Abstract
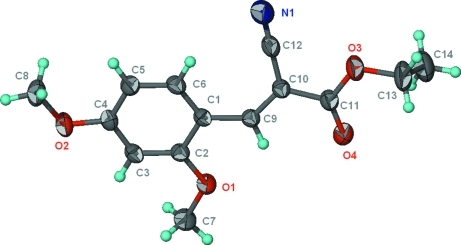
The C=C bond in the title compound, C14H15NO4, is in an E configuration. With the exception of the methyl C atoms, the non-H atoms of the molecule all lie approximately on a plane (r.m.s. deviation = 0.096 Å). π–π stacking is observed between parallel benzene rings of adjacent molecules, the centroid–centroid distance being 3.7924 (8) Å.
Related literature
For benzylidenecyanoacetate, see: Bodrikov et al. (1992 ▶) and for 3,4-dimethoxybenzylidenecyanoacetate, see: Nesterov et al. (2001 ▶).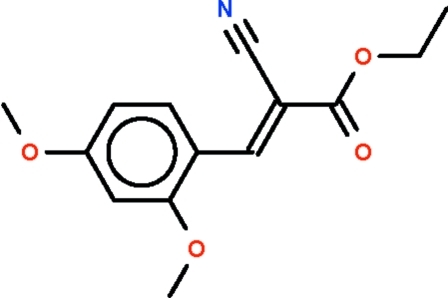
Experimental
Crystal data
C14H15NO4
M r = 261.27
Monoclinic,
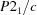
a = 10.5661 (6) Å
b = 6.9715 (4) Å
c = 18.4141 (10) Å
β = 101.858 (1)°
V = 1327.47 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 295 K
0.20 × 0.20 × 0.20 mm
Data collection
Bruker SMART APEXII diffractometer
14924 measured reflections
3330 independent reflections
2382 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.046
wR(F 2) = 0.134
S = 1.03
3330 reflections
172 parameters
H-atom parameters constrained
Δρmax = 0.23 e Å−3
Δρmin = −0.21 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811040013/xu5338sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040013/xu5338Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040013/xu5338Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Manchester Metropolitan University, Baku State University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
The synthesis of benzylidenecyanoacetate was reported by Bodrikov et al. in 1992; the compound was synthesized by a conventional route. In the present study, microwave radiation was used to initiate the condensation; 2,4-dimethoxylbenzaldehyde was used in place of the unsubstituted homolog. The carbon-carbon double-bond in C14H15NO4 is of an E-configuration (Scheme I, Fig. 1). With the exception of the methyl C, the non-hydrogen atoms all lie on a plane. The features are similar to those of 3,4-dimethoxybenzylidenecyanoacetate (Bodrikov et al., 1992).
Experimental
2,4-Dimethoxy benzaldehyde (10 mmol), ethyl cyanoacetate (10 mmol), and 2,4-pentanedione (100 mmol, aprox. 10 ml) dissolved in ethanol (50 ml) and the solution was irradiated by microwave irradiation for 5 minutes. The mixture was cooled and the product was recrystalized from ethanol in 90% yield; m.p. 405 K.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C–H 0.93 to 0.97 Å; U(H) 1.2 to 1.5U(C)] and were included in the refinement in the riding model approximation, with U(H) set to 1.2 to 1.5U(C).
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C14H15NO4 at the 50% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C14H15NO4 | F(000) = 552 |
| Mr = 261.27 | Dx = 1.307 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3565 reflections |
| a = 10.5661 (6) Å | θ = 2.3–27.7° |
| b = 6.9715 (4) Å | µ = 0.10 mm−1 |
| c = 18.4141 (10) Å | T = 295 K |
| β = 101.858 (1)° | Prism, colorless |
| V = 1327.47 (13) Å3 | 0.20 × 0.20 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII diffractometer | 2382 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.026 |
| graphite | θmax = 28.4°, θmin = 2.0° |
| φ and ω scans | h = −14→14 |
| 14924 measured reflections | k = −9→9 |
| 3330 independent reflections | l = −24→24 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.046 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.134 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0663P)2 + 0.2366P] where P = (Fo2 + 2Fc2)/3 |
| 3330 reflections | (Δ/σ)max = 0.001 |
| 172 parameters | Δρmax = 0.23 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.31913 (10) | 0.27747 (18) | 0.55366 (6) | 0.0536 (3) | |
| O2 | 0.29391 (10) | 0.12247 (17) | 0.30174 (6) | 0.0537 (3) | |
| O3 | 0.87131 (11) | 0.3785 (2) | 0.71774 (6) | 0.0628 (4) | |
| O4 | 0.66774 (11) | 0.4110 (2) | 0.73311 (6) | 0.0627 (3) | |
| N1 | 0.89121 (15) | 0.2321 (3) | 0.55058 (9) | 0.0804 (6) | |
| C1 | 0.51259 (12) | 0.25722 (18) | 0.50966 (7) | 0.0351 (3) | |
| C2 | 0.37586 (13) | 0.2438 (2) | 0.49505 (8) | 0.0377 (3) | |
| C3 | 0.30758 (13) | 0.1990 (2) | 0.42512 (8) | 0.0411 (3) | |
| H3 | 0.2179 | 0.1899 | 0.4163 | 0.049* | |
| C4 | 0.37162 (14) | 0.1674 (2) | 0.36783 (8) | 0.0401 (3) | |
| C5 | 0.50565 (14) | 0.1815 (2) | 0.37981 (8) | 0.0434 (3) | |
| H5 | 0.5489 | 0.1616 | 0.3413 | 0.052* | |
| C6 | 0.57268 (13) | 0.2257 (2) | 0.45012 (8) | 0.0417 (3) | |
| H6 | 0.6623 | 0.2350 | 0.4582 | 0.050* | |
| C7 | 0.18101 (15) | 0.2627 (3) | 0.54175 (10) | 0.0631 (5) | |
| H7A | 0.1535 | 0.2895 | 0.5873 | 0.095* | |
| H7B | 0.1548 | 0.1353 | 0.5254 | 0.095* | |
| H7C | 0.1422 | 0.3535 | 0.5046 | 0.095* | |
| C8 | 0.35279 (19) | 0.0981 (3) | 0.23941 (9) | 0.0636 (5) | |
| H8A | 0.2877 | 0.0668 | 0.1965 | 0.095* | |
| H8B | 0.4151 | −0.0038 | 0.2492 | 0.095* | |
| H8C | 0.3953 | 0.2150 | 0.2305 | 0.095* | |
| C9 | 0.57905 (13) | 0.30444 (19) | 0.58405 (7) | 0.0371 (3) | |
| H9 | 0.5242 | 0.3340 | 0.6160 | 0.045* | |
| C10 | 0.70570 (13) | 0.3137 (2) | 0.61586 (8) | 0.0383 (3) | |
| C11 | 0.74281 (14) | 0.3727 (2) | 0.69485 (8) | 0.0446 (3) | |
| C12 | 0.80883 (14) | 0.2688 (2) | 0.57949 (8) | 0.0497 (4) | |
| C13 | 0.92092 (19) | 0.4426 (4) | 0.79351 (10) | 0.0773 (6) | |
| H13A | 0.8687 | 0.5488 | 0.8047 | 0.093* | |
| H13B | 1.0088 | 0.4886 | 0.7977 | 0.093* | |
| C14 | 0.9198 (2) | 0.2881 (4) | 0.84808 (12) | 0.0922 (8) | |
| H14A | 0.9534 | 0.3361 | 0.8971 | 0.138* | |
| H14B | 0.9725 | 0.1835 | 0.8378 | 0.138* | |
| H14C | 0.8327 | 0.2442 | 0.8449 | 0.138* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0315 (5) | 0.0867 (8) | 0.0439 (6) | −0.0039 (5) | 0.0112 (4) | −0.0102 (5) |
| O2 | 0.0473 (6) | 0.0751 (8) | 0.0352 (5) | −0.0065 (5) | 0.0001 (4) | 0.0012 (5) |
| O3 | 0.0433 (6) | 0.0943 (10) | 0.0461 (6) | −0.0115 (6) | −0.0018 (5) | −0.0126 (6) |
| O4 | 0.0539 (7) | 0.0885 (9) | 0.0455 (6) | 0.0063 (6) | 0.0098 (5) | −0.0101 (6) |
| N1 | 0.0359 (7) | 0.1402 (17) | 0.0662 (10) | −0.0018 (9) | 0.0134 (7) | −0.0185 (10) |
| C1 | 0.0305 (6) | 0.0374 (7) | 0.0368 (7) | −0.0007 (5) | 0.0054 (5) | 0.0033 (5) |
| C2 | 0.0339 (7) | 0.0416 (7) | 0.0385 (7) | 0.0003 (5) | 0.0095 (5) | 0.0027 (6) |
| C3 | 0.0296 (6) | 0.0488 (8) | 0.0432 (7) | −0.0020 (6) | 0.0036 (6) | 0.0044 (6) |
| C4 | 0.0413 (7) | 0.0416 (7) | 0.0348 (7) | −0.0021 (6) | 0.0020 (5) | 0.0049 (6) |
| C5 | 0.0408 (7) | 0.0549 (9) | 0.0361 (7) | 0.0000 (6) | 0.0117 (6) | 0.0035 (6) |
| C6 | 0.0301 (7) | 0.0525 (8) | 0.0425 (7) | −0.0001 (6) | 0.0076 (6) | 0.0035 (6) |
| C7 | 0.0323 (8) | 0.1026 (15) | 0.0569 (10) | −0.0057 (8) | 0.0149 (7) | −0.0097 (10) |
| C8 | 0.0674 (11) | 0.0851 (13) | 0.0361 (8) | −0.0066 (9) | 0.0059 (7) | −0.0056 (8) |
| C9 | 0.0342 (7) | 0.0405 (7) | 0.0371 (7) | 0.0004 (5) | 0.0081 (5) | 0.0013 (6) |
| C10 | 0.0336 (7) | 0.0421 (7) | 0.0387 (7) | −0.0025 (6) | 0.0065 (5) | 0.0004 (6) |
| C11 | 0.0398 (8) | 0.0509 (9) | 0.0412 (8) | −0.0030 (6) | 0.0041 (6) | −0.0011 (6) |
| C12 | 0.0329 (7) | 0.0704 (11) | 0.0432 (8) | −0.0058 (7) | 0.0017 (6) | −0.0036 (7) |
| C13 | 0.0638 (12) | 0.1100 (17) | 0.0506 (10) | −0.0214 (11) | −0.0059 (8) | −0.0201 (11) |
| C14 | 0.0774 (15) | 0.134 (2) | 0.0547 (11) | −0.0001 (14) | −0.0103 (10) | −0.0037 (13) |
Geometric parameters (Å, °)
| O1—C2 | 1.3584 (16) | C6—H6 | 0.9300 |
| O1—C7 | 1.4342 (18) | C7—H7A | 0.9600 |
| O2—C4 | 1.3573 (17) | C7—H7B | 0.9600 |
| O2—C8 | 1.4238 (19) | C7—H7C | 0.9600 |
| O3—C11 | 1.3370 (18) | C8—H8A | 0.9600 |
| O3—C13 | 1.456 (2) | C8—H8B | 0.9600 |
| O4—C11 | 1.1945 (18) | C8—H8C | 0.9600 |
| N1—C12 | 1.139 (2) | C9—C10 | 1.3475 (19) |
| C1—C6 | 1.3925 (19) | C9—H9 | 0.9300 |
| C1—C2 | 1.4172 (18) | C10—C12 | 1.426 (2) |
| C1—C9 | 1.4431 (19) | C10—C11 | 1.485 (2) |
| C2—C3 | 1.376 (2) | C13—C14 | 1.475 (3) |
| C3—C4 | 1.383 (2) | C13—H13A | 0.9700 |
| C3—H3 | 0.9300 | C13—H13B | 0.9700 |
| C4—C5 | 1.391 (2) | C14—H14A | 0.9600 |
| C5—C6 | 1.377 (2) | C14—H14B | 0.9600 |
| C5—H5 | 0.9300 | C14—H14C | 0.9600 |
| C2—O1—C7 | 117.86 (12) | O2—C8—H8B | 109.5 |
| C4—O2—C8 | 117.79 (12) | H8A—C8—H8B | 109.5 |
| C11—O3—C13 | 116.96 (13) | O2—C8—H8C | 109.5 |
| C6—C1—C2 | 116.87 (12) | H8A—C8—H8C | 109.5 |
| C6—C1—C9 | 124.86 (12) | H8B—C8—H8C | 109.5 |
| C2—C1—C9 | 118.26 (12) | C10—C9—C1 | 132.04 (13) |
| O1—C2—C3 | 123.40 (12) | C10—C9—H9 | 114.0 |
| O1—C2—C1 | 115.90 (12) | C1—C9—H9 | 114.0 |
| C3—C2—C1 | 120.70 (12) | C9—C10—C12 | 124.87 (13) |
| C2—C3—C4 | 120.35 (12) | C9—C10—C11 | 118.55 (12) |
| C2—C3—H3 | 119.8 | C12—C10—C11 | 116.57 (12) |
| C4—C3—H3 | 119.8 | O4—C11—O3 | 124.19 (14) |
| O2—C4—C3 | 114.83 (12) | O4—C11—C10 | 124.48 (14) |
| O2—C4—C5 | 124.54 (13) | O3—C11—C10 | 111.33 (12) |
| C3—C4—C5 | 120.63 (13) | N1—C12—C10 | 179.7 (2) |
| C6—C5—C4 | 118.36 (13) | O3—C13—C14 | 112.15 (18) |
| C6—C5—H5 | 120.8 | O3—C13—H13A | 109.2 |
| C4—C5—H5 | 120.8 | C14—C13—H13A | 109.2 |
| C5—C6—C1 | 123.09 (13) | O3—C13—H13B | 109.2 |
| C5—C6—H6 | 118.5 | C14—C13—H13B | 109.2 |
| C1—C6—H6 | 118.5 | H13A—C13—H13B | 107.9 |
| O1—C7—H7A | 109.5 | C13—C14—H14A | 109.5 |
| O1—C7—H7B | 109.5 | C13—C14—H14B | 109.5 |
| H7A—C7—H7B | 109.5 | H14A—C14—H14B | 109.5 |
| O1—C7—H7C | 109.5 | C13—C14—H14C | 109.5 |
| H7A—C7—H7C | 109.5 | H14A—C14—H14C | 109.5 |
| H7B—C7—H7C | 109.5 | H14B—C14—H14C | 109.5 |
| O2—C8—H8A | 109.5 | ||
| C7—O1—C2—C3 | 0.9 (2) | C4—C5—C6—C1 | −0.1 (2) |
| C7—O1—C2—C1 | −179.11 (14) | C2—C1—C6—C5 | −0.6 (2) |
| C6—C1—C2—O1 | −179.18 (12) | C9—C1—C6—C5 | −179.78 (13) |
| C9—C1—C2—O1 | 0.05 (19) | C6—C1—C9—C10 | −6.4 (2) |
| C6—C1—C2—C3 | 0.8 (2) | C2—C1—C9—C10 | 174.47 (15) |
| C9—C1—C2—C3 | −179.93 (13) | C1—C9—C10—C12 | −2.2 (3) |
| O1—C2—C3—C4 | 179.66 (13) | C1—C9—C10—C11 | 178.48 (14) |
| C1—C2—C3—C4 | −0.4 (2) | C13—O3—C11—O4 | −2.3 (3) |
| C8—O2—C4—C3 | 176.73 (14) | C13—O3—C11—C10 | 177.39 (15) |
| C8—O2—C4—C5 | −3.5 (2) | C9—C10—C11—O4 | 0.9 (2) |
| C2—C3—C4—O2 | 179.36 (13) | C12—C10—C11—O4 | −178.46 (16) |
| C2—C3—C4—C5 | −0.4 (2) | C9—C10—C11—O3 | −178.79 (14) |
| O2—C4—C5—C6 | −179.10 (14) | C12—C10—C11—O3 | 1.84 (19) |
| C3—C4—C5—C6 | 0.6 (2) | C11—O3—C13—C14 | 81.1 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5338).
References
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Bodrikov, I. V., Bel’skii, V. K., Krasnov, V. L. & Pigin, O. V. (1992). Zh. Org. Khim. 28, 2228–2238.
- Bruker (2005). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Nesterov, V. N., Kuleshova, L. N. & Antipin, M. Yu. (2001). Kristallografiya, 46, 452–460.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811040013/xu5338sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040013/xu5338Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040013/xu5338Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report