Abstract
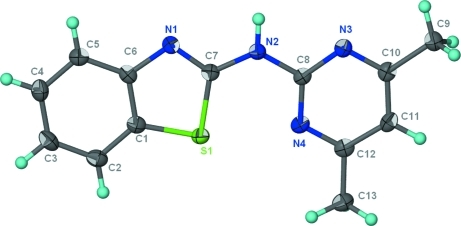
In the title compound, C13H12N4S, an amino N atom is connected to a 1,3-benzothiazole fused-ring system and a dimethyl-substituted pyrimidine ring, these components being aligned [interplanar dihedral angle = 1.9 (1)°]. The secondary amino N atom forms an intermolecular N—H⋯N hydrogen bond to an N atom of the fused ring of an adjacent molecule, generating a centrosymmetric cyclic hydrogen-bonded dimer [graph set R 2 2(8)].
Related literature
For the structure of N-(4,6-dimethylpyrimidin-2-yl)-1H-benzimidazol-2-amine, see: Mohamed et al. (2011 ▶). For graph-set analysis, see: Etter et al. (1990 ▶).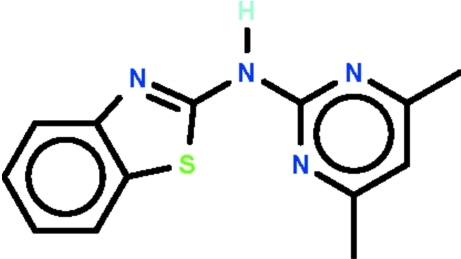
Experimental
Crystal data
C13H12N4S
M r = 256.34
Monoclinic,

a = 6.7608 (2) Å
b = 8.5154 (2) Å
c = 20.6503 (9) Å
β = 97.237 (2)°
V = 1179.39 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.26 mm−1
T = 120 K
0.24 × 0.14 × 0.08 mm
Data collection
Bruker–Nonius Roper CCD camera on κ-goniostat diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.940, T max = 0.980
11943 measured reflections
2704 independent reflections
2100 reflections with I > 2σ(I)
R int = 0.061
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.126
S = 1.02
2704 reflections
169 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.29 e Å−3
Δρmin = −0.46 e Å−3
Data collection: COLLECT (Hooft, 1998 ▶); cell refinement: DENZO (Otwinowski & Minor, 1997 ▶) and COLLECT; data reduction: DENZO and COLLECT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811044631/zs2156sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044631/zs2156Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811044631/zs2156Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯N1i | 0.89 (3) | 2.27 (3) | 3.142 (2) | 168 (2) |
Symmetry code: (i) 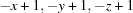 .
.
Acknowledgments
The use of the EPSRC X-ray crystallographic facilities at the University of Southampton, England, is gratefully acknowledged. We thank Manchester Metropolitan University, Sohag University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
In an earlier study, we reported the structure of N-(4,6-dimethylpyrimidin-2-yl)-1H-benzimidazol-2-amine (Mohamed et al., 2011). The benzimidazole portion of that molecule was replaced by a benzothiazole unit in the present study, giving the title compound C13H12N4S (Scheme I). In this molecule, an amino N atom is connected to a benzothiazole fused-ring system and a dimethyl-substituted pyrimidine ring, these being aligned [inter-ring dihedral angle, 1.9 (1)°] (Fig. 1). The amino N atom forms an intermolecular N—H···N hydrogen bond to the N atom of the fused-ring of an adjacent molecule (Table 1) to generate a centrosymmetric cyclic hydrogen-bonded dimer [graph set R</i<>22(8) (Etter et al., 1990)].
Experimental
2-(1,3-Benzothiazol-2-yl)guanidine (0.05 mol) was heated in acetylacetone solution (0.10 mol, approx. 10 ml) in the presence of a few drops of acetic acid at 473 K for 1 h. The mixture was cooled and the product was collected and recrystalized from ethanol to give the title compound (m.p. 513 K) in 85% yield.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H, 0.95 to 0.98 Å) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 to 1.5Ueq(C). The amino H-atom was located in a difference Fourier map, and was freely refined. The reflections (-1 2 3) and (0 1 2) were omitted because to bad agreement.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C13H12N4S at the 70% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C13H12N4S | F(000) = 536 |
| Mr = 256.34 | Dx = 1.444 Mg m−3 |
| Monoclinic, P21/n | Melting point: 513 K |
| Hall symbol: -P 2yn | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.7608 (2) Å | Cell parameters from 2630 reflections |
| b = 8.5154 (2) Å | θ = 2.9–27.5° |
| c = 20.6503 (9) Å | µ = 0.26 mm−1 |
| β = 97.237 (2)° | T = 120 K |
| V = 1179.39 (7) Å3 | Block, colorless |
| Z = 4 | 0.24 × 0.14 × 0.08 mm |
Data collection
| Bruker–Nonius Roper CCD camera on κ-goniostat diffractometer | 2704 independent reflections |
| Radiation source: Bruker–Nonius FR591 rotating anode | 2100 reflections with I > 2σ(I) |
| graphite | Rint = 0.061 |
| Detector resolution: 9.091 pixels mm-1 | θmax = 27.6°, θmin = 3.1° |
| φ and ω scans | h = −8→8 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | k = −11→10 |
| Tmin = 0.940, Tmax = 0.980 | l = −25→26 |
| 11943 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.126 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0525P)2 + 0.7927P] where P = (Fo2 + 2Fc2)/3 |
| 2704 reflections | (Δ/σ)max = 0.001 |
| 169 parameters | Δρmax = 0.29 e Å−3 |
| 0 restraints | Δρmin = −0.46 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.47670 (7) | 0.76071 (6) | 0.65817 (3) | 0.01754 (17) | |
| N1 | 0.6118 (2) | 0.55355 (17) | 0.58054 (8) | 0.0158 (4) | |
| N2 | 0.2981 (2) | 0.64918 (19) | 0.53944 (9) | 0.0170 (4) | |
| H2 | 0.305 (4) | 0.586 (3) | 0.5054 (13) | 0.034 (7)* | |
| N3 | −0.0130 (2) | 0.71820 (18) | 0.48927 (8) | 0.0165 (4) | |
| N4 | 0.1226 (2) | 0.83658 (18) | 0.59065 (8) | 0.0167 (4) | |
| C1 | 0.7100 (3) | 0.6766 (2) | 0.68212 (10) | 0.0167 (4) | |
| C2 | 0.8414 (3) | 0.7057 (2) | 0.73865 (10) | 0.0203 (5) | |
| H2A | 0.8097 | 0.7796 | 0.7702 | 0.024* | |
| C3 | 1.0194 (3) | 0.6232 (2) | 0.74717 (10) | 0.0226 (5) | |
| H3 | 1.1108 | 0.6402 | 0.7854 | 0.027* | |
| C4 | 1.0668 (3) | 0.5154 (2) | 0.70042 (10) | 0.0215 (5) | |
| H4 | 1.1898 | 0.4602 | 0.7075 | 0.026* | |
| C5 | 0.9374 (3) | 0.4875 (2) | 0.64396 (10) | 0.0184 (4) | |
| H5 | 0.9708 | 0.4147 | 0.6122 | 0.022* | |
| C6 | 0.7561 (3) | 0.5692 (2) | 0.63488 (10) | 0.0160 (4) | |
| C7 | 0.4608 (3) | 0.6453 (2) | 0.58687 (10) | 0.0152 (4) | |
| C8 | 0.1274 (3) | 0.7392 (2) | 0.54049 (10) | 0.0150 (4) | |
| C9 | −0.3381 (3) | 0.7869 (2) | 0.43244 (11) | 0.0201 (4) | |
| H9A | −0.2945 | 0.7105 | 0.4017 | 0.030* | |
| H9B | −0.3627 | 0.8884 | 0.4105 | 0.030* | |
| H9C | −0.4611 | 0.7497 | 0.4479 | 0.030* | |
| C10 | −0.1786 (3) | 0.8053 (2) | 0.48952 (10) | 0.0168 (4) | |
| C11 | −0.2001 (3) | 0.9088 (2) | 0.54009 (10) | 0.0181 (4) | |
| H11 | −0.3180 | 0.9694 | 0.5400 | 0.022* | |
| C12 | −0.0455 (3) | 0.9217 (2) | 0.59088 (10) | 0.0168 (4) | |
| C13 | −0.0551 (3) | 1.0316 (2) | 0.64689 (11) | 0.0217 (5) | |
| H13A | 0.0326 | 0.9934 | 0.6851 | 0.032* | |
| H13B | −0.1924 | 1.0371 | 0.6573 | 0.032* | |
| H13C | −0.0117 | 1.1364 | 0.6351 | 0.032* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0175 (3) | 0.0186 (3) | 0.0166 (3) | 0.00010 (19) | 0.0027 (2) | −0.00291 (19) |
| N1 | 0.0172 (8) | 0.0131 (8) | 0.0166 (9) | −0.0006 (6) | 0.0008 (7) | 0.0007 (6) |
| N2 | 0.0170 (9) | 0.0173 (8) | 0.0163 (9) | 0.0018 (7) | 0.0008 (7) | −0.0029 (7) |
| N3 | 0.0158 (8) | 0.0159 (8) | 0.0181 (9) | −0.0013 (6) | 0.0033 (7) | 0.0016 (7) |
| N4 | 0.0183 (8) | 0.0145 (8) | 0.0181 (9) | −0.0003 (6) | 0.0051 (7) | 0.0009 (7) |
| C1 | 0.0189 (10) | 0.0165 (9) | 0.0153 (10) | −0.0022 (8) | 0.0040 (8) | 0.0017 (8) |
| C2 | 0.0241 (11) | 0.0222 (10) | 0.0152 (11) | −0.0031 (8) | 0.0048 (9) | −0.0002 (8) |
| C3 | 0.0240 (11) | 0.0272 (11) | 0.0153 (11) | −0.0037 (9) | −0.0021 (9) | 0.0023 (8) |
| C4 | 0.0188 (10) | 0.0229 (10) | 0.0221 (12) | 0.0016 (8) | −0.0002 (9) | 0.0044 (9) |
| C5 | 0.0212 (10) | 0.0164 (9) | 0.0180 (11) | −0.0012 (8) | 0.0038 (8) | 0.0005 (8) |
| C6 | 0.0179 (10) | 0.0145 (9) | 0.0153 (10) | −0.0035 (7) | 0.0011 (8) | 0.0022 (8) |
| C7 | 0.0168 (10) | 0.0131 (9) | 0.0160 (10) | −0.0025 (7) | 0.0027 (8) | −0.0001 (7) |
| C8 | 0.0160 (9) | 0.0129 (9) | 0.0167 (11) | −0.0014 (7) | 0.0047 (8) | 0.0014 (7) |
| C9 | 0.0189 (10) | 0.0208 (10) | 0.0203 (11) | −0.0001 (8) | 0.0013 (9) | 0.0012 (8) |
| C10 | 0.0175 (9) | 0.0133 (9) | 0.0201 (11) | −0.0017 (7) | 0.0044 (8) | 0.0042 (8) |
| C11 | 0.0164 (10) | 0.0166 (9) | 0.0222 (11) | 0.0021 (8) | 0.0060 (8) | 0.0039 (8) |
| C12 | 0.0199 (10) | 0.0131 (9) | 0.0184 (11) | −0.0033 (7) | 0.0065 (8) | 0.0028 (8) |
| C13 | 0.0233 (11) | 0.0188 (10) | 0.0238 (12) | −0.0001 (8) | 0.0068 (9) | −0.0030 (8) |
Geometric parameters (Å, °)
| S1—C1 | 1.746 (2) | C3—H3 | 0.9500 |
| S1—C7 | 1.762 (2) | C4—C5 | 1.387 (3) |
| N1—C7 | 1.305 (2) | C4—H4 | 0.9500 |
| N1—C6 | 1.397 (2) | C5—C6 | 1.401 (3) |
| N2—C7 | 1.378 (2) | C5—H5 | 0.9500 |
| N2—C8 | 1.388 (2) | C9—C10 | 1.502 (3) |
| N2—H2 | 0.89 (3) | C9—H9A | 0.9800 |
| N3—C8 | 1.342 (3) | C9—H9B | 0.9800 |
| N3—C10 | 1.343 (2) | C9—H9C | 0.9800 |
| N4—C8 | 1.330 (3) | C10—C11 | 1.388 (3) |
| N4—C12 | 1.349 (3) | C11—C12 | 1.389 (3) |
| C1—C6 | 1.400 (3) | C11—H11 | 0.9500 |
| C1—C2 | 1.397 (3) | C12—C13 | 1.496 (3) |
| C2—C3 | 1.385 (3) | C13—H13A | 0.9800 |
| C2—H2A | 0.9500 | C13—H13B | 0.9800 |
| C3—C4 | 1.399 (3) | C13—H13C | 0.9800 |
| C1—S1—C7 | 88.04 (9) | N1—C7—S1 | 116.81 (14) |
| C7—N1—C6 | 109.77 (16) | N2—C7—S1 | 122.68 (15) |
| C7—N2—C8 | 126.35 (18) | N4—C8—N3 | 127.80 (18) |
| C7—N2—H2 | 115.6 (16) | N4—C8—N2 | 117.28 (17) |
| C8—N2—H2 | 118.1 (16) | N3—C8—N2 | 114.91 (17) |
| C8—N3—C10 | 115.50 (17) | C10—C9—H9A | 109.5 |
| C8—N4—C12 | 116.16 (17) | C10—C9—H9B | 109.5 |
| C6—C1—C2 | 121.59 (18) | H9A—C9—H9B | 109.5 |
| C6—C1—S1 | 110.06 (15) | C10—C9—H9C | 109.5 |
| C2—C1—S1 | 128.35 (16) | H9A—C9—H9C | 109.5 |
| C3—C2—C1 | 117.85 (19) | H9B—C9—H9C | 109.5 |
| C3—C2—H2A | 121.1 | N3—C10—C11 | 121.32 (18) |
| C1—C2—H2A | 121.1 | N3—C10—C9 | 117.08 (18) |
| C2—C3—C4 | 121.07 (19) | C11—C10—C9 | 121.60 (18) |
| C2—C3—H3 | 119.5 | C10—C11—C12 | 118.60 (18) |
| C4—C3—H3 | 119.5 | C10—C11—H11 | 120.7 |
| C5—C4—C3 | 121.16 (19) | C12—C11—H11 | 120.7 |
| C5—C4—H4 | 119.4 | N4—C12—C11 | 120.60 (18) |
| C3—C4—H4 | 119.4 | N4—C12—C13 | 117.25 (18) |
| C4—C5—C6 | 118.41 (19) | C11—C12—C13 | 122.14 (18) |
| C4—C5—H5 | 120.8 | C12—C13—H13A | 109.5 |
| C6—C5—H5 | 120.8 | C12—C13—H13B | 109.5 |
| C1—C6—N1 | 115.32 (17) | H13A—C13—H13B | 109.5 |
| C1—C6—C5 | 119.92 (18) | C12—C13—H13C | 109.5 |
| N1—C6—C5 | 124.76 (18) | H13A—C13—H13C | 109.5 |
| N1—C7—N2 | 120.51 (18) | H13B—C13—H13C | 109.5 |
| C7—S1—C1—C6 | −0.08 (15) | C8—N2—C7—S1 | −0.8 (3) |
| C7—S1—C1—C2 | 179.4 (2) | C1—S1—C7—N1 | −0.22 (16) |
| C6—C1—C2—C3 | −0.8 (3) | C1—S1—C7—N2 | 179.59 (17) |
| S1—C1—C2—C3 | 179.75 (16) | C12—N4—C8—N3 | 1.7 (3) |
| C1—C2—C3—C4 | 0.6 (3) | C12—N4—C8—N2 | −179.41 (16) |
| C2—C3—C4—C5 | 0.1 (3) | C10—N3—C8—N4 | −0.8 (3) |
| C3—C4—C5—C6 | −0.5 (3) | C10—N3—C8—N2 | −179.65 (16) |
| C2—C1—C6—N1 | −179.22 (17) | C7—N2—C8—N4 | 3.1 (3) |
| S1—C1—C6—N1 | 0.3 (2) | C7—N2—C8—N3 | −177.90 (17) |
| C2—C1—C6—C5 | 0.4 (3) | C8—N3—C10—C11 | −0.4 (3) |
| S1—C1—C6—C5 | 179.93 (15) | C8—N3—C10—C9 | 179.09 (17) |
| C7—N1—C6—C1 | −0.5 (2) | N3—C10—C11—C12 | 0.4 (3) |
| C7—N1—C6—C5 | 179.94 (18) | C9—C10—C11—C12 | −179.04 (18) |
| C4—C5—C6—C1 | 0.3 (3) | C8—N4—C12—C11 | −1.6 (3) |
| C4—C5—C6—N1 | 179.82 (18) | C8—N4—C12—C13 | 179.75 (17) |
| C6—N1—C7—N2 | −179.37 (17) | C10—C11—C12—N4 | 0.6 (3) |
| C6—N1—C7—S1 | 0.4 (2) | C10—C11—C12—C13 | 179.24 (18) |
| C8—N2—C7—N1 | 179.05 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···N1i | 0.89 (3) | 2.27 (3) | 3.142 (2) | 168 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZS2156).
References
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Hooft, R. (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Mohamed, S. K., El-Remaily, M. A. A., Gurbanov, A. V., Khalilov, A. N. & Ng, S. W. (2011). Acta Cryst. E67, o719. [DOI] [PMC free article] [PubMed]
- Otwinowski, O. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811044631/zs2156sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811044631/zs2156Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811044631/zs2156Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report