Abstract
In the two independent but very similar molecules (A and B) of the title compound, C14H16N2O8, both six-membered pyrimidine rings are nearly planar [maximum deviations = 0.010 (3) Å in A and 0.028 (3) Å in B]. The five-membered furanose ring in molecule A adopts an envelope conformation, while the same ring in molecule B has a twisted conformation. In the crystal, the A molecules are linked via a pair of intermolecular N—H⋯O hydrogen bonds, forming dimers. Each A molecule is further linked to a B molecule via a second N—H⋯O hydrogen bond. There are also a number of C—H⋯·O interactions present, leading to the formation of a three-dimensional network.
Related literature
For the bioactivity of 5-substituted pyrimidine nucleosides, see: De Clercq (2005 ▶); Agrofoglio et al. (2003 ▶); Lee et al. (2009 ▶). For the use of the title compound as a synthon for the preparation of a variety of nucleoside derivatives, see: Fan et al. (2006a
▶,b
▶, 2010 ▶, 2011 ▶); Zhang et al. (2009 ▶). For related structures of uridines, see: Luo et al. (2007 ▶); Low & Wilson (1984 ▶).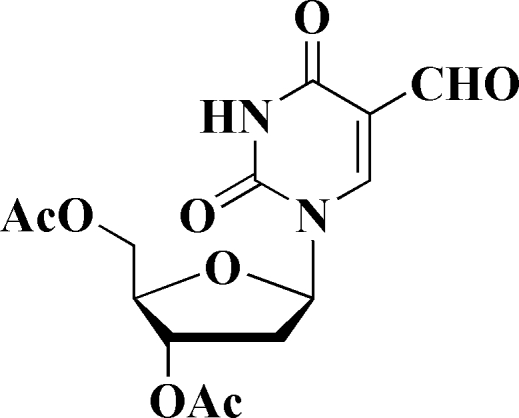
Experimental
Crystal data
C28H32N4O16
M r = 680.58
Orthorhombic,

a = 15.5268 (18) Å
b = 29.977 (4) Å
c = 6.6207 (8) Å
V = 3081.6 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.12 mm−1
T = 296 K
0.24 × 0.18 × 0.09 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.971, T max = 0.989
23677 measured reflections
5719 independent reflections
3519 reflections with I > 2σ(I)
R int = 0.060
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.110
S = 1.01
5719 reflections
437 parameters
H-atom parameters constrained
Δρmax = 0.14 e Å−3
Δρmin = −0.17 e Å−3
Absolute structure: Flack (1983 ▶), 2320 Friedel pairs
Flack parameter: −0.6 (12)
Data collection: SMART (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041304/su2320sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041304/su2320Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811041304/su2320Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O6i | 0.86 | 1.96 | 2.817 (3) | 172 |
| N4—H4A⋯O5 | 0.86 | 2.08 | 2.935 (4) | 173 |
| C3—H3⋯O13 | 0.98 | 2.57 | 3.489 (4) | 156 |
| C13—H13⋯O2 | 0.93 | 2.54 | 3.402 (4) | 155 |
| C16—H16A⋯O7ii | 0.97 | 2.41 | 3.312 (4) | 155 |
| C18—H18⋯O10iii | 0.98 | 2.46 | 3.257 (4) | 138 |
| C21—H21A⋯O4iv | 0.96 | 2.51 | 3.441 (5) | 162 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20972042) and the Natural Science Foundation of Department of Education of Henan Province (No. 2009 A150017).
supplementary crystallographic information
Comment
Many pyrimidine nucleosides with modification on the 5-position of the pyrimidine ring have drawn much attention due to their interesting pharmacological properties, such as antitumor, antiviral, and antimicrobial activities (De Clercq et al., 2005; Agrofoglio et al., 2003, Lee et al., 2009). The title compound has been used as a powerful synthon for the preparation of a variety of nucleoside derivatives due to the rich and extensive chemistry of the aldehyde carbonyl (Fan et al., 2006a, 2006b, 2010, 2011; Zhang et al., 2009). However, its crystal structure has not been reported as yet.
The absolute structure of the title compound is known because the synthetic procedure does not affect stereogenic atoms of the starting compound. In the two independent (A & B) but very similar molecules of the title compound (Fig. 1) all the bond lengths and bond angles are within normal ranges. In molecule A the O1—C4 bond is a little longer than bond O1—C1, as is bond O16-C15 compared to bond O16-C18 in molecule B. This is similar to the situation in 2'-deoxy-3',5'-di-O-acetyluridine (Luo et al., 2007), but different to that in 2,3,5-triacetyluridine (Low & Wilson, 1984).
The pyrimidine rings in both molecules are planar [maximum deviations being 0.010 (3) Å in A and 0.028 (3) Å in B]. The atoms connected directly with the pyrimidine ring and the atoms in the aldehyde carbonyl group in the 5-position of the pyrimidine ring are coplanar with the pyrimidine ring, which means there is an exstensive conjugated system in each molecule. The five-membered furanose ring in molecule A adopts an envelope conformation with atom C2 at the flap, while in molecule B the five-membered ring is twisted on bond C15-C16.
In the crystal, two A molecules form a pseudosymmetric dimer connected via N—H···O hydrogen bonds, involving the N atom of the pyrimidine base and the adjacent carbonyl O atom of the pyrimidine base. Each A molecule is further connected to a B molecule via an N—H···O hydrogen bond involving the N atom of the pyrimidine base and carbonyl O atom of the acetoxy groups in the 3'-position of the furanose ring (see Table 1 and Fig. 2 for details). There are also a number of C-H···O interactions present leading to the formation of a three-dimensional network (Table 1).
Experimental
The title compound was synthesized following the previously reported procedure (Fan, et al., 2006b). Single crystals, suitable for X-ray diffraction analysis, were obtained by slow evaporation of the solvents from a dichloromethane-petroleum ether (1:1 v/v) solution of the title compound.
Refinement
The H atoms were positioned geometrically and refined as riding atoms: N—H = 0.86 Å, and C—H = 0.93, 0.98, 0.97, and 0.96 Å for aromatic, methine, methylene, and methyl H atoms, respectively, with Uiso(H) = x × Ueq(N,C), where x = 1.5 for methyl H atoms, and x = 1.2 for all other H atoms. The absolute structure of the title compound is known as the synthetic procedure did not affect the stereogenic atoms of the reactant.
Figures
Fig. 1.
Molecular structure of the two independent molecules (A left; B right) of the title compound, with displacement ellipsoids drawn at the 30% probability level.
Fig. 2.
Crystal packing of the title compound, viewed along the c axis. The intermolecular N—H···O hydrogen bonds are shown as dashed lines.
Crystal data
| C28H32N4O16 | Dx = 1.467 Mg m−3 |
| Mr = 680.58 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P21212 | Cell parameters from 2422 reflections |
| a = 15.5268 (18) Å | θ = 2.4–18.6° |
| b = 29.977 (4) Å | µ = 0.12 mm−1 |
| c = 6.6207 (8) Å | T = 296 K |
| V = 3081.6 (6) Å3 | Block, colourless |
| Z = 4 | 0.24 × 0.18 × 0.09 mm |
| F(000) = 1424 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 5719 independent reflections |
| Radiation source: fine-focus sealed tube | 3519 reflections with I > 2σ(I) |
| graphite | Rint = 0.060 |
| φ and ω scans | θmax = 25.5°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Bruker, 2007) | h = −18→18 |
| Tmin = 0.971, Tmax = 0.989 | k = −36→36 |
| 23677 measured reflections | l = −8→8 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.110 | w = 1/[σ2(Fo2) + (0.0461P)2 + 0.0767P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 5719 reflections | Δρmax = 0.14 e Å−3 |
| 437 parameters | Δρmin = −0.17 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 2320 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: −0.6 (12) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell esds are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR andgoodness of fit S are based on F2, conventional R-factors R are basedon F, with F set to zero for negative F2. The threshold expression ofF2 > σ(F2) is used only for calculating R-factors(gt) etc. and isnot relevant to the choice of reflections for refinement. R-factors basedon F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.86095 (13) | 0.15062 (7) | −0.1052 (3) | 0.0481 (8) | |
| O2 | 0.92305 (14) | 0.23904 (7) | −0.0130 (4) | 0.0536 (8) | |
| O3 | 0.9059 (2) | 0.31223 (8) | −0.0547 (5) | 0.0973 (13) | |
| O4 | 0.69953 (13) | 0.16685 (7) | 0.1642 (3) | 0.0460 (8) | |
| O5 | 0.68476 (16) | 0.19298 (8) | 0.4790 (4) | 0.0634 (9) | |
| O6 | 0.91976 (14) | 0.03929 (7) | 0.0972 (4) | 0.0597 (9) | |
| O7 | 1.20552 (15) | 0.06592 (8) | 0.0540 (4) | 0.0742 (10) | |
| O8 | 1.17118 (16) | 0.20098 (9) | 0.0293 (5) | 0.0822 (11) | |
| N1 | 0.96297 (14) | 0.11188 (8) | 0.0831 (4) | 0.0430 (9) | |
| N2 | 1.06276 (15) | 0.05417 (8) | 0.0818 (4) | 0.0488 (10) | |
| C1 | 0.87385 (18) | 0.12800 (9) | 0.0809 (5) | 0.0428 (11) | |
| C2 | 0.85180 (19) | 0.16088 (10) | 0.2454 (5) | 0.0440 (11) | |
| C3 | 0.78276 (17) | 0.18915 (10) | 0.1481 (5) | 0.0418 (11) | |
| C4 | 0.80634 (19) | 0.18894 (10) | −0.0762 (5) | 0.0421 (11) | |
| C5 | 0.8510 (2) | 0.23042 (11) | −0.1460 (5) | 0.0540 (12) | |
| C6 | 0.9448 (2) | 0.28190 (13) | 0.0199 (6) | 0.0603 (14) | |
| C7 | 1.0206 (2) | 0.28489 (12) | 0.1526 (7) | 0.0803 (19) | |
| C8 | 0.6580 (2) | 0.17068 (11) | 0.3399 (6) | 0.0467 (11) | |
| C9 | 0.5770 (2) | 0.14428 (12) | 0.3443 (6) | 0.0717 (16) | |
| C10 | 0.9779 (2) | 0.06618 (10) | 0.0875 (5) | 0.0448 (11) | |
| C11 | 1.1334 (2) | 0.08179 (11) | 0.0667 (5) | 0.0493 (12) | |
| C12 | 1.11258 (18) | 0.12880 (10) | 0.0623 (5) | 0.0419 (11) | |
| C13 | 1.03017 (19) | 0.14162 (10) | 0.0667 (5) | 0.0446 (11) | |
| C14 | 1.1828 (2) | 0.16136 (13) | 0.0510 (5) | 0.0550 (12) | |
| O9 | 1.01699 (14) | 0.43238 (7) | 0.3062 (3) | 0.0513 (8) | |
| O10 | 1.01223 (18) | 0.44857 (9) | −0.0227 (4) | 0.0786 (11) | |
| O11 | 0.78072 (14) | 0.48878 (7) | 0.5019 (4) | 0.0563 (9) | |
| O12 | 0.76091 (17) | 0.55682 (9) | 0.6310 (5) | 0.0820 (11) | |
| O13 | 0.83321 (16) | 0.28502 (7) | 0.4225 (4) | 0.0648 (10) | |
| O14 | 0.54241 (17) | 0.29279 (9) | 0.4668 (5) | 0.0914 (14) | |
| O15 | 0.54287 (16) | 0.42833 (10) | 0.4218 (5) | 0.0932 (13) | |
| O16 | 0.85943 (14) | 0.40460 (7) | 0.5944 (3) | 0.0543 (8) | |
| N3 | 0.77201 (16) | 0.35465 (8) | 0.4263 (4) | 0.0470 (10) | |
| N4 | 0.68780 (19) | 0.29072 (9) | 0.4549 (4) | 0.0581 (11) | |
| C15 | 0.85591 (19) | 0.37705 (10) | 0.4219 (5) | 0.0488 (11) | |
| C16 | 0.8695 (2) | 0.40735 (11) | 0.2421 (5) | 0.0483 (12) | |
| C17 | 0.9270 (2) | 0.44370 (11) | 0.3260 (5) | 0.0446 (11) | |
| C18 | 0.9070 (2) | 0.44455 (10) | 0.5522 (5) | 0.0444 (11) | |
| C19 | 0.8576 (2) | 0.48391 (10) | 0.6264 (5) | 0.0533 (12) | |
| C20 | 1.0522 (2) | 0.43648 (12) | 0.1203 (6) | 0.0550 (14) | |
| C21 | 1.1458 (2) | 0.42467 (15) | 0.1230 (7) | 0.0840 (18) | |
| C22 | 0.7370 (2) | 0.52717 (13) | 0.5227 (6) | 0.0597 (14) | |
| C23 | 0.6581 (2) | 0.52785 (13) | 0.3963 (8) | 0.0840 (19) | |
| C24 | 0.7698 (2) | 0.30800 (11) | 0.4335 (5) | 0.0503 (12) | |
| C25 | 0.6102 (2) | 0.31309 (13) | 0.4535 (5) | 0.0610 (14) | |
| C26 | 0.6196 (2) | 0.36078 (11) | 0.4387 (5) | 0.0497 (12) | |
| C27 | 0.6985 (2) | 0.37920 (11) | 0.4317 (5) | 0.0477 (12) | |
| C28 | 0.5422 (2) | 0.38840 (15) | 0.4283 (6) | 0.0667 (16) | |
| H1 | 0.83450 | 0.10250 | 0.08870 | 0.0510* | |
| H2 | 1.07330 | 0.02600 | 0.08830 | 0.0590* | |
| H2A | 0.90150 | 0.17870 | 0.28230 | 0.0530* | |
| H2B | 0.83010 | 0.14580 | 0.36460 | 0.0530* | |
| H3 | 0.78130 | 0.21940 | 0.20390 | 0.0500* | |
| H4 | 0.75370 | 0.18490 | −0.15560 | 0.0510* | |
| H5A | 0.87120 | 0.22660 | −0.28350 | 0.0650* | |
| H5B | 0.81130 | 0.25540 | −0.14320 | 0.0650* | |
| H7A | 1.03510 | 0.31570 | 0.17400 | 0.1210* | |
| H7B | 1.06840 | 0.26990 | 0.09040 | 0.1210* | |
| H7C | 1.00790 | 0.27110 | 0.28000 | 0.1210* | |
| H9A | 0.58980 | 0.11390 | 0.37930 | 0.1080* | |
| H9B | 0.55020 | 0.14520 | 0.21360 | 0.1080* | |
| H9C | 0.53850 | 0.15670 | 0.44290 | 0.1080* | |
| H13 | 1.01770 | 0.17190 | 0.05840 | 0.0530* | |
| H14 | 1.23920 | 0.15110 | 0.06090 | 0.0660* | |
| H4A | 0.68480 | 0.26230 | 0.47120 | 0.0700* | |
| H15 | 0.90210 | 0.35480 | 0.42780 | 0.0590* | |
| H16A | 0.81530 | 0.41950 | 0.19440 | 0.0580* | |
| H16B | 0.89750 | 0.39160 | 0.13220 | 0.0580* | |
| H17 | 0.91440 | 0.47260 | 0.26310 | 0.0540* | |
| H18 | 0.96160 | 0.44320 | 0.62610 | 0.0530* | |
| H19A | 0.84140 | 0.47960 | 0.76650 | 0.0640* | |
| H19B | 0.89270 | 0.51060 | 0.61720 | 0.0640* | |
| H21A | 1.15370 | 0.39560 | 0.06520 | 0.1270* | |
| H21B | 1.16630 | 0.42470 | 0.25980 | 0.1270* | |
| H21C | 1.17760 | 0.44620 | 0.04560 | 0.1270* | |
| H23A | 0.61210 | 0.51320 | 0.46680 | 0.1260* | |
| H23B | 0.66910 | 0.51260 | 0.27130 | 0.1260* | |
| H23C | 0.64210 | 0.55820 | 0.36890 | 0.1260* | |
| H27 | 0.70300 | 0.41010 | 0.43060 | 0.0580* | |
| H28 | 0.48900 | 0.37410 | 0.42670 | 0.0800* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0548 (13) | 0.0426 (12) | 0.0468 (14) | 0.0117 (10) | 0.0016 (12) | 0.0004 (11) |
| O2 | 0.0525 (14) | 0.0384 (13) | 0.0699 (16) | −0.0016 (11) | 0.0001 (12) | 0.0071 (11) |
| O3 | 0.106 (2) | 0.0449 (15) | 0.141 (3) | 0.0053 (15) | −0.016 (2) | 0.0169 (19) |
| O4 | 0.0391 (12) | 0.0470 (13) | 0.0520 (14) | −0.0058 (11) | 0.0035 (11) | −0.0026 (11) |
| O5 | 0.0716 (16) | 0.0605 (15) | 0.0581 (17) | −0.0130 (13) | 0.0144 (14) | −0.0046 (14) |
| O6 | 0.0457 (13) | 0.0382 (12) | 0.0953 (19) | 0.0004 (11) | 0.0056 (14) | 0.0012 (13) |
| O7 | 0.0418 (14) | 0.0747 (17) | 0.106 (2) | 0.0114 (12) | 0.0074 (14) | 0.0057 (16) |
| O8 | 0.0690 (17) | 0.0746 (19) | 0.103 (2) | −0.0171 (15) | 0.0077 (16) | −0.0053 (18) |
| N1 | 0.0351 (14) | 0.0342 (14) | 0.0596 (18) | 0.0014 (12) | −0.0003 (14) | 0.0043 (14) |
| N2 | 0.0433 (16) | 0.0388 (14) | 0.0642 (19) | 0.0056 (12) | 0.0039 (15) | 0.0038 (15) |
| C1 | 0.0379 (18) | 0.0356 (17) | 0.055 (2) | 0.0017 (14) | −0.0010 (17) | 0.0048 (18) |
| C2 | 0.0378 (19) | 0.050 (2) | 0.0441 (19) | −0.0007 (16) | −0.0002 (15) | 0.0034 (17) |
| C3 | 0.0336 (18) | 0.0367 (17) | 0.055 (2) | −0.0043 (14) | 0.0011 (15) | 0.0000 (16) |
| C4 | 0.0408 (17) | 0.0406 (18) | 0.045 (2) | 0.0074 (15) | −0.0028 (16) | 0.0008 (17) |
| C5 | 0.057 (2) | 0.051 (2) | 0.054 (2) | 0.0015 (17) | −0.0032 (18) | 0.0143 (17) |
| C6 | 0.062 (2) | 0.048 (2) | 0.071 (3) | −0.0061 (19) | 0.012 (2) | 0.004 (2) |
| C7 | 0.073 (3) | 0.064 (3) | 0.104 (4) | −0.014 (2) | 0.002 (3) | −0.005 (2) |
| C8 | 0.045 (2) | 0.0421 (19) | 0.053 (2) | 0.0000 (16) | 0.0072 (18) | 0.0053 (18) |
| C9 | 0.054 (2) | 0.082 (3) | 0.079 (3) | −0.019 (2) | 0.015 (2) | 0.002 (2) |
| C10 | 0.0403 (19) | 0.045 (2) | 0.049 (2) | 0.0067 (16) | 0.0017 (17) | −0.0023 (17) |
| C11 | 0.044 (2) | 0.058 (2) | 0.046 (2) | 0.0049 (18) | 0.0029 (17) | −0.0002 (18) |
| C12 | 0.0345 (17) | 0.055 (2) | 0.0363 (19) | −0.0038 (15) | 0.0058 (15) | −0.0023 (17) |
| C13 | 0.048 (2) | 0.0408 (17) | 0.045 (2) | −0.0033 (16) | −0.0003 (17) | −0.0025 (17) |
| C14 | 0.047 (2) | 0.061 (2) | 0.057 (2) | −0.0062 (18) | 0.0075 (18) | −0.006 (2) |
| O9 | 0.0400 (13) | 0.0585 (15) | 0.0554 (15) | 0.0020 (11) | −0.0022 (11) | −0.0017 (12) |
| O10 | 0.0779 (19) | 0.101 (2) | 0.0569 (17) | 0.0079 (16) | 0.0026 (15) | 0.0083 (16) |
| O11 | 0.0516 (14) | 0.0516 (15) | 0.0658 (16) | 0.0085 (12) | −0.0076 (12) | −0.0083 (12) |
| O12 | 0.083 (2) | 0.0629 (17) | 0.100 (2) | 0.0161 (15) | 0.0022 (17) | −0.0230 (17) |
| O13 | 0.0690 (16) | 0.0471 (14) | 0.0783 (19) | 0.0118 (13) | 0.0046 (15) | 0.0023 (14) |
| O14 | 0.0703 (19) | 0.092 (2) | 0.112 (3) | −0.0347 (16) | 0.0091 (17) | 0.0173 (18) |
| O15 | 0.0596 (18) | 0.092 (2) | 0.128 (3) | 0.0150 (16) | 0.0063 (17) | 0.003 (2) |
| O16 | 0.0700 (15) | 0.0476 (13) | 0.0452 (14) | −0.0095 (12) | −0.0084 (13) | 0.0002 (11) |
| N3 | 0.0451 (16) | 0.0400 (16) | 0.0560 (18) | −0.0013 (13) | −0.0042 (15) | 0.0018 (14) |
| N4 | 0.070 (2) | 0.0442 (16) | 0.060 (2) | −0.0129 (16) | 0.0094 (17) | 0.0077 (15) |
| C15 | 0.0461 (19) | 0.0424 (18) | 0.058 (2) | −0.0013 (15) | −0.0072 (18) | −0.0066 (19) |
| C16 | 0.043 (2) | 0.056 (2) | 0.046 (2) | −0.0068 (17) | −0.0020 (16) | −0.0090 (17) |
| C17 | 0.039 (2) | 0.0429 (19) | 0.052 (2) | 0.0010 (16) | −0.0065 (16) | 0.0002 (17) |
| C18 | 0.0422 (18) | 0.0400 (18) | 0.051 (2) | 0.0000 (15) | −0.0083 (16) | −0.0029 (17) |
| C19 | 0.051 (2) | 0.053 (2) | 0.056 (2) | 0.0004 (17) | −0.0097 (18) | −0.0086 (17) |
| C20 | 0.053 (2) | 0.055 (2) | 0.057 (3) | −0.0045 (18) | 0.003 (2) | −0.012 (2) |
| C21 | 0.052 (2) | 0.123 (4) | 0.077 (3) | 0.003 (2) | 0.010 (2) | −0.029 (3) |
| C22 | 0.056 (2) | 0.057 (2) | 0.066 (3) | 0.010 (2) | 0.016 (2) | −0.002 (2) |
| C23 | 0.061 (3) | 0.082 (3) | 0.109 (4) | 0.019 (2) | −0.014 (3) | −0.005 (3) |
| C24 | 0.062 (2) | 0.045 (2) | 0.044 (2) | −0.0091 (18) | 0.0033 (19) | −0.0013 (19) |
| C25 | 0.057 (2) | 0.076 (3) | 0.050 (2) | −0.012 (2) | 0.0037 (19) | 0.005 (2) |
| C26 | 0.046 (2) | 0.063 (2) | 0.040 (2) | −0.0008 (18) | −0.0004 (17) | 0.0042 (18) |
| C27 | 0.049 (2) | 0.050 (2) | 0.044 (2) | 0.0031 (17) | −0.0029 (17) | −0.0006 (17) |
| C28 | 0.055 (2) | 0.088 (3) | 0.057 (3) | −0.004 (2) | 0.007 (2) | 0.008 (3) |
Geometric parameters (Å, °)
| O1—C1 | 1.421 (4) | C12—C13 | 1.336 (4) |
| O1—C4 | 1.441 (4) | C12—C14 | 1.465 (5) |
| O2—C5 | 1.447 (4) | C1—H1 | 0.9800 |
| O2—C6 | 1.346 (4) | C2—H2B | 0.9700 |
| O3—C6 | 1.198 (5) | C2—H2A | 0.9700 |
| O4—C3 | 1.459 (3) | C3—H3 | 0.9800 |
| O4—C8 | 1.335 (4) | C4—H4 | 0.9800 |
| O5—C8 | 1.212 (4) | C5—H5A | 0.9700 |
| O6—C10 | 1.212 (4) | C5—H5B | 0.9700 |
| O7—C11 | 1.220 (4) | C7—H7A | 0.9600 |
| O8—C14 | 1.210 (5) | C7—H7C | 0.9600 |
| O9—C20 | 1.352 (4) | C7—H7B | 0.9600 |
| O9—C17 | 1.444 (4) | C9—H9B | 0.9600 |
| O10—C20 | 1.189 (5) | C9—H9C | 0.9600 |
| O11—C22 | 1.343 (4) | C9—H9A | 0.9600 |
| O11—C19 | 1.458 (4) | C13—H13 | 0.9300 |
| O12—C22 | 1.201 (5) | C14—H14 | 0.9300 |
| O13—C24 | 1.204 (4) | C15—C16 | 1.512 (5) |
| O14—C25 | 1.219 (4) | C16—C17 | 1.514 (5) |
| O15—C28 | 1.198 (5) | C17—C18 | 1.530 (5) |
| O16—C18 | 1.435 (4) | C18—C19 | 1.491 (4) |
| O16—C15 | 1.411 (4) | C20—C21 | 1.496 (4) |
| N1—C10 | 1.390 (4) | C22—C23 | 1.484 (5) |
| N1—C13 | 1.377 (4) | C25—C26 | 1.440 (5) |
| N1—C1 | 1.466 (4) | C26—C27 | 1.345 (4) |
| N2—C10 | 1.366 (4) | C26—C28 | 1.461 (5) |
| N2—C11 | 1.378 (4) | C15—H15 | 0.9800 |
| N2—H2 | 0.8600 | C16—H16A | 0.9700 |
| N3—C15 | 1.466 (4) | C16—H16B | 0.9700 |
| N3—C24 | 1.400 (4) | C17—H17 | 0.9800 |
| N3—C27 | 1.359 (4) | C18—H18 | 0.9800 |
| N4—C24 | 1.382 (4) | C19—H19A | 0.9700 |
| N4—C25 | 1.379 (4) | C19—H19B | 0.9700 |
| N4—H4A | 0.8600 | C21—H21A | 0.9600 |
| C1—C2 | 1.508 (4) | C21—H21B | 0.9600 |
| C2—C3 | 1.511 (4) | C21—H21C | 0.9600 |
| C3—C4 | 1.530 (5) | C23—H23A | 0.9600 |
| C4—C5 | 1.497 (4) | C23—H23B | 0.9600 |
| C6—C7 | 1.471 (5) | C23—H23C | 0.9600 |
| C8—C9 | 1.486 (5) | C27—H27 | 0.9300 |
| C11—C12 | 1.446 (4) | C28—H28 | 0.9300 |
| C1—O1—C4 | 110.4 (2) | H9A—C9—H9B | 110.00 |
| C5—O2—C6 | 117.6 (3) | H9A—C9—H9C | 109.00 |
| C3—O4—C8 | 116.9 (2) | H9B—C9—H9C | 109.00 |
| C17—O9—C20 | 116.9 (2) | C8—C9—H9A | 110.00 |
| C19—O11—C22 | 116.2 (3) | C8—C9—H9B | 109.00 |
| C15—O16—C18 | 110.6 (2) | N1—C13—H13 | 119.00 |
| C10—N1—C13 | 120.9 (2) | C12—C13—H13 | 119.00 |
| C1—N1—C10 | 118.9 (2) | O8—C14—H14 | 118.00 |
| C1—N1—C13 | 120.1 (2) | C12—C14—H14 | 118.00 |
| C10—N2—C11 | 127.7 (3) | O16—C15—C16 | 106.3 (2) |
| C11—N2—H2 | 116.00 | N3—C15—C16 | 114.5 (3) |
| C10—N2—H2 | 116.00 | O16—C15—N3 | 106.7 (2) |
| C24—N3—C27 | 121.3 (3) | C15—C16—C17 | 103.1 (3) |
| C15—N3—C24 | 118.7 (2) | O9—C17—C18 | 106.8 (2) |
| C15—N3—C27 | 119.9 (2) | O9—C17—C16 | 111.6 (3) |
| C24—N4—C25 | 128.5 (3) | C16—C17—C18 | 104.6 (3) |
| C24—N4—H4A | 116.00 | O16—C18—C19 | 109.4 (2) |
| C25—N4—H4A | 116.00 | C17—C18—C19 | 116.1 (3) |
| O1—C1—N1 | 107.4 (2) | O16—C18—C17 | 106.3 (2) |
| N1—C1—C2 | 115.0 (2) | O11—C19—C18 | 108.3 (3) |
| O1—C1—C2 | 106.4 (2) | O9—C20—C21 | 111.1 (3) |
| C1—C2—C3 | 102.7 (3) | O9—C20—O10 | 122.8 (3) |
| O4—C3—C4 | 106.3 (2) | O10—C20—C21 | 126.1 (4) |
| C2—C3—C4 | 104.0 (2) | O12—C22—C23 | 125.6 (3) |
| O4—C3—C2 | 109.9 (2) | O11—C22—C23 | 111.8 (3) |
| O1—C4—C3 | 105.9 (2) | O11—C22—O12 | 122.6 (3) |
| O1—C4—C5 | 110.4 (2) | O13—C24—N3 | 123.3 (3) |
| C3—C4—C5 | 114.0 (3) | O13—C24—N4 | 123.0 (3) |
| O2—C5—C4 | 108.6 (3) | N3—C24—N4 | 113.6 (3) |
| O2—C6—C7 | 110.8 (3) | O14—C25—N4 | 120.7 (3) |
| O2—C6—O3 | 122.1 (3) | N4—C25—C26 | 113.2 (3) |
| O3—C6—C7 | 127.1 (4) | O14—C25—C26 | 126.0 (3) |
| O5—C8—C9 | 124.7 (3) | C25—C26—C28 | 118.8 (3) |
| O4—C8—O5 | 123.0 (3) | C25—C26—C27 | 120.2 (3) |
| O4—C8—C9 | 112.3 (3) | C27—C26—C28 | 121.0 (3) |
| N1—C10—N2 | 114.8 (3) | N3—C27—C26 | 123.0 (3) |
| O6—C10—N2 | 123.0 (3) | O15—C28—C26 | 124.1 (3) |
| O6—C10—N1 | 122.2 (3) | O16—C15—H15 | 110.00 |
| N2—C11—C12 | 114.2 (3) | N3—C15—H15 | 110.00 |
| O7—C11—N2 | 120.1 (3) | C16—C15—H15 | 110.00 |
| O7—C11—C12 | 125.7 (3) | C15—C16—H16A | 111.00 |
| C13—C12—C14 | 121.5 (3) | C15—C16—H16B | 111.00 |
| C11—C12—C14 | 118.9 (3) | C17—C16—H16A | 111.00 |
| C11—C12—C13 | 119.6 (3) | C17—C16—H16B | 111.00 |
| N1—C13—C12 | 122.8 (3) | H16A—C16—H16B | 109.00 |
| O8—C14—C12 | 123.3 (3) | O9—C17—H17 | 111.00 |
| N1—C1—H1 | 109.00 | C16—C17—H17 | 111.00 |
| O1—C1—H1 | 109.00 | C18—C17—H17 | 111.00 |
| C2—C1—H1 | 109.00 | O16—C18—H18 | 108.00 |
| H2A—C2—H2B | 109.00 | C17—C18—H18 | 108.00 |
| C3—C2—H2B | 111.00 | C19—C18—H18 | 108.00 |
| C3—C2—H2A | 111.00 | O11—C19—H19A | 110.00 |
| C1—C2—H2B | 111.00 | O11—C19—H19B | 110.00 |
| C1—C2—H2A | 111.00 | C18—C19—H19A | 110.00 |
| O4—C3—H3 | 112.00 | C18—C19—H19B | 110.00 |
| C4—C3—H3 | 112.00 | H19A—C19—H19B | 108.00 |
| C2—C3—H3 | 112.00 | C20—C21—H21A | 110.00 |
| C5—C4—H4 | 109.00 | C20—C21—H21B | 109.00 |
| O1—C4—H4 | 109.00 | C20—C21—H21C | 110.00 |
| C3—C4—H4 | 109.00 | H21A—C21—H21B | 110.00 |
| O2—C5—H5B | 110.00 | H21A—C21—H21C | 109.00 |
| O2—C5—H5A | 110.00 | H21B—C21—H21C | 109.00 |
| H5A—C5—H5B | 108.00 | C22—C23—H23A | 110.00 |
| C4—C5—H5A | 110.00 | C22—C23—H23B | 109.00 |
| C4—C5—H5B | 110.00 | C22—C23—H23C | 109.00 |
| C6—C7—H7B | 109.00 | H23A—C23—H23B | 109.00 |
| C6—C7—H7C | 110.00 | H23A—C23—H23C | 109.00 |
| H7A—C7—H7B | 109.00 | H23B—C23—H23C | 110.00 |
| H7A—C7—H7C | 109.00 | N3—C27—H27 | 118.00 |
| H7B—C7—H7C | 110.00 | C26—C27—H27 | 119.00 |
| C6—C7—H7A | 110.00 | O15—C28—H28 | 118.00 |
| C8—C9—H9C | 109.00 | C26—C28—H28 | 118.00 |
| C4—O1—C1—N1 | −142.9 (2) | C24—N3—C15—O16 | −120.1 (3) |
| C4—O1—C1—C2 | −19.2 (3) | C27—N3—C15—O16 | 57.3 (4) |
| C1—O1—C4—C5 | 122.9 (3) | C24—N3—C15—C16 | 122.7 (3) |
| C1—O1—C4—C3 | −1.0 (3) | C24—N4—C25—C26 | −3.8 (5) |
| C5—O2—C6—C7 | −178.2 (3) | C24—N4—C25—O14 | 177.2 (3) |
| C6—O2—C5—C4 | −147.9 (3) | C25—N4—C24—N3 | 5.6 (5) |
| C5—O2—C6—O3 | 1.0 (5) | C25—N4—C24—O13 | −174.5 (3) |
| C8—O4—C3—C4 | −167.3 (2) | O1—C1—C2—C3 | 31.3 (3) |
| C3—O4—C8—O5 | 3.2 (4) | N1—C1—C2—C3 | 150.1 (2) |
| C8—O4—C3—C2 | 80.8 (3) | C1—C2—C3—C4 | −31.0 (3) |
| C3—O4—C8—C9 | −176.2 (3) | C1—C2—C3—O4 | 82.5 (3) |
| C17—O9—C20—C21 | 178.7 (3) | C2—C3—C4—C5 | −101.1 (3) |
| C20—O9—C17—C18 | −170.0 (3) | O4—C3—C4—O1 | −95.5 (2) |
| C17—O9—C20—O10 | −0.3 (5) | C2—C3—C4—O1 | 20.5 (3) |
| C20—O9—C17—C16 | 76.3 (3) | O4—C3—C4—C5 | 142.9 (2) |
| C19—O11—C22—O12 | 2.9 (5) | C3—C4—C5—O2 | 51.7 (3) |
| C22—O11—C19—C18 | −168.1 (3) | O1—C4—C5—O2 | −67.3 (3) |
| C19—O11—C22—C23 | −177.5 (3) | O7—C11—C12—C13 | 176.4 (3) |
| C18—O16—C15—N3 | −146.0 (2) | O7—C11—C12—C14 | −3.0 (5) |
| C18—O16—C15—C16 | −23.4 (3) | N2—C11—C12—C14 | 178.7 (3) |
| C15—O16—C18—C19 | 132.3 (3) | N2—C11—C12—C13 | −2.0 (5) |
| C15—O16—C18—C17 | 6.1 (3) | C13—C12—C14—O8 | −5.9 (5) |
| C10—N1—C1—O1 | −116.1 (3) | C11—C12—C13—N1 | 2.5 (5) |
| C13—N1—C1—C2 | −58.8 (4) | C11—C12—C14—O8 | 173.5 (3) |
| C13—N1—C10—N2 | 2.3 (4) | C14—C12—C13—N1 | −178.1 (3) |
| C10—N1—C1—C2 | 125.7 (3) | O16—C15—C16—C17 | 30.7 (3) |
| C13—N1—C10—O6 | −178.3 (3) | N3—C15—C16—C17 | 148.1 (3) |
| C1—N1—C10—N2 | 177.7 (3) | C15—C16—C17—O9 | 88.9 (3) |
| C1—N1—C13—C12 | −178.1 (3) | C15—C16—C17—C18 | −26.2 (3) |
| C1—N1—C10—O6 | −2.8 (5) | C16—C17—C18—O16 | 13.4 (3) |
| C10—N1—C13—C12 | −2.7 (5) | O9—C17—C18—O16 | −105.0 (3) |
| C13—N1—C1—O1 | 59.4 (3) | C16—C17—C18—C19 | −108.6 (3) |
| C11—N2—C10—N1 | −2.0 (5) | O9—C17—C18—C19 | 133.0 (3) |
| C11—N2—C10—O6 | 178.6 (3) | C17—C18—C19—O11 | 52.1 (3) |
| C10—N2—C11—O7 | −176.6 (3) | O16—C18—C19—O11 | −68.2 (3) |
| C10—N2—C11—C12 | 1.8 (5) | O14—C25—C26—C27 | 177.7 (4) |
| C15—N3—C24—N4 | 174.7 (3) | N4—C25—C26—C28 | 177.8 (3) |
| C15—N3—C24—O13 | −5.2 (5) | O14—C25—C26—C28 | −3.2 (5) |
| C27—N3—C24—N4 | −2.6 (4) | N4—C25—C26—C27 | −1.2 (5) |
| C27—N3—C24—O13 | 177.5 (3) | C25—C26—C27—N3 | 3.9 (5) |
| C24—N3—C27—C26 | −1.8 (5) | C27—C26—C28—O15 | −3.3 (6) |
| C27—N3—C15—C16 | −60.0 (4) | C28—C26—C27—N3 | −175.1 (3) |
| C15—N3—C27—C26 | −179.1 (3) | C25—C26—C28—O15 | 177.7 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O6i | 0.86 | 1.96 | 2.817 (3) | 172 |
| N4—H4A···O5 | 0.86 | 2.08 | 2.935 (4) | 173 |
| C3—H3···O13 | 0.98 | 2.57 | 3.489 (4) | 156 |
| C13—H13···O2 | 0.93 | 2.54 | 3.402 (4) | 155 |
| C16—H16A···O7ii | 0.97 | 2.41 | 3.312 (4) | 155 |
| C18—H18···O10iii | 0.98 | 2.46 | 3.257 (4) | 138 |
| C21—H21A···O4iv | 0.96 | 2.51 | 3.441 (5) | 162 |
Symmetry codes: (i) −x+2, −y, z; (ii) x−1/2, −y+1/2, −z; (iii) x, y, z+1; (iv) x+1/2, −y+1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2320).
References
- Agrofoglio, L. A., Gillaizeau, I. & Saito, Y. (2003). Chem. Rev. 103, 1875–1916. [DOI] [PubMed]
- Bruker (2007). SADABS, SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- De Clercq, E. (2005). Antivir. Res. 67, 56–75. [DOI] [PubMed]
- Fan, X.-S., Feng, D., Qu, Y.-Y., Zhang, X.-Y., Wang, J.-J., Loiseau, P.-M., Andrei, G., Snoeck, R. & De Clercq, E. (2010). Bioorg. Med. Chem. Lett. 20, 809–813. [DOI] [PubMed]
- Fan, X.-S., Wang, Y.-Y., Qu, Y.-Y., Xu, H.-Y., He, Y., Zhang, X.-Y. & Wang, J.-J. (2011). J. Org. Chem. 76, 982–985. [DOI] [PubMed]
- Fan, X.-S., Zhang, X.-Y., Zhou, L.-H., Keith, K. A., Prichard, M. N., Kern, E. R. & Torrence, P. F. (2006a). Antivir. Res. 71, 201–205. [DOI] [PubMed]
- Fan, X.-S., Zhang, X.-Y., Zhou, L.-H., Keith, K. A., Prichard, M. N., Kern, E. R. & Torrence, P. F. (2006b). J. Med. Chem. 49, 3377–3382. [DOI] [PMC free article] [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Lee, Y.-S., Park, S. M. & Kim, B. H. (2009). Bioorg. Med. Chem. Lett. 19, 1126–1128. [DOI] [PMC free article] [PubMed]
- Low, J. N. & Wilson, C. C. (1984). Acta Cryst. C40, 1030–1032.
- Luo, Q., Tang, D.-H., Zhen, Z. & Liu, X.-H. (2007). Acta Cryst. E63, o4–o6.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhang, X.-Y., Li, X.-Y., Li, D.-F., Qu, G.-R., Wang, J.-J. & Loiseau, P. M. (2009). Bioorg. Med. Chem. Lett. 19, 6280–6283. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811041304/su2320sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811041304/su2320Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811041304/su2320Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




