Abstract
The molecule of the title compound, C14H9NO2S, is nearly planar, the maximum atomic deviation being 0.081 (2) Å. An intramolecular O—H⋯N bond generates an S(6) ring motif. In the crystal, inversion-related molecules linked by a pair of weak C—H⋯O hydrogen bonds form a supramolecular dimer. π–π stacking is observed between the thiazole and benzene rings of adjacent molecules, the centroid–centroid distance being 3.7679 (9) Å.
Related literature
For the spectroscopy and preparation of the title compound, see: Hsieh et al. (2008 ▶). For the spectroscopy and applications of benzoxazole and benzothiazole derivatives, see: Chen & Pang (2009 ▶, 2010 ▶); Hrobáriková et al. (2010 ▶); Kim et al. (2010a
▶,b
▶); Lijima et al. (2010 ▶); Lim et al. (2011 ▶); López-Ruiz et al. (2011 ▶); Tanaka et al. (2001 ▶). For related structures, see: Tong (2005 ▶); Hahn et al. (1998 ▶). For graph-set theory, see: Bernstein et al. (1995 ▶).
Experimental
Crystal data
C14H9NO2S
M r = 255.28
Monoclinic,

a = 8.2645 (3) Å
b = 5.6449 (2) Å
c = 23.8341 (9) Å
β = 98.147 (2)°
V = 1100.69 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.29 mm−1
T = 150 K
0.38 × 0.14 × 0.04 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2001 ▶) T min = 0.882, T max = 0.992
8427 measured reflections
1943 independent reflections
1333 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.029
wR(F 2) = 0.058
S = 0.90
1943 reflections
168 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.22 e Å−3
Δρmin = −0.27 e Å−3
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811040712/xu5345sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040712/xu5345Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040712/xu5345Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯N1 | 0.89 (2) | 1.81 (2) | 2.6228 (18) | 150 (2) |
| C5—H5⋯O2i | 0.93 | 2.61 | 3.293 (2) | 130 |
Symmetry code: (i)  .
.
Acknowledgments
This work was supported by the National Science Council and Feng Chia University in Taiwan.
supplementary crystallographic information
Comment
The excited-state intramolecular proton transfer (ESIPT) reaction of 2-(2-hydroxyphenyl)benzoxazole and 2-(2-hydroxyphenyl)benzothiazole derivatives has been investigated for past years (Hsieh et al., 2008; Kim et al., 2010a,b; Lijima et al., 2010; López-Ruiz et al., 2011), which incorporates transfer of a hydroxy proton to the imine nitrogen through a intramolecular six-membered-ring hydrogen-bonding system (Chen et al., 2009, 2010). The unusual photophysical property of the resulting proton-transfer tautomer has found many important applications (Hrobáriková et al., 2010; Lim et al., 2011; Tanaka et al., 2001).
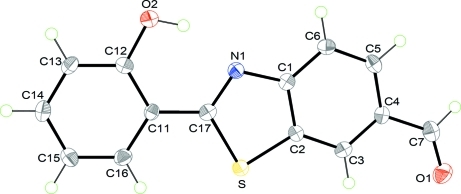
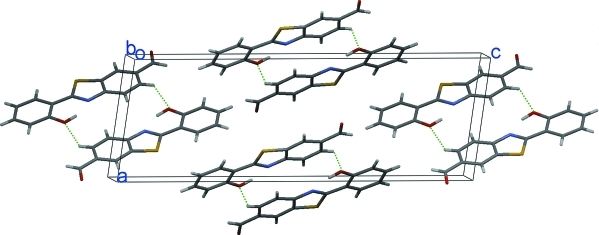
The molecular structure of the title compound (HBT) is shown in Figure 1. The molecule is nearly planar, which is consistent with previous studies (Tong, 2005; Hahn et al., 1998). HBT possesses an intramolecular O—H···N hydrogen bond (Table 1), which generates an S(6) ring motif (Bernstein et al., 1995). In the crystal (Figure 2), inversion-related molecules are linked by a pair of weak C—H···O hydrogen bonds, forming a cyclic dimers with R22(18) graph-set motif. π-π stacing is observed between thiazole and C1i-benzene rings of adjacent molecules [symmetry code: (i): 2-x,2-y,1-z], the centroid-to-centroid distance being 3.7679 (9) Å.
Experimental
The title compound was synthesized according to the literature (Hsieh et al., 2008). Yellow needle-shaped crystals suitable for the crystallographic studies reported here were isolated over a period of five weeks by slow evaporation from the chloroform solution.
Refinement
H atoms bonded to O and C atoms were located in a difference electron density map. The hydroxy H atom and the Csp3 H atoms were freely refined, and the Csp2 H atoms repositioned geometrically and refined using a riding model, [C—H = 0.93 Å and Uiso(H) = 1.2Ueq(C)].
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids.
Fig. 2.
A section of the crystal packing of the title compound, viewed down the b axis. Green dashed lines denote the intermolecular C—H···O hydrogen bonds.
Crystal data
| C14H9NO2S | F(000) = 528 |
| Mr = 255.28 | Dx = 1.541 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -p 2yn | Cell parameters from 2297 reflections |
| a = 8.2645 (3) Å | θ = 2.5–25.7° |
| b = 5.6449 (2) Å | µ = 0.29 mm−1 |
| c = 23.8341 (9) Å | T = 150 K |
| β = 98.147 (2)° | Plate, yellow |
| V = 1100.69 (7) Å3 | 0.38 × 0.14 × 0.04 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 1943 independent reflections |
| Radiation source: fine-focus sealed tube | 1333 reflections with I > 2σ(I) |
| graphite | Rint = 0.042 |
| φ and ω scans | θmax = 25.0°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Bruker, 2001) | h = −9→9 |
| Tmin = 0.882, Tmax = 0.992 | k = −6→3 |
| 8427 measured reflections | l = −28→28 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.029 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.058 | w = 1/[σ2(Fo2) + (0.0251P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.90 | (Δ/σ)max < 0.001 |
| 1943 reflections | Δρmax = 0.22 e Å−3 |
| 168 parameters | Δρmin = −0.27 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0014 (2) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S | 0.74344 (6) | 0.79801 (8) | 0.392532 (18) | 0.02213 (16) | |
| O1 | 0.51794 (15) | 0.6205 (2) | 0.59718 (5) | 0.0317 (4) | |
| O2 | 1.05490 (15) | 1.4470 (2) | 0.35839 (5) | 0.0272 (3) | |
| N1 | 0.88683 (16) | 1.1973 (2) | 0.42282 (6) | 0.0183 (3) | |
| C1 | 0.81872 (19) | 1.1165 (3) | 0.46928 (7) | 0.0167 (4) | |
| C2 | 0.7362 (2) | 0.8986 (3) | 0.46067 (7) | 0.0169 (4) | |
| C3 | 0.66326 (19) | 0.7940 (3) | 0.50315 (7) | 0.0189 (4) | |
| H3 | 0.6076 | 0.6510 | 0.4970 | 0.023* | |
| C4 | 0.67509 (19) | 0.9071 (3) | 0.55528 (7) | 0.0183 (4) | |
| C5 | 0.7578 (2) | 1.1247 (3) | 0.56406 (7) | 0.0211 (4) | |
| H5 | 0.7644 | 1.1987 | 0.5992 | 0.025* | |
| C6 | 0.82896 (19) | 1.2299 (3) | 0.52162 (7) | 0.0204 (4) | |
| H6 | 0.8830 | 1.3741 | 0.5277 | 0.024* | |
| C7 | 0.5997 (2) | 0.7988 (3) | 0.60128 (7) | 0.0252 (4) | |
| H7 | 0.6162 | 0.8736 | 0.6364 | 0.030* | |
| C11 | 0.91907 (19) | 1.0821 (3) | 0.32631 (7) | 0.0168 (4) | |
| C12 | 1.0157 (2) | 1.2802 (3) | 0.31764 (7) | 0.0190 (4) | |
| C13 | 1.07588 (19) | 1.3083 (3) | 0.26659 (7) | 0.0219 (4) | |
| H13 | 1.1400 | 1.4394 | 0.2611 | 0.026* | |
| C14 | 1.04146 (19) | 1.1439 (3) | 0.22413 (7) | 0.0229 (5) | |
| H14 | 1.0826 | 1.1644 | 0.1901 | 0.027* | |
| C15 | 0.9459 (2) | 0.9477 (3) | 0.23149 (7) | 0.0239 (5) | |
| H15 | 0.9225 | 0.8371 | 0.2026 | 0.029* | |
| C16 | 0.8862 (2) | 0.9183 (3) | 0.28209 (7) | 0.0222 (4) | |
| H16 | 0.8223 | 0.7863 | 0.2870 | 0.027* | |
| C17 | 0.85778 (19) | 1.0478 (3) | 0.38021 (7) | 0.0180 (4) | |
| H2 | 1.005 (2) | 1.407 (4) | 0.3877 (7) | 0.071 (8)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S | 0.0278 (3) | 0.0195 (3) | 0.0199 (3) | −0.0044 (2) | 0.0062 (2) | −0.0013 (2) |
| O1 | 0.0349 (8) | 0.0318 (8) | 0.0295 (8) | −0.0074 (7) | 0.0085 (7) | 0.0053 (6) |
| O2 | 0.0346 (8) | 0.0245 (8) | 0.0233 (8) | −0.0092 (6) | 0.0070 (7) | −0.0026 (6) |
| N1 | 0.0198 (9) | 0.0164 (8) | 0.0186 (8) | 0.0005 (7) | 0.0025 (7) | 0.0009 (7) |
| C1 | 0.0154 (10) | 0.0160 (10) | 0.0181 (10) | 0.0040 (8) | 0.0003 (8) | 0.0037 (8) |
| C2 | 0.0172 (10) | 0.0169 (10) | 0.0165 (10) | 0.0029 (8) | 0.0016 (8) | 0.0006 (8) |
| C3 | 0.0162 (10) | 0.0168 (10) | 0.0233 (11) | 0.0011 (8) | 0.0016 (8) | 0.0021 (9) |
| C4 | 0.0170 (10) | 0.0194 (10) | 0.0187 (10) | 0.0043 (8) | 0.0034 (8) | 0.0037 (8) |
| C5 | 0.0226 (11) | 0.0225 (11) | 0.0179 (11) | 0.0051 (9) | 0.0019 (9) | −0.0020 (8) |
| C6 | 0.0209 (11) | 0.0178 (10) | 0.0220 (11) | −0.0004 (8) | 0.0011 (8) | −0.0001 (8) |
| C7 | 0.0241 (12) | 0.0299 (11) | 0.0215 (11) | 0.0069 (10) | 0.0030 (9) | 0.0008 (9) |
| C11 | 0.0162 (10) | 0.0173 (10) | 0.0171 (10) | 0.0008 (8) | 0.0028 (8) | 0.0023 (8) |
| C12 | 0.0183 (10) | 0.0184 (10) | 0.0194 (10) | 0.0019 (9) | −0.0003 (8) | −0.0006 (9) |
| C13 | 0.0207 (11) | 0.0220 (10) | 0.0233 (11) | −0.0037 (8) | 0.0048 (9) | 0.0048 (9) |
| C14 | 0.0202 (11) | 0.0292 (12) | 0.0201 (11) | 0.0032 (9) | 0.0060 (9) | 0.0051 (9) |
| C15 | 0.0279 (12) | 0.0240 (11) | 0.0198 (11) | 0.0016 (9) | 0.0040 (9) | −0.0030 (8) |
| C16 | 0.0238 (11) | 0.0184 (10) | 0.0244 (11) | −0.0023 (9) | 0.0038 (9) | 0.0011 (9) |
| C17 | 0.0146 (10) | 0.0163 (10) | 0.0228 (11) | 0.0030 (8) | 0.0016 (8) | 0.0022 (8) |
Geometric parameters (Å, °)
| S—C2 | 1.7296 (16) | C5—H5 | 0.9300 |
| S—C17 | 1.7454 (17) | C6—H6 | 0.9300 |
| O1—C7 | 1.2087 (19) | C7—H7 | 0.9300 |
| O2—C12 | 1.3588 (19) | C11—C16 | 1.400 (2) |
| O2—H2 | 0.890 (14) | C11—C12 | 1.406 (2) |
| N1—C17 | 1.3160 (18) | C11—C17 | 1.459 (2) |
| N1—C1 | 1.3882 (19) | C12—C13 | 1.387 (2) |
| C1—C6 | 1.394 (2) | C13—C14 | 1.373 (2) |
| C1—C2 | 1.407 (2) | C13—H13 | 0.9300 |
| C2—C3 | 1.382 (2) | C14—C15 | 1.386 (2) |
| C3—C4 | 1.388 (2) | C14—H14 | 0.9300 |
| C3—H3 | 0.9300 | C15—C16 | 1.376 (2) |
| C4—C5 | 1.407 (2) | C15—H15 | 0.9300 |
| C4—C7 | 1.469 (2) | C16—H16 | 0.9300 |
| C5—C6 | 1.375 (2) | ||
| C2—S—C17 | 89.10 (8) | C4—C7—H7 | 117.4 |
| C12—O2—H2 | 107.2 (14) | C16—C11—C12 | 117.93 (15) |
| C17—N1—C1 | 110.79 (14) | C16—C11—C17 | 121.39 (15) |
| N1—C1—C6 | 125.67 (16) | C12—C11—C17 | 120.67 (15) |
| N1—C1—C2 | 114.43 (15) | O2—C12—C13 | 117.94 (15) |
| C6—C1—C2 | 119.88 (16) | O2—C12—C11 | 121.94 (15) |
| C3—C2—C1 | 121.30 (16) | C13—C12—C11 | 120.11 (16) |
| C3—C2—S | 128.62 (14) | C14—C13—C12 | 120.42 (16) |
| C1—C2—S | 110.07 (12) | C14—C13—H13 | 119.8 |
| C2—C3—C4 | 118.44 (16) | C12—C13—H13 | 119.8 |
| C2—C3—H3 | 120.8 | C13—C14—C15 | 120.61 (16) |
| C4—C3—H3 | 120.8 | C13—C14—H14 | 119.7 |
| C3—C4—C5 | 120.40 (16) | C15—C14—H14 | 119.7 |
| C3—C4—C7 | 119.55 (16) | C16—C15—C14 | 119.28 (16) |
| C5—C4—C7 | 120.05 (16) | C16—C15—H15 | 120.4 |
| C6—C5—C4 | 121.13 (16) | C14—C15—H15 | 120.4 |
| C6—C5—H5 | 119.4 | C15—C16—C11 | 121.64 (16) |
| C4—C5—H5 | 119.4 | C15—C16—H16 | 119.2 |
| C5—C6—C1 | 118.84 (16) | C11—C16—H16 | 119.2 |
| C5—C6—H6 | 120.6 | N1—C17—C11 | 123.12 (15) |
| C1—C6—H6 | 120.6 | N1—C17—S | 115.59 (12) |
| O1—C7—C4 | 125.29 (17) | C11—C17—S | 121.27 (12) |
| O1—C7—H7 | 117.4 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···N1 | 0.89 (2) | 1.81 (2) | 2.6228 (18) | 150 (2) |
| C5—H5···O2i | 0.93 | 2.61 | 3.293 (2) | 130 |
Symmetry codes: (i) −x+2, −y+3, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: XU5345).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2001). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, W.-H. & Pang, Y. (2009). Tetrahedron Lett. 50, 6680–6683.
- Chen, W.-H. & Pang, Y. (2010). Tetrahedron Lett. 51, 1914–1918.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Hahn, F. E., Imhof, L. & Lügger, T. (1998). Acta Cryst. C54, 668–669.
- Hrobáriková, V., Hrobárik, P., Gajdos, P., Fitilis, I., Fakis, M., Persephonis, P. & Zahradnik, P. (2010). J. Org. Chem. 75, 3053–3068. [DOI] [PubMed]
- Hsieh, C.-C., Cheng, Y.-M., Hsu, C.-J., Chen, K.-Y. & Chou, P.-T. (2008). J. Phys. Chem. A, 112, 8323–8332. [DOI] [PubMed]
- Kim, T. H., Kwon, N. Y. & Lee, T. S. (2010a). Tetrahedron Lett. 51, 5596–5600.
- Kim, C. H., Park, J., Seo, J., Park, S. Y. & Joo, T. (2010b). J. Phys. Chem. A, 114, 5618–5629. [DOI] [PubMed]
- Lijima, T., Momotake, A., Shinohara, Y., Sato, T., Nishimura, Y. & Arai, T. (2010). J. Phys. Chem. A, 114, 1603–1609. [DOI] [PubMed]
- Lim, C. K., Seo, J., Kim, S., Kwon, I. C., Ahn, C. H. & Park, S. Y. (2011). Dyes Pigments, 90, 284–289.
- López-Ruiz, H., Briseno-Ortega, H., Rojas-Lima, S., Santillan, R. & Farfán, N. (2011). Tetrahedron Lett. 52, 4308–4312.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tanaka, K., Kumagai, T., Aoki, H., Deguchi, M. & Iwata, S. (2001). J. Org. Chem. 66, 7328–7333. [DOI] [PubMed]
- Tong, Y.-P. (2005). Acta Cryst. E61, o3076–o3078.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811040712/xu5345sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040712/xu5345Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040712/xu5345Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report