) |
| C1 | 0.0197 (12) | 0.0213 (11) | 0.0176 (11) | −0.0005 (9) | 0.0025 (9) | 0.0011 (8) |
| C2 | 0.0250 (12) | 0.0190 (11) | 0.0202 (12) | −0.0016 (9) | 0.0033 (10) | 0.0023 (9) |
| C3 | 0.0221 (12) | 0.0245 (12) | 0.0191 (12) | 0.0008 (9) | −0.0009 (10) | 0.0019 (9) |
| C4 | 0.0211 (12) | 0.0246 (11) | 0.0219 (12) | −0.0038 (9) | 0.0028 (10) | −0.0033 (9) |
| C5 | 0.0272 (13) | 0.0216 (11) | 0.0242 (12) | −0.0034 (9) | 0.0025 (10) | 0.0038 (9) |
| C6 | 0.0214 (12) | 0.0264 (11) | 0.0171 (11) | 0.0024 (9) | 0.0001 (9) | 0.0008 (9) |
| C7 | 0.0186 (12) | 0.0301 (13) | 0.0271 (13) | 0.0016 (10) | −0.0004 (10) | −0.0049 (10) |
| C8 | 0.0340 (15) | 0.0240 (12) | 0.0306 (14) | −0.0011 (11) | 0.0021 (11) | −0.0023 (10) |
| C9 | 0.0308 (14) | 0.0219 (11) | 0.0301 (13) | −0.0002 (10) | 0.0026 (11) | 0.0050 (10) |
| C10 | 0.060 (2) | 0.0535 (18) | 0.0340 (16) | −0.0199 (15) | 0.0169 (14) | −0.0058 (13) |
| C11 | 0.0211 (12) | 0.0224 (11) | 0.0212 (12) | −0.0003 (9) | 0.0041 (10) | −0.0016 (9) |
| C12 | 0.0210 (12) | 0.0209 (11) | 0.0215 (12) | −0.0012 (9) | 0.0035 (9) | 0.0006 (9) |
| C13 | 0.0237 (12) | 0.0184 (11) | 0.0202 (12) | −0.0010 (9) | 0.0039 (9) | −0.0001 (8) |
| C14 | 0.0197 (12) | 0.0226 (11) | 0.0186 (11) | 0.0002 (9) | 0.0024 (9) | −0.0017 (9) |
| C15 | 0.0209 (12) | 0.0212 (12) | 0.0215 (12) | −0.0023 (9) | 0.0031 (10) | −0.0018 (9) |
| C16 | 0.0263 (13) | 0.0184 (10) | 0.0170 (11) | 0.0005 (9) | 0.0045 (9) | −0.0002 (9) |
| C17 | 0.0264 (13) | 0.0200 (11) | 0.0167 (12) | 0.0031 (10) | 0.0017 (9) | −0.0046 (9) |
| C18 | 0.0218 (12) | 0.0234 (12) | 0.0213 (12) | −0.0014 (9) | 0.0031 (10) | −0.0021 (9) |
| C19 | 0.0252 (12) | 0.0240 (12) | 0.0175 (11) | −0.0032 (10) | 0.0035 (9) | −0.0026 (9) |
| C20 | 0.0347 (15) | 0.0288 (13) | 0.0299 (14) | −0.0098 (11) | 0.0019 (12) | 0.0041 (10) |
| C21 | 0.0247 (13) | 0.0295 (13) | 0.0247 (13) | 0.0034 (10) | −0.0044 (10) | −0.0021 (10) |
| C22 | 0.0335 (15) | 0.0368 (15) | 0.0245 (13) | 0.0003 (12) | 0.0012 (11) | −0.0058 (11) |
| C23 | 0.0235 (12) | 0.0223 (11) | 0.0211 (12) | 0.0015 (9) | 0.0015 (9) | 0.0000 (9) |
| C24 | 0.0242 (13) | 0.0217 (11) | 0.0230 (12) | 0.0013 (9) | 0.0022 (10) | −0.0007 (9) |
| C25 | 0.0230 (12) | 0.0250 (12) | 0.0216 (12) | −0.0005 (10) | −0.0022 (10) | −0.0018 (9) |
| C26 | 0.0193 (12) | 0.0263 (12) | 0.0222 (12) | 0.0010 (9) | 0.0010 (9) | 0.0036 (9) |
| C27 | 0.0250 (13) | 0.0216 (11) | 0.0219 (12) | 0.0021 (9) | 0.0029 (10) | −0.0022 (9) |
| C28 | 0.0248 (13) | 0.0216 (11) | 0.0178 (11) | −0.0028 (9) | 0.0020 (9) | −0.0001 (8) |
| C29 | 0.0186 (12) | 0.0276 (12) | 0.0317 (14) | 0.0008 (10) | −0.0030 (10) | 0.0000 (10) |
| C30 | 0.063 (2) | 0.0250 (13) | 0.0297 (14) | 0.0034 (13) | 0.0042 (14) | 0.0063 (11) |
| C31 | 0.0282 (14) | 0.0279 (12) | 0.0246 (13) | 0.0033 (10) | 0.0028 (11) | −0.0073 (10) |
| C32 | 0.0335 (16) | 0.0358 (14) | 0.0536 (19) | −0.0032 (12) | 0.0074 (13) | −0.0035 (13) |
| C33 | 0.0236 (12) | 0.0232 (12) | 0.0235 (12) | 0.0030 (10) | 0.0022 (10) | 0.0002 (9) |
| C34 | 0.0242 (13) | 0.0269 (12) | 0.0217 (12) | 0.0035 (10) | 0.0022 (10) | 0.0005 (9) |
| C35 | 0.0232 (12) | 0.0186 (11) | 0.0203 (12) | 0.0002 (9) | 0.0034 (9) | 0.0001 (9) |
| C36 | 0.0194 (12) | 0.0213 (11) | 0.0168 (11) | −0.0020 (9) | 0.0030 (9) | 0.0015 (8) |
| C37 | 0.0233 (12) | 0.0211 (11) | 0.0191 (12) | 0.0012 (9) | 0.0050 (9) | −0.0010 (9) |
| C38 | 0.0162 (11) | 0.0229 (11) | 0.0228 (12) | 0.0035 (9) | 0.0031 (9) | 0.0020 (9) |
| C39 | 0.0174 (11) | 0.0227 (11) | 0.0174 (11) | −0.0033 (9) | 0.0015 (9) | 0.0022 (9) |
| C40 | 0.0239 (12) | 0.0156 (10) | 0.0189 (11) | −0.0021 (9) | 0.0075 (9) | −0.0004 (8) |
| C41 | 0.0175 (11) | 0.0193 (11) | 0.0249 (12) | 0.0018 (9) | 0.0043 (9) | 0.0027 (9) |
| C42 | 0.0374 (15) | 0.0249 (12) | 0.0285 (14) | 0.0083 (11) | 0.0074 (12) | −0.0050 (10) |
| C43 | 0.0190 (12) | 0.0306 (13) | 0.0242 (13) | −0.0009 (10) | −0.0011 (10) | −0.0010 (10) |
| C44 | 0.0316 (14) | 0.0289 (13) | 0.0284 (14) | 0.0000 (11) | 0.0012 (11) | 0.0031 (10) |
Geometric parameters (Å, °)
| O1—C4 | 1.367 (3) | C14—C19 | 1.389 (3) |
| O1—C7 | 1.432 (3) | C14—C15 | 1.406 (3) |
| O2—C7 | 1.385 (3) | C15—C16 | 1.380 (3) |
| O2—C8 | 1.433 (3) | C15—H15 | 0.9500 |
| O3—C6 | 1.375 (3) | C16—C17 | 1.409 (3) |
| O3—C9 | 1.440 (3) | C17—C18 | 1.389 (3) |
| O4—C9 | 1.384 (3) | C18—C19 | 1.381 (3) |
| O4—C10 | 1.436 (3) | C18—H18 | 0.9500 |
| O5—C2 | 1.353 (3) | C19—H19 | 0.9500 |
| O5—H5 | 0.8400 | C20—H20A | 0.9800 |
| O6—C11 | 1.256 (3) | C20—H20B | 0.9800 |
| O7—C16 | 1.368 (2) | C20—H20C | 0.9800 |
| O7—C20 | 1.431 (3) | C21—H21A | 0.9900 |
| O8—C17 | 1.370 (3) | C21—H21B | 0.9900 |
| O8—C21 | 1.428 (3) | C22—H22A | 0.9800 |
| O9—C21 | 1.391 (3) | C22—H22B | 0.9800 |
| O9—C22 | 1.437 (3) | C22—H22C | 0.9800 |
| O10—C26 | 1.366 (3) | C23—C24 | 1.430 (3) |
| O10—C29 | 1.423 (3) | C23—C28 | 1.433 (3) |
| O11—C29 | 1.385 (3) | C23—C33 | 1.468 (3) |
| O11—C30 | 1.430 (3) | C24—C25 | 1.386 (3) |
| O12—C28 | 1.373 (3) | C25—C26 | 1.376 (3) |
| O12—C31 | 1.436 (2) | C25—H25 | 0.9500 |
| O13—C31 | 1.390 (3) | C26—C27 | 1.395 (3) |
| O13—C32 | 1.420 (3) | C27—C28 | 1.373 (3) |
| O14—C24 | 1.347 (3) | C27—H27 | 0.9500 |
| O14—H14 | 0.8400 | C29—H29A | 0.9900 |
| O15—C33 | 1.261 (3) | C29—H29B | 0.9900 |
| O16—C40 | 1.371 (2) | C30—H30A | 0.9800 |
| O16—C42 | 1.424 (3) | C30—H30B | 0.9800 |
| O17—C39 | 1.367 (2) | C30—H30C | 0.9800 |
| O17—C43 | 1.427 (3) | C31—H31A | 0.9900 |
| O18—C43 | 1.395 (3) | C31—H31B | 0.9900 |
| O18—C44 | 1.432 (3) | C32—H32A | 0.9800 |
| C1—C2 | 1.419 (3) | C32—H32B | 0.9800 |
| C1—C6 | 1.430 (3) | C32—H32C | 0.9800 |
| C1—C11 | 1.468 (3) | C33—C34 | 1.473 (3) |
| C2—C3 | 1.387 (3) | C34—C35 | 1.328 (3) |
| C3—C4 | 1.376 (3) | C34—H34 | 0.9500 |
| C3—H3 | 0.9500 | C35—C36 | 1.463 (3) |
| C4—C5 | 1.406 (3) | C35—H35 | 0.9500 |
| C5—C6 | 1.367 (3) | C36—C37 | 1.383 (3) |
| C5—H5A | 0.9500 | C36—C41 | 1.410 (3) |
| C7—H7A | 0.9900 | C37—C38 | 1.393 (3) |
| C7—H7B | 0.9900 | C37—H37 | 0.9500 |
| C8—H8A | 0.9800 | C38—C39 | 1.389 (3) |
| C8—H8B | 0.9800 | C38—H38 | 0.9500 |
| C8—H8C | 0.9800 | C39—C40 | 1.414 (3) |
| C9—H9A | 0.9900 | C40—C41 | 1.383 (3) |
| C9—H9B | 0.9900 | C41—H41 | 0.9500 |
| C10—H10A | 0.9800 | C42—H42A | 0.9800 |
| C10—H10B | 0.9800 | C42—H42B | 0.9800 |
| C10—H10C | 0.9800 | C42—H42C | 0.9800 |
| C11—C12 | 1.479 (3) | C43—H43A | 0.9900 |
| C12—C13 | 1.335 (3) | C43—H43B | 0.9900 |
| C12—H12 | 0.9500 | C44—H44A | 0.9800 |
| C13—C14 | 1.462 (3) | C44—H44B | 0.9800 |
| C13—H13 | 0.9500 | C44—H44C | 0.9800 |
| C4—O1—C7 | 118.07 (18) | O8—C21—H21B | 108.9 |
| C7—O2—C8 | 112.09 (17) | H21A—C21—H21B | 107.7 |
| C6—O3—C9 | 118.27 (18) | O9—C22—H22A | 109.5 |
| C9—O4—C10 | 111.77 (18) | O9—C22—H22B | 109.5 |
| C2—O5—H5 | 109.5 | H22A—C22—H22B | 109.5 |
| C16—O7—C20 | 116.70 (18) | O9—C22—H22C | 109.5 |
| C17—O8—C21 | 117.35 (18) | H22A—C22—H22C | 109.5 |
| C21—O9—C22 | 113.39 (19) | H22B—C22—H22C | 109.5 |
| C26—O10—C29 | 117.62 (18) | C24—C23—C28 | 115.58 (19) |
| C29—O11—C30 | 113.18 (18) | C24—C23—C33 | 118.2 (2) |
| C28—O12—C31 | 119.19 (17) | C28—C23—C33 | 126.1 (2) |
| C31—O13—C32 | 112.67 (19) | O14—C24—C25 | 116.0 (2) |
| C24—O14—H14 | 109.5 | O14—C24—C23 | 121.6 (2) |
| C40—O16—C42 | 116.60 (18) | C25—C24—C23 | 122.5 (2) |
| C39—O17—C43 | 117.11 (17) | C26—C25—C24 | 119.1 (2) |
| C43—O18—C44 | 113.86 (17) | C26—C25—H25 | 120.5 |
| C2—C1—C6 | 115.69 (19) | C24—C25—H25 | 120.5 |
| C2—C1—C11 | 119.03 (19) | O10—C26—C25 | 124.4 (2) |
| C6—C1—C11 | 125.28 (19) | O10—C26—C27 | 114.43 (19) |
| O5—C2—C3 | 116.5 (2) | C25—C26—C27 | 121.2 (2) |
| O5—C2—C1 | 120.8 (2) | C28—C27—C26 | 120.1 (2) |
| C3—C2—C1 | 122.7 (2) | C28—C27—H27 | 119.9 |
| C4—C3—C2 | 118.8 (2) | C26—C27—H27 | 119.9 |
| C4—C3—H3 | 120.6 | C27—C28—O12 | 121.77 (19) |
| C2—C3—H3 | 120.6 | C27—C28—C23 | 121.5 (2) |
| O1—C4—C3 | 125.0 (2) | O12—C28—C23 | 116.70 (19) |
| O1—C4—C5 | 113.91 (19) | O11—C29—O10 | 114.05 (19) |
| C3—C4—C5 | 121.1 (2) | O11—C29—H29A | 108.7 |
| C6—C5—C4 | 119.6 (2) | O10—C29—H29A | 108.7 |
| C6—C5—H5A | 120.2 | O11—C29—H29B | 108.7 |
| C4—C5—H5A | 120.2 | O10—C29—H29B | 108.7 |
| C5—C6—O3 | 122.4 (2) | H29A—C29—H29B | 107.6 |
| C5—C6—C1 | 121.8 (2) | O11—C30—H30A | 109.5 |
| O3—C6—C1 | 115.75 (19) | O11—C30—H30B | 109.5 |
| O2—C7—O1 | 112.93 (18) | H30A—C30—H30B | 109.5 |
| O2—C7—H7A | 109.0 | O11—C30—H30C | 109.5 |
| O1—C7—H7A | 109.0 | H30A—C30—H30C | 109.5 |
| O2—C7—H7B | 109.0 | H30B—C30—H30C | 109.5 |
| O1—C7—H7B | 109.0 | O13—C31—O12 | 112.08 (18) |
| H7A—C7—H7B | 107.8 | O13—C31—H31A | 109.2 |
| O2—C8—H8A | 109.5 | O12—C31—H31A | 109.2 |
| O2—C8—H8B | 109.5 | O13—C31—H31B | 109.2 |
| H8A—C8—H8B | 109.5 | O12—C31—H31B | 109.2 |
| O2—C8—H8C | 109.5 | H31A—C31—H31B | 107.9 |
| H8A—C8—H8C | 109.5 | O13—C32—H32A | 109.5 |
| H8B—C8—H8C | 109.5 | O13—C32—H32B | 109.5 |
| O4—C9—O3 | 112.51 (18) | H32A—C32—H32B | 109.5 |
| O4—C9—H9A | 109.1 | O13—C32—H32C | 109.5 |
| O3—C9—H9A | 109.1 | H32A—C32—H32C | 109.5 |
| O4—C9—H9B | 109.1 | H32B—C32—H32C | 109.5 |
| O3—C9—H9B | 109.1 | O15—C33—C23 | 119.3 (2) |
| H9A—C9—H9B | 107.8 | O15—C33—C34 | 117.2 (2) |
| O4—C10—H10A | 109.5 | C23—C33—C34 | 123.6 (2) |
| O4—C10—H10B | 109.5 | C35—C34—C33 | 121.1 (2) |
| H10A—C10—H10B | 109.5 | C35—C34—H34 | 119.4 |
| O4—C10—H10C | 109.5 | C33—C34—H34 | 119.4 |
| H10A—C10—H10C | 109.5 | C34—C35—C36 | 127.5 (2) |
| H10B—C10—H10C | 109.5 | C34—C35—H35 | 116.2 |
| O6—C11—C1 | 119.6 (2) | C36—C35—H35 | 116.2 |
| O6—C11—C12 | 118.4 (2) | C37—C36—C41 | 118.4 (2) |
| C1—C11—C12 | 121.93 (19) | C37—C36—C35 | 120.2 (2) |
| C13—C12—C11 | 120.7 (2) | C41—C36—C35 | 121.4 (2) |
| C13—C12—H12 | 119.6 | C36—C37—C38 | 121.8 (2) |
| C11—C12—H12 | 119.6 | C36—C37—H37 | 119.1 |
| C12—C13—C14 | 128.0 (2) | C38—C37—H37 | 119.1 |
| C12—C13—H13 | 116.0 | C39—C38—C37 | 119.8 (2) |
| C14—C13—H13 | 116.0 | C39—C38—H38 | 120.1 |
| C19—C14—C15 | 118.2 (2) | C37—C38—H38 | 120.1 |
| C19—C14—C13 | 119.2 (2) | O17—C39—C38 | 125.2 (2) |
| C15—C14—C13 | 122.6 (2) | O17—C39—C40 | 115.65 (19) |
| C16—C15—C14 | 121.0 (2) | C38—C39—C40 | 119.16 (19) |
| C16—C15—H15 | 119.5 | O16—C40—C41 | 124.3 (2) |
| C14—C15—H15 | 119.5 | O16—C40—C39 | 115.41 (19) |
| O7—C16—C15 | 124.5 (2) | C41—C40—C39 | 120.24 (19) |
| O7—C16—C17 | 115.6 (2) | C40—C41—C36 | 120.6 (2) |
| C15—C16—C17 | 119.9 (2) | C40—C41—H41 | 119.7 |
| O8—C17—C18 | 125.0 (2) | C36—C41—H41 | 119.7 |
| O8—C17—C16 | 115.94 (19) | O16—C42—H42A | 109.5 |
| C18—C17—C16 | 119.0 (2) | O16—C42—H42B | 109.5 |
| C19—C18—C17 | 120.4 (2) | H42A—C42—H42B | 109.5 |
| C19—C18—H18 | 119.8 | O16—C42—H42C | 109.5 |
| C17—C18—H18 | 119.8 | H42A—C42—H42C | 109.5 |
| C18—C19—C14 | 121.4 (2) | H42B—C42—H42C | 109.5 |
| C18—C19—H19 | 119.3 | O18—C43—O17 | 113.16 (19) |
| C14—C19—H19 | 119.3 | O18—C43—H43A | 108.9 |
| O7—C20—H20A | 109.5 | O17—C43—H43A | 108.9 |
| O7—C20—H20B | 109.5 | O18—C43—H43B | 108.9 |
| H20A—C20—H20B | 109.5 | O17—C43—H43B | 108.9 |
| O7—C20—H20C | 109.5 | H43A—C43—H43B | 107.8 |
| H20A—C20—H20C | 109.5 | O18—C44—H44A | 109.5 |
| H20B—C20—H20C | 109.5 | O18—C44—H44B | 109.5 |
| O9—C21—O8 | 113.23 (19) | H44A—C44—H44B | 109.5 |
| O9—C21—H21A | 108.9 | O18—C44—H44C | 109.5 |
| O8—C21—H21A | 108.9 | H44A—C44—H44C | 109.5 |
| O9—C21—H21B | 108.9 | H44B—C44—H44C | 109.5 |
| C6—C1—C2—O5 | −176.5 (2) | C28—C23—C24—O14 | 179.8 (2) |
| C11—C1—C2—O5 | 4.1 (3) | C33—C23—C24—O14 | 3.1 (3) |
| C6—C1—C2—C3 | 4.6 (3) | C28—C23—C24—C25 | 0.3 (3) |
| C11—C1—C2—C3 | −174.7 (2) | C33—C23—C24—C25 | −176.4 (2) |
| O5—C2—C3—C4 | −178.5 (2) | O14—C24—C25—C26 | −178.03 (19) |
| C1—C2—C3—C4 | 0.4 (3) | C23—C24—C25—C26 | 1.5 (3) |
| C7—O1—C4—C3 | −10.9 (3) | C29—O10—C26—C25 | 10.2 (3) |
| C7—O1—C4—C5 | 169.68 (18) | C29—O10—C26—C27 | −170.93 (18) |
| C2—C3—C4—O1 | 176.7 (2) | C24—C25—C26—O10 | 177.4 (2) |
| C2—C3—C4—C5 | −3.9 (3) | C24—C25—C26—C27 | −1.4 (3) |
| O1—C4—C5—C6 | −178.4 (2) | O10—C26—C27—C28 | −179.44 (19) |
| C3—C4—C5—C6 | 2.1 (3) | C25—C26—C27—C28 | −0.6 (3) |
| C4—C5—C6—O3 | −175.10 (19) | C26—C27—C28—O12 | −176.98 (19) |
| C4—C5—C6—C1 | 3.3 (3) | C26—C27—C28—C23 | 2.5 (3) |
| C9—O3—C6—C5 | −3.8 (3) | C31—O12—C28—C27 | 4.3 (3) |
| C9—O3—C6—C1 | 177.69 (18) | C31—O12—C28—C23 | −175.19 (18) |
| C2—C1—C6—C5 | −6.5 (3) | C24—C23—C28—C27 | −2.3 (3) |
| C11—C1—C6—C5 | 172.8 (2) | C33—C23—C28—C27 | 174.1 (2) |
| C2—C1—C6—O3 | 172.05 (18) | C24—C23—C28—O12 | 177.19 (19) |
| C11—C1—C6—O3 | −8.7 (3) | C33—C23—C28—O12 | −6.4 (3) |
| C8—O2—C7—O1 | 72.9 (2) | C30—O11—C29—O10 | −67.2 (3) |
| C4—O1—C7—O2 | 83.6 (2) | C26—O10—C29—O11 | −73.6 (2) |
| C10—O4—C9—O3 | −65.3 (2) | C32—O13—C31—O12 | −68.7 (2) |
| C6—O3—C9—O4 | −67.5 (2) | C28—O12—C31—O13 | −72.7 (2) |
| C2—C1—C11—O6 | −14.3 (3) | C24—C23—C33—O15 | 4.5 (3) |
| C6—C1—C11—O6 | 166.5 (2) | C28—C23—C33—O15 | −171.9 (2) |
| C2—C1—C11—C12 | 162.98 (19) | C24—C23—C33—C34 | −176.9 (2) |
| C6—C1—C11—C12 | −16.3 (3) | C28—C23—C33—C34 | 6.8 (3) |
| O6—C11—C12—C13 | −13.5 (3) | O15—C33—C34—C35 | 6.1 (3) |
| C1—C11—C12—C13 | 169.2 (2) | C23—C33—C34—C35 | −172.6 (2) |
| C11—C12—C13—C14 | −178.7 (2) | C33—C34—C35—C36 | 178.6 (2) |
| C12—C13—C14—C19 | −178.5 (2) | C34—C35—C36—C37 | 172.2 (2) |
| C12—C13—C14—C15 | 1.0 (4) | C34—C35—C36—C41 | −7.6 (3) |
| C19—C14—C15—C16 | 1.6 (3) | C41—C36—C37—C38 | 1.5 (3) |
| C13—C14—C15—C16 | −177.9 (2) | C35—C36—C37—C38 | −178.38 (19) |
| C20—O7—C16—C15 | −2.5 (3) | C36—C37—C38—C39 | 0.3 (3) |
| C20—O7—C16—C17 | 176.30 (19) | C43—O17—C39—C38 | 5.8 (3) |
| C14—C15—C16—O7 | 178.64 (19) | C43—O17—C39—C40 | −174.90 (18) |
| C14—C15—C16—C17 | −0.1 (3) | C37—C38—C39—O17 | 177.26 (19) |
| C21—O8—C17—C18 | −4.0 (3) | C37—C38—C39—C40 | −2.1 (3) |
| C21—O8—C17—C16 | 178.21 (19) | C42—O16—C40—C41 | 3.0 (3) |
| O7—C16—C17—O8 | −3.0 (3) | C42—O16—C40—C39 | −177.43 (19) |
| C15—C16—C17—O8 | 175.86 (18) | O17—C39—C40—O16 | 3.1 (3) |
| O7—C16—C17—C18 | 179.07 (18) | C38—C39—C40—O16 | −177.50 (18) |
| C15—C16—C17—C18 | −2.0 (3) | O17—C39—C40—C41 | −177.32 (18) |
| O8—C17—C18—C19 | −174.9 (2) | C38—C39—C40—C41 | 2.1 (3) |
| C16—C17—C18—C19 | 2.8 (3) | O16—C40—C41—C36 | 179.23 (19) |
| C17—C18—C19—C14 | −1.3 (3) | C39—C40—C41—C36 | −0.3 (3) |
| C15—C14—C19—C18 | −0.8 (3) | C37—C36—C41—C40 | −1.5 (3) |
| C13—C14—C19—C18 | 178.70 (19) | C35—C36—C41—C40 | 178.39 (19) |
| C22—O9—C21—O8 | 68.4 (2) | C44—O18—C43—O17 | −66.9 (2) |
| C17—O8—C21—O9 | 66.4 (2) | C39—O17—C43—O18 | −66.7 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5···O6 | 0.84 | 1.75 | 2.506 (2) | 148. |
| O14—H14···O15 | 0.84 | 1.72 | 2.475 (2) | 148. |
| C8—H8C···O8i | 0.98 | 2.57 | 3.312 (3) | 132. |
| C9—H9A···O5ii | 0.99 | 2.52 | 3.444 (3) | 155. |
Symmetry codes: (i) x−1, y, z−1; (ii) −x+1, y−1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5103).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Chu, H. W., Wu, H. T. & Lee, Y. J. (2004). Tetrahedron, 60, 2647–2655.
- Duan, Y. B., Qi, Y., Ji, Z., Fang, G., Cheng, Y. H. & Wu, S. (2006). J. Chin. J. Med. Chem. 16, 342–346.
- Jung, U. J., Lee, M. K., Park, Y. B., Kang, M. A. & Choi, M. S. (2006). Biochem. Cell Biol. 38, 1134–1145. [DOI] [PubMed]
- Ong, K. C. & Khoo, H. E. (1996). J. Biochem. Pharmacol. 51, 423–429. [DOI] [PubMed]
- Rigaku/MSC (2009). CrystalClear-SM Expert and CrystalStructure Rigaku/MSC, The Woodlands, Texas, USA
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sousa, E. D., Zanatta, L., Seifriz, I., Creczynski-Pasa, T. B., Pizzolatti, M. G., Szpoganicz, B. & Silva, F. R. (2004). J. Nat. Prod. 67, 829–832. [DOI] [PubMed]
- Vessal, M., Hemmati, M. & VaseiM, M. (2003). Biochem. Physiol. C, 135, 357–364. [DOI] [PubMed]
- Zhang, Y., Zhang, Y.-N., Liu, M.-M., Ryu, K.-C. & Ye, D.-Y. (2011). Acta Cryst. E67, o912–o913. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041213/hg5103sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041213/hg5103Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811041213/hg5103Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



