Abstract
The title compound, C11H9NO5, was prepared by the reaction of 2-furanacrylic acid and N-hydroxysuccinimide. The molecule consists of two approximately planar moieties, viz. a succinimide group and the rest of the molecule [the largest deviations from the least-squares planes are 0.120 (1) and 0.210 (1) Å, respectively]. The dihedral angle between these fragments is 63.70 (5)°. In the crystal, molecules are linked by C—H⋯O hydrogen bonds into two-dimensional nets.
Related literature
For derivatives of N-hydroxysuccinimide, see: Anderson et al. (1964 ▶); Blumberg & Vallee (1975 ▶); Brown et al. (2005 ▶); Cheng et al. (2007 ▶); Jones (2003 ▶).
Experimental
Crystal data
C11H9NO5
M r = 235.19
Orthorhombic,

a = 10.3054 (13) Å
b = 9.2376 (12) Å
c = 21.892 (3) Å
V = 2084.0 (5) Å3
Z = 8
Mo Kα radiation
μ = 0.12 mm−1
T = 296 K
0.30 × 0.30 × 0.10 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.977, T max = 0.977
16939 measured reflections
2399 independent reflections
1900 reflections with I > 2σ(I)
R int = 0.041
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.082
S = 1.02
2399 reflections
154 parameters
H-atom parameters constrained
Δρmax = 0.20 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811040566/yk2020sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040566/yk2020Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040566/yk2020Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C12—H12A⋯O1i | 0.93 | 2.48 | 3.3533 (17) | 156 |
| C14—H14A⋯O5ii | 0.93 | 2.45 | 3.3672 (18) | 169 |
Symmetry codes: (i) 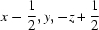 ; (ii)
; (ii)  .
.
Acknowledgments
This project was supported by Zhejiang Provincial Natural Science Foundation of China (grant No. Y4100113), the National Natural Science Foundation of China (grant No. 20903058), the Natural Science Foundation of Ningbo City (grant Nos. 2009 A610047, 2010 A610025, 2010 A610027) and the K. C. Wong Magna Fund of Ningbo University.
supplementary crystallographic information
Comment
N-Hydroxysuccinimide is frequently used in organic chemistry as an activating reagent, which readily form crystalline adducts with amines or acids (Jones, 2003). N-Hydroxysuccinimide esters are widely used as leaving groups to activate carboxylic acids (Cheng et al., 2007). The title compound N-(2-furanacryloyl)-succinimide ester is an intermediate of FAPGG which is the substrate of diagnostic reagent. We have used a simple procedure to synthesize the title compound in reasonable yields (Blumberg & Vallee, 1975; Brown et al., 2005).
The molecular structure of the title compound (I) is shown in Fig.1. In the molecule, the dihedral angle between the furan and succinimide rings is 56.26 (43)°. In the crystal structure, molecules are linked by the C12—H12···O1i hydrogen bonds to form chains along a and C14—H14···O5ii hydrogen bonds to form chains along b directions.
Experimental
2-Furanacrylic acid (13.81 g, 0.10 mol), N-hydroxysuccinimide (11.51 g, 0.10 mol) and dicyclohexylcarbodiimide (20.63 g, 0.10 mol) were added to 200 ml dioxane in a round flask. This mixture was stirred at 4°C for 14 h before the dicyclohexylurea was removed by filtration. Then the resulting dark brown filtrate was evaporated in vacuum to give the dark brown residue. Slight brown crystals were obtained by recrystallization from 2-propanol (15.29 g, 65%). 1H NMR(400 MHz, CDCl3): \d 7.62 (d, J=16 Hz, 1H), 7.56 (d, J=1.6 Hz, 1H), 6.77 (d, J=3.6 Hz, 1H), 6.53–6.52 (dd, J=3.6 Hz, J=1.6 Hz, 1H), 6.47 (d, J=16 Hz, 1H), 2.87 (s, 4H).
Figures
Fig. 1.
The molecular structure of (I) showing displacement ellipsoids at the 30% probability level.
Crystal data
| C11H9NO5 | F(000) = 976 |
| Mr = 235.19 | Dx = 1.499 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -p 2ac 2ab | Cell parameters from 3779 reflections |
| a = 10.3054 (13) Å | θ = 2.7–27.4° |
| b = 9.2376 (12) Å | µ = 0.12 mm−1 |
| c = 21.892 (3) Å | T = 296 K |
| V = 2084.0 (5) Å3 | Needle, brown |
| Z = 8 | 0.30 × 0.30 × 0.10 mm |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 2399 independent reflections |
| Radiation source: fine-focus sealed tube | 1900 reflections with I > 2σ(I) |
| graphite | Rint = 0.041 |
| Detector resolution: 0 pixels mm-1 | θmax = 27.6°, θmin = 1.9° |
| φ and ω scans | h = −13→13 |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | k = −12→12 |
| Tmin = 0.977, Tmax = 0.977 | l = −28→28 |
| 16939 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.082 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0285P)2 + 0.9232P] where P = (Fo2 + 2Fc2)/3 |
| 2399 reflections | (Δ/σ)max < 0.001 |
| 154 parameters | Δρmax = 0.20 e Å−3 |
| 0 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.80658 (9) | 0.22692 (10) | 0.28201 (4) | 0.0261 (2) | |
| O2 | 0.89760 (9) | 0.55049 (10) | 0.10826 (4) | 0.0273 (2) | |
| O3 | 0.67881 (9) | 0.58836 (11) | 0.11454 (5) | 0.0314 (2) | |
| O4 | 0.81461 (10) | 0.44760 (11) | −0.00431 (5) | 0.0356 (3) | |
| O5 | 1.01727 (10) | 0.81990 (11) | 0.09170 (5) | 0.0349 (3) | |
| C6 | 0.68207 (13) | 0.36659 (14) | 0.21024 (6) | 0.0237 (3) | |
| H6A | 0.6008 | 0.3944 | 0.1960 | 0.028* | |
| C7 | 0.78634 (13) | 0.42777 (14) | 0.18443 (6) | 0.0244 (3) | |
| H7A | 0.8686 | 0.4048 | 0.1989 | 0.029* | |
| N8 | 0.89355 (11) | 0.63213 (12) | 0.05495 (5) | 0.0246 (3) | |
| C9 | 0.68679 (12) | 0.26114 (14) | 0.25831 (6) | 0.0220 (3) | |
| C10 | 0.77299 (13) | 0.52965 (14) | 0.13407 (6) | 0.0233 (3) | |
| C11 | 0.96337 (13) | 0.76125 (14) | 0.04991 (6) | 0.0243 (3) | |
| C12 | 0.59420 (14) | 0.18035 (14) | 0.28687 (6) | 0.0255 (3) | |
| H12A | 0.5054 | 0.1829 | 0.2793 | 0.031* | |
| C13 | 0.85503 (13) | 0.56972 (15) | 0.00020 (6) | 0.0250 (3) | |
| C14 | 0.65847 (14) | 0.09163 (15) | 0.33025 (6) | 0.0270 (3) | |
| H14A | 0.6205 | 0.0249 | 0.3566 | 0.032* | |
| C15 | 0.78534 (14) | 0.12362 (15) | 0.32556 (6) | 0.0279 (3) | |
| H15A | 0.8503 | 0.0810 | 0.3489 | 0.034* | |
| C16 | 0.87575 (15) | 0.68378 (15) | −0.04765 (6) | 0.0289 (3) | |
| H16A | 0.7934 | 0.7229 | −0.0614 | 0.035* | |
| H16B | 0.9214 | 0.6439 | −0.0826 | 0.035* | |
| C17 | 0.95729 (14) | 0.80146 (15) | −0.01663 (6) | 0.0286 (3) | |
| H17A | 1.0438 | 0.8046 | −0.0341 | 0.034* | |
| H17B | 0.9170 | 0.8956 | −0.0217 | 0.034* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0231 (5) | 0.0287 (5) | 0.0264 (5) | −0.0003 (4) | −0.0014 (4) | 0.0072 (4) |
| O2 | 0.0248 (5) | 0.0329 (5) | 0.0242 (5) | −0.0006 (4) | −0.0008 (4) | 0.0097 (4) |
| O3 | 0.0272 (5) | 0.0337 (5) | 0.0334 (5) | 0.0032 (4) | −0.0018 (4) | 0.0105 (4) |
| O4 | 0.0398 (6) | 0.0254 (5) | 0.0416 (6) | −0.0037 (5) | −0.0043 (5) | −0.0042 (5) |
| O5 | 0.0351 (6) | 0.0322 (5) | 0.0376 (6) | −0.0055 (5) | −0.0022 (5) | −0.0085 (5) |
| C6 | 0.0264 (7) | 0.0222 (6) | 0.0226 (6) | 0.0026 (5) | −0.0019 (5) | −0.0008 (5) |
| C7 | 0.0253 (7) | 0.0243 (7) | 0.0235 (7) | 0.0016 (6) | −0.0028 (5) | 0.0022 (5) |
| N8 | 0.0284 (6) | 0.0253 (6) | 0.0199 (5) | −0.0037 (5) | −0.0009 (5) | 0.0052 (5) |
| C9 | 0.0222 (6) | 0.0232 (6) | 0.0208 (6) | 0.0025 (5) | −0.0012 (5) | −0.0009 (5) |
| C10 | 0.0245 (7) | 0.0215 (6) | 0.0238 (6) | −0.0014 (6) | −0.0009 (5) | 0.0000 (5) |
| C11 | 0.0211 (6) | 0.0211 (6) | 0.0309 (7) | 0.0008 (5) | 0.0032 (6) | −0.0016 (6) |
| C12 | 0.0245 (7) | 0.0251 (7) | 0.0268 (7) | −0.0017 (6) | 0.0018 (5) | −0.0016 (5) |
| C13 | 0.0239 (7) | 0.0243 (7) | 0.0269 (7) | 0.0037 (6) | −0.0016 (6) | −0.0025 (5) |
| C14 | 0.0363 (8) | 0.0227 (6) | 0.0220 (6) | −0.0047 (6) | 0.0031 (6) | 0.0010 (5) |
| C15 | 0.0359 (8) | 0.0253 (7) | 0.0226 (6) | 0.0014 (6) | −0.0030 (6) | 0.0065 (6) |
| C16 | 0.0355 (8) | 0.0283 (7) | 0.0229 (7) | 0.0055 (6) | 0.0013 (6) | 0.0007 (6) |
| C17 | 0.0280 (7) | 0.0246 (7) | 0.0333 (8) | 0.0012 (6) | 0.0048 (6) | 0.0068 (6) |
Geometric parameters (Å, °)
| O1—C15 | 1.3664 (16) | C9—C12 | 1.3632 (18) |
| O1—C9 | 1.3759 (15) | C11—C17 | 1.5044 (19) |
| O2—N8 | 1.3900 (13) | C12—C14 | 1.4185 (19) |
| O2—C10 | 1.4162 (16) | C12—H12A | 0.9300 |
| O3—C10 | 1.1912 (16) | C13—C16 | 1.5011 (19) |
| O4—C13 | 1.2066 (16) | C14—C15 | 1.344 (2) |
| O5—C11 | 1.1996 (16) | C14—H14A | 0.9300 |
| C6—C7 | 1.3391 (18) | C15—H15A | 0.9300 |
| C6—C9 | 1.4348 (18) | C16—C17 | 1.533 (2) |
| C6—H6A | 0.9300 | C16—H16A | 0.9700 |
| C7—C10 | 1.4561 (18) | C16—H16B | 0.9700 |
| C7—H7A | 0.9300 | C17—H17A | 0.9700 |
| N8—C13 | 1.3880 (17) | C17—H17B | 0.9700 |
| N8—C11 | 1.3974 (17) | ||
| C15—O1—C9 | 106.25 (10) | C14—C12—H12A | 126.4 |
| N8—O2—C10 | 112.43 (9) | O4—C13—N8 | 123.89 (13) |
| C7—C6—C9 | 124.66 (12) | O4—C13—C16 | 130.41 (12) |
| C7—C6—H6A | 117.7 | N8—C13—C16 | 105.69 (11) |
| C9—C6—H6A | 117.7 | C15—C14—C12 | 106.01 (12) |
| C6—C7—C10 | 121.09 (12) | C15—C14—H14A | 127.0 |
| C6—C7—H7A | 119.5 | C12—C14—H14A | 127.0 |
| C10—C7—H7A | 119.5 | C14—C15—O1 | 111.26 (12) |
| C13—N8—O2 | 120.55 (11) | C14—C15—H15A | 124.4 |
| C13—N8—C11 | 115.69 (11) | O1—C15—H15A | 124.4 |
| O2—N8—C11 | 120.93 (11) | C13—C16—C17 | 105.46 (11) |
| C12—C9—O1 | 109.22 (11) | C13—C16—H16A | 110.6 |
| C12—C9—C6 | 133.19 (13) | C17—C16—H16A | 110.6 |
| O1—C9—C6 | 117.58 (11) | C13—C16—H16B | 110.6 |
| O3—C10—O2 | 122.25 (12) | C17—C16—H16B | 110.6 |
| O3—C10—C7 | 130.02 (13) | H16A—C16—H16B | 108.8 |
| O2—C10—C7 | 107.72 (11) | C11—C17—C16 | 106.07 (11) |
| O5—C11—N8 | 124.31 (13) | C11—C17—H17A | 110.5 |
| O5—C11—C17 | 130.25 (13) | C16—C17—H17A | 110.5 |
| N8—C11—C17 | 105.42 (11) | C11—C17—H17B | 110.5 |
| C9—C12—C14 | 107.25 (12) | C16—C17—H17B | 110.5 |
| C9—C12—H12A | 126.4 | H17A—C17—H17B | 108.7 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C12—H12A···O1i | 0.93 | 2.48 | 3.3533 (17) | 156. |
| C14—H14A···O5ii | 0.93 | 2.45 | 3.3672 (18) | 169. |
Symmetry codes: (i) x−1/2, y, −z+1/2; (ii) x−1/2, y−1, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: YK2020).
References
- Anderson, G. W., Callahan, F. M. & Zimmerman, J. E. (1964). J. Am. Chem. Soc. 86, 1839–1842.
- Blumberg, S. & Vallee, B. L. (1975). Biochemistry, 14, 2410–2419. [DOI] [PubMed]
- Brown, C. L., Atkinson, S. J. & Healy, P. C. (2005). Acta Cryst. E61, o1203–o1204.
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cheng, F., Gamble, L. J. & Grainger, D. W. (2007). Anal. Chem. 79, 8781–8788. [DOI] [PMC free article] [PubMed]
- Jones, P. G. (2003). Acta Cryst. E59, o1951–o1952.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811040566/yk2020sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811040566/yk2020Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811040566/yk2020Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



