Abstract
In the title molecule, C15H18N2OS, a small twist is noted, with the dihedral angle between the central carbohydrazone residue (r.m.s. deviation = 0.029 Å) and the thiophene ring being 12.47 (10)°. The syn arrangement of the amide H and carbonyl O atoms allows for the formation of centrosymmetric dimers via N—H⋯O hydrogen bonds. These are linked in the three-dimensional structure by C—H⋯π interactions. The thiophene ring is disordered over two co-planar orientations, the major component having a site-occupancy factor of 0.833 (2).
Related literature
For the biological activity of adamantane derivatives see: Vernier et al. (1969 ▶); El-Emam et al. (2004 ▶). For background to our work into the biological activity of adamantane derivatives, see: Kadi et al. (2010 ▶); Al-Omar et al. (2010 ▶). For a related structure, see: Al-Tamimi et al. (2010 ▶).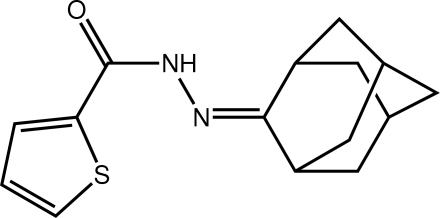
Experimental
Crystal data
C15H18N2OS
M r = 274.37
Monoclinic,
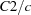
a = 16.7262 (2) Å
b = 12.5663 (1) Å
c = 13.5562 (2) Å
β = 102.473 (1)°
V = 2782.08 (6) Å3
Z = 8
Cu Kα radiation
μ = 2.01 mm−1
T = 100 K
0.30 × 0.25 × 0.20 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010 ▶) T min = 0.584, T max = 0.690
5687 measured reflections
2849 independent reflections
2671 reflections with I > 2σ(I)
R int = 0.015
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.087
S = 1.04
2849 reflections
189 parameters
10 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.38 e Å−3
Δρmin = −0.39 e Å−3
Data collection: CrysAlis PRO (Agilent, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811044758/hg5127sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044758/hg5127Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811044758/hg5127Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the S1,C1–C4 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1i | 0.93 (2) | 1.92 (2) | 2.844 (1) | 173 (2) |
| C13—H13⋯Cg1ii | 1.00 | 2.61 | 3.5791 (16) | 163 |
| C15—H15a⋯Cg1iii | 0.99 | 2.69 | 3.5683 (16) | 148 |
Symmetry codes: (i) 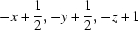 ; (ii)
; (ii) 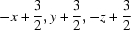 ; (iii)
; (iii) 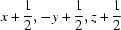 .
.
Acknowledgments
We thank the Deanship of Scientific Research and the Research Center of the College of Pharmacy, King Saud University, and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
Derivatives of adamantane have long been known for their diverse biological activities including anti-viral activity against the influenza (Vernier et al., 1969) and HIV viruses (El-Emam et al., 2004). In continuation of our interest in the chemical and pharmacological properties of adamantane derivatives (Kadi et al., 2010; Al-Omar et al., 2010; Al-Tamimi et al., 2010), we synthesized the title compound, N'-(2-thienylcarbonyl)-2-adamantanone hydrazone, (I), as a potential chemotherapeutic agent. Herein, the crystal and molecular structures are described.
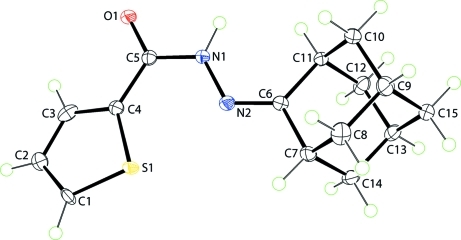
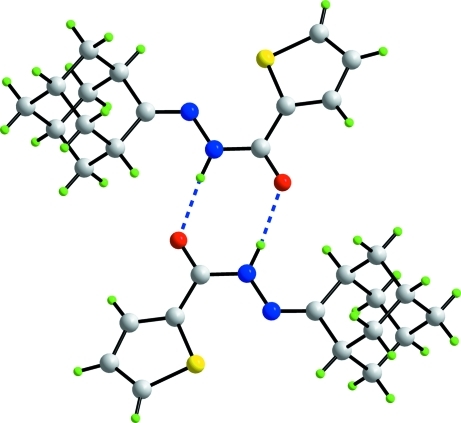
A small twist is noted in the molecule of (I), Fig. 1, as seen in the value of the dihedral between the thiophene ring and the central carbohydrazone residue (O1,N1,N2,C4,C5; r.m.s. deviation = 0.029 Å) being 12.47 (10)°. In the major component of the disordered molecule, the thiophene-S atom is proximate to the hydrazone-N atom, S1···N1 = 2.7797 (11) Å, whereas in the minor component, the C3'—H3' atom is 2.29 Å from N2. The amide-H and carbonyl-O atoms are syn. This arrangement allows for the formation of centrosymmetric dimers, Fig. 2 and Table 1, and eight-membered {···HNCO}2 synthons. The dimers thus formed are consolidated in the crystal packing by C—H···π interactions, Fig. 3 and Table 1.
Experimental
Thiophene-2-carbohydrazide (1.42 g, 0.01 mol) and 2-adamantanone (1.5 g, 0.01 mol) were heated in ethanol (10 ml) for 4 h. The solid that separated upon cooling was collected and recrystallized from ethanol to yield 2.44 g (98%) of C15H18N2OS as colourless crystals, M.pt. 464–467 K. The formulation was established by solution NMR spectroscopy. 1H-NMR (CDCl3): δ 1.91–2.05 (m, 14 adamantyl-H), 7.12 (s, 1 thienyl H), 7.60–7.63 (m, 1 thienyl H), 8.17 (s, 1 thienyl H), 10.04 (s, 1 amino H) p.p.m.. 13C-NMR: 27.78, 30.86, 36.38, 37.81, 39.21, 163.32 (adamantyl), 129.25, 133.78,134.30, 134.90 (thienyl)), 164.15 (carbonyl) p.p.m..
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H 0.95 to 1.00 Å, Uiso(H) 1.2–1.5Ueq(C)] and were included in the refinement in the riding model approximation. The amino H-atom was located in a difference Fourier map, and was freely refined. The thienyl ring was disordered over two positions in respect of the sulfur atom and three of the four carbon atoms; the carbon atom connected to the carbonyl group is ordered. The C—S single bond distances were restrained to 1.71±0.01 Å, the formal C—C double-bond distances were restrained to 1.36±0.01 Å and the formal C—C single-bond distances to 1.46±0.01 Å. The anisotropic displacement parameters of the primed atoms were set to those of the unprimed ones. The major component refined to a site occupancy factor = 0.833 (1).
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level. Only the major component of the disordered thiophene ring is shown for reasons of clarity.
Fig. 2.
Centrosymmetric dimer in (I) sustained by N—H···O hydrogen bonds shown as blue dashed lines.
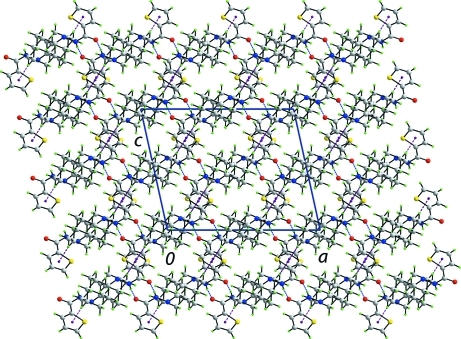
Fig. 3.
Unit-cell contents for (I) shown in projection down the b axis. The N—H···O hydrogen bonds and C—H···π interactions are shown as blue and purple dashed lines, respectively.
Crystal data
| C15H18N2OS | F(000) = 1168 |
| Mr = 274.37 | Dx = 1.310 Mg m−3 |
| Monoclinic, C2/c | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: -C 2yc | Cell parameters from 4116 reflections |
| a = 16.7262 (2) Å | θ = 3.3–76.3° |
| b = 12.5663 (1) Å | µ = 2.01 mm−1 |
| c = 13.5562 (2) Å | T = 100 K |
| β = 102.473 (1)° | Prism, colourless |
| V = 2782.08 (6) Å3 | 0.30 × 0.25 × 0.20 mm |
| Z = 8 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 2849 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 2671 reflections with I > 2σ(I) |
| Mirror | Rint = 0.015 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 76.5°, θmin = 4.4° |
| ω scan | h = −20→21 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2010) | k = −15→15 |
| Tmin = 0.584, Tmax = 0.690 | l = −16→11 |
| 5687 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.087 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0511P)2 + 1.8544P] where P = (Fo2 + 2Fc2)/3 |
| 2849 reflections | (Δ/σ)max = 0.001 |
| 189 parameters | Δρmax = 0.38 e Å−3 |
| 10 restraints | Δρmin = −0.39 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.32529 (2) | 0.46310 (4) | 0.24150 (3) | 0.01360 (14) | 0.833 (2) |
| C1 | 0.26341 (15) | 0.55387 (19) | 0.1659 (2) | 0.0172 (5) | 0.833 (2) |
| H1A | 0.2777 | 0.5884 | 0.1098 | 0.021* | 0.833 (2) |
| C2 | 0.1909 (2) | 0.5709 (3) | 0.1958 (2) | 0.0187 (4) | 0.833 (2) |
| H2 | 0.1493 | 0.6191 | 0.1646 | 0.022* | 0.833 (2) |
| C3 | 0.18762 (10) | 0.5073 (2) | 0.2781 (2) | 0.0223 (5) | 0.833 (2) |
| H3 | 0.1417 | 0.5081 | 0.3088 | 0.027* | 0.833 (2) |
| S1' | 0.16790 (19) | 0.5096 (3) | 0.2790 (2) | 0.01360 (14) | 0.167 (2) |
| C1' | 0.2016 (12) | 0.5693 (15) | 0.1820 (12) | 0.0172 (5) | 0.167 (2) |
| H1' | 0.1703 | 0.6204 | 0.1384 | 0.021* | 0.167 (2) |
| C2' | 0.2765 (11) | 0.5375 (13) | 0.1731 (14) | 0.0187 (4) | 0.167 (2) |
| H2' | 0.3044 | 0.5617 | 0.1234 | 0.022* | 0.167 (2) |
| C3' | 0.3071 (7) | 0.4624 (12) | 0.2493 (10) | 0.0223 (5) | 0.167 (2) |
| H3' | 0.3588 | 0.4285 | 0.2565 | 0.027* | 0.167 (2) |
| O1 | 0.19394 (5) | 0.34953 (7) | 0.42862 (7) | 0.0180 (2) | |
| N1 | 0.32838 (6) | 0.32219 (8) | 0.44331 (8) | 0.0143 (2) | |
| H1 | 0.3254 (10) | 0.2681 (13) | 0.4887 (12) | 0.023 (4)* | |
| N2 | 0.39858 (6) | 0.33902 (8) | 0.40601 (7) | 0.0147 (2) | |
| C4 | 0.25352 (7) | 0.44346 (9) | 0.31283 (8) | 0.0135 (2) | |
| C5 | 0.25708 (7) | 0.36818 (9) | 0.39748 (9) | 0.0137 (2) | |
| C6 | 0.46677 (7) | 0.30469 (9) | 0.45855 (9) | 0.0137 (2) | |
| C7 | 0.54146 (7) | 0.31718 (10) | 0.41432 (9) | 0.0172 (3) | |
| H7 | 0.5274 | 0.3575 | 0.3493 | 0.021* | |
| C8 | 0.57170 (8) | 0.20454 (11) | 0.39590 (10) | 0.0216 (3) | |
| H8A | 0.5290 | 0.1666 | 0.3464 | 0.026* | |
| H8B | 0.6214 | 0.2095 | 0.3676 | 0.026* | |
| C9 | 0.59118 (7) | 0.14246 (10) | 0.49565 (10) | 0.0194 (3) | |
| H9 | 0.6107 | 0.0694 | 0.4835 | 0.023* | |
| C10 | 0.51402 (7) | 0.13459 (10) | 0.53943 (10) | 0.0195 (3) | |
| H10A | 0.5265 | 0.0941 | 0.6035 | 0.023* | |
| H10B | 0.4706 | 0.0962 | 0.4914 | 0.023* | |
| C11 | 0.48369 (7) | 0.24706 (10) | 0.55881 (9) | 0.0147 (2) | |
| H11 | 0.4329 | 0.2428 | 0.5862 | 0.018* | |
| C12 | 0.55174 (7) | 0.30665 (11) | 0.63301 (9) | 0.0193 (3) | |
| H12A | 0.5328 | 0.3792 | 0.6449 | 0.023* | |
| H12B | 0.5644 | 0.2687 | 0.6985 | 0.023* | |
| C13 | 0.62905 (7) | 0.31363 (10) | 0.58972 (9) | 0.0177 (3) | |
| H13 | 0.6731 | 0.3514 | 0.6388 | 0.021* | |
| C14 | 0.60877 (7) | 0.37598 (10) | 0.49038 (10) | 0.0196 (3) | |
| H14A | 0.6585 | 0.3830 | 0.4623 | 0.024* | |
| H14B | 0.5897 | 0.4483 | 0.5028 | 0.024* | |
| C15 | 0.65806 (7) | 0.20126 (10) | 0.57098 (9) | 0.0184 (3) | |
| H15A | 0.6712 | 0.1615 | 0.6355 | 0.022* | |
| H15B | 0.7084 | 0.2056 | 0.5439 | 0.022* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0146 (3) | 0.0164 (2) | 0.0113 (2) | −0.00024 (18) | 0.00607 (15) | 0.00223 (12) |
| C1 | 0.0211 (13) | 0.0172 (11) | 0.0118 (8) | 0.0019 (8) | 0.0004 (8) | 0.0070 (7) |
| C2 | 0.0190 (12) | 0.0181 (7) | 0.0184 (12) | 0.0026 (7) | 0.0026 (6) | 0.0013 (8) |
| C3 | 0.0147 (11) | 0.0256 (8) | 0.0287 (8) | −0.0040 (10) | 0.0096 (9) | −0.0030 (7) |
| S1' | 0.0146 (3) | 0.0164 (2) | 0.0113 (2) | −0.00024 (18) | 0.00607 (15) | 0.00223 (12) |
| C1' | 0.0211 (13) | 0.0172 (11) | 0.0118 (8) | 0.0019 (8) | 0.0004 (8) | 0.0070 (7) |
| C2' | 0.0190 (12) | 0.0181 (7) | 0.0184 (12) | 0.0026 (7) | 0.0026 (6) | 0.0013 (8) |
| C3' | 0.0147 (11) | 0.0256 (8) | 0.0287 (8) | −0.0040 (10) | 0.0096 (9) | −0.0030 (7) |
| O1 | 0.0134 (4) | 0.0194 (4) | 0.0217 (4) | 0.0002 (3) | 0.0053 (3) | 0.0062 (3) |
| N1 | 0.0125 (5) | 0.0153 (5) | 0.0153 (5) | 0.0001 (4) | 0.0036 (4) | 0.0035 (4) |
| N2 | 0.0139 (5) | 0.0153 (5) | 0.0159 (5) | 0.0005 (4) | 0.0052 (4) | 0.0016 (4) |
| C4 | 0.0149 (5) | 0.0128 (5) | 0.0125 (5) | −0.0014 (4) | 0.0024 (4) | −0.0010 (4) |
| C5 | 0.0141 (5) | 0.0118 (5) | 0.0147 (5) | −0.0010 (4) | 0.0021 (4) | −0.0015 (4) |
| C6 | 0.0149 (5) | 0.0128 (5) | 0.0138 (5) | 0.0000 (4) | 0.0041 (4) | 0.0006 (4) |
| C7 | 0.0160 (6) | 0.0213 (6) | 0.0160 (6) | 0.0026 (4) | 0.0068 (4) | 0.0059 (5) |
| C8 | 0.0214 (6) | 0.0266 (7) | 0.0176 (6) | 0.0041 (5) | 0.0062 (5) | −0.0034 (5) |
| C9 | 0.0187 (6) | 0.0145 (6) | 0.0249 (6) | 0.0031 (4) | 0.0045 (5) | 0.0001 (5) |
| C10 | 0.0167 (6) | 0.0152 (6) | 0.0251 (6) | −0.0025 (4) | 0.0009 (5) | 0.0059 (5) |
| C11 | 0.0115 (5) | 0.0189 (6) | 0.0141 (5) | −0.0009 (4) | 0.0035 (4) | 0.0040 (4) |
| C12 | 0.0155 (6) | 0.0274 (7) | 0.0148 (6) | −0.0014 (5) | 0.0029 (5) | −0.0027 (5) |
| C13 | 0.0130 (5) | 0.0202 (6) | 0.0196 (6) | −0.0035 (4) | 0.0028 (4) | −0.0011 (5) |
| C14 | 0.0153 (5) | 0.0161 (6) | 0.0289 (7) | −0.0009 (4) | 0.0081 (5) | 0.0051 (5) |
| C15 | 0.0132 (5) | 0.0204 (6) | 0.0215 (6) | 0.0012 (4) | 0.0037 (5) | 0.0060 (5) |
Geometric parameters (Å, °)
| S1—C4 | 1.7142 (12) | C7—C8 | 1.5415 (18) |
| S1—C1 | 1.7215 (15) | C7—C14 | 1.5412 (17) |
| C1—C2 | 1.377 (2) | C7—H7 | 1.0000 |
| C1—H1A | 0.9500 | C8—C9 | 1.5340 (18) |
| C2—C3 | 1.382 (3) | C8—H8A | 0.9900 |
| C2—H2 | 0.9500 | C8—H8B | 0.9900 |
| C3—C4 | 1.362 (2) | C9—C15 | 1.5319 (17) |
| C3—H3 | 0.9500 | C9—C10 | 1.5358 (17) |
| S1'—C4 | 1.634 (3) | C9—H9 | 1.0000 |
| S1'—C1' | 1.712 (9) | C10—C11 | 1.5432 (17) |
| C1'—C2' | 1.344 (9) | C10—H10A | 0.9900 |
| C1'—H1' | 0.9500 | C10—H10B | 0.9900 |
| C2'—C3' | 1.411 (10) | C11—C12 | 1.5406 (16) |
| C2'—H2' | 0.9500 | C11—H11 | 1.0000 |
| C3'—C4 | 1.391 (8) | C12—C13 | 1.5336 (16) |
| C3'—H3' | 0.9500 | C12—H12A | 0.9900 |
| O1—C5 | 1.2414 (14) | C12—H12B | 0.9900 |
| N1—C5 | 1.3499 (15) | C13—C14 | 1.5313 (17) |
| N1—N2 | 1.3912 (13) | C13—C15 | 1.5321 (17) |
| N1—H1 | 0.926 (17) | C13—H13 | 1.0000 |
| N2—C6 | 1.2820 (15) | C14—H14A | 0.9900 |
| C4—C5 | 1.4784 (16) | C14—H14B | 0.9900 |
| C6—C7 | 1.5062 (15) | C15—H15A | 0.9900 |
| C6—C11 | 1.5118 (15) | C15—H15B | 0.9900 |
| C4—S1—C1 | 91.53 (11) | C7—C8—H8A | 109.7 |
| C2—C1—S1 | 112.5 (3) | C9—C8—H8B | 109.7 |
| C2—C1—H1A | 123.7 | C7—C8—H8B | 109.7 |
| S1—C1—H1A | 123.7 | H8A—C8—H8B | 108.2 |
| C1—C2—C3 | 109.8 (3) | C15—C9—C8 | 109.14 (10) |
| C1—C2—H2 | 125.1 | C15—C9—C10 | 109.09 (10) |
| C3—C2—H2 | 125.1 | C8—C9—C10 | 109.70 (10) |
| C4—C3—C2 | 116.73 (19) | C15—C9—H9 | 109.6 |
| C4—C3—H3 | 121.6 | C8—C9—H9 | 109.6 |
| C2—C3—H3 | 121.6 | C10—C9—H9 | 109.6 |
| C4—S1'—C1' | 91.4 (7) | C9—C10—C11 | 109.97 (10) |
| C2'—C1'—S1' | 114.0 (15) | C9—C10—H10A | 109.7 |
| C2'—C1'—H1' | 123.0 | C11—C10—H10A | 109.7 |
| S1'—C1'—H1' | 123.0 | C9—C10—H10B | 109.7 |
| C1'—C2'—C3' | 109.4 (17) | C11—C10—H10B | 109.7 |
| C1'—C2'—H2' | 125.3 | H10A—C10—H10B | 108.2 |
| C3'—C2'—H2' | 125.3 | C6—C11—C12 | 108.82 (10) |
| C4—C3'—C2' | 112.8 (12) | C6—C11—C10 | 106.87 (10) |
| C4—C3'—H3' | 123.6 | C12—C11—C10 | 109.43 (10) |
| C2'—C3'—H3' | 123.6 | C6—C11—H11 | 110.5 |
| C5—N1—N2 | 119.91 (10) | C12—C11—H11 | 110.5 |
| C5—N1—H1 | 116.8 (10) | C10—C11—H11 | 110.5 |
| N2—N1—H1 | 121.6 (10) | C13—C12—C11 | 110.07 (10) |
| C6—N2—N1 | 117.79 (10) | C13—C12—H12A | 109.6 |
| C3—C4—C3' | 105.5 (6) | C11—C12—H12A | 109.6 |
| C3—C4—C5 | 122.63 (13) | C13—C12—H12B | 109.6 |
| C3'—C4—C5 | 131.8 (6) | C11—C12—H12B | 109.6 |
| C3'—C4—S1' | 112.5 (6) | H12A—C12—H12B | 108.2 |
| C5—C4—S1' | 115.42 (13) | C14—C13—C15 | 110.10 (10) |
| C3—C4—S1 | 109.44 (12) | C14—C13—C12 | 108.72 (10) |
| C5—C4—S1 | 127.90 (9) | C15—C13—C12 | 109.52 (10) |
| S1'—C4—S1 | 116.54 (13) | C14—C13—H13 | 109.5 |
| O1—C5—N1 | 119.64 (10) | C15—C13—H13 | 109.5 |
| O1—C5—C4 | 119.35 (10) | C12—C13—H13 | 109.5 |
| N1—C5—C4 | 120.98 (10) | C13—C14—C7 | 109.52 (10) |
| N2—C6—C7 | 117.30 (10) | C13—C14—H14A | 109.8 |
| N2—C6—C11 | 129.20 (11) | C7—C14—H14A | 109.8 |
| C7—C6—C11 | 113.42 (9) | C13—C14—H14B | 109.8 |
| C6—C7—C8 | 107.35 (10) | C7—C14—H14B | 109.8 |
| C6—C7—C14 | 109.42 (10) | H14A—C14—H14B | 108.2 |
| C8—C7—C14 | 109.31 (10) | C13—C15—C9 | 110.08 (10) |
| C6—C7—H7 | 110.2 | C13—C15—H15A | 109.6 |
| C8—C7—H7 | 110.2 | C9—C15—H15A | 109.6 |
| C14—C7—H7 | 110.2 | C13—C15—H15B | 109.6 |
| C9—C8—C7 | 109.81 (10) | C9—C15—H15B | 109.6 |
| C9—C8—H8A | 109.7 | H15A—C15—H15B | 108.2 |
| C4—S1—C1—C2 | 1.5 (2) | S1—C4—C5—N1 | 15.62 (17) |
| S1—C1—C2—C3 | −1.4 (3) | N1—N2—C6—C7 | 176.23 (10) |
| C1—C2—C3—C4 | 0.4 (4) | N1—N2—C6—C11 | −0.37 (18) |
| C4—S1'—C1'—C2' | 1.7 (16) | N2—C6—C7—C8 | −115.56 (12) |
| S1'—C1'—C2'—C3' | −1(2) | C11—C6—C7—C8 | 61.57 (13) |
| C1'—C2'—C3'—C4 | −1(2) | N2—C6—C7—C14 | 125.92 (11) |
| C5—N1—N2—C6 | 171.74 (11) | C11—C6—C7—C14 | −56.95 (13) |
| C2—C3—C4—C3' | −0.8 (7) | C6—C7—C8—C9 | −58.56 (13) |
| C2—C3—C4—C5 | −177.5 (2) | C14—C7—C8—C9 | 60.04 (13) |
| C2—C3—C4—S1' | −161 (2) | C7—C8—C9—C15 | −60.03 (13) |
| C2—C3—C4—S1 | 0.8 (3) | C7—C8—C9—C10 | 59.45 (13) |
| C2'—C3'—C4—C3 | −0.4 (14) | C15—C9—C10—C11 | 59.76 (12) |
| C2'—C3'—C4—C5 | 175.8 (10) | C8—C9—C10—C11 | −59.75 (13) |
| C2'—C3'—C4—S1' | 2.4 (16) | N2—C6—C11—C12 | −126.71 (13) |
| C2'—C3'—C4—S1 | −160 (10) | C7—C6—C11—C12 | 56.58 (13) |
| C1'—S1'—C4—C3 | 18.8 (18) | N2—C6—C11—C10 | 115.21 (13) |
| C1'—S1'—C4—C3' | −2.3 (11) | C7—C6—C11—C10 | −61.49 (12) |
| C1'—S1'—C4—C5 | −176.8 (7) | C9—C10—C11—C6 | 58.68 (12) |
| C1'—S1'—C4—S1 | −0.8 (7) | C9—C10—C11—C12 | −58.99 (13) |
| C1—S1—C4—C3 | −1.28 (18) | C6—C11—C12—C13 | −57.90 (13) |
| C1—S1—C4—C3' | 19 (9) | C10—C11—C12—C13 | 58.55 (13) |
| C1—S1—C4—C5 | 176.83 (14) | C11—C12—C13—C14 | 61.27 (13) |
| C1—S1—C4—S1' | 1.40 (18) | C11—C12—C13—C15 | −59.07 (13) |
| N2—N1—C5—O1 | 177.15 (10) | C15—C13—C14—C7 | 59.00 (12) |
| N2—N1—C5—C4 | −4.97 (16) | C12—C13—C14—C7 | −60.97 (13) |
| C3—C4—C5—O1 | 11.4 (2) | C6—C7—C14—C13 | 58.13 (13) |
| C3'—C4—C5—O1 | −164.3 (9) | C8—C7—C14—C13 | −59.16 (13) |
| S1'—C4—C5—O1 | 9.0 (2) | C14—C13—C15—C9 | −59.43 (12) |
| S1—C4—C5—O1 | −166.49 (9) | C12—C13—C15—C9 | 60.06 (13) |
| C3—C4—C5—N1 | −166.50 (17) | C8—C9—C15—C13 | 59.55 (13) |
| C3'—C4—C5—N1 | 17.8 (9) | C10—C9—C15—C13 | −60.30 (13) |
| S1'—C4—C5—N1 | −168.90 (17) |
Hydrogen-bond geometry (Å, °)
| Cg1 is the centroid of the S1,C1–C4 ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1i | 0.93 (2) | 1.92 (2) | 2.844 (1) | 173 (2) |
| C13—H13···Cg1ii | 1.00 | 2.61 | 3.5791 (16) | 163 |
| C15—H15a···Cg1iii | 0.99 | 2.69 | 3.5683 (16) | 148 |
Symmetry codes: (i) −x+1/2, −y+1/2, −z+1; (ii) −x+3/2, y+3/2, −z+3/2; (iii) x+1/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5127).
References
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Al-Omar, M. A., Al-Abdullah, E. S., Shehata, I. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2010). Molecules, 15, 2526-2550. [DOI] [PMC free article] [PubMed]
- Al-Tamimi, A.-M. S., Bari, A., Al-Omar, M. A., Alrashood, K. A. & El-Emam, A. A. (2010). Acta Cryst. E66, o1756. [DOI] [PMC free article] [PubMed]
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- El-Emam, A. A., Al-Deeb, O. A., Al-Omar, M. A. & Lehmann, J. (2004). Bioorg. Med. Chem. 12, 5107–5113. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Kadi, A. A., Al-Abdullah, E. S., Shehata, I. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2010). Eur. J. Med. Chem. 45, 5006–5011. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Vernier, V. G., Harmon, J. B., Stump, J. M., Lynes, T. L., Marvel, M. P. & Smith, D. H. (1969). Toxicol. Appl. Pharmacol. 15, 642–665. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811044758/hg5127sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044758/hg5127Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811044758/hg5127Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report